Abstract
Objective
To test relationships between adipokines, adiposity, and vasomotor symptoms (VMS), including how these associations vary by menopause stage.
Design
A sub-cohort of the longitudinal cohort study the Study of Women’s Health Across the Nation completed questionnaires, physical measures, and a fasting blood draw annually for 8 years. Associations between a poorer adipokine profile [lower adiponectin, lower high molecular weight (HMW) adiponectin, higher leptin, lower soluble leptin receptor, higher monocyte chemoattractant protein-1 (MCP-1)] and VMS were tested using generalized estimating equations adjusting for potential confounders. Interactions by menopause stage (pre-/early perimenopause, late peri-/postmenopause) were tested.
Setting
Community
Patients
536 women ages 42–52 at baseline
Interventions
None
Main Outcome Measures
VMS
Results
Associations between adipokines and hot flashes varied by menopause stage, with a poorer adipokine profile associated with higher odds of hot flashes early in the transition [adiponectinlog, OR(95% CI): 0.68 (0.51–0.90); HMW adiponectinlog, OR(95% CI): 0.70 (0.58–0.85); leptinlog, OR(95% CI): 1.23 (0.99–1.54), multivariable models including BMI], but not later in the transition. The direction of associations between BMI and VMS also varied by menopausal stage. Higher MCP-1 was associated with more night sweats [OR(95% CI): 1.37 (1.06–1.76)] across menopausal stages.
Conclusions
An adverse adipokine profile was associated with more VMS, particularly early in the menopause transition.
Key Terms: Adipokines, adiposity, vasomotor symptoms, hot flashes
INTRODUCTION
Vasomotor symptoms (VMS; hot flashes, night sweats) are experienced by most women during the menopause transition (1). Hot flashes are associated with impaired quality of life (2), poor sleep (3), and depressed mood (4), and therefore, women often seek medical treatment of them (5). Despite their prevalence and impact on women’s lives, the underlying physiology and risk factors for VMS are poorly understood.
One risk factor of interest is adiposity. Overweight/obesity is a major issue for midlife women, as most US midlife women are overweight or obese (6). Although higher adiposity adversely impacts multiple health outcomes, its relationship to VMS is more complex. In fact, higher body fat has traditionally been assumed to be protective against VMS due to the peripheral aromatization of androgens into estrogens in body fat. However, findings from large cohort studies challenge this perspective (1), showing higher adiposity linked to more VMS. Reconciling these two perspectives is the suggestion that associations between adiposity and VMS may be menopause stage-dependent (7), with body fat possibly acting as a risk factor for VMS early in the menopause transition (8), and protective later (9). Notably, findings also indicate stage-dependent associations between body fat and estradiol (E2), with higher body mass index (BMI) associated with lower endogenous E2 early in the transition, but higher E2 later in the transition (10–12).
Mechanisms linking adiposity to VMS are not understood. However, body fat is an active endocrine organ and secretes multiple cytokines and inflammatory factors that may be related to VMS occurrence and/or ovarian function (13). One cross sectional study (14) provides preliminary evidence of relationships between hot flashes and higher leptin levels, a key pro-inflammatory adipokine and regulator of energy homeostasis (15). Several other adipokines may be relevant. For example, leptin’s bioavailability may be determined by its binding to its soluble leptin receptor (sOB-R) (16, 17) which is downregulated with obesity (18). Further, adiponectin is the most abundant adipokine in circulation (19), is inversely related to BMI, and exerts anti-inflammatory effects (20). The high molecular weight (HMW) isoform of adiponectin may be its most biologically active (21). The chemokine monocyte chemoattractant protein-1 (MCP-1) attracts macrophages into adipose tissue and is pro-inflammatory (22). Thus, a range of adipokines can be considered in relation to VMS.
We examined associations between several adipokines (adiponectin, HMW adiponectin, leptin, sOB-R, MCP-1) and hot flashes and night sweats over 8 years in the Study of Women’s Health Across the Nation (SWAN), a large, prospective study of women transitioning through the menopause. We hypothesized that a more adverse adipokine profile (lower adiponectin, lower HMW adiponectin, higher leptin, lower sOB-R, higher MCP-1) would be associated with greater VMS occurrence. We also considered the relation between BMI and VMS, and the role of adipokines in the relation between BMI and VMS. Finally, given suggestion of a potential stage dependence of associations between adiposity and VMS, we considered whether relationships between adipokines, BMI, and VMS varied by menopause stage. This analysis represents the first study examining links between adipokines and VMS in a prospective study of women transitioning through the menopause.
MATERIALS AND METHODS
Study Population
The current study included a sub-cohort of participants from SWAN, a community-based, longitudinal study of the menopause transition (23). Briefly, SWAN is conducted at seven sites: Boston; Chicago; the Detroit area; Los Angeles; Newark, NJ; Pittsburgh, PA; and Oakland, CA. From 1996 to 1997, 3,302 women aged 42–52 years were enrolled. Each site recruited Caucasian women plus one racial-ethnic group: African American (Pittsburgh, Chicago, Michigan, Boston), Chinese (Oakland), Japanese (UCLA) and Hispanic (Newark). Baseline eligibility criteria for SWAN included being aged 42–52 years, having a uterus and ≥one ovary, not being pregnant or lactating, not using oral contraceptives or hormone therapy (HT), and having ≥one menstrual cycle in the prior 3 months.
Adipokines were collected in ancillary study conducted at the Michigan SWAN site. Eligibility for this study included being aged 42–52 years, African American or white, having menstruated in the preceding 3 months, and having not used oral contraceptives or HT within 3 months of enrollment. Procedures included annual examinations, questionnaires, and a blood draw. Written informed consent was obtained from all participants and procedures approved by the University of Michigan’s Institutional Review Board. A cohort of 536 women was enrolled for this ancillary study; all 536 women were included in primary models.
Adipokines
Adipokines were assayed in serum collected from the baseline and follow-up visits in years 1, 3, 4, 5, 6, and 7. A 12-hour fasting blood draw was performed within days 2–5 of the menstrual cycle if a woman was still menstruating. For women not regularly menstruating or if a blood sample was not obtainable in the day 2–5 window, a random fasting blood draw was obtained. Leptin, sOB-R, total and HMW adiponectin were determined at the University of Michigan, in duplicate, using commercially available colorimetric enzyme immunoassay kits according to the manufacturer’s instructions (leptin, adiponectin, and HMW adiponectin: Millipore, St. Charles, MO; sOB-R and MCP-1: R&D systems, Minneapolis, MN). The mean coefficient of variation percent (CV%) for duplicate samples for each subject and lower limit of detection (LLD), respectively, were: adiponectin: 5.4%, 0.78 ng/ml; HMW adiponectin: 8.1%, 0.5 ng/ml; leptin: 4%, 0.5 ng/mL; sOB-R: 3.7%, 0.31 ng/ml; MCP-1: 1.7%, 5.0 pg/ml).
Anthropometric measures
Height and weight were measured in light clothing without shoes. BMI was calculated as weight (kilograms)/height (meter)2 and considered primarily as a continuous variable but additionally as a categorical variable (normal weight: <25, overweight: 25–<30; obese: ≥30) in all models. Waist circumference was measured at the natural waist (the narrowest part of the torso as seen from the anterior aspect). If a waist narrowing was difficult to identify, the measure was taken at the smallest horizontal circumference in the area between the ribs and the iliac crest. Bioelectrical impedance analysis quantified fat mass and percentage of body fat (24, 25).
Vasomotor symptoms (VMS)
At each SWAN visit VMS were assessed via questionnaire. Women responded to two questions which asked separately how often they experienced 1) hot flashes and 2) night sweats in the past two weeks (not at all, 1–5 days, 6–8 days, 9–13 days, every day; categorized as none vs. any for analysis). Hot flashes and night sweats were considered separately due to their potentially differential pattern of associations with adipokines.
Other covariates
Self-defined race/ethnicity and education (categorized as < vs. ≥college) were obtained at the baseline SWAN interview. Age, smoking status (current vs. past/never), physical activity, menopause status, depressive symptoms, blood pressure, health status, and medication use were derived from standard questionnaires, interviews, and physical measures administered at annual visits. Physical activity was assessed via a modified Kaiser Permanente Health Plan Activity Survey (26). Diabetes status and overall self-rated health (response options: poor, fair, good, excellent) were reported at each visit. Depressive symptoms were assessed via the Center for Epidemiologic Studies Depression Scale (27), considered as a continuous scale score. Systolic and diastolic blood pressure (DBP) were assessed annually via seated measurements. The blood pressure index most strongly related to the outcome (DBP) was included in models here. Menopause status was obtained annually from self-reported bleeding patterns over the prior year, categorized as premenopausal (bleeding in the previous three months with no change in cycle predictability in the past year) early perimenopausal (bleeding in the previous three months with decrease in cycle predictability in the past year), late perimenopausal (<12 and >3 months of amenorrhea), postmenopausal (≥12 of amenorrhea), surgical menopause (hysterectomy with/without oophorectomy prior to reaching postmenopause), and unknown (past-year use of HT before the documentation of a final menstrual period). HT use was reported use of HT during the past year.
E2 assays were performed on the ACS-180 automated analyzer (Bayer Diagnostics Corporation, Tarrytown, NY) utilizing a double-antibody chemiluminescent immunoassay with a solid phase anti-IgG immunoglobulin conjugated to paramagnetic particles, anti-ligand antibody, and competitive ligand labeled with dimethylacridinium ester (DMAE). The E2 assay modifies the rabbit anti-E2-6 ACS-180 immunoassay to increase sensitivity, with a LLD of 6.6 pg/mL and inter- and intra-assay CV’s of 10.6% and 6.4%, respectively (28). Duplicate E2 assays were conducted and results reported as the arithmetic mean.
Data analysis
Adipokine and E2 values were log transformed to normalize distributions. Longitudinal associations between adipokines and hot flashes were estimated using generalized estimating equations (GEE), a method that accounts for repeated measures within subjects and potential attrition from the study. Covariates were selected based upon association with the outcome at p<0.10. Models were adjusted for age, ethnicity, education, smoking, and menopause stage, depressive symptoms, diabetes, DBP, self-rated health, physical activity, HT use, and additionally BMI (all but ethnicity and education time-varying). Interaction terms between menopause stage and each adipokine were tested. Where a significant interaction was detected, models stratified by time-varying menopause stage (pre/early perimenopause versus late/post menopause). Visits in which women were unable to be staged (unknown status: n=83; surgically menopausal: n=43) were excluded from stage-stratified models. Associations between BMI and hot flashes and any stage-dependence of these associations were considered similarly. Each adipokine was added to multivariable menopause stage-stratified models of relationships between BMI and hot flashes. Sensitivity analyses considered fat mass, waist circumference, or percent body fat instead of BMI. The role of E2 was determined by examining overall associations between E2 and hot flashes in GEE models, as well as E2 in relationships between adipokines and/or BMI and hot flashes in overall and stage-dependent models. Cycle day of blood draw (in/out of cycle days 2–5) was included as a covariate in E2 models. Interactions between adipokines and race/ethnicity in relation to hot flashes were examined as cross product terms in multivariable models. All models were repeated for night sweats. Models were 2-sided, alpha=0.05 and performed with SAS v9.2 (Cary, NC).
RESULTS
At baseline, women were on average 46 years old, obese, and nonsmoking (Table 1). Most of the women were African American. By design, approximately half of the sample was premenopausal and half was early perimenopausal at baseline.
Table 1.
Baseline sample characteristics (N=528)
| Age, M(SD) | 46.11 (2.73) |
| Race/ethnicity, N(%) | |
| African American | 318 (60.23) |
| White | 210 (39.77) |
| Menopausal stage, N(%) | |
| Premenopausal | 264 (50.29) |
| Early perimenopausal | 261 (49.71) |
| Educational attainment, N(%) | |
| Less than college | 390 (76.2) |
| College degree or higher | 122 (23.83) |
| Smoking status, N(%) yes/current | 139 (26.7) |
| Overall self-rated health, N(%) | |
| Good or excellent | 428 (82.5) |
| Fair or poor | 91 (17.5) |
| Diabetes status, N(%) yes | 46 (8.9) |
| BMI (continuous), M(SD) | 32.06 (8.09) |
| BMI (categorical), N(%) | |
| Normal weight (<25) | 107 (20.7) |
| Overweight (25–<30) | 120 (23.3) |
| Obese (≥30) | 289 (56.0) |
| % body fat, M(SD) | 40.68 (9.15) |
| Depressive symptomsa, M(SD) | 12.26 (10.83) |
| SBP, M(SD) | 119.85 (19.61) |
| DBP, M(SD) | 71.64 (11.14) |
| Physical activityb, M(SD) | 7.32 (1.84) |
| Adiponectin, (μg/mL), M(SD) | 10.11 (6.04) |
| HMW adiponectin, (μg/mL), M(SD) | 4.03 (3.59) |
| Leptin, (ng/mL), M(SD) | 31.44 (19.92) |
| Soluble leptin receptor, (ng/mL), M(SD) | 24.89 (10.45) |
| MCP-1 (pg/mL), mean (SD) | 306.34 (150.34) |
| Hot flashes, N(%) yes | 201 (38.36) |
| Night sweats, N(%) yes | 206 (39.32) |
Note: N=536 for primary analyses included; 8 women missing baseline data
Center for Epidemiologic Studies Depression scale score
modified Kaiser Permanente Health Plan Activity Survey scale score
We examined the overall association between adipokines and hot flashes. Controlling for all covariates except for BMI revealed marginal associations between higher HMW adiponectin (OR (95% CI): 0.86 (0.72–1.01), p=0.07) and lower odds of hot flashes and higher leptin (OR (95% CI): 1.14 (0.99–1.32), p=0.07) and higher sOB-R (OR (95% CI): 1.45 (0.98–2.13), p=0.06) and higher odds of hot flashes. Addition of BMI had minimal impact on models (HMW adiponectin: OR (95% CI): 0.84 (0.70–1.00), p=0.05; leptin: OR (95% CI): 1.17 (0.97–1.40), p=0.10; sOB-R: OR (95% CI): 1.51 (1.00–2.30), p=0.05). Associations between adiponectin (OR (95% CI): 0.82 (0.64–1.05), p=0.11) or MCP-1 (OR (95% CI): 1.11 (0.85–1.45), p=0.43) and hot flashes were not significant in either model (here multivariable models with BMI).
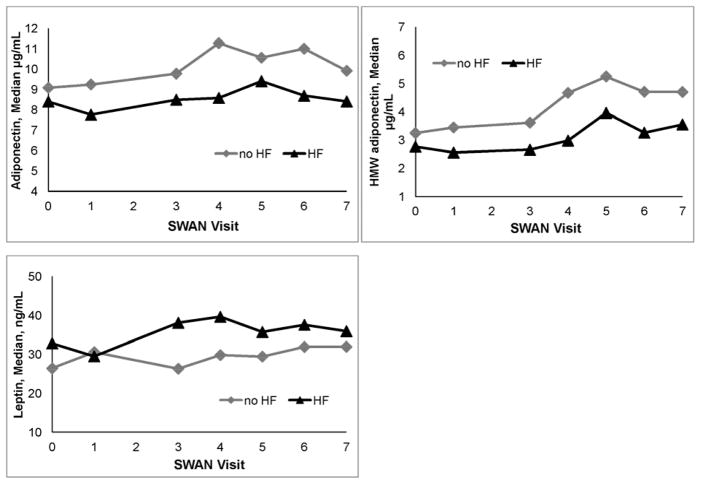
Highly significant interactions between adipokines and menopause stage in relation to hot flashes were noted for adiponectin (p=0.0001), HMW adiponectin (p=0.001), leptin (p=0.0002), and sOB-R (p=0.02), indicating that the associations between adipokines and hot flashes varied strongly by stage. Stratified models revealed that in the pre- and early perimenopause, higher adiponectin and HMW adiponectin were related to lower odds of hot flashes, and higher leptin with marginally higher odds of hot flashes (Tables 2, 3, Figure 1). Later in the transition (late peri- and postmenopause), these associations were not apparent.
Table 2.
Relation between adipokines and hot flashes by menopausal stage
| Hot flashes OR (95% CI) | Menopausal stage-by-adipokine interaction | ||
|---|---|---|---|
| Pre-/early perimenopause | Late peri-/postmenopause | ||
| Adiponectin | 0.68 (0.51–0.90)a | 1.01 (0.65–1.56) | p=0.0001 |
| HMW adiponectin | 0.70 (0.58–0.85)b | 0.98 (0.72–1.32) | p=0.001 |
| Leptin | 1.23 (0.99–1.54)c | 0.92 (0.60–1.40) | p=0.0002 |
| Soluble leptin receptor | 1.32 (0.82–2.15) | 1.85 (0.88–3.88) | p=0.02 |
p<0.01;
p<0.0001;
p<0.10
Note: Pre/early perimenopausal models: N=506 women, 1447 observations; late peri-/postmenopausal models: N=259 women, 753 observations; All adipokines log transformed for analysis
Covariates: age, race/ethnicity, BMI, education, HT use, smoking, self-rated health, diabetes status, DBP, physical activity, depressive symptoms
Table 3.
Median (interquartile range) adipokine levels by hot flash status and menopausal stage over 7 years
| Pre-/early perimenopause | Late peri-/postmenopause | |||
|---|---|---|---|---|
| Hot flashes | No Hot Flashes | Hot flashes | No Hot Flashes | |
| Adiponectin, μg/mL | 8.44 (6.27–11.08) | 9.72 (6.83–13.5) | 9.38 (6.65–13.68) | 9.55 (6.43–13.89) |
| HMW adiponectin, μg/mL | 3.02 (1.83–4.75) | 3.76 (2.14–6.28) | 3.71 (2.17–6.26) | 3.99 (2.22–6.78) |
| Leptin, ng/mL | 34.15 (20.83–49.09) | 27.98 (15.05–44.35) | 34.26 (19.03–49.55) | 39.64 (24.77–57.34) |
| Soluble leptin receptor, ng/mL | 24.41 (19.74–29.61) | 24.67 (19.91–29.89) | 27.33 (22.40–33.96) | 25.53 (20.40–31.16) |
Note: Pre/early perimenopausal: N=505 women, 1449 observations; late peri-/postmenopausal: N=259 women, 754 observations
Figure 1.
Adiponectin, HMW adiponectin, and leptin by hot flash (HF) status among pre- and early perimenopausal women (N=505 women, 1449 observations)
We also found a highly significant interaction between BMI and menopause stage in relation to hot flashes (p=0.004). Earlier in the transition (pre- and early perimenopause), obesity was related to higher odds of hot flashes (overweight, OR (95% CI): 1.50 (0.98–2.30), p=0.07; obese, OR (95% CI): 1.91 (1.25–2.90), p=0.003; relative to normal weight, multivariable models). Later in the transition (late peri- and postmenopause), these associations were not apparent (overweight, OR (95% CI): 0.76 (0.41–1.39), p=0.37; obese, OR (95% CI): 0.55 (0.29–1.04), p=0.07, relative to normal weight, multivariable models). Findings were similar when BMI was considered as a continuous variable, yet with a clearer reversal in the BMI-hot flashes associations later in the transition (early in the transition per unit increase in BMI: OR (95% CI): 1.02 (1.00–1.04), p=0.05; later in the transition per unit increase in BMI: OR (95% CI): 0.96 (0.94–0.99), p=0.005, multivariable models).
In an exploratory fashion, we added adipokines to models of links between obesity and hot flashes. We found that positive relationships between BMI and hot flashes were reduced with addition of HMW adiponectin, with BMI considered as a continuous (OR(95% CI): 1.01 (0.99–1.04), p=0.28) or categorical variable (overweight, OR (95% CI): 1.26 (0.77–2.04), p=0.36; obese, OR (95% CI): 1.53 (0.96–2.46), p=0.08). Similar yet less pronounced patterns were observed with addition of adiponectin (continuous BMI: OR (95% CI): 1.01 (0.99–1.04), p=0.24; categorical BMI: overweight, OR (95% CI): 1.36 (0.88–2.11), p=0.17; obese, OR (95% CI) 1.62 (1.05–2.51), p=0.03) or leptin (continuous BMI: OR (95% CI): 1.01 (0.98–1.03), p=0.68; categorical BMI: overweight, OR (95% CI): 1.38 (0.89–2.14), p=0.15; obese, OR (95% CI): 1.64 (1.00–2.69), p=0.05; multivariable models). In the later stages of the transition, relationships between BMI and hot flashes were largely unchanged with the addition of adipokines with the exception of leptin, which reduced associations between BMI and hot flashes (continuous BMI: OR=0.97; 95% CI 0.93–1.00, p=0.08; categorical BMI: overweight, OR (95% CI): 0.91 (0.46–1.80), p=0.79; obese, OR (95% CI) 0.73 (0.30–1.78), p=0.49; multivariable models).
We next considered night sweats. The only adipokine related to night sweats was MCP-1, which was robustly associated with higher odds of night sweats (multivariable models with adjustment for BMI: OR (95% CI): 1.37 (1.06–1.76), p=0.02; without adjustment for BMI: OR (95% CI): 1.37 (1.07–1.76), p=0.01). Associations between adipokines and night sweats did not vary by menopause stage, and there was no evidence of a relation between BMI and night sweats for the total sample or by menopause stage.
We conducted several additional analyses. Since our conceptual models concerned adiposity (rather than simply body size), we considered bioimpedance measures of fat mass and percentage of body fat or waist circumference instead of BMI. Results were comparable to those with BMI (data not shown). Moreover, one mechanism by which adipose-derived inflammatory factors may play a role in hot flashes early in the transition is a potential adverse effect of adipokines on ovarian E2 production. Although E2 was strongly inversely related to hot flashes (OR (95% CI): 0.85 (0.77–0.95), p=0.003) and to a lesser extent night sweats (OR (95% CI): 0.92 (0.83–1.01), p=0.095), E2 had little impact on associations between adipokines and/or BMI in relation to hot flashes, nor the stage dependence of these relationships (data not shown). Finally, we considered any interactions by race/ethnicity in relationships between adipokines and hot flashes or night sweats, with no effect modification apparent (data not shown).
DISCUSSION
In this study, associations between adiponectin, HMW adiponectin, and leptin and hot flashes were menopause stage-dependent. Lower adiponectin, lower HMW adiponectin, and to a lesser extent higher leptin were associated with higher odds of hot flashes early, but not later, in the menopause transition. These findings paralleled the finding that BMI showed a stage-dependent relationship with hot flashes, with higher BMI associated with higher odds of hot flashes early, but not later in the transition, when BMI-hot flash associations may reverse direction. Higher MCP-1 was associated with higher odds of night sweats irrespective of menopause stage.
Despite prior work postulating a potential role for adipokines in hot flashes (13), few studies have examined associations between hot flashes and adipokines. Alexander et al. (14) found that hot flashes were associated with higher leptin, the only marker examined, among relatively young middle aged women (mean age 48–49 years). Yasui et al (29) examined MCP-1 and several other cytokines, finding hot flashes associated with elevated interleukin-8 and macrophage inflammatory protein (MIP)-1β (but not MCP-1). However, both studies were limited by their cross-sectional design, lack of consideration of potential confounders, and lack of assessment of night sweats. Further, Alexander et al. assessed a single adipokine, and adipokines were not the focus of Yasui et al. study. Thus, the present study was the first examination of associations between adipokines and VMS in a prospective cohort study, with a wide range of adipokines, consideration of potential confounders, and examination of how these variations varied by menopause stage. The present work provided some indication that higher adiponectin and HMW adiponectin were related to lower odds of hot flashes in the early transition, over and above confounders. This study is the first to clearly demonstrate the stage-dependent relationships between BMI and hot flashes. Finally, there was some limited support for the idea that certain adipokines (HMW adiponectin) may be one mechanism linking BMI and hot flashes early in the transition, although further examination of this question is required.
Variations by menopause stage figured prominently into the present findings, both for associations between adipokines and hot flashes as well as between BMI and hot flashes. Only among the premenopausal and early perimenopausal women was a higher BMI and a more adverse adipokine profile related to more hot flashes. Among women later in the transition, there was suggestion that BMI may play a protective role. These novel findings are consistent with other findings stage-dependent associations between BMI/adiposity and E2, with higher BMI/adiposity related to lower E2 earlier in the transition, but higher E2 later in the transition (10–12). One interpretation of this pattern of associations has been that body fat may have a deleterious effect on ovarian function early in the transition when ovarian function is still intact, but not later in the transition after ovarian function had ceased, when adiposity may be the primary estrogen source (albeit primarily for estrone, a weaker estrogen).
These findings may add to the understanding of the physiology of adipose-hot flash relationships. Although incompletely understood, hot flashes are thought to be endocrine and/or thermoregulatory events, or dramatic heat dissipating events originating in the hypothalamus and occurring due to the withdrawal of ovarian hormones. At first glance, our findings may be considered consistent with an endocrine perspective, as relationships appeared dependent on the functioning of the ovary. There is some evidence that a more pro-inflammatory adipokine profile may adversely impact ovarian function (30–33), which may be mechanisms by which adipokines could increase hot flashes early in the transition. However, it is notable that controlling for E2 had little impact on the results observed here. Thus, several other factors not assessed here may be important. The pro-inflammatory properties of adipose tissue may act directly on the central nervous system, with potential thermoregulatory impact to increase hot flashes (34). Further, body fat may act as an insulator (35), impairing the heat dissipating function of hot flashes and increasing their likelihood. Finally, multiple pathways may be operating at once, with their relative predominance possibly changing with age.
We also considered night sweats. Pronounced associations were observed between night sweats and MCP-1, and these relationships were not stage-dependent. No associations were observed for BMI, adiponectin, HMW adiponectin and leptin with respect to night sweats. The reasons for this pattern of results are not immediately apparent, and the two other studies examining relevant adipokines/cytokines and VMS reported solely on hot flashes (14, 29). However, our finding is broadly consistent with findings of clearer associations for hot flashes than night sweats in relation to other physical health indices (13, 36, 37). It is possible that women may also have more difficulty accurately recalling night sweats, given their occurrence during the sleep period, impacting the reliability of their self-report (38). However, it is also possible that the underlying physiology of hot flashes and night sweats differ, thus, they may show a differential pattern of associations with adiposity and adipokines. These differences should be further examined in future work. In particular, the pronounced association between MCP-1 and night sweats should be further explored in future research.
The present work may further add to the emerging literature hot flashes and cardiovascular disease (CVD) risk. Evidence shows increased atherosclerosis (37, 39); poorer vascular endothelial function (37); a more pro-thrombotic profile (40); insulin resistance (41); elevated lipids (42); and altered sympathovagal balance (43) with hot flashes, above and beyond obesity. A pro-inflammatory profile is consistent with higher CVD risk, and the somewhat more adverse adipokine profile associated with hot flashes is consistent this prior work.
These results should be interpreted in the context of several limitations. First, our hot flash measure queried about their occurrence over the prior two weeks. Future work should consider these associations with improved measures, such as diaries or physiologic measures. The magnitudes of associations here were modest. Thus, there are clearly other predictors of VMS and pathways by which adiposity may be linked to VMS. Moreover, the observational nature of these data do not allow for conclusions about the causal nature of these relationships. Adipokines, E2, and hot flashes measures were obtained annually, and fluctuations within a year were not captured. Particularly relevant is E2, which fluctuates markedly during the menopause transition, and therefore the lack of an impact of E2 on the present results cannot be considered conclusive. Estrone was not assessed here, relevant to adiposity. While this study considered multiple adipokines, representing the most comprehensive test of this question to date, additional markers may be considered in future work. Further, the number of women in the later menopause stage group was smaller than that of the early stage group, and therefore significance testing in stratified models in the later stage group may have been relatively underpowered and should be interpreted with caution. However, the significance of menopause stage interactions strongly supports the stage-dependent nature of these associations. Finally, the study included African American and Caucasian women from one SWAN site. The degree to which findings generalize to other groups and in other samples should be tested in future work.
This study had several strengths. Questions were addressed within a relatively large prospective cohort study. Measures of hot flashes and adipokines were obtained approximately annually over eight years. Several adipokines were considered as well as a range of potential confounders. Moreover, a conceptual model of relationships between adiposity and hot flashes, including how these associations vary by menopause stage, guided study questions. The present study represents the strongest test of relationships between adipokines, BMI, and hot flashes to date.
The present study indicated that early in the menopause transition, lower adiponectin, lower HMW adiponectin, and to a lesser extent higher leptin were associated with elevated odds of reporting hot flashes. Additionally, relationships between BMI and hot flashes varied by stage, with positive associations early in the transition, and inverse associations later in the transition. Higher MCP-1 was related to increased night sweats across the transition. These results can contribute to our understanding of VMS and the ways in which relationships between adipokines, adiposity, and VMS may vary over the menopause transition.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The adipokine data utilized in this report were generated through NIH-National Heart, Lung, and Blood Institute (NHLBI) grant HL086858 (to Dr. Wildman). This work was also supported by NIH grant AG029216 (to Dr. Thurston). We thank Dr. Sowers (deceased) and Dr. Wildman for their contributions to this work. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN.
Financial Support: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495), Bethesda, MD. The adipokine data utilized in this report were generated through NIH-National Heart, Lung, and Blood Institute (NHLBI) grant HL086858 (to Dr. Wildman). This work was also supported by NIH grant AG029216 (to Dr. Thurston).
Footnotes
Financial Disclosures: All authors have nothing to disclose.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Chris Gallagher, Former Chair; Susan Johnson, Current Chair
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gold E, Colvin A, Avis N, Bromberger J, Greendale G, Powell L, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, Ory M, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16:860–9. doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–58. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Hyde Riley E, Inui TS, Kleinman K, Connelly MT. Differential association of modifiable health behaviors with hot flashes in perimenopausal and postmenopausal women. J Gen Intern Med. 2004;19:740–6. doi: 10.1007/s11606-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston RC, Sowers MR, Chang Y, Sternfeld B, Gold EB, Johnston JM, et al. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women’s health across the nation. Am J Epidemiol. 2008;167:78–85. doi: 10.1093/aje/kwm244. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Santoro N, Matthews KA. Adiposity and hot flashes in midlife women: A modifying role of age. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96:746–54. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildman RP, Tepper PG, Crawford S, Finkelstein JS, Sutton-Tyrrell K, Thurston RC, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from The Study of Women’s Health Across the Nation. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1695–704. doi: 10.1210/jc.2012-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17:718–26. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Grisso JA, Battistini M, Garcia-Espagna B, Hollander L. Hot flashes in the late reproductive years: risk factors for African American and Caucasian women. J Womens Health Gend Based Med. 2001;10:67–76. doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 14.Alexander C, Cochran CJ, Gallicchio L, Miller SR, Flaws JA, Zacur H. Serum leptin levels, hormone levels, and hot flashes in midlife women. Fertil Steril. 2010;94:1037–43. doi: 10.1016/j.fertnstert.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama I, Nakata M, Yamaji K. Effect of leptin in platelet and endothelial cells. Obesity and arterial thrombosis. Ann N Y Acad Sci. 2000;902:315–9. doi: 10.1111/j.1749-6632.2000.tb06330.x. [DOI] [PubMed] [Google Scholar]
- 16.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–8. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 17.Landt M, Horowitz JF, Coppack SW, Klein S. Effect of short-term fasting on free and bound leptin concentrations in lean and obese women. J Clin Endocrinol Metab. 2001;86:3768–71. doi: 10.1210/jcem.86.8.7771. [DOI] [PubMed] [Google Scholar]
- 18.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–12. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 19.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. European journal of endocrinology/European Federation of Endocrine Societies. 2005;153:409–17. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 22.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta physiologica Scandinavica. 2005;184:285–93. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 23.Sowers M, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and pathobiology. Academic Press; 2000. pp. 175–188. [Google Scholar]
- 24.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 25.Boulier A, Fricker J, Thomasset AL, Apfelbaum M. Fat-free mass estimation by the two-electrode impedance method. The American journal of clinical nutrition. 1990;52:581–5. doi: 10.1093/ajcn/52.4.581. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32:1327–38. doi: 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48:1584–6. [PubMed] [Google Scholar]
- 29.Yasui T, Uemura H, Tomita J, Miyatani Y, Yamada M, Kuwahara A, et al. Association of interleukin-8 with hot flashes in premenopausal, perimenopausal, and postmenopausal women and bilateral oophorectomized women. J Clin Endocrinol Metab. 2006;91:4805–8. doi: 10.1210/jc.2006-1100. [DOI] [PubMed] [Google Scholar]
- 30.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–44. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 31.Metwally M, Ledger WL, Li TC. Reproductive endocrinology and clinical aspects of obesity in women. Ann NY Acad Sci. 2008;1127:140–6. doi: 10.1196/annals.1434.000. [DOI] [PubMed] [Google Scholar]
- 32.Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘beneficial’ adipokines in reproduction and fertility. Int J Obesity. 2008;32:223–31. doi: 10.1038/sj.ijo.0803719. [DOI] [PubMed] [Google Scholar]
- 33.Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. 2010;94:1949–57. doi: 10.1016/j.fertnstert.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7047–52. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson GS, Martin AD. Calculated thermal conductivities and heat flux in man. Undersea Hyperb Med. 1994;21:431–41. [PubMed] [Google Scholar]
- 36.Thurston RC, Sowers MR, Sutton-Tyrrell K, Everson-Rose SA, Lewis TT, Edmundowicz D, et al. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15:429–34. doi: 10.1097/gme.0b013e31815879cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. The association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. International Journal of Behavioral Medicine. 2006;13:163–72. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 39.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18:352–8. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, et al. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women’s Health Across the Nation. Menopause. 2011;18:1044–51. doi: 10.1097/gme.0b013e31821f5d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Sternfeld B, Joffe H, et al. Vasomotor symptoms and insulin resistance in the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold EB, Sternfeld B, et al. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstetrics and gynecology. 2012;119:753–61. doi: 10.1097/AOG.0b013e31824a09ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control during women’s daily lives. Menopause. 2012;19:406–12. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]