Abstract
White matter disease (WMD) of the brain is associated with incident stroke. Similarly subclinical calcified coronary artery plaque has been associated with incident coronary artery disease (CAD) events. Although atherogenesis in both vascular beds may share some common mechanisms, the extent to which subclinical CAD is associated with WMD across age ranges in individuals with a family history of early onset CAD remains unknown. We screened 405 apparently healthy participants in the Genetic Study of Atherosclerotic Risk (GeneSTAR) for CAD risk factors, and for the presence of noncalcified and calcified coronary plaque using dual-source multi-detector cardiac CT angiography. The presence and volumes of WMD were assessed by 3 Tesla brain MRI. Participants were 60% female, 36% African American; mean age 51.6 ± 10.6 years. The prevalence of coronary plaque overall was 43.0%. Individuals with coronary plaque had significantly higher WMD volumes (median 1222 mm3, IQR [448 to 3871]) compared to those without coronary plaque (median 551 mm3, IQR [105 to 1523], p<0.001). In multivariable regression analysis, adjusting for age, sex, race, traditional risk factors, total brain volume, and intrafamilial correlations, the presence of coronary plaque was independently associated with WMD volume (p=0.05). This study shows a significant association between WMD and noncalcified and calcified coronary plaque in healthy individuals, independent of age and risk factors. In conclusion, these findings support the premise of possible shared causal pathways in two vascular beds in families at increased risk for early-onset vascular disease.
Keywords: coronary artery disease, brain white matter disease, subclinical
INTRODUCTION
Apparently healthy persons with a family history of early-onset coronary artery disease (CAD) are at marked increased risk of developing clinically manifest CAD, independent of traditional risk factors.1 We recently demonstrated a high prevalence of early silent CAD in younger age apparently healthy siblings of persons with early-onset CAD as well as a high 10-year incidence of clinically manifest CAD events.2 We have also reported a high prevalence of cerebral white matter disease (WMD) on magnetic resonance imaging (MRI) in young healthy siblings of early-onset CAD probands that was comparable to that of older individuals participating in the Atherosclerosis Risk in Communities (ARIC) study,3 suggesting an early subclinical atherosclerotic disease of the brains in people with a strong family history of CAD. Thus, this study was designed to determine the association between coronary plaque on computed tomographic angiography (CTA) and WMD in young apparently healthy asymptomatic persons with a strong family history of early-onset CAD.
METHODS
Participants (n=405) were recruited from the ongoing Genetic Study of Atherosclerosis Risk (GeneSTAR), a prospective study begun in 1982 to characterize genetic and biological factors associated with incident cardiovascular and cerebrovascular disease in families with early-onset CAD.2 Briefly, hospitalized probands with acute myocardial infarction, unstable angina with coronary revascularization, or acute angina with flow-limiting stenosis of >50% diameter in at least one coronary artery at age <60 years were identified and their healthy siblings < 60 years of age were recruited and screened for risk factors and occult CAD using nuclear perfusion imaging.2 Commencing in 2002, adult offspring of both the probands and siblings were also enrolled and underwent CTA at the same time as the original siblings. For this study, both initially healthy siblings and offspring were included if they were 30 to 75 years of age and had no known history of CAD, stroke, or documented transient ischemic attacks. At the time of return for the CTA measurements, siblings and offspring were excluded if they had any serious chronic illness, such as systemic autoimmune disease, chronic kidney disease, neurologic diseases (dementia, Parkinson’s disease or multiple sclerosis or life-threatening co-morbidity (AIDS, cancer). Subjects were excluded if they reported a history of allergy to iodinated contrast material or implanted metal precluding MRI testing, The study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants gave informed consent.
Subjects underwent a comprehensive screening with all testing performed on the same day. Medical history and current medication use were assessed and a physical examination was performed by a study physician. Anthropometric measures included height in inches and weight in kilograms; body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Current cigarette smoking was assessed using a standardized questionnaire and/or by expired carbon monoxide (CO) levels of ≥8 ppm on two measurements. Blood pressure was measured according to the American Heart Association guidelines three times over an 8 hour screening visit. Hypertension was defined as an average blood pressure ≥140 mmHg systolic, or ≥90 mmHg diastolic, and/or use of an antihypertensive drug. Blood was taken for measurement of lipid and glucose levels after subjects had fasted overnight for 8–12 hours. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were measured using the United States Centers for Disease Control standardized methods. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald formula4 for persons with triglyceride levels <400 mg/dl. Direct measurement of LDL cholesterol using ultracentrifugation was used for persons with triglyceride levels ≥400 mg/dL (n=5). Glucose concentration was measured using the glucose oxidase method;5 type 2 diabetes was defined as a physician diagnosed history, a fasting glucose level ≥126 mg/dl, and/or use of hypoglycemic medications.
All participants underwent intracranial magnetic resonance imaging (MRI) and coronary CTA imaging. MRI was performed using a Philips 3.0 Tesla scanner. The series included the following imaging sequences. 1) Axial T1-weighted MPRAGE (magnetization prepared rapid gradient echo): TR (repetition time) 10 ms, TE (time to echo) −6ms, TI (inversion time) voxel size 0.75 × 0.75 × 1.0 mm3, contiguous slices, with field of view imaging (FOV) 240 mm, matrix 256×256×160 mm. 2) Axial turbo spin echo FLAIR (fluid attenuation inversion recovery): TR 11000ms, TI 2800 ms, TE 68 ms, voxel size 0.47 × 0.47 × 3.0 mm3, contiguous slices, FOV 240mm, matrix 256 × 256mm. An expert neuroradiologist evaluated all images for clinical pathology (DY). Volumetric analysis of white matter hyperintensities was performed using Medical Image Processing, Analysis, and Visualization (MIPAV) software as previously described.6 We implemented the topology-preserving anatomical segmentation algorithm (Lesion- TOADS software) as a module in the Medic Automated Pipeline Scheduler (MAPS) in order to simultaneously segment major brain structures and delineate white matter lesions.7 Segmented brain volumes and the WMD volume were quantified automatically using a multichannel classifier based on a support vector machine approach. The total volume of WMD was the primary dependent variable.
All participants underwent coronary CTA using a dual-source multi-detector scanner (Definition Flash, Siemens Medical Solutions, Forchheim, Germany) to detect coronary artery plaque. A noncontrast scan was first performed to determine the coronary artery calcium score. Coronary CTA was then performed with prospective ECG-gating, 128 × 0.6-mm detector collimation, 280 ms gantry rotation, 850 mAs and 120 kV. Subsequently, 0.75-mm-thick axial slices were reconstructed at 0.5-mm intervals with B26 kernel using a half-scan reconstruction algorithm with resulting temporal resolution of 75 ms. All CTA scans were evaluated with the reader blinded to the participants’ risk factor, clinical, and MRI profiles. Using the noncontrast enhanced images, the extent of coronary artery calcium (CAC), defined as pixels of >130 HU, using the Agatston method8 was measured on a workstation (Leonardo Multimodality Workstation, Syngo, Siemens Medical Solutions, Malvern, PA). The contrast-enhanced MDCT scans were then evaluated for plaque by examining the axial slices, curved multiplanar reformations, and thin-slab maximum intensity projections. Plaques were classified as exclusively noncalcified, primarily calcified, or of mixed composition.
All variables were examined using standard descriptive statistics, T-tests were used for group comparisons for normally distributed variables and Wilcoxon rank sum tests for non-normally distributed variables; the chi2 statistic was used for testing categorical variables. Previous studies have examined associations of CAC with WMD only in older individuals using the age cutpoint of ≥55 years.9 We thus examined WMD volumes by strata of sex and age dichotomized at <55 or ≥55 years to garner new information about the younger age group, and also by CAC Agatston score groups (0, 1–99, 100–299, ≥300). White matter lesion volume was logarithmically transformed to achieve normality for multivariable linear regression analysis. One-half the minimum detectable lesion volume (i.e., 0.5*13 = 6.5) was added to zero readings to allow for logarithmic transformation (39/405 individuals with zero detectable lesion volume). The multivariable model using generalized estimating equations to correct for nonindependence of families was performed to determine the association of CAC with WMD volume, adjusting for total brain volume and traditional risk factors, including age, sex race, hypertension, diabetes, current smoking, and LDL cholesterol, Sensitivity analyses were performed using alternate transformations of WMD volume: (1) a random number between 1–12 was added to the zero values to allow for logarithmic transformation, (2) Tobit analysis was used where zero WMD volume was considered censored, with 1000 bootstrapped iterations with family resampling to correct for intrafamilial correlations.
RESULTS
The study population consisted of 405 apparently healthy individuals identified from 245 families with early-onset CAD (one proband per family). Probands were 68.2% male, with a mean age of 46.7 ± 6.8 years for the first CAD event. Study subjects were siblings (n=217) of the probands or adult offspring (n=188) of the probands or siblings. The mean age of offspring was 43.6 ± 7.6 range, 30 to 62 years, while siblings were 58.5 ± 7.4 range, 38 to 74 years. Sample characteristics are shown in Table 1 by the absence or presence of any coronary plaque and by WMD volume dichotomized at the median. The overall prevalence of calcified and/or noncalcified coronary plaque was 43.0%. Most participants had some degree of WMD with an overall prevalence of 90.4%. Older age and hypertension were significantly associated with both the presence of coronary plaque and WMD volume above the median. Additionally, triglycerides, HDL-C, and diabetes were significantly associated with the presence of coronary plaque. Nothing beyond age and hypertension was associated with WMD volume above or below the median level. Subjects on statin therapy had a higher prevalence of coronary plaque compared to those not on statin therapy (Table 1) as well as higher median WMD volumes (1356 [422, 3696] versus 687 [207, 1774], p=0.0002.)
Table 1.
| Variable | Coronary Plaque Absent (n=231) |
Coronary Plaque Present (n=174) |
p-value | White matter disease volume ≤799 mm3 (median) (n= 203) |
White matter disease volume >799 mm3 (median) (n=202) |
p-value |
|---|---|---|---|---|---|---|
| Age (years) | 47.3 ± 9.5 | 57.4 ± 8.9 | <0.001 | 47.8 ± 9.8 | 55.5 ± 9.8 | <0.001 |
| Men | 30.7% | 53.4% | <0.001 | 41.9% | 39.1% | 0.57 |
| African American | 36.4% | 35.1% | 0.78 | 32.0% | 39.1% | 0.14 |
| Hypertensive | 30.3% | 58.6% | <0.001 | 34.0% | 51.0% | 0.001 |
| Diabetic | 7.4% | 16.1% | 0.006 | 9.4% | 12.9% | 0.26 |
| Current smoking | 16.5% | 20.1% | 0.34 | 18.7% | 17.3% | 0.72 |
| Statin therapy | 34.3% | 65.7% | <0.001 | 35.4% | 64.7% | <0.001 |
| LDL cholesterol (mg/dL) | 114.1 ± 34.7 | 114.5 ± 40.1 | 0.91 | 117.3 ± 37.4 | 111.2 ± 36.5 | 0.10 |
| HDL cholesterol (mg/dL) | 60.3 ± 17.8 | 56.1 ± 17.3 | 0.02 | 57.8 ± 16.9 | 59.7 ± 18.6 | 0.28 |
| Triglycerides (mg/dL)‡ | 92.0 [66.0, 135.0] | 103.0 [73.0, 151.0] | 0.002 | 93.0 [70.0, 141.0] | 95.0 [66.0, 138.3] | 0.81 |
| Body-mass index (kg/m2) | 29.7 ± 5.9 | 30.3 ± 5.4 | 0.34 | 30.2 ± 5.8 | 29.7 ± 5.5 | 0.42 |
| hsCRP (mg/dL)‡ | 2.8 [1.1, 9.3] | 2.3 [1.1, 7.9] | 0.73 | 2.8 [1.2, 9.2] | 2.3 [1.1, 7.8] | 0.36 |
Coronary plaque defined by the presence of calcified or noncalcified plaque on computed tomographic angiography
Continuous variables presented as mean ± 1 standard deviation
Non-normally distributed continuous variables presented as median [interquartile range]
HDL = High density lipoprotein
LDL = Low density lipoprotein
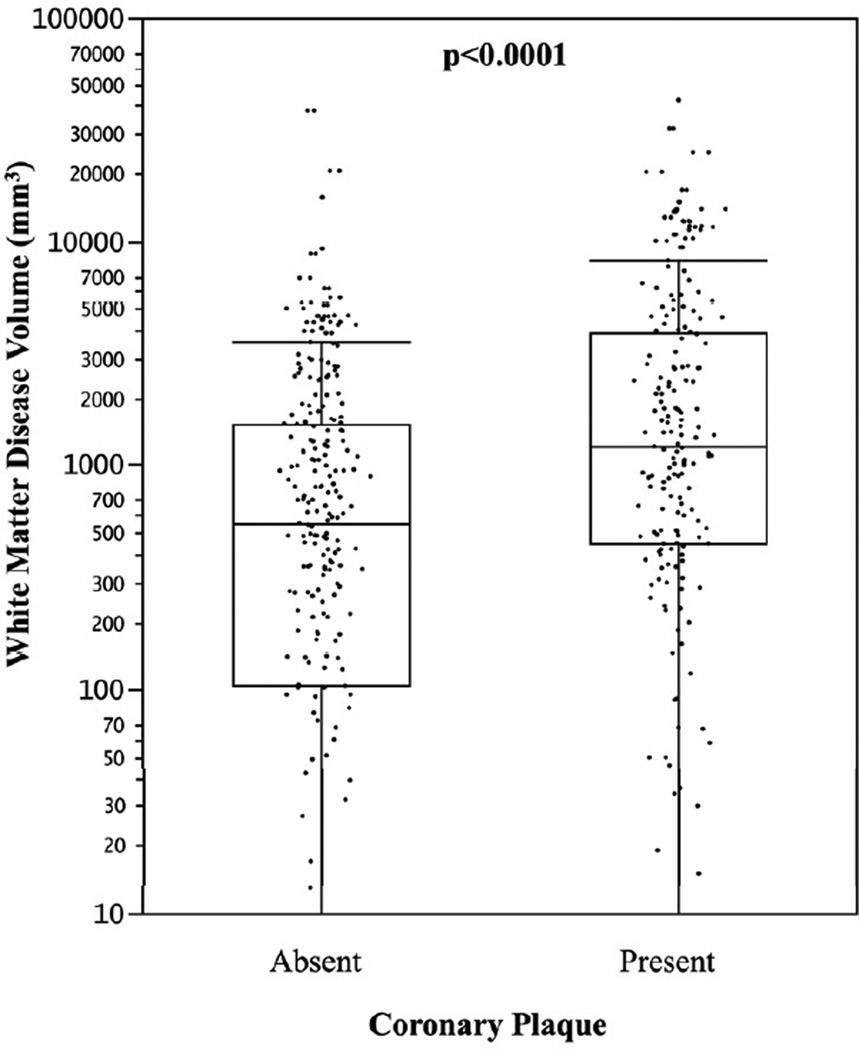
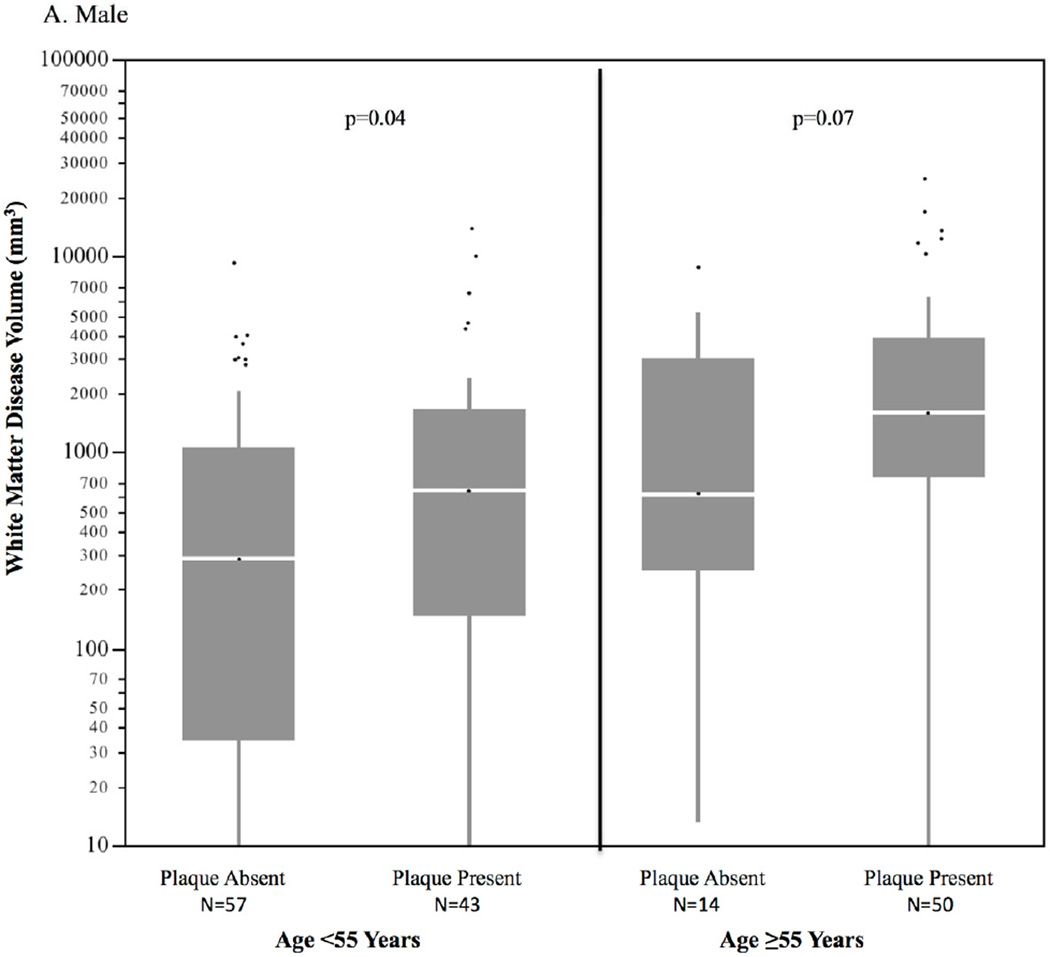
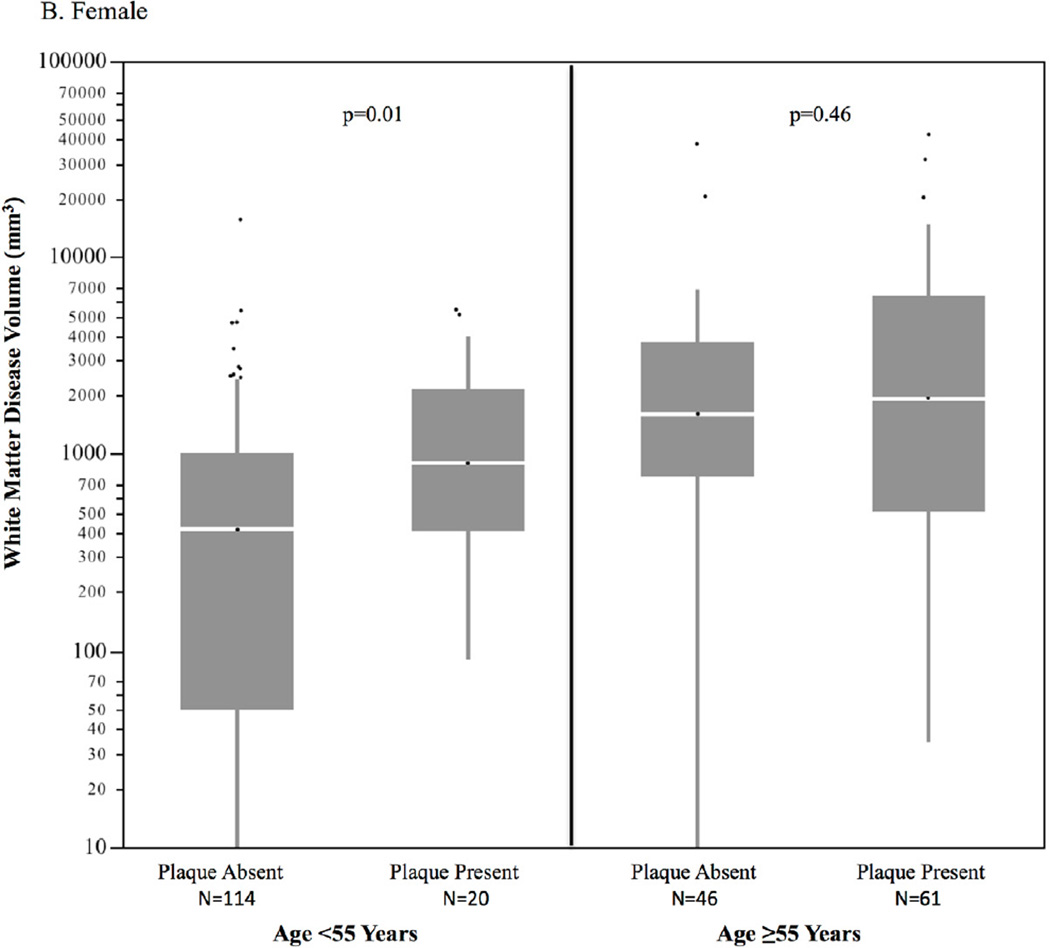
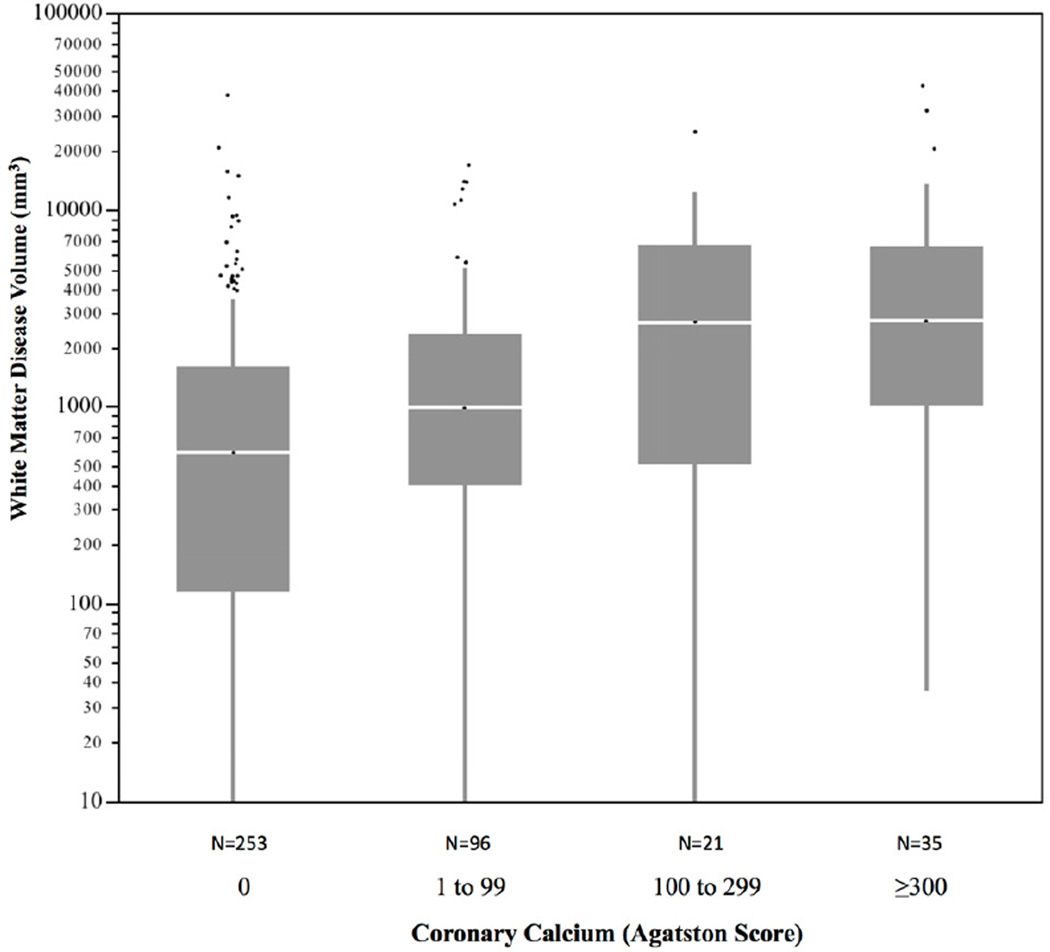
Of the 174 individuals with coronary plaque on CTA, 11.3% had exclusively noncalcified plaque (no calcium), 31.2% had primarily calcified plaque, and 57.5% had both calcified and noncalcified plaque. In 63 subjects <55 years of age with coronary plaque, 14.4% had exclusively noncalcified plaque, compared to only 6.6% of those subjects ≥55 years of age. Subjects with coronary plaque had significantly higher volumes of WMD compared to those with no coronary plaque (median 1222; IQR [105,1523] and median 551 IQR [448, 3871], respectively, p<0.001), as shown in Figure 1. The sex-specific distributions of WMD volume by age group and the absence or presence of subclinical CAD are shown in Figure 2. In individuals <55 years of age, WMD volume was significantly higher in males and females with coronary plaque compared to those without coronary plaque. There was a similar pattern in older males ≥55 years of age but not in older females. Overall the association of coronary plaque with higher WMD volume remained highly significant when adjusting for age, sex, and intrafamilial correlation (p=0.004). This association remained significant after additional adjustment with statin use (p=0.03). The distribution of WMD volume by calcified plaque extent defined by incremental categories of CAC (Agatston) is shown in Figure 3. There was a strong association of incremental coronary calcium score category with WMD volume (p<0.001 for trend).
Figure 1. White matter disease volume and presence of coronary plaque (N=405).
* Horizontal lines represents median; bars represent interquartile range; whiskers represent upper and lower 25%
Figure 2. Sex-specific distribution of white matter disease volume by age and coronary plaque*†.
A. Male
B. Female
* Horizontal lines represents median; bars represent interquartile range; whiskers represent upper and lower 25%; dots represent outliers
†Overall, p=0.004 controlling for age decade, sex, and intrafamilial correlation (GEE)
Figure 3. Distribution of white matter disease volume by severity categories of coronary calcium (Agatston) score*† (N=405).
* Horizontal lines represents median; bars represent interquartile range; whiskers represent upper and lower 25%; dots represent outliers
†p<0.001 for trend by increasing categories of coronary calcium score
Results from the multivariate linear regression analysis predicting WMD volume is shown in Table 2. Older age, female sex, and the presence of hypertension were associated with higher WMD volume. Additionally, subjects with coronary plaque had on average, 49% greater volumes of WMD compared to those without coronary plaque. The independent association of WMD with coronary plaque did not change in the sensitivity analyses with alternative handling of zero WMD volume (beta-coefficient change was <10%).
Table 2.
Multivariable regression analysis predicting white matter disease volume*
| Variable | Relative Difference in White Matter Disease Volume† (95% Confidence) |
p-value |
|---|---|---|
| Presence of coronary plaque | 1.49 (1.00–2.23) | 0.05 |
| Female sex | 1.99 (1.32–.00) | 0.001 |
| Black race | 1.29 (0.87–92) | 0.21 |
| Hypertension | 1.51 (1.03–2.21) | 0.03 |
| Diabetes | 0.78 (0.45–1.33) | 0.36 |
| Current smoking | 1.17 (0.74–1.85) | 0.49 |
| Log Scale Beta ± SE | ||
| Age | 0.093 ± 0.010 | <0.001 |
| LDL cholesterol | −0.003 ± 0.002 | 0.17 |
Adjusted for intrafamilial correlation (GEE) and total brain volume
Geometric mean
DISCUSSION
This is the first study to our knowledge to show an independent association of subclinical coronary plaque with white matter disease of the brain in young apparently healthy individuals with a family history of early-onset CAD, a group at known excess risk for subsequent CAD. The findings support the premise of shared genetic and biological pathways in families at increased risk for both coronary and cerebrovascular disease.
Overt cerebrovascular disease and CAD appear to share many risk factors, aggregate in families, and often co-exist, although the relative herarchy of risk factors for each vascular bed may differ.10, 11 Cerebral WMD is associated with increased risk for subsequent ischemic stroke and transient ischemic attacks.12, 13 Similarly, CAC has been shown to be associated with incident CAD events.14 The association of clinical cerebrovascular disease and CAD suggests an overlap in the pathogenesis of vascular disease in both the brain and heart, although both are strongly associated with older age.15, 16 This premise of shared biological mechanisms is further supported by our finding of an association of preclinical coronary plaque with higher WMD in younger individuals. The higher prevalence of exclusively noncalcified plaque in younger individuals, reflecting an earlier stage of atherogenesis than CAC, would have been missed by CAC screening alone. CAC has been previously associated with the presence of WMD in elderly populations older than age 55 years.9,17–19 We found a similar trend in older males but not in females ≥55 years of age. It is possible that this lack of association was due to our relatively small sample size of older adults or perhaps related to survival bias with an earlier onset of subclinical and clinically manifest disease in both vascular beds in this population ascertained on family history.
The precise mechanisms contributing to microvascular disease in the brain and atherosclerosis of the larger epicardial coronary arteries of the heart are unknown, although hypertension, endothelial dysfunction, and localized inflammation are strongly implicated in both,20,21 consistent with a systemic atherogenic process. Early-onset CAD is more heritable than CAD occurring at older ages.22 Multiple genetic susceptibility loci have been identified that are strongly associated with CAD.23 Similarly, WMD volume is known to be highly heritable with up to 72% of intersubject variability attributed to genetic factors,24 although unlike CAD, familial aggregation of WMD and its clinical manifestations are observed primarily in the elderly.25 Kochunov et al25 recently found a strong association of a locus on chromosome 1q24 that harbors a number of adhesion molecules including P-selectin and E-selectin, with WMD, systolic blood pressure, and pulse pressure. Serum levels and genetic polymorphisms of P-selectin and E-selectin have also been implicated in both stroke and CAD,26–30 suggesting that shared inflammatory pathways may contribute to the common development of silent vascular disease in the brain and heart in families at risk for stroke and myocardial infarction.
Although this study was cross-sectional in design, the findings suggest that early primary prevention is warranted for individuals with a family history of early-onset CAD. Such therapies addressing similar risk factor cascades may benefit two different vascular beds, and two different outcomes, acute CAD events, and stroke.
Acknowledgments
Funding: This work was supported by grants from the National Heart, Lung, and Blood Institute (Grants RC1HL099747 and K23HL094747), the National Institute of Neurological Disorders and Stroke (Grant R01NS062059) and the Institute for Clinical and Translational Research (Grant TR000424).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Horne BD, Camp NJ, Muhlestein JB, Cannon-Albright LA. Identification of excess clustering of coronary heart diseases among extended pedigrees in a genealogical population database. Am Heart J. 2006;152:305–311. doi: 10.1016/j.ahj.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Kral BG, Becker LC, Vaidya D, Yanek LR, Becker DM. Silent myocardial ischaemia and long-term coronary artery disease outcomes in apparently healthy people from families with early-onset ischaemic heart disease. Eur Heart J. 2011;32:2766–2772. doi: 10.1093/eurheartj/ehr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyquist PA, Wityk R, Yanek LR, Vaidya D, Yousem DM, Becker LC, Becker DM. Silent small-vessel cerebrovascular disease and silent myocardial ischemia in families with premature coronary disease. Neuroepidemiol. 2009;33:66–67. doi: 10.1159/000215831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 5.Pesce AJ, Kaplan LA. Methods in clinical chemistry. Mosby: St. Louis; 1987. [Google Scholar]
- 6.Bazin PL, Cuzzocreo JL, Yassa MA, Gandler W, McAuliffe MJ, Bassett SS, Pham DL. Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods. 2007;165:111–121. doi: 10.1016/j.jneumeth.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage. 2010;49:1524–1535. doi: 10.1016/j.neuroimage.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agatston AS, Janowitz WR, Kaplan G, Gasso J, Hildner F, Viamonte M., Jr Ultrafast computed tomography-detected coronary calcium reflects the angiographic extent of coronary arterial atherosclerosis. Am J Cardiol. 1994;74:1272–1274. doi: 10.1016/0002-9149(94)90563-0. [DOI] [PubMed] [Google Scholar]
- 9.Bos D, Ikram MA, Elias-Smale SE, Krestin GP, Hofman A, Witteman JC, van der Lugt A, Vernooij MW. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol. 2011;31:2331–2337. doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 10.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 11.Williams RR. Understanding genetic and environmental risk factors in susceptible persons. West J Med. 1984;141:799–806. [PMC free article] [PubMed] [Google Scholar]
- 12.Fazekas F, Fazekas G, Schmidt R, Kapeller P, Offenbacher H. Magnetic resonance imaging correlates of transient cerebral ischemic attacks. Stroke. 1996;27:607–611. doi: 10.1161/01.str.27.4.607. [DOI] [PubMed] [Google Scholar]
- 13.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 14.Clouse ME. How useful is computed tomography for screening for coronary artery disease? Noninvasive screening for coronary artery disease with computed tomography is useful. Circulation. 2006;113:125–146. doi: 10.1161/CIRCULATIONAHA.104.478354. discussion 125–146. [DOI] [PubMed] [Google Scholar]
- 15.Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging. 2004;15:365–367. doi: 10.1097/01.rmr.0000168216.98338.8d. [DOI] [PubMed] [Google Scholar]
- 16.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 17.Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr, Newman AB. Coronary artery calcium: associations with brain magnetic resonance imaging abnormalities and cognitive status. J Am Geriatr Soc. 2005;53:609–615. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim BJ, Lee SH, Kim CK, Ryu WS, Kwon HM, Choi SY, Yoon BW. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ J. 2011;75:451–456. doi: 10.1253/circj.cj-10-0762. [DOI] [PubMed] [Google Scholar]
- 19.Vidal JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, Garcia ME, van Buchem MA, Harris TB, Gudnason V, Launer LJ. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke. 2010;41:891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 22.Nora JJ, Lortscher RH, Spangler RD, Nora AH, Kimberling WJ. Genetic--epidemiologic study of early-onset ischemic heart disease. Circulation. 1980;61:503–508. doi: 10.1161/01.cir.61.3.503. [DOI] [PubMed] [Google Scholar]
- 23.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann J, Samani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochunov P, Glahn D, Winkler A, Duggirala R, Olvera RL, Cole S, Dyer TD, Almasy L, Fox PT, Blangero J. Analysis of genetic variability and whole genome linkage of whole-brain, subcortical, and ependymal hyperintense white matter volume. Stroke. 2009;40:3685–3690. doi: 10.1161/STROKEAHA.109.565390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochunov P, Glahn D, Lancaster J, Winkler A, Kent JW, Jr, Olvera RL, Cole SA, Dyer TD, Almasy L, Duggirala R, Fox PT, Blangero J. Whole brain and regional hyperintense white matter volume and blood pressure: overlap of genetic loci produced by bivariate, whole-genome linkage analyses. Stroke. 2010;41:2137–2142. doi: 10.1161/STROKEAHA.110.590943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auer J, Weber T, Berent R, Lassnig E, Lamm G, Eber B. Genetic polymorphisms in cytokine and adhesion molecule genes in coronary artery disease. Am J Pharmacogenomics. 2003;3:317–328. doi: 10.2165/00129785-200303050-00003. [DOI] [PubMed] [Google Scholar]
- 27.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Zee RY, Cook NR, Cheng S, Reynolds R, Erlich HA, Lindpaintner K, Ridker PM. Polymorphism in the P-selectin and interleukin-4 genes as determinants of stroke: a population-based, prospective genetic analysis. Hum Mol Genet. 2004;13:389–396. doi: 10.1093/hmg/ddh039. [DOI] [PubMed] [Google Scholar]
- 29.Volcik KA, Ballantyne CM, Coresh J, Folsom AR, Boerwinkle E. Specific P-selectin and P-selectin glycoprotein ligand-1 genotypes/haplotypes are associated with risk of incident CHD and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2007;195:e76–e82. doi: 10.1016/j.atherosclerosis.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flex A, Gaetani E, Papaleo P, Straface G, Proia AS, Pecorini G, Tondi P, Pola P, Pola R. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke. 2004;35:2270–2275. doi: 10.1161/01.STR.0000140740.19421.fe. [DOI] [PubMed] [Google Scholar]