Abstract
Protein synthesis and degradation are dynamically regulated processes that act in concert to control the accretion or loss of muscle mass. The present article focuses on the mechanisms involved in the impairment of protein synthesis that are associated with skeletal muscle atrophy. The vast majority of mechanisms known to regulate protein synthesis involve modulation of the initiation phase of mRNA translation, which comprises a series of reactions that result in the binding of initiator methionyl-tRNAi and mRNA to the 40S ribosomal subunit. The function of the proteins involved in both events has been shown to be repressed under atrophic conditions such as sepsis, cachexia, chronic kidney disease, sarcopenia, and disuse atrophy. The basis for the inhibition of protein synthesis under such conditions is likely to be multifactorial and includes insulin/insulin-like growth factor 1 resistance, pro-inflammatory cytokine expression, malnutrition, corticosteroids, and/or physical inactivity. The present article provides an overview of the existing literature regarding mechanisms and signaling pathways involved in the regulation of mRNA translation as they apply to skeletal muscle wasting, as well as the efficacy of potential clinical interventions such as nutrition and exercise in the maintenance of skeletal muscle protein synthesis under atrophic conditions.
Keywords: mRNA translation, mTOR, muscle atrophy, inflammation, insulin resistance, leucine
1. Introduction
Protein synthesis and degradation are dynamically regulated processes that act in concert to control the accretion or loss of muscle mass. Muscle hypertrophy occurs when the rate of protein synthesis exceeds the rate of degradation, or, conversely, muscle atrophy occurs under conditions wherein the rate of protein synthesis is repressed relative to that of degradation. Thus, muscle atrophy can be caused by either a reduction in the rate of protein synthesis, a rise in the rate of degradation, or a simultaneous decline in synthesis in combination with an increase in degradation. Although protein degradation is undoubtedly an important contributor to the development and progression of muscle wasting under a variety of conditions, the focus of the present article will be on mechanisms involved in the impairment of protein synthesis associated with muscle atrophy in selected atrophic conditions, with the primary emphasis being on those conditions that are the focus of other articles in this issue of the journal.
2. mRNA translation
The process of mRNA translation occurs through a series of reactions that can be functionally divided into three phases: initiation, elongation, and termination (Hershey et al., 2012). The majority of mechanisms known to regulate mRNA translation involve the initiation phase, with many fewer occurring at elongation or termination (Hershey et al., 2012). Therefore, this review will focus primarily on modulation of translation initiation in muscle wasting.
2.1. The met-tRNAi binding step in translation initiation
One of the first steps in translation initiation involves formation of a ternary complex consisting of eukaryotic initiation factor (eIF) 2, GTP, and initiator methionyl-tRNA (Met- tRNAi) (Hinnebusch and Lorsch, 2012). The ternary complex then associates with other initiation factors to form a multifactor complex that subsequently binds to the 40S ribosomal subunit to form the 43S preinitiation complex. During a later step in initiation, the GTP bound to eIF2 is hydrolyzed, and the eIF2•GDP complex is released from the preinitiation complex. The GDP bound to eIF2 must then be exchanged for GTP to permit formation of the ternary complex and efficient recycling of eIF2, because the affinity of Met-tRNAi for the eIF2•GDP complex is significantly lower compared to its affinity for eIF2•GTP. The GDP-GTP exchange on eIF2 is mediated by the heteropentameric complex, eIF2B. Interestingly, only one of the five eIF2B subunits (referred to as eIF2Bε) exhibits guanine nucleotide exchange activity, the remaining subunits are thought to act to modulate the activity of the complex (Reid et al., 2012).
2.2. The mRNA binding step in translation initiation
The binding of mRNA to the 43S preinitiation complex can occur through two functionally dissimilar mechanisms (Hinnebusch and Lorsch, 2012). In the first, eIF4E binds to the m7GTP cap structure at the 5’-end of the mRNA, and the eIF4E•mRNA complex then interacts with eIF4G in association with eIF4A to form the eIF4F•mRNA complex. As part of the eIF4F complex, eIF4G subsequently interacts with eIF3 that is associated with the 43S pre-initiation complex to form the 48S preinitiation complex. Thus, eIF4G acts as a molecular bridge linking the eIF4E•mRNA complex to the eIF3•40S ribosomal subunit complex. The 48S pre-initiation complex then scans along the 5’-untranslated region (5’-UTR) of the mRNA, and stops at the AUG start codon.
An alternative mechanism through which the 43S pre-initiation complex can bind to mRNA involves its association with mRNA at or near the AUG start codon in a cap-independent manner (Martínez-Salas et al., 2012). This interaction may require eIF4A and eIF4G, but does is independent of eIF4E. Many mRNAs that initiate translation through this mechanism have long, highly-structured 5’-UTRs and contain a domain, referred to as an internal ribosome entry site (IRES), to which the 43S pre-initiation complex binds.
Regardless of whether association of the mRNA with the 43S pre-initiation complex occurs through a cap-dependent or –independent mechanism, the binding of the anti-codon loop of the Met-tRNAi to the AUG start codon of the mRNA halts the scanning of the 40S ribosomal subunit (Hinnebusch and Lorsch, 2012). The GTP bound to eIF2 is then hydrolyzed and the eIF2•GDP complex along with most of the other initiation factors are released from the 40S ribosomal subunit. The 60S ribosomal subunit then joins, and the resulting 80S initiation complex is competent to proceed with peptide chain elongation.
3. Regulatory mechanisms
3.1. Regulation involving eIF2 and eIF2B
One of the best-characterized mechanisms for regulating translation initiation involves the α-subunit of eIF2 (Wek et al., 2006). Phosphorylation of eIF2α on Ser51 indirectly inhibits translation initiation by transforming eIF2 from a substrate of the guanine nucleotide exchange factor (GEF) eIF2B, into a competitive inhibitor. Inhibition of eIF2B GEF activity by phosphorylated eIF2 results in accumulation of the eIF2•GDP complex that has limited affinity for Met-tRNAi, leading to a decrease in the amount of the active Met-tRNAi•eIF2•GTP complex available to participate in assembly of the 43S pre-initiation complex. Consequently, translation of most mRNAs is repressed under conditions that promote phosphorylation of eIF2α. Counterintuitively, the translation of some mRNAs is not repressed, but is instead enhanced in response to phosphorylation of eIF2α. Such mRNAs typically have multiple short upstream open reading frames (uORF) in the 5’-UTR that are required for enhanced translation when eIF2α is phosphorylated. A detailed discussion of the mechanism involved in this type of regulation is beyond the scope of this article, and the reader is referred to a recent review on this topic for further information (Wethmar et al., 2010).
Four kinases have been shown to phosphorylate eIF2α: double-stranded RNA-dependent protein kinase (PKR), PKR-like endoplasmic reticulum-associated protein kinase (PERK), general-control nonderepressible (GCN2), and heme-regulated inhibitor (HRI) (Wek et al., 2006). All four kinases phosphorylate eIF2α on Ser51, leading to inhibition of eIF2B activity, but are activated in response to diverse stresses. For example, PKR is activated by pro-inflammatory cytokines (Kang and Tang, 2012) and GCN2 is activated in response to deprivation of essential amino acids (Dever and Hinnebusch, 2005).
In addition to regulation by changes in phosphorylation of eIF2α, eIF2B GEF activity is also modulated in response to phosphorylation of the catalytic eIF2Bε subunit. For example, amino acid deprivation results in phosphorylation of eIF2Bε on Ser525 and repression of eIF2B GEF activity independent of changes in eIF2α phosphorylation (Wang and Proud, 2008). Although the kinase that phosphorylates eIF2Bε on Ser525 is unknown, phosphorylation occurs independent of GCN2. The ε-subunit of eIF2B is phosphorylated on a number of additional residues, including Ser540. Phosphorylation of Ser540 by glycogen synthase kinase 3 (GSK-3) leads to inhibition of eIF2B GEF activity (Welsh et al., 1998). Thus, inhibition of GSK-3 in response to insulin or IGF-1 treatment promotes eIF2B GEF activity in part through inhibition of GSK-3 (Welsh and Proud, 1993).
3.2. Regulation involving eIF4F and eIF4B
Because little, if any, research has been performed on cap-independent mRNA translation in muscle wasting, the focus herein will be primarily on cap-dependent translation. Cap-dependent translation is contingent upon assembly of eIF4A, eIF4E, and eIF4G into the active eIF4F complex (Hinnebusch and Lorsch, 2012). The binding of eIF4E to eIF4G is predominantly regulated by a family of eIF4E binding proteins (4E-BP) that bind to the same domain on eIF4E as does eIF4G. Thus, the binding of 4E-BP to eIF4E prevents its association with eIF4G, and therefore attenuates cap-dependent binding of mRNA to the 43S preinitiation complex. The association of 4E-BPs with eIF4E is controlled by phosphorylation, whereby hyperphosphorylated forms of the proteins do not bind to eIF4E, but unphosphorylated and hypophosphorylated forms do. Phosphorylation of 4E-BP1, e.g. in response to provision of amino acids, insulin, or IGF-1 to amino acid- and serum-starved cells, is blocked by the immunosuppressant rapamycin (Shigemitsu et al., 1999), suggesting that the primary kinase responsible for 4E-BP1 phosphorylation is the mechanistic target of rapamycin in complex 1 (mTORC1). The phosphorylation of 4E-BP1, as well as the increase in protein synthesis, that occurs in muscle in response to either consumption of amino acids (Dickinson et al., 2011) or resistance exercise (Drummond et al., 2009) is also blocked by rapamycin treatment.
In addition to 4E-BP1, mTORC1 also phosphorylates the 70kDa ribosomal protein S6 kinase 1 (p70S6K1) in a rapamycin-sensitive manner, resulting in its activation (Hara et al., 1998). Activated p70S6K1 subsequently phosphorylates two proteins that modulate cap-dependent mRNA translation, eIF4B and programmed cell death 4 (PDCD4). Similar to the function of 4E-BP1 to inhibit assembly of the eIF4E•eIF4G complex, PDCD4 interacts with eIF4A and prevents its association with eIF4G (Yang et al., 2003). Phosphorylation of PDCD4 by p70S6K1 causes it to be released from eIF4A, allowing eIF4A to interact with eIF4G. Assembly of eIF4A into the eIF4F complex is also modulated by eIF4B (Park et al., 2013). Moreover, eIF4B enhances the RNA helicase activity of eIF4A (Rogers et al., 1999). Thus, mTORC1 stimulates cap-dependent mRNA translation through at least three mechanisms: release of 4E-BP1 from eIF4E and PDCD4 from eIF4A, allowing them to bind to eIF4G and form the active eIF4F complex, and phosphorylation of eIF4B. As demonstrated in a recent study (Dennis et al., 2012), all three events contribute to maintenance of optimal rates of mRNA translation.
3.3. Regulation of signaling through mTORC1
Signaling through mTORC1 is modulated through changes in activation of multiple upstream pathways, many of which converge on the tuberous sclerosis complex (TSC) proteins TSC1 and TSC2. Together, TSC1 and TSC2 function to promote GTP hydrolysis by the small GTPase referred to as ras homolog enriched in brain (Rheb) (Inoki et al., 2003). When it is associated with GTP, Rheb activates mTORC1, whereas Rheb•GDP does not (Long et al., 2005). By stimulating the GTPase activity of Rheb, the TSC complex converts Rheb from a GTP- to a GDP-bound form, thereby inhibiting mTORC1 activity. The GTPase-activating function of the TSC complex is repressed through phosphorylation of TSC2 by Akt and ERK, and activated through phosphorylation by the AMP-activated protein kinase (AMPK) (Huang and Manning, 2008). Thus, hormones such as insulin and IGF-1 promote mTORC1 signaling, in part, through Akt- and ERK-mediated inhibition of the TSC complex, whereas energy deficit, e.g. during hypoxia, has the opposite effect. Amino acids, and in particular the branched-chain amino acid leucine, also stimulate mTORC1 signaling, but do so in a TSC1•TSC2 independent manner. Instead, amino acids promote the assembly of a mTORC1 complex with the Rag small GTPases and a heteropentameric complex referred to as Ragulator at the late endosomal/lysosomal membrane (Sancak et al., 2010). Localization of mTORC1 at the membrane is thought to promote its activation by allowing for its interaction with Rheb.
4. Regulation of mRNA translation in muscle wasting conditions
4.1. Sepsis
Sepsis causes a rapid and significant loss of body protein, in part due to development of an impairment of protein synthesis in skeletal muscle (Fig. 1). The molecular basis for the decline in protein synthesis appears to be multifactorial in origin, and, at least in animal models, is associated with inhibition of both eIF2B GEF activity and repressed signaling through mTORC1. Early studies using a rodent model of chronic abdominal sepsis demonstrated that induction of sepsis leads to a repression of 43S preinitiation complex assembly (Vary et al., 1998) and inhibition of eIF2B GEF activity in skeletal muscle, with no change in eIF2a phosphorylation (Vary et al., 1994). Instead, repressed eIF2B GEF activity is associated with decreased expression of eIF2Bs, with no change in expression of the regulatory subunit eIF2Bβ (Voisin et al., 1996). Notably, changes in expression of eIF2Bβ are directly proportional to alterations in eIF2B activity and muscle protein synthesis during the development of and recovery from the septic insult. Moreover, decreasing serum tumor necrosis factor (TNF)α concentrations using amrinone ameliorates not only the sepsis-induced repression of protein synthesis, but also the inhibition of eIF2B GEF activity. Overall, the results of the early studies provide strong support for the hypothesis that sepsis-induced inhibition of protein synthesis is a consequence of repressed eIF2Bε expression. The results of a more recent study (Tuckow et al., 2010) provide further support for this idea. In that study, eIF2Bε was exogenously expressed in skeletal muscle of septic rats by electroporating an expression plasmid into the tibialis anterior muscle. The resulting increase in eIF2Bε expression prevented both the sepsis-induced fall in eIF2B GEF activity as well as the inhibition of protein synthesis.
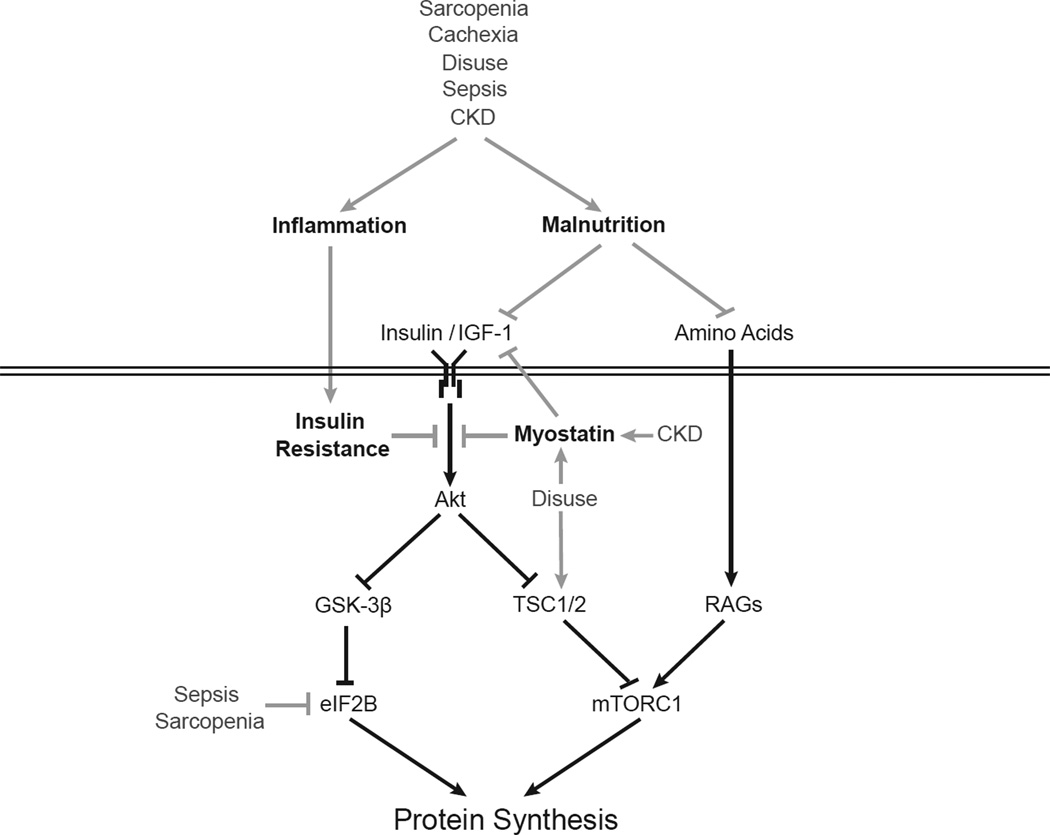
Figure 1. Impairment of protein synthesis in various skeletal muscle wasting diseases is associated with inflammation, malnutrition, myostatin, and insulin resistance.
Hormones such as insulin and insulin-like growth factor 1 (IGF-1) stimulate protein synthesis through both the Akt/mTORC1 signaling pathway and activation of eIF2B, while amino acids activate mTORC1 signaling through the Rag proteins. Cell signaling pathways are depicted by black lines (arrows represent stimulatory pathways; lines with blunt ends represent inhibitory). Skeletal muscle wasting diseases, including sarcopenia, cachexia, disuse, sepsis, and chronic kidney disease (CKD), impair signaling through these pathways at specific steps depicted by gray lines.
Decreased expression of eIF2Bε may not be the sole mechanism through which sepsis represses eIF2B GEF activity. Thus, in the rat model of chronic abdominal sepsis, phosphorylation of eIF2Bε on Ser535 (Ser540 in the human protein) changes in proportion to the fall in eIF2B activity during the development of and recovery from sepsis (Vary et al., 2002). Changes in eIF2Bε phosphorylation are mirrored by alterations in GSK-3 phosphorylation, suggesting that decreased eIF2B activity might be attributable in part to activation of GSK-3. Indeed, the sepsis-induced changes in eIF2Bε and GSK-3 phosphorylation are both blocked by treatment with TNFα-binding protein, suggesting that, similar to the alterations in eIF2B GEF activity and eIF2Bε expression, phosphorylation of eIF2Bε is regulated by inflammatory cytokines. Repressed eIF2B GEF activity has also been observed in other rodent models of sepsis. For example, eIF2B activity is repressed in rat gastrocnemius muscle four hours after intravenous injection of E. coli lipopolysaccharide (LPS) (Lang et al., 2000). The repression is not associated with altered expression of eIF2Bε, but instead may be due to increased phosphorylation of eIF2α. However, inhibition of eIF2B GEF activity is not observed in all studies using LPS. For example, repressed eIF2B activity is not observed in LPS-treated neonatal pigs (Orellana et al., 2004).
In addition to perturbations in eIF2B activity, chronic abdominal sepsis also leads to impaired signaling through mTORC1, and the reader is referred to a recent review for further information on this topic (Frost and Lang, 2011). However, it should be noted that the repression of muscle protein synthesis in septic rodents is resistant to stimulation by either insulin or leucine, although the response to IGF-1 appears to be intact. Whether IGF-1 is acting solely through mTORC1 to stimulate protein synthesis, or might in addition result in activation of eIF2B, e.g. through inhibition of GSK-3 mediated phosphorylation of eIF2Bε, is unknown.
In septic patients there is a rapid and profound loss of body protein that initially occurs primarily due to loss of muscle protein (e.g. Plank et al., 1998). However, few studies have examined the relative role of protein degradation compared to synthesis in sepsis-induced muscle atrophy in humans, and fewer still have assessed the mechanism(s) involved in regulating mRNA translation. To address this deficit, a recent study using muscle biopsies taken from healthy volunteers and critically ill patients within 6–8 hours of admission to the Intensive Care Unit (ICU, Constantin et al., 2011) assessed a number of biomarkers associated with the control of mRNA translation. Expression of TNFα and interleukin-6 are significantly elevated in muscle of critically ill compared to healthy subjects, whereas phosphorylation of mTOR, 4E-BP1, p70S6K1, and GSK-3 is reduced. Since dephosphorylation of GSK-3 leads to its activation, this finding is consistent with the idea that eIF2B GEF activity might be inhibited through GSK-3-mediated phosphorylation of eIF2Bε. Unfortunately, phosphorylation of eIF2B was not assessed. Overall, the results of the study by Constantin et al. (Constantin et al., 2011) suggest that muscle protein synthesis in critically ill patients might be mediated through mechanisms similar to those identified using animal models of sepsis. However, the results of a second study using critically ill patients (Jespersen et al., 2011) appear to contradict those reported by Constantin et al. (Constantin et al., 2011). In that study, mTORC1 signaling is significantly increased in muscle of critically ill patients compared to controls. The reason for the discrepancy between the two studies is unknown, but is likely due to the “intensive insulin therapy” that patients in the second study received. Indeed, in the second study, phosphorylation of Akt was positively correlated with the rate of insulin infusion. Since Akt is a positive upstream regulator of mTORC1, it is possible that the increase in mTORC1 signaling observed in that study is a consequence of insulin therapy.
4.2. Sarcopenia
Most studies assessing the basis for the loss of muscle mass that occurs with aging have focused primarily on reduced rates of protein synthesis as being causative (Rasmussen and Volpi, 2012). However, the results are inconsistent, with some studies suggesting that basal, i.e. postabsorptive, rates of protein synthesis are reduced in muscle of aged compared to younger individuals (Guillet et al., 2004; Welle et al., 1993; Yarasheski et al., 1993) whereas others show no change (Fry et al., 2011; Fujita et al., 2007; Rasmussen et al., 2006; Rasmussen and Volpi, 2012; Toth et al., 2005). Despite this paradox, there is substantial evidence that skeletal muscle is resistant to anabolic stimuli in aged animals and humans (Balage et al., 2010; Fry et al., 2011; Fujita et al., 2009; Fujita et al., 2007; Guillet et al., 2004; Hwee and Bodine, 2009; Katsanos et al., 2005; Rasmussen et al., 2006). For example, muscle exhibits age-related resistance to exercise-induced hypertrophy, as shown in studies using the synergistic ablation model of resistance exercise in old compared to young rats (Hwee and Bodine, 2009) and exercise can augment the anabolic response in elderly men (Fujita et al., 2007; Pennings et al., 2011; Timmerman et al., 2012).
Skeletal muscle in the elderly is also resistant to the anabolic effect of amino acids. Thus, the concentration of essential amino acids or leucine necessary to stimulate muscle protein synthesis is greater in older compared to young humans (Katsanos et al., 2005; Katsanos et al., 2006; Rasmussen and Volpi, 2012). The resistance to amino acids is aggravated by two compounding factors. First, the elderly are often malnourished, particularly with regard to dietary protein. It has been estimated that up to 38% of adult men and 41% of adult women have dietary protein intakes below the current recommended daily allowance of 0.8 grams • kg−1 • day−1 (Paddon-Jones et al., 2008). However, the use of protein supplementation has yielded mixed results with some studies showing increased rates of protein synthesis in response to amino acid supplementation (Guillet et al., 2004; Paddon-Jones et al., 2006; Paddon-Jones et al., 2003) whereas others have not (Katsanos et al., 2005; Katsanos et al., 2006). This discrepancy may be related to the type or composition of the protein supplement, as essential amino acids stimulate protein synthesis to a greater degree than whey protein (Paddon-Jones et al., 2006). Second, the relative insensitivity of muscle protein synthesis to amino acid-induced stimulation may be further complicated by the age-related decline in the rate of amino acid absorption and subsequent secretion of insulin (Katsanos et al., 2005; Paddon-Jones et al., 2003). However, it should be noted that in one study (Paddon-Jones et al., 2003), amino acid delivery to the muscle in the elderly was not compromised and in another study no difference between young and old humans in the kinetics of amino acid digestion and absorption was observed (Koopman et al., 2009).
Anabolic resistance in skeletal muscle of the elderly also manifests as resistance to insulin-induced stimulation of protein synthesis (Fujita et al., 2009; Fujita et al., 2007; Guillet et al., 2004; Rasmussen et al., 2006). Thus, although physiological or postprandial levels of insulin are sufficient to induce protein synthesis in young muscle, supra-physiological levels of the hormone are necessary to stimulate protein synthesis in the elderly (Fujita et al., 2009; Fujita et al., 2007; Guillet et al., 2004). Moreover, activation of the PI3K/Akt/mTORC1 pathway exhibits age-related insulin resistance, with supra-physiological levels of insulin needed to potently increase phosphorylation of Akt on Ser473 and p70S6K1 on Thr389 (Fujita et al., 2009). Resistance to insulin-induced protein synthesis may be exacerbated by reduced secretion of the hormone in response to amino acid ingestion in aged individuals providing another potential mechanism for reduced protein synthesis with amino acid supplementation (Paddon-Jones et al., 2003). However, this phenomenon may be specific to amino acid supplementation as insulin secretion in frail elderly women is increased compared to young healthy women following a fixed meal demonstrating that dietary composition may affect insulin secretion (Goulet et al., 2010). Notably, one group found that a single bout of aerobic exercise prior to infusion of insulin to raise its concentration to postprandial levels stimulated muscle protein synthesis in association with phosphorylation of Akt, mTOR, and p70S6K1, whereas the hormone had no effect in the non-exercised control group (Fujita et al., 2007).
IGF-1 may also play a role in sarcopenia. Though IGF-1 may not be necessary for muscle hypertrophy that occurs following resistance exercise in young, healthy individuals (West et al., 2010; West and Phillips, 2012), its over expression in muscle can induce significant hypertrophy (Coleman et al., 1995). Moreover, plasma levels of IGF-1 are significantly lower in older individuals than young (Toth et al., 2005). Additional evidence shows that there is a significant negative correlation between age and plasma IGF-1 concentration in both men and women (Copeland et al., 1990), and the amount of fat-free mass can be predicted by plasma IGF-1 concentrations even after adjusting for age, baseline fat-free mass, fat mass, and two-year weight changes (Payette et al., 2003). Despite these findings, direct evidence regarding a precise role for IGF-1 in human sarcopenia is not available.
Another potential mechanism underlying sarcopenia is inflammation. Old rats with low levels of inflammation have higher rates of protein synthesis in the postprandial state compared to those with high inflammation (Balage et al., 2010). Additionally, daily administration of ibuprofen to old rats increases rates of protein synthesis and phosphorylation of 4E-BP1 in the postprandial state while decreasing serum IL-6 and IL-1 (Rieu et al., 2009). Human data show significantly higher levels of circulating IL-6 and IL-6 receptor in old individuals (Roubenoff et al., 1998), with IL-6 levels being a significant predictor of sarcopenia (Payette et al., 2003). Lastly, there is a negative correlation between the level of C Reactive Protein and IL-6 to rates of mixed muscle and myosin heavy chain protein synthesis further illustrating the likelihood of inflammation contributing to reduced protein synthesis in sarcopenia (Toth et al., 2005).
The molecular basis for the diminished protein synthetic response to anabolic stimuli appears to be multifactorial. As observed in septic rats, both the expression of eIF2Bε and signaling through mTORC1 are reduced in muscle of aged compared to young rats (Hwee and Bodine, 2009). In addition, in humans, the stimulation of muscle protein synthesis following a single bout of resistance exercise is significantly decreased in aged compared to young individuals, with no activation of the mTORC1 signaling pathway in the muscle of the elderly (Fry et al., 2011). However, the relative contribution of the observed changes in eIF2Bε expression compared to perturbations in mTORC1 signaling to the unresponsiveness of muscle protein synthesis to anabolic stimuli is as yet undefined.
4.3. Cancer cachexia
Rates of muscle protein synthesis are significantly reduced in patients with cancer cachexia (Emery et al., 1984) and muscle of cachectic patients is resistant anabolic stimulation (e.g. Williams et al., 2012). Surgical removal of the tumor in some cases, e.g. nonmetastatic colorectal cancer, reverses the reduced synthesis rate (Williams et al., 2012). Moreover, many of the cachexia-induced transcriptome alterations in cancer patients are reversed following tumor resection (Gallagher et al., 2012), suggesting that the optimal treatment for cachexia may be complete removal of the tumor. However, in many cases complete tumor removal is not possible, and therefore there is a need for a better understanding of the mechanisms that regulate protein synthesis during cancer cachexia.
Due to the difficult nature of studying the regulation of protein synthesis in cachectic subjects, most of our understanding of the mechanisms involved in the effect have been delineated using mouse models, both during cancer onset as well as during advanced stages of the disease. Recent work investigating the regulation of protein turnover at the onset of cachexia in the Apcmin/+ mouse model of colon cancer shows rates of myofibrillar protein synthesis to be significantly decreased (White et al., 2011). The dysregulation of protein synthesis is associated with suppressed mTORC1 signaling as assessed by decreased phosphorylation of both 4E-BP1 and p70S6K1. However, no change in phosphorylation of Akt, mTOR, or AMPK is observed, suggesting that mTORC1 signaling might be regulated by another upstream regulator, e.g. the Rag complex. Overall, the results suggest that the regulation of protein synthesis during the early stages of cachexia occurs through suppression of mTORC1 signaling resulting in decreased rates of protein synthesis, although the underlying mechanism is still not understood.
A further decline in the rate of myofibrillar protein synthesis, beyond that seen in early cachexia, occurs in advanced cachexia (White et al., 2011), suggesting that the regulation of protein synthesis at more advanced stages may occur through mechanisms distinct from those observed in early cachexia. For example, the phosphorylation of AMPK, as well as AMPK activity, increase significantly during later stages (White et al., 2011). Moreover, repressed phosphorylation of mTORC1 manifests during advanced cachexia and phosphorylation of its substrates show further declines compared to early cachexia, despite increased phosphorylation of the upstream mTORC1 regulator, Akt (White et al., 2011). The apparent disconnect that occurs between Akt and mTORC1 during advanced cachexia may be related to activation of AMPK because its activation in muscle overcomes insulin-induced activation of mTORC1 with no effect on insulin-induced phosphorylation of Akt (Deshmukh et al., 2008).
One possible explanation for the cachexia-associated activation AMPK could be mitochondrial dysfunction. Mitochondrial size is significantly reduced in mice with colon cancer prior to the onset of cachexia (White et al., 2012), and the expression of mitofusin 1 and 2, which promote mitochondrial fusion and maintain metabolic function (Santel et al., 2003; Sebastian et al., 2012), are also significantly decreased during early cachexia (White et al., 2011; White et al., 2012). Furthermore, the protein expression of fis1, which promotes fragmenting of mitochondria (Stojanovski et al., 2004), is increased during advanced cachexia progression coinciding with AMPK activation (White et al., 2012). Thus, mitochondrial dysfunction may activate AMPK during later stages of cachexia resulting in further reductions in the rates of protein synthesis and mTORC1 signaling.
Additional mechanisms that may be involved in regulation of protein synthesis include resistance to insulin/IGF-1 signaling. Expression of the IGF-1 mRNA in skeletal muscle is significantly reduced upon initiation of cachexia in a mouse model of colon cancer (White et al., 2011), and administration of IGF-1 to rats inoculated with AH-130 cancer cells helps maintain bodyweight, lean body mass, and muscle weight (Schmidt et al., 2011). However, in humans, serum IGF-1 concentrations do not change during cachexia (Fouladiun et al., 2005). A possible explanation for this dichotomy may be that the cachexia-induced decrease in muscle IGF-1 mRNA expression in animal models results in reduced intramuscular concentrations of the hormone, leading to repressed autocrine/paracrine function, while circulating IGF-1 is not affected. It is also possible that the sensitivity of the muscle to IGF-1 is impaired, as there is evidence of skeletal muscle insulin resistance in human cancer patients (Lundholm et al., 1978). In support of this idea, plasma insulin concentrations in cachectic patients are increased >50% (Fouladiun et al., 2005). Moreover, plasma insulin levels as well as the phosphorylation of Akt are significantly elevated in mice with colon cancer despite reduced protein synthesis (Puppa et al., 2012; White et al., 2011). As noted above, impaired insulin and Akt signaling to mTORC1 may be a consequence of concomitant activation of AMPK.
In addition to impaired signaling through mTORC1 to proteins involved in 48S preinitiation complex assembly, cancer cachexia also impinges on proteins that control assembly of the 43S preinitiation complex. Mice injected with MAC16 tumor cells exhibit increased phosphorylation of both the eIF2α kinase, PKR, and the α-subunit of eIF2 (Eley et al., 2007a; Tisdale, 2009). Elevated phosphorylation of both proteins is also observed in the skeletal muscle of cancer patients exhibiting cachexia (Eley et al., 2008). The increase in PKR phosphorylation is positively correlated with changes in eIF2α phosphorylation, suggesting that activation of PKR leads to eIF2 phosphorylation in human cachexia (Eley et al., 2008). Lastly, an inverse relationship exists between muscle myosin content and levels of eIF2α phosphorylation, providing further support for the idea that reduced rates of protein synthesis during cachexia are directly attributable to PKR-mediated phosphorylation of eIF2 (Eley et al., 2008). Although untested, a possible mechanism that might account for the observed activation of PKR is inflammation, as this pathological feature is increased in both humans and in animal models of cancer and PKR has been shown to be activated in other disease models where inflammation is evident (Cao et al., 2012; McMillan et al., 1994; Puppa et al., 2012; White et al., 2011; White et al., 2012).
Although it is unlikely to be the only cause (Fouladiun et al., 2005; Tisdale, 2009), malnutrition may contribute to the development of cancer cachexia, as anorexia has been noted in 61% of cancer patients nearing the end of life (Tisdale, 2009). Indeed, one study noted decreased plasma levels of free amino acids in cachectic patients (Eley et al., 2007b), and dietary protein supplementation to cancer patients can maintain bodyweight (Eley et al., 2007a; Eley et al., 2007b; Fearon et al., 2003; Tisdale, 2009). Of particular note, dietary supplementation with leucine in mice bearing MAC16 tumors both maintains muscle weight and also attenuates phosphorylation of PKR and eIF2α (Eley et al., 2007b). In addition, leucine supplementation also increases the phosphorylation of 4E-BP1 (Eley et al., 2007b). Similarly, administration of -hydroxy- -methylbutyrate to MAC16 tumor mice increases rates of muscle protein synthesis, decreases phosphorylation of PKR and eIF2α, and increases signaling through mTORC1 (Eley et al., 2007a). Thus malabsorption of amino acids, particularly leucine, may be partially responsible for reduced rates of protein synthesis in cancer cachexia.
4.4. Disuse atrophy
Loss of skeletal muscle mass in response to limb immobilization, limb unloading/suspension, and bed rest, referred to as disuse atrophy, is attributed to changes in the rate of protein synthesis in skeletal muscle. During disuse atrophy, rates of protein synthesis are attenuated under postabsorptive conditions and are resistant to stimulation by nutrients (Phillips et al., 2009). In animal models of hindlimb immobilization, postabsorptive rates of protein synthesis are attenuated as early as 6 hours post-immobilization (Booth and Seider, 1979; Goldspink, 1977). Human models of bed rest and limb immobilization also exhibit attenuated postabsorptive rates of protein synthesis after 7 and 14 days, respectively (Glover et al., 2008; Shangraw et al., 1988).
In addition to reduced postabsorptive rates of protein synthesis, disuse leads to attenuated nutrient-induced stimulation of muscle protein synthesis (Rennie, 2009). In response to both low- and high-dose amino acid infusions, stimulation of protein synthesis was attenuated in vastus lateralis muscle from immobilized compared to non-immobilized limbs (Glover et al., 2008). The resistance to nutrient-induced stimulation of protein synthesis is termed ‘anabolic resistance’ and is observed in other human models of disuse, including bed rest (Biolo et al., 2004; Drummond et al., 2012). The mechanisms responsible for attenuated rates of protein synthesis and anabolic resistance in disuse atrophy are currently unknown, but attenuated mTORC1 signaling and suppression of insulin/IGF-I signaling are possible contributors.
As described above, mTORC1 signaling mediates mitogenic-, exercise-, and nutrient-induced stimulation of protein synthesis in skeletal muscle. Although postabsorptive signaling through mTORC1 does not appear to be attenuated in human disuse atrophy (de Boer et al., 2007; Marimuthu et al., 2011), mTORC1 signaling to p70S6K1 is repressed under postabsorptive conditions in animal models of hindlimb immobilization (Kelleher et al., 2013; Morris et al., 2004; You et al., 2010) and hindlimb suspension (Bajotto et al., 2011; Hornberger et al., 2001). The mechanism responsible for attenuated mTORC1 signaling is unknown, but may be a consequence of upregulated expression of mTORC1 inhibitors such as the regulated in DNA damage and development 1 and 2 (REDD1/2) proteins (Kelleher et al., 2013). In both animal (Kelleher et al., 2013) and human models (Drummond et al., 2012; Glover et al., 2008) of disuse atrophy, nutrient-induced phosphorylation of p70S6K1 is delayed or resistant to stimulation. For example, in response to leucine administration (Kelleher et al., 2013), p70S6K1 is resistant to phosphorylation on Thr389 and Thr229 in soleus muscle from immobilized rat hindlimbs. Phosphorylation of p70S6K1 on Thr229, which is mediated by 3-phosphoinositol-dependent kinase 1 (PDK1), is required for mTORC1-mediated phosphorylation of p70S6K1 on Thr389 (Keshwani et al., 2011). Therefore, the resistance to leucine-induced phosphorylation of p70S6K1 by mTORC1 in the immobilized hindlimb may be consequence of defective PDK1 signaling. A possible explanation for repressed phosphorylation of p70S6K1 on Thr229 is the development of insulin resistance in muscle in the immobilized hindlimb that would perturb activation of PDK1. Notably, insulin resistance and reduced skeletal muscle glucose uptake are observed in people undergoing bed rest (Shangraw et al., 1988; Stuart et al., 1988), limb immobilization (Richter et al., 1989), and spaceflight (Allen et al., 2009; Stein and Wade, 2005). Moreover, IGF-1 resistance occurs in rats after 14 days of hindlimb suspension in skeletal muscle (Allen et al., 1997). Thus, insulin and IGF-1 resistance-induced attenuation of Akt/TSC2 signaling to mTORC1 may provide a mechanism for attenuated protein synthesis in disuse atrophy. The effects of insulin/IGF-1 resistance on mTORC1 signaling may be compounded by changes in expression of Akt. Thus, in a recent study (Bienso et al., 2012), seven days of bed rest reduced Akt1 and Akt2 expression as well as Akt phosphorylation on Thr308 in vastus lateralis biopsies from human participants both before and after hyperinsulinemic-euglycemic clamp. In contrast, similar reductions in Akt protein and phosphorylation have not been observed in other models of disuse atrophy that exhibit insulin resistance (de Boer et al., 2007; Glover et al., 2008; O’Keefe M et al., 2004). Therefore, the precise mechanism by which insulin/IGF-1 resistance contributes to impaired mTORC1 signaling and protein synthesis remains unclear.
Possible mechanisms for suppression of insulin/IGF-1 signaling in models of disuse atrophy include increases in myostatin expression, activation of the p38 MAPK signaling pathway, and upregulated pro-inflammatory cytokine expression. Myostatin suppresses skeletal muscle protein synthesis through inhibition of IGF-1 expression and signaling to Akt (Sun et al., 2006; Zhang et al., 2011) as well as activation of p38 MAPK signaling (Zhang et al., 2011). A caveat to this idea is that myostatin expression is not increased in mice (Dong et al., 2008) or humans (Jones et al., 2004) in other models of disuse, and fails to prevent disuse atrophy in myostatin-deficient mice (McMahon et al., 2003). Regardless of the involvement of myostatin, increases in p38 MAPK expression and phosphorylation are associated with suppression of Akt phosphorylation and development of insulin resistance in rats after one day of hindlimb suspension (O’Keefe M et al., 2004). p38 MAPK is also implicated in promoting pro-inflammatory cytokine expression (Zhang et al., 2011), which is linked to the development of insulin resistance and skeletal muscle wasting (Cai et al., 2004; Li et al., 2008).
Upregulated expression of pro-inflammatory cytokines may also play a role in the development of muscle wasting during disuse atrophy. Exogenous expression of a dominant negative IκB kinase spares skeletal muscle mass in rats following 7 days of hindlimb suspension (Van Gammeren et al., 2009), suggesting that pro-inflammatory cytokine signaling through the nuclear factor kappa-B (NF-κB) pathway may play a major role in skeletal muscle disuse atrophy. Further support for a mechanism based on NF-κB signaling is provided in studies where overexpression of heat shock protein 70 prevents both NF-κB activation and skeletal muscle atrophy in immobilized rats (Senf et al., 2010; Senf et al., 2008). Overall, myostatin, p38 MAPK and pro-inflammatory cytokines are key players in the induction of insulin resistance and attenuation of protein synthesis in animal models of disuse atrophy, but these players have yet to be validated in human models of disuse atrophy.
In conclusion, attenuated rates of skeletal muscle protein synthesis in models of disuse atrophy are associated with attenuated mTORC1 signaling and insulin/IGF-1 resistance. However, more research is necessary to determine the precise mechanism of attenuated protein synthesis in human models of disuse atrophy.
4.5. Chronic Kidney Disease
Patients with chronic kidney disease leading to chronic and end-stage renal failure suffer debilitating skeletal muscle wasting due in part to attenuated rates of protein synthesis. Both chronic (Castellino et al., 1992) and end-stage (Castellino et al., 1999) renal failure patients exhibit attenuated whole-body protein synthesis under postabsorptive conditions. Whole-body protein synthesis increases in response to either hyperinsulinemia or infusion of a combination of insulin and amino acids, but it is resistant to stimulation by hyperaminoacidemia alone (Castellino et al., 1999; Castellino et al., 1992).
In skeletal muscle of chronic renal failure patients, protein synthesis is attenuated without an increase in skeletal muscle protein degradation (Adey et al., 2000). Similar attenuations in skeletal muscle protein synthesis have been reported in healthy participants made metabolically acidotic by ammonium chloride supplementation (Kleger et al., 2001), suggesting that development of metabolic acidosis in chronic kidney disease likely contributes to the repression of skeletal muscle protein synthesis and development of skeletal muscle wasting. Interestingly, although end-stage renal failure patients without metabolic acidosis do not exhibit attenuated rates of skeletal muscle protein synthesis (Raj et al., 2004), they still experience skeletal muscle wasting due in part to hemodialysis-induced skeletal muscle protein catabolism (Ikizler, 2005; Raj et al., 2004).
One mechanism through which metabolic acidosis may attenuate protein synthesis in patients with chronic kidney disease is through suppression of insulin/IGF-1 signaling. Metabolic acidosis and uremia in response to kidney dysfunction stimulate the release of glucocorticoids (Slee, 2012), leading to inhibition of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), suppression of Akt phosphorylation (Hu et al., 2009), and upregulated expression of the mTORC1 repressor REDD1 (Wang et al., 2006). Metabolic acidosis further inhibits PI3K signaling to Akt by increasing expression of its p85 subunit without a concomitant increase in the expression of the p110 catalytic subunit (Bailey et al., 2006). Upregulated expression of p85 alone results in assembly of a p85•IRS-1 complex that interferes with the binding of the p85•p110 complex to IRS-1 thereby preventing its activation under acidotic conditions.
Increases in pro-inflammatory cytokine expression with chronic kidney disease may also attenuate protein synthesis and suppress insulin/IGF-1 signaling through multiple mechanisms. Pro-inflammatory cytokines lead to increased expression of both myostatin (Sun et al., 2006; Zhang et al., 2011) and suppressors of cytokine signaling (SOCS), which inhibit insulin/IGF-1 signaling through decreased IGF-1 expression (Mak and Rotwein, 2006) and IRS-1 tyrosine phosphorylation (Cheung et al., 2008; Suchy et al., 2013; Tanti et al., 2012). In mice with chronic kidney disease, anti-myostatin peptibody administration improves IGF-1 signaling to Akt and increases skeletal muscle protein synthesis (Zhang et al., 2011). Moreover, increases in skeletal muscle mass are associated with suppression of myostatin expression and induction of IGF-1 expression in work-induced overloaded uremic rats (Chen et al., 2008; Sun et al., 2006). Insulin/IGF-1 resistance is likely to attenuate protein synthesis through suppression of IRS-1/PI3K/Akt signaling to mTORC1. Despite evidence of SOCS-mediated insulin resistance under diabetic conditions and myostatin-mediated suppression of IGF-1 signaling in animal models, the mechanism by which insulin/IGF-1 resistance attenuates skeletal muscle protein synthesis in humans is unclear (Mak and Rotwein, 2006; Siew et al., 2007; Suchy et al., 2013). Therefore, more research is necessary to determine the roles of SOCS and myostatin in attenuating skeletal muscle protein synthesis in humans with chronic kidney disease.
Attenuated rates of skeletal muscle protein synthesis with chronic kidney disease are likely to be compounded by malnutrition as a result of anorexia. Systemic inflammation associated with chronic kidney disease disrupts the hypothalamic response to the appetite-regulating hormones leptin, ghrelin and melanocortin, resulting in persistent activation of neural anorexigenic pathways (Cheung et al., 2008; Slee, 2012). The resulting anorexia limits nutrient intake of amino acids (Mak et al., 2011), and amino acids can become further limited in patients receiving hemodialysis if they are not replaced (Ikizler et al., 2002). Low protein intake can also decrease circulating IGF-1 concentrations (Donahue and Phillips, 1989). Combined, reduced concentrations of plasma amino acids and IGF-1 likely result in repression of skeletal muscle mTORC1 signaling and protein synthesis. In conclusion, a possible mechanism for attenuated skeletal muscle protein synthesis with chronic kidney disease may be due to suppression of the insulin/IGF-1 signaling due to metabolic acidosis, upregulated pro-inflammatory cytokine expression, and malnutrition due to anorexia.
5. Conclusions and future directions
Loss of muscle mass is a debilitating feature that is a common manifestation of a variety of diseases, and leads to reduced muscle function and increased morbidity and mortality. Maintenance of skeletal muscle mass relies on a dynamic balance between protein synthesis and degradation. In atrophic muscle, the balance between synthesis and degradation is disrupted in favor of reduced synthesis and/or increased degradation. At a molecular level, the reduced rate of protein synthesis that is commonly observed in muscle wasting diseases is associated with impaired assembly of the 43S and/or 48S preinitiation complex (Fig. 1). Assembly of the 48S preinitiation complex is in large part controlled by signaling through mTORC1, and in many cases therapeutic interventions are targeted toward activating the kinase. As an example, nutritional supplementation with leucine or mixtures of branched-chain amino acids and/or resistance exercise are used to stimulate signaling through the kinase. However, the concurrent development of insulin resistance, increased corticosteroid production, and/or inflammation may limit the effectiveness of amino acids and resistance exercise in stimulating protein synthesis. It is also important to note that a general reduction in overall physical activity may be an underlying contributor to the impaired rates of protein synthesis and subsequent reductions in muscle mass observed in many of the conditions discussed above. For example, aerobic exercise can spare muscle mass in a mouse model of colon cancer, and a single bout of aerobic exercise increases nutrient-induced rates of protein synthesis in aged individuals (Fujita et al., 2007; Puppa et al., 2012). If physical activity is not an option, such as with bed rest or limb immobilization, development of therapies that target the mechanisms through which these repressors act may be requisite in designing more effective treatments to increase mTORC1 signaling and maintain muscle mass. Devising effective strategies for reversing the inhibition of protein synthesis is also complicated by the perturbation of 43S preinitiation complex assembly that occurs concomitantly with repressed mTORC1 signaling in many atrophic conditions. Although to our knowledge the relative contribution of repressed 43S compared to 48S preinitiation complex assembly in the control of global rates of protein synthesis has not been addressed in vivo, in cells in culture inhibition of 43S preinitiation complex assembly appears to be dominant (Kimball et al., 1998). Assuming this phenomenon also occurs in muscle in vivo, delineating the mechanisms involved in the regulation of eIF2α phosphorylation and eIF2B GEF activity during muscle wasting will be critical in identifying effective strategies for therapeutic intervention. The studies that are described above have greatly expanded our knowledge concerning the mechanisms through which protein synthesis is regulated during muscle wasting. However, many questions remain unanswered, and more work is necessary to fully understand the regulation of protein synthesis in human disease states as much of our current knowledge is derived from animal studies. Although these studies provide valuable information about the mechanistic details involved in the development of muscle wasting, there is sometimes a disconnect between results from cell culture/animal studies and human disease situations. Moreover, although in most cases changes in mTORC1 signaling and phosphorylation of its downstream targets correlate well with alterations in human muscle protein synthesis, in some studies modulation of upstream regulators of mTORC1 signaling exhibit inconsistent patterns of change (Beelen et al., 2011; Greenhaff et al., 2008; Rasmussen, 2011). Thus, a more detailed understanding of the regulatory mechanisms controlling protein synthesis in humans will aid in the development of effective and personalized therapies that preserve muscle mass.
Acknowledgements
The authors would like to thank Dr. Leonard S. Jefferson for critical reading and helpful comments during preparation of the manuscript. Work described in this document that was performed in the authors’ laboratory was supported by NIH grant DK15658.
Abbreviations
- 4E-BP1
eIF4E binding proteins
- 5’-UTR
5’-untranslated region
- AMPK
AMP-activated protein kinase
- eIF
eukaryotic initiation factor
- GCN2
general-control nonderepressible
- GEF
guanine nucleotide exchange factor
- GSK-3
glycogen synthase kinase-3
- HRI
heme-regulated inhibitor
- ICU
Intensive Care Unit
- IGF-1
insulin-like growth factor-1
- IRES
internal ribosome entry site
- LPS
lipopolysaccharide
- Met-tRNAi
initiator methionyl-tRNA
- mTORC1
mechanistic target of rapamycin in complex 1
- NF-κB
nuclear factor kappa-B
- p70S6K1
70 kDa ribosomal protein S6 kinase 1
- PDCD4
programmed cell death 4
- PDK1
3-phosphoinositol-dependent kinase 1
- PERK
PKR-like endoplasmic reticulum-associated protein kinase
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKR
double-stranded RNA-dependent protein kinase
- REDD
regulated in DNA damage and development
- Rheb
ras homolog enriched in brain
- TSC
tuberous sclerosis complex
- TNF
tumor necrosis factor
- uORF
upstream open reading frame
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278:E219–E225. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, et al. Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol. 2009;106:582–595. doi: 10.1152/japplphysiol.90780.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol. 1997;83:1857–1861. doi: 10.1152/jappl.1997.83.6.1857. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- Bajotto G, Sato Y, Kitaura Y, Shimomura Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur J Appl Physiol. 2011;111:1815–1828. doi: 10.1007/s00421-010-1825-8. [DOI] [PubMed] [Google Scholar]
- Balage M, Averous J, Remond D, Bos C, Pujos-Guillot E, Papet I, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2010;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Beelen M, Zorenc A, Pennings B, Senden JM, Kuipers H, van Loon LJ. Impact of protein coingestion on muscle protein synthesis during continuous endurance type exercise. Am J Physiol Endocrinol Metab. 2011;300:E945–E954. doi: 10.1152/ajpendo.00446.2010. [DOI] [PubMed] [Google Scholar]
- Bienso RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, et al. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes. 2012;61:1090–1099. doi: 10.2337/db11-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Ciocchi B, Lebenstedt M, Barazzoni R, Zanetti M, Platen P, et al. Short-term bed rest impairs amino acid-induced protein anabolism in humans. J Physiol. 2004;558:381–388. doi: 10.1113/jphysiol.2004.066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol. 1979;47:974–977. doi: 10.1152/jappl.1979.47.5.974. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cao SS, Song B, Kaufman RJ. PKR protects colonic epithelium against colitis through the unfolded protein response and prosurvival signaling. Inflamm Bowel Dis. 2012;18:1735–1742. doi: 10.1002/ibd.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino P, Luzi L, Giordano M, Defronzo RA. Effects of insulin and amino acids on glucose and leucine metabolism in CAPD patients. J Am Soc Nephrol. 1999;10:1050–1058. doi: 10.1681/ASN.V1051050. [DOI] [PubMed] [Google Scholar]
- Castellino P, Solini A, Luzi L, Barr JG, Smith DJ, Petrides A, et al. Glucose and amino acid metabolism in chronic renal failure: effect of insulin and amino acids. Am J Physiol Renal Physiol. 1992;262:F168–F176. doi: 10.1152/ajprenal.1992.262.2.F168. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sood S, Biada J, Roth R, Rabkin R. Increased workload fully activates the blunted IRS-1/PI3-kinase/Akt signaling pathway in atrophied uremic muscle. Kidney Int. 2008;73:848–855. doi: 10.1038/sj.ki.5002801. [DOI] [PubMed] [Google Scholar]
- Cheung WW, Rosengren S, Boyle DL, Mak RH. Modulation of melanocortin signaling ameliorates uremic cachexia. Kidney Int. 2008;74:180–186. doi: 10.1038/ki.2008.150. [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol. 2011;589:3883–3895. doi: 10.1113/jphysiol.2011.206193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KC, Colletti RB, Devlin JT, McAuliffe TL. The relationship between insulin-like growth factor-1, adiposity, and aging. Metabolism. 1990;39:584–587. doi: 10.1016/0026-0495(90)90022-5. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Maganaris CN, Seynnes OR, Rennie MJ, Narici MV. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MD, Jefferson LS, Kimball SR. Role of p70S6K1-mediated phosphorylation of eIF4B and PDCD4 proteins in the regulation of protein synthesis. J Biol Chem. 2012;287:42890–42899. doi: 10.1074/jbc.M112.404822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AS, Treebak JT, Long YC, Viollet B, Wojtaszewski JFP, Zierath JR. Role of adenosine 5′-monophosphate-activated protein kinase subunits in skeletal muscle mammalian target of rapamycin signaling. Mol Endocrinol. 2008;22:1105–1112. doi: 10.1210/me.2007-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Hinnebusch AG. GCN2 whets the appetite for amino acids. Mol Cell. 2005;18:141–142. doi: 10.1016/j.molcel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue SP, Phillips LS. Response of IGF-1 to nutritional support in malnourished hospital patients: a possible indicator of short-term changes in nutritional status. Am J Clin Nutr. 1989;50:962–969. doi: 10.1093/ajcn/50.5.962. [DOI] [PubMed] [Google Scholar]
- Dong F, Kandadi MR, Ren J, Sreejayan N. Chromium (D-phenylalanine)3 supplementation alters glucose disposal, insulin signaling, and glucose transporter-4 membrane translocation in insulin-resistant mice. J Nutr. 2008;138:1846–1851. doi: 10.1093/jn/138.10.1846. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007a;293:E923–E931. doi: 10.1152/ajpendo.00314.2007. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Tisdale MJ. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007b;407:113–120. doi: 10.1042/BJ20070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley HL, Skipworth RJ, Deans DA, Fearon KC, Tisdale MJ. Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2alpha may signal skeletal muscle atrophy in weight-losing cancer patients. Br J Cancer. 2008;98:443–449. doi: 10.1038/sj.bjc.6604150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984;289:584–586. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care--correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103:2189–2198. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda, Md) 2011;26:83–96. doi: 10.1152/physiol.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:1–11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinemia is necessary to stimulate skeletal muscle protein anabolism in older adults: Evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52:1889–1898. doi: 10.1007/s00125-009-1430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improvingendothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher IJ, Stephens NA, MacDonald AJ, Skipworth RJ, Husi H, Greig CA, et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clin Cancer Res. 2012;18:2817–2827. doi: 10.1158/1078-0432.CCR-11-2133. [DOI] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet ED, Khursigara Z, Gougeon R, Morais JA. Postprandial insulin sensitivity and thermogenesis in frail elderly women. Appl Physiol Nutr Metab. 2010;35:526–533. doi: 10.1139/H10-041. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly human. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Weng Q-P, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Hershey JW, Sonenberg N, Mathews MB. Principles of translational control: an overview. Cold Spring Harb Perspect Biol. 2012;4:a011528. doi: 10.1101/cshperspect.a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol. 2012;4:a011544. doi: 10.1101/cshperspect.a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Hunter RB, Kandarian SC, Esser KA. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol. 2001;281:C179–C187. doi: 10.1152/ajpcell.2001.281.1.C179. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest. 2009;119:3059–3069. doi: 10.1172/JCI38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64:618–628. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikizler TA. Effects of hemodialysis on protein metabolism. J Ren Nutr. 2005;15:39–43. doi: 10.1053/j.jrn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Ikizler TA, Pupim LB, Brouillette JR, Levenhagen DK, Farmer K, Hakim RM, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan K-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, et al. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS One. 2011;6:e18090. doi: 10.1371/journal.pone.0018090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18:1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- Kang R, Tang D. PKR-dependent inflammatory signals. Sci Signal. 2012;5:e47. doi: 10.1126/scisignal.2003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab. 2013;304:E229–E236. doi: 10.1152/ajpendo.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshwani MM, von Daake S, Newton AC, Harris TK, Taylor SS. Hydrophobic motif phosphorylation is not required for activation loop phosphorylation of p70 ribosomal protein S6 kinase 1 (S6K1) J Biol Chem. 2011;286:23552–23558. doi: 10.1074/jbc.M111.258004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J Biol Chem. 1998;273:30945–30953. doi: 10.1074/jbc.273.47.30945. [DOI] [PubMed] [Google Scholar]
- Kleger GR, Turgay M, Imoberdorf R, McNurlan MA, Garlick PJ, Ballmer PE. Acute metabolic acidosis decreases muscle protein synthesis but not albumin synthesis in humans. Am J Kidney Dis. 2001;38:1199–1207. doi: 10.1053/ajkd.2001.29215. [DOI] [PubMed] [Google Scholar]
- Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, et al. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139:1707–1713. doi: 10.3945/jn.109.109173. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol. 2000;278:E1133–E1143. doi: 10.1152/ajpendo.2000.278.6.E1133. [DOI] [PubMed] [Google Scholar]
- Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Lundholm K, Holm G, Schersten T. Insulin resistance in patients with cancer. Cancer Res. 1978;38:4665–4670. [PubMed] [Google Scholar]
- Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2:9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak RH, Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int. 2006;70:410–412. doi: 10.1038/sj.ki.5001622. [DOI] [PubMed] [Google Scholar]
- Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J Appl Physiol. 2011;110:555–560. doi: 10.1152/japplphysiol.00962.2010. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E, Piñeiro D, Fernández N. Alternative mechanisms to initiate translation in eukaryotic mRNAs. Comp Funct Genomics. 2012;2012:12. doi: 10.1155/2012/391546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CD, Popovic L, Oldham JM, Jeanplong F, Smith HK, Kambadur R, et al. Myostatin-deficient mice lose more skeletal muscle mass than wild-type controls during hindlimb suspension. Am J Physiol Endocrinol Metab. 2003;285:E82–E87. doi: 10.1152/ajpendo.00275.2002. [DOI] [PubMed] [Google Scholar]
- McMillan DC, Preston T, Fearon KC, Burns HJ, Slater C, Shenkin A. Protein synthesis in cancer patients with inflammatory response: investigations with [15N]glycine. Nutrition. 1994;10:232–240. [PubMed] [Google Scholar]
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J Appl Physiol. 2004;96:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- O’Keefe MP, Perez FR, Kinnick TR, Tischler ME, Henriksen EJ. Development of whole-body and skeletal muscle insulin resistance after one day of hindlimb suspension. Metabolism. 2004;53:1215–1222. doi: 10.1016/j.metabol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Orellana RA, Kimball SR, Nguyen HV, Bush JA, Suryawan A, Thivierge MC, et al. Regulation of muscle protein synthesis in neonatal pigs during prolonged endotoxemia. Pediatr Res. 2004;55:442–449. doi: 10.1203/01.PDR.0000110526.02282.F3. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2003;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- Park E-H, Walker SE, Zhou F, Lee JM, Rajagopal V, Lorsch JR, et al. Yeast eukaryotic initiation factor 4B (eIF4B) enhances complex assembly between eIF4A and eIF4G in vivo. J Biol Chem. 2013;288:2340–2354. doi: 10.1074/jbc.M112.398537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PWF, Abad LW, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the framingham heart study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:645–654. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- Plank LD, Connolly AB, Hill GL. Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann Surg. 1998;228:146–158. doi: 10.1097/00000658-199808000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, et al. The effect of exercise on IL-6-induced cachexia in the Apc min/+ mouse. J Cachexia Sarcopenia Muscle. 2012;3:117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj DS, Dominic EA, Wolfe R, Shah VO, Bankhurst A, Zager PG, et al. Coordinated increase in albumin, fibrinogen, and muscle protein synthesis during hemodialysis: role of cytokines. Am J Physiol Endocrinol Metab. 2004;286:E658–E664. doi: 10.1152/ajpendo.00444.2003. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB. The missing Akt in the mechanical regulation of skeletal muscle mTORC1 signalling and growth. J Physiol. 2011;589:1507. doi: 10.1113/jphysiol.2011.207837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Volpi E. In: Muscle biology and mTORC1 signaling in aging. Cruz-Jenfoft AJ, Morley JE, editors. Sarcopenia: John Wiley & Sons; 2012. [Google Scholar]
- Reid PJ, Mohammad-Qureshi SS, Pavitt GD. Identification of intersubunit domain interactions within eukaryotic initiation factor (eIF) 2B, the nucleotide exchange factor for translation initiation. J Biol Chem. 2012;287:8275–8285. doi: 10.1074/jbc.M111.331645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- Richter EA, Kiens B, Mizuno M, Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol. 1989;67:19–23. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Payron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GW, Jr, Richter NJ, Merrick WC. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J Biol Chem. 1999;274:12236–12244. doi: 10.1074/jbc.274.18.12236. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, Springer J. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle. 2011;2:105–109. doi: 10.1007/s13539-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol. 2010;298:C38–C45. doi: 10.1152/ajpcell.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangraw RE, Stuart CA, Prince MJ, Peters EJ, Wolfe RR. Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am J Physiol. 1988;255:E548–E558. doi: 10.1152/ajpendo.1988.255.4.E548. [DOI] [PubMed] [Google Scholar]
- Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acids mammalian target of rapamycin signaling pathways Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007;71:146–152. doi: 10.1038/sj.ki.5001984. [DOI] [PubMed] [Google Scholar]
- Slee AD. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond) 2012;9:36. doi: 10.1186/1743-7075-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TP, Wade CE. Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S. doi: 10.1093/jn/135.7.1824S. [DOI] [PubMed] [Google Scholar]
- Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Suchy D, Labuzek K, Machnik G, Kozlowski M, Okopien B. SOCS and diabetes-ups and downs of a turbulent relationship. Cell Biochem Funct. 2013;31:181–195. doi: 10.1002/cbf.2940. [DOI] [PubMed] [Google Scholar]
- Sun DF, Chen Y, Rabkin R. Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int. 2006;70:453–459. doi: 10.1038/sj.ki.5001532. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne) 2012;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- Tuckow AP, Vary TC, Kimball SR, Jefferson LS. Ectopic expression of eIF2Bε in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E241–E248. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009;23:362–370. doi: 10.1096/fj.08-114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary TC, Deiter G, Kimball SR. Phosphorylation of eukaryotic initiation factor eIF2Be in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 2002;283:E1032–E1039. doi: 10.1152/ajpendo.00171.2002. [DOI] [PubMed] [Google Scholar]
- Vary TC, Jurasinski C, Kimball SR. Reduced 40S initiation complex formation in skeletal muscle during sepsis. Mol Cell Biochem. 1998;178:81–86. doi: 10.1023/a:1006826331115. [DOI] [PubMed] [Google Scholar]
- Vary TC, Jurasinski CV, Karinch AM, Kimball SR. Regulation of eukaryotic initiation factor 2 expression in sepsis. Am J Physiol Endocrinol Metab. 1994;266:E193–E201. doi: 10.1152/ajpendo.1994.266.2.E193. [DOI] [PubMed] [Google Scholar]
- Voisin L, Gray K, Flowers KM, Kimball SR, Jefferson LS, Vary TC. Altered expression of eukaryotic initiation factor 2B in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 1996;270:E43–E50. doi: 10.1152/ajpendo.1996.270.1.E43. [DOI] [PubMed] [Google Scholar]
- Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281:39128–39134. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- Wang X, Proud CG. A novel mechanism for the control of translation initiation by amino acids, mediated by phosphorylation of eukaryotic Initiation factor 2B. Molec Cell Biol. 2008;28:1429–1442. doi: 10.1128/MCB.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am JPhysiol Endocrinol Metab. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, et al. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophynor strength of the elbow flexors. J Appl Physiol. 2010;108:60–67. doi: 10.1152/japplphysiol.01147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DW, Phillips SM. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol. 2012;112:2693–2702. doi: 10.1007/s00421-011-2246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethmar K, Smink JJ, Leutz A. Upstream open reading frames: molecular switches in (patho)physiology. BioEssays. 2010;32:885–893. doi: 10.1002/bies.201000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6:e24650. doi: 10.1371/journal.pone.0024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. 2012;2:14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Phillips BE, Smith K, Atherton PJ, Rankin D, Selby AL, et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96:1064–1070. doi: 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- Yang H-S, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 Is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Molec Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- You JS, Park MN, Song W, Lee YS. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl Physiol Nutr Metab. 2010;35:310–318. doi: 10.1139/H10-022. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011;25:1653–1663. doi: 10.1096/fj.10-176917. [DOI] [PMC free article] [PubMed] [Google Scholar]