Abstract
Background
The mid-gestational fetus is capable of regenerative healing. We have recently demonstrated a novel role for the anti-inflammatory cytokine, IL-10, as a regulator of hyaluronan (HA) in the extracellular matrix (ECM). The signaling pathway of IL-10 has been studied in monocytes but is unknown in dermal fibroblasts. We hypothesized IL-10 signals through its primary receptor IL-10R1 to activate STAT3 resulting in HA synthesis.
Methods
Murine mid-gestation (E14.5) fetal fibroblasts (FFb) were evaluated in vitro. Pericellular matrix (PCM) was quantified using a particle exclusion assay. STAT3 levels and cellular localization were evaluated by Western blot/band densitometry and immunocytochemistry/confocal microscopy. HA levels were quantified by ELISA. The effects of IL-10R1 signal blockade by a neutralizing antibory and STAT3 inhibition were evaluated. An ex vivo mid-gestation fetal forearm culture incisional wound model in control and transgenic IL-10−/− mice was used to evaluate the role of STAT3 on the ECM.
Results
FFb produce a robust hyaluronan-rich PCM which is IL-10R1 and STAT3 dependent. Inhibition of IL-10R1 signaling results in decreased phosphorylated STAT3 levels and inhibition of nuclear localization. Inhibition of STAT3 results in decreased HA production. At day 3, Mid-gestation fetal wounds have efficient re-epithelialization, which is significantly slowed in IL-10−/− wounds at the same gestation and with inhibition of STAT3.
Conclusions
Our data demonstrates that IL-10 regulates HA synthesis through its primary receptor IL-10R1 and STAT3 activation. This supports a novel-non-immunoregulatory mechanism of IL-10 in its role in fetal regenerative wound healing.
Keywords: fetal wound healing, IL-10, hyaluronan, extracellular matrix, STAT3
Introduction
It has been known for over 30 years, the mid-gestation fetus is capable of cutaneous wound healing without scar formation with complete regeneration of dermal appendages, including hair follicles and neurovasculature[1]. The fetal regenerative response has been observed in multiple mammalian models, and is characterized by an attenuated inflammatory response and distinct extracellular matrix (ECM) rich in hyaluronan[2–4]. These regenerative capabilities are lost as the fetus continues to develop in utero with late-gestation fetal wounds healing with characteristic scar formation with absence of dermal appendages and a disorganized collagen-rich ECM similar to post-natal wound healing[5].
Our laboratory has demonstrated a significant role for IL-10 in the fetal regenerative response. There are clearly elevated levels of IL-10 noted in mid-gestation fetal skin, in comparison to scar forming post-natal skin[6]. Additionally, studies of incisional wounds in transgenic fetal mice heal with scar formation in fetal IL-10 knockout mice at a gestational age that typically heals regeneratively[7]. Most convincingly, multiple studies have demonstrated over-expression of IL-10 in postnatal tissue induces the fetal regenerative response, with healing progressing without scar formation[6,8]. As a potent anti-inflammatory cytokine, IL-10 has been shown to attenuate the inflammatory response in wound healing[6]. We have recently reported a novel role of IL-10, beyond immunoregulation, as a regulator of the extracellular matrix through an increase in hyaluronan production[9].
The fetal extracellular matrix (ECM) has a distinct profile with persistent and elevated levels of hyaluronan, in contrast to postnatal tissue. Hyaluronan synthesis is regulated by three isoforms of hyaluronan synthase (HAS1-3)[5]. Fibroblasts are the main effector cells in wound healing and are likely the cellular source of hyaluronan synthesis[10]. When cultured in vivo, fetal fibroblasts generate a large hyaluronan-rich pericullar matrix (PCM) compared to a relatively scant PCM produced by adult fibroblasts. We have demonstrated IL-10 is critical to the production of this HA-rich PCM, with mid-gestation IL-10 knockout fetal fibroblasts producing a scant PCM, as well as the ability of IL-10 to induce large HA-rich PCM in adult fibroblasts.
Although, the importance of IL-10 and HA have been demonstrated in the fetal regenerative response, there have been no studies evaluating IL-10 signaling in fetal dermal fibroblasts. IL-10 signal transduction pathways have been extensively studied in monocytes. IL-10 exerts its effect on cells through interaction with its primary receptor IL-10R1, a 110-kDA polypeptide that binds IL-10 with high affinity[11]. A second low affinity subunit, IL-10R2, then binds to modulate signaling. This receptor complex engages JAK1 and induces the activation of the transcription factor STAT3 which becomes phosphorylated, dimerizes and localizes to the nucleus[12]. This is the major signaling mechanism of IL-10 with few minor alternate pathways[13].
Taken together, we hypothesize that IL-10 regulates the hyaluronan-rich fetal extracellular matrix through its primary receptor IL-10R1 and a STAT3-dependent signal transduction pathway. To test this hypothesis, we performed a series of loss-of-function experiments in vitro through the use of a neutralizing antibody against IL-10R1 (anti-IL-10R1) and a small molecule inhibitor of STAT3, STA21, which inhibits STAT3 dimerization and DNA binding[14]. To examine its role in fetal wound healing, we then used an ex vivo fetal forearm organ culture model to evaluate the effects of STAT3 inhibition.
Methods
Cell Culture
All protocols were approved by the Cincinnati Children’s Hospital IACUC committee (9D10087). Primary dermal fibroblasts were isolated from mid-gestation age fetuses (day 14.5) from control C57Bl/6 (Jackson Laboratories, Strain 000664) and transgenic IL-10−/− mice (Jackson Laboratories, Strain 002251) per Hiramatsu et al 2011[15]. Fibroblasts were maintained in culture in Dulbecco’s modified Eagle’s Media (DMEM) supplemented with 10% bovine growth serum (BGS) at 37°C with 5% CO2. All experiments were conducted between passage 5 and 15.
Pericellular Matrix
Particle exclusion assay was performed to evaluate PCM formation. Fibroblasts were plated at 1 × 105 cells/well in a 6-well plate and allowed to settle overnight. Cells were serum starved in DMEM with 2% BGS for 24 hours. Treatment to inhibit IL-10R1 signaling (1 ug/ml, Abcam, Cambridge, MA) or STAT3 (STA21, 20 ug/ml) were added at the initiation of serum starvation. These experiments were repeated with appropriate experimental controls, including antibody control (Rabbit IgG, 1 ug/ml, Abcam, Cambridge) and vehicle control (DMSO) and similarly evaluated. 500 μl of suspended glutaraldehyde stabilized sheep erythrocytes (1 × 108 cells/ml) were added and allowed to settle. Randomly selected individual fibroblasts were photomicrographed and evaluated with computer-assisted morphometric analysis. Each group was evaluated in triplicate with at least twenty cells evaluated from random high power fields. The PCM ratio is expressed as the ratio of PCM area to cell body area.
Western Blot
Western blot analysis was performed to evaluate protein levels of STAT3 and phosphorylated STAT3 (pSTAT3) (n=3/group). Twenty μg of protein were resolved by SDS-PAGE on a 4–20% Tris glycine gradient gel (Invitrogen Inc., Carlsbad, CA) and transferred to nitrocellulose membrane using the iBlot™ Gel Transfer Device (Invitrogen, Carlsbad, CA). The membranes were blocked 1 hours at room temperature with 5% (w/v) dried skim milk in 25mM Tris-HCl, pH 7.4 containing 0.13 M NaCl and 0.0027M KCl and 0.01% Tween. The membrane was washed and then incubated overnight at 4 °C with rabbit monoclonal antibody to STAT3 (1:2000) or pSTAT3 (1:500, Cell Signaling Technologies, Danvers, MA) or b-actin (1:5000, Abcam, Cambridge, MA), and washed again. Horseradish peroxidase-goat anti-rabbit or anti-mouse IgG was used as secondary antibody for all primary antibodies. The protein bands were visualized with an enhanced chemoluminescence kit (ECL, Pierce, Rockford, IL), followed by exposure to Hyperfilm ECL. Band densitometry was performed using Totallab software and standardized to b-actin as a loading control (New Castle, UK).
Immunocytochemistry
Immunocytochemistry staining for STAT3 was performed to evaluate nuclear localization. Fibroblasts were plated at 1×103 cells/well in 4-chamber slides (BD Falcon, Bedford, MA) and allowed to settle overnight. Cells were serum starved, as above, for 24 hours. Treatment to inhibit IL-10R1 signaling (1 ug/ml) was added at 75 min and then fixed in 1% paraformaldehyde for 10 minutes at room temperature. Immunocytochemistry was preformed as previously described [14] with the following modifications. Anti-Stat3 antibody (Cell Signaling Technology, 1:100) was incubated overnight at 4°C followed by Alexa Fluor-633 goat anti-rabbit IgG secondary (Invitrogen, Carlsbad, CA). Cell nuclei (POPO3, 1:400, Invitrogen) and filamentous actin (Alexa Fluor-488-Phallodin, 1:40, Invitrogen) were stained simultaneously for 30 minutes at room temperature. The slides were mounted with Prolong Gold (Invitrogen) and imaged with confocal microscopy (Carl Zeiss, Zen 2009).
HA ELISA
Fibroblasts were plated at 2 × 105 cells and allowed to settle overnight (n=3/group). Cells were serum starved, as above, for 24 hours. Treatment to inhibit STAT3 was added on initiation of serum starvation. Media was collected at 48 hours and HA ELISA was performed according to manufacturer’s protocol (Corgenix). HA ELISA was standardized to total protein content evaluated by Coomassie blue assay per manufacturers protocol (Pierce, Rockford, IL).
Ex vivo culture
All protocols were approved by the Cincinnati Children’s Hospital IACUC committee (9D10087). Methods of euthanizing animals were in accordance with recommendations of the Panel on Euthanasia of the American Veterinrary Medical Association. Forelimb organ culture was performed as previously described by Iocono et al[16]. Briefly, time-date pregnant C57Bl/6 and transgenic IL-10 knockout mice (term, 21 days) were killed at mid-gestational age day 14.5 (E14.5) fetuses were harvested via laparotomy (n=5 per group). An incisional wound was made in each forelimb with a 1-mm microscalpel. The wound was closed with a single 9–0 nylon suture. The forelimbs were amputated at the level of the shoulder and placed in the center-well of organ culture dishes on top of a stainless steel mesh (BD Falcon 353037) with serum free BGJb media for three days. After 3 days, the forelimbs were washed and fixed in 10% neutral buffered formalin and mechanically processed and paraffin embedded.
Epithelial Gap Analysis
5 μm wound sections were deparaffinized and rehydrated and stained by hemotoxyllin and eosin. Following staining, slides were dehydrated and mounted. Epithelial gap was evaluated with computer-assisted morphometric analysis. Five wounds were evaluated per group.
Statistical Analysis
Data are expressed as the mean±S.D. values. Statistical analysis of data was performed using analysis of variance (ANOVA), followed by post hoc tests (Tukey) and Student t-test when appropriate using Excel (Microsoft). A p-value of <0.05 was considered statistically significant.
Results
In-vitro - HA-rich PCM formation is mediated through IL-10R1 and STAT3
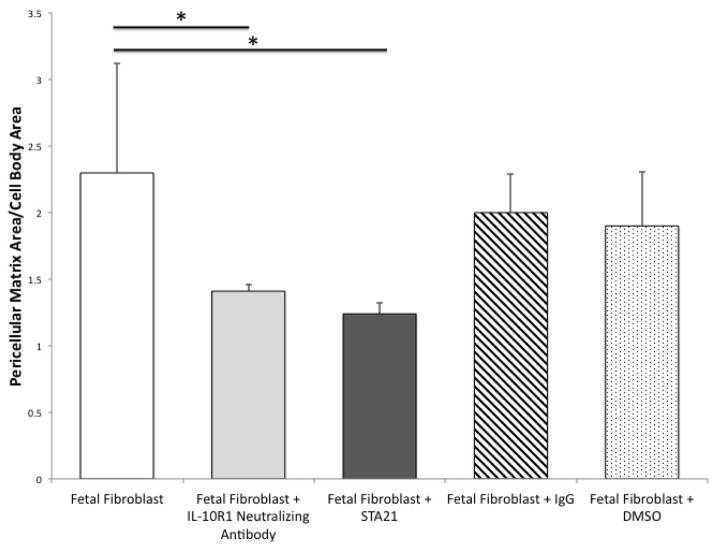
Mid-gestational fetal fibroblasts produce a robust HA-rich PCM (FFb 2.82+1.02) (Figure 1). We have previously reported that IL-10 plays an essential role in the HA-rich PCM. To determine if IL-10 signals through it’s primary receptor, IL-10R1, FFb were cultured with a neutralizing antibody (anti-IL-10R1 Ab). Blocking signaling through IL-10R1 results in significantly smaller PCM than control (FFb 2.82+1.02 vs FFb+anti-IL-10R1 1.58+0.16, p=0.01). To determine the role of STAT3, FFb were treated with a STAT3 inhibitor (STA21). Inhibition of STAT3 results in attenuated PCM formation (FFb 2.3+0.82 vs FFb+STA21 1.24+0.08, p<0.001). There were no differences noted between FFb and those treated with IgG control antibody or DMSO vehicle control (FFb 2.3+0.82 vs FFb+IgG 2.0+0.29, p=0.20; FFb 2.3+0.82 vs FFb+DMSO 1.9+0.41, p=0.27).
Figure 1.
Fetal fibroblasts (FFb, E14.5) produce a robust hyaluronan rich PCM. PCM is significantly attenuated with blockade of primary receptor IL-10R1 signaling using a neutralizing antibody and with inhibition of STAT3, using a small molecule inhibitor, STA21. There were no differences noted with fetal fibroblasts treated with antibody or vehicle control. (* p<0.05)
In-vitro - Inhibition of IL-10R1 decreases STAT3 phosphorylation
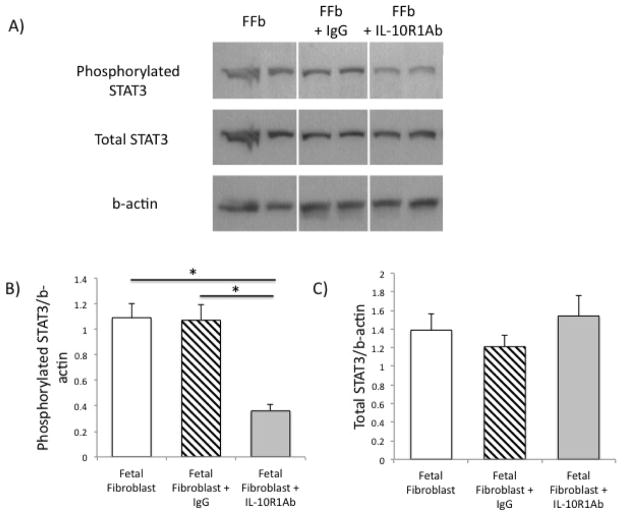
To determine if IL-10R1 signals through STAT3, we performed a Western blot/band densitometry on mid-gestation fetal fibroblasts treated with IL-10R1 neutralizing antibody. Blockade of IL-10R1 signaling results in a decrease in STAT3 phosphorylation compared to controls (FFb 1.09+0.11 vs FFb+IL-10R1 Ab 0.36+0.05, p<0.05) (Figure 2). There is no difference noted in total STAT3 phosphorylation (FFb 1.39+0.17 vs FFb+anti-IL-10R1 Ab 1.54+0.22, p=NS). There were no differences in levels of phosphorylated STAT3 and total STAT3 with nonspecific antibody control (phosphorylated STAT3: FFb 1.09+0.11 vs FFb 1.07+0.12; total STAT3: FFb 1.39+0.17 vs 1.21+0.12, p=NS).
Figure 2.
Blockade of signaling through IL-10R1 results in a significant decrease in phosphorylated STAT3 with no differences noted in total STAT3 levels (Western blot, A) quantified by band densitometry (Phosphorylated STAT3, B; and total STAT3, C). There were no differences noted with fetal fibroblasts treated with antibody control. (* p <0.05)
In vitro - IL-10R1 facilitates nuclear localization of STAT3
To determine if IL-10R1 signaling results in nuclear localization of STAT3, we performed immunocytochemistry on fibroblasts to determine STAT3 localization. Mid-gestational fetal fibroblasts demonstrate nuclear localization at baseline, with relatively low levels of STAT3 noted in the cytosol. Inhibition of IL-10R1 results in a qualitative decrease in nuclear localization, with STAT3 remaining cytosolic and perinuclear with a paucity of STAT3 within the nucleus (Figure 3). There were no differences noted between mid-gestational fetal fibroblasts at baseline and following treatment with IgG control.
Figure 3.
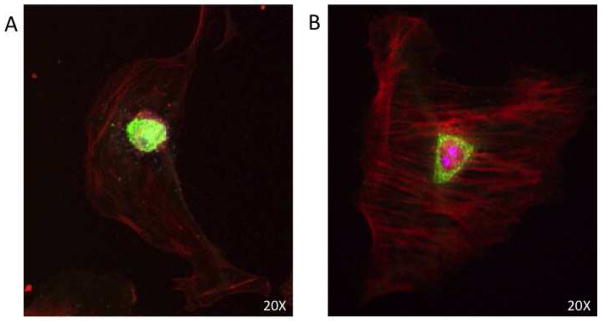
Confocal microscopy of fetal fibroblasts with staining for STAT3 (green) and phalloidin (red). Fetal fibroblasts demonstrate nuclear localization of STAT3 (A). Blockade of signaling through IL-10R1 using a neutralizing antibody inhibits nuclear translocation of STAT3, resulting in peri-nuclear localization (B) (Magnification 20X).
In vitro - HA production is STAT3-dependent
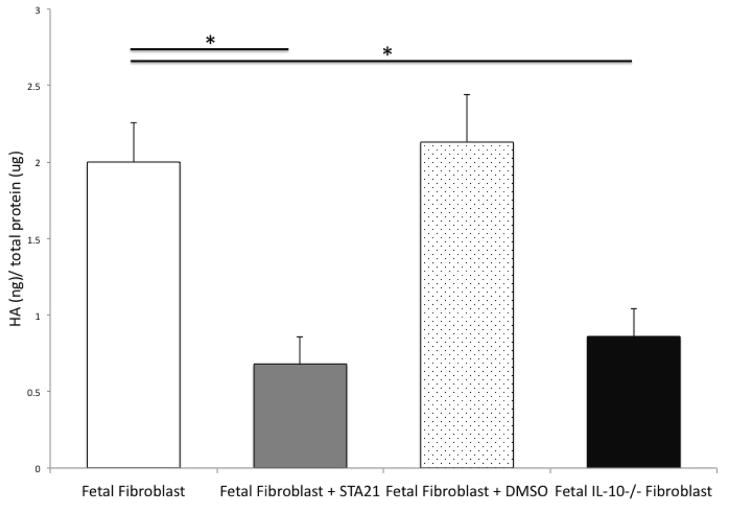
In comparison with mid-gestation fetal fibroblasts grown in serum starvation conditions for 48 hours, the inhibition of STAT3, using the small molecular inhibitor STA21, results in the significant decrease of hyaluronan production (FFb 2.0+0.26 ng HA/μg total protein vs FFb+STA21 0.68+0.18 ng HA/μg total protein, p=0.002). Interestingly, this attenuated level of hyaluronan production following inhibition of STAT3 is very similar to the decreased hyaluronan production, previously reported, by IL-10−/− fibroblasts (IL-10−/− FFb 0.86+0.18 ng HA/μg total protein vs FFb+STA21 0.68+0.18 ng HA/μg total protein, p=0.06) (Figure 4). There were no differences noted between FFb and those treated with vehicle control (FFb 2.00+0.26 vs FFb+DMSO 2.13+0.31, p=0.62).
Figure 4.
Hyaluronan (HA) in media normalized to total protein content. Fetal fibroblasts (E14.5) produce significantly more HA than IL-10−/− fibroblasts of the same gestational age. Inhibition of STAT3 with STA21 significantly attenuates fetal production of HA. There were no differences noted with fetal fibroblasts treated with vehicle control. (* p <0.05)
Ex vivo – Day 3 Fetal Wound Morphology
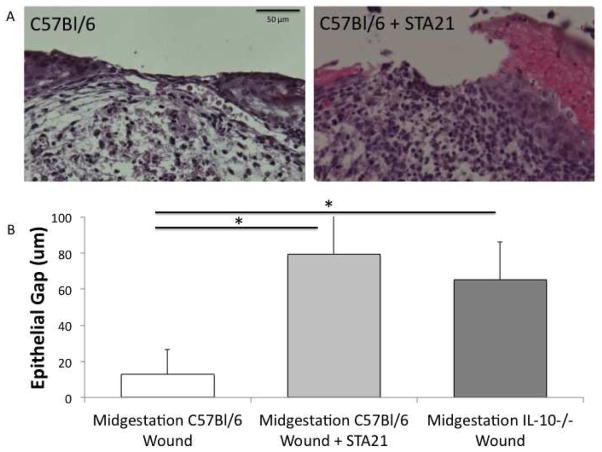
All fetal forearm specimens remain morphologically intact following fixation and staining. Mid-gestation fetal wounds maintain normal architecture throughout the section with sparse cellular infiltrate at the local wound (Figure 5A). The extracellular matrix maintains a reticular pattern within the wound bed and is indistinguishable from the surrounding uninjured tissue with a thin epithelial ridge that is almost completely healed. Inhibition of STAT3 results in disruption of the wound architecture with increased cellular infiltrate localizing to the wound bed. The extracellular matrix has a less organized pattern, distinct from the control fetal forearm and uninjured tissue. Also, there is a thickened epithelial ridge and larger epithelial gap.
Figure 5.
A) Mid-gestation fetal forelimb incision wounds, cultured ex vivo for 3 days. Control C57Bl/6 wounds re-epithelialize quickly with sparse cellular infiltrate. Inhibition of STAT3 results in slowed re-epithelialization with a widened epithelial gap and increased cellular infiltrate. B) Epithelial gap of mid-gestation (E14.5) fetal forelimb incisions cultured ex vivo 3 days in control C57Bl/6 and transgenic IL-10−/− mice (n=5/group). Control mid-gestation fetal wounds re-epithelialize efficiently at 3 days. This re-epithelialization is decreased with inhibition of STAT3 and knockout of IL-10. (* p<0.05)
Ex vivo - IL-10 and STAT3 are essential for efficient re-epithelialization
Mid-gestation fetal wounds demonstrate efficient re-epithelialization of incisional wounds by 3 days following injury. In contrast to control mid-gestational wounds, transgenic IL-10 knockout incisional wounds at the same gestational age, demonstrate a larger epithelial gap (C57Bl/6 epithelial gap 12.8+13.8 μm vs IL-10−/− epithelial gap 65.2+20.7 μm, p=0.003) (Figure 5B). Similarly, STAT3 inhibition in mid-gestation wounds have significantly slowed re-epithelialization (C57Bl/6 epithelial gap 12.8+13.8 μm vs C57Bl/6+STA21 epithelial gap 79.3+21.2 μm, p=0.004). Interestingly, IL-10−/− wounds and control wounds following STAT3 inhibition demonstrate similar impairment of re-epithelialization (IL-10−/− epithelial gap 65.2+20.7 μm vs C57Bl/6+STA21 epithelial gap 79.3+21.2 μm, p=0.58).
Discussion
We have demonstrated a novel-role of IL-10 as a regulator of the hyaluronan-rich fetal extracellular matrix through its primary receptor IL-10R1 and a STAT3 dependent signal transduction pathway. The robust HA-rich ECM produced by fetal fibroblasts is dependent on IL-10R1 signaling, as evidenced by blockade of IL-10R1 in fibroblasts which results in attenuated pericellular matrices. Similarly, inhibition of STAT3 results in a decrease in HA synthesis with a decreased pericellular matrix. We confirmed IL-10R1 signaling proceeds through STAT3 activation by inhibiting IL-10R1 signaling using a neutralizing antibody which results in decreased STAT3-phosphorylation and prevents localization of the transcription factor into the nucleus. We then confirmed the importance of STAT3 signaling in an ex vivo incisional wound model. Mid-gestation fetal forelimbs, which have been previously shown to be capable of regenerative healing, are detrimentally effected with STAT3 inhibition, demonstrating impaired re-epithelization and disruption of tissue architecture, as well as an increase in cellular infiltrate.
While the precise mechanism of scarless wound repair remain unclear, IL-10 has been implicated to play an importance role in the fetal regenerative response. IL-10 has been primarily studied as a potent anti-inflammatory cytokine. IL-10 has been shown to deactivate macrophages and neutrophils and down-regulate pro-inflammatory cytokine production, such as IL-6 and IL-8. We have previously reported the ability of IL-10 overexpression to recapitulate fetal scarless wound healing in postnatal tissue accompanied by a decrease in inflammatory recruitment. IL-10 is a promising agent in facilitating regenerative healing, however, the process of fetal wound healing is a complex sequence of orchestrated events. Our recent work has demonstrated IL-10 likely exerts its regenerative effects with a novel role as a regulator the extracellular matrix with increased hyaluronan content, in addition to its well-established role as an anti-inflammatory agent as we have previously reported[6].
In contrast to adult wound healing where hyaluronan production is initially elevated but quickly degraded, fetal regenerative healing is characterized by persistent and elevated levels of hyaluronan [5], As the fetus progresses through gestation, the level of hyaluronan declines, corresponding temporally with the loss of regenerative healing capabilities. Addition of hyaluronan to scar-forming late-gestation fetal wounds, results in an improvement of extracellular matrix quality within the wound bed[16]. Conversely, removal of hyaluronan through administration of hyaluronidase results in increased granulation tissue and scarring [17]. Hyaluronan supplementation induced an increase in basket weave patterned, reticular collagen bundles similar to local uninjured dermis, as opposed to the thick collagen fibers arranged in parallel arrays, characteristic of scar formation. Hyaluronan is a negatively charged glycosaminoglycan composed of repeating disaccharides of D-glucuronic acid and N-acetylglucosomine, with highly variable molecular composition ranging from ~2,000–25,000 disaccharides[15]. The HA-rich extracellular matrix may confer regenerative capabilities by providing a well-hydrated environment permitting more efficient cell migration throughout wound healing, thereby minimizing the time for fibroblasts to produce excess collagen, as seen in scar formation[18]. Additionally, regenerative mid-gestation fetal wounds are known to produce predominantly high-molecular weight HA, which has been shown to be anti-inflammatory in nature.
This study extends our previous observations that the anti-inflammatory cytokine IL-10 functions as a regulator of the extracellular matrix. Further, this demonstrates in dermal fibroblasts, IL-10 signals through its primary receptor IL-10R1 to activate STAT3, leading to phosphorylation and translocation to the nucleus, resulting in an increase in hyaluronan production. Interestingly, other studies have also shown that STAT3 is also an important transcription factor for interleukin-6 (IL-6), which as a pro-inflammatory cytokine exerts multiple effects in direct opposition to IL-10, such as increased inflammatory cell infiltration[19]. These differing downstream actions via a shared transcription factor may be the result of members of the SOCS protein family comprised of eight proteins. SOCS proteins regulate the cellular response to cytokines through multiple actions including competitive inhibition of STAT recruitment to receptors and targeted polyubiquitination and proteosomal degradation[20,21]. SOCS3 in particular has been shown to be critical in physiological regulation of IL-6, negatively regulating the anti-inflammatory cytokine in vivo[21]. Additional studies to investigate the possible role of SOCS proteins in dermal wound healing are needed to better understand the complicated signaling cascades initiated by tissue injury.
Although we have known for over 30 years the fetus is capable of scarless wound healing, we now have the molecular tools to elucidate the underlying mechanism of the fetal regenerative response[5]. This study makes use of multiple models to elucidate the signaling pathway by which IL-10 mediates fibroblast hyaluronan synthesis in fetal wound healing. Better understanding of fetal wound healing has implications beyond cosmetic benefit with potential to restore integrity to tissue and possibly to any pathology characterized by excessive fibrosis including pulmonary fibrosis, glomerulosclerosis, intra-abdominal adhesions and hypertrophic scarring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson J, Terenghi G, Ferguson MW. The reinnervation and revascularisation pattern of scarless murine fetal wounds. J Anat. 2011;218:660–667. doi: 10.1111/j.1469-7580.2011.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krummel TM, Nelson JM, Diegelmann RF, et al. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- 3.Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–68. doi: 10.1016/s0022-3468(05)80165-4. discussion 68–69. [DOI] [PubMed] [Google Scholar]
- 4.Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–319. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- 5.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24:371–378. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon A, Kozin ED, Keswani SG, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70–79. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 7.Liechty KW, Kim HB, Adzick NS, et al. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–872. doi: 10.1053/jpsu.2000.6868. discussion 872–863. [DOI] [PubMed] [Google Scholar]
- 8.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 9.Leung A, Balaji S, Le LD, et al. An In Vitro And Ex Vivo Study of Fetal Wound Healing: A Novel Role for IL-10 As A Regulator of the Extracellular Matrix. Wound Repair Regen. 2012;20:A29. [Google Scholar]
- 10.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 11.Riley JK, Takeda K, Akira S, et al. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–16521. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 12.Yoon SI, Logsdon NJ, Sheikh F, et al. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex. J Biol Chem. 2006;281:35088–35096. doi: 10.1074/jbc.M606791200. [DOI] [PubMed] [Google Scholar]
- 13.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi K, Takaishi M, Nakajima K, et al. Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest Dermatol. 2011;131:108–117. doi: 10.1038/jid.2010.255. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Sasagawa S, Outani H, et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011;121:640–657. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iocono JA, Ehrlich HP, Keefer KA, et al. Hyaluronan induces scarless repair in mouse limb organ culture. J Pediatr Surg. 1998;33:564–567. doi: 10.1016/s0022-3468(98)90317-7. [DOI] [PubMed] [Google Scholar]
- 17.Iocono JA, Krummel TM, Keefer KA, et al. Repeated additions of hyaluronan alters granulation tissue deposition in sponge implants in mice. Wound Repair Regen. 1998;6:442–448. doi: 10.1046/j.1524-475x.1998.60506.x. [DOI] [PubMed] [Google Scholar]
- 18.Estes JM, Adzick NS, Harrison MR, et al. Hyaluronate metabolism undergoes an ontogenic transition during fetal development: implications for scar-free wound healing. J Pediatr Surg. 1993;28:1227–1231. doi: 10.1016/s0022-3468(05)80303-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Li Y, Wu Y, et al. Interleukin-6/STAT3 pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem. 2012 doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Badgwell DB, Bevers JJ, 3rd, et al. IL-6 signaling via the STAT3/SOCS3 pathway: functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem. 2006;288:179–189. doi: 10.1007/s11010-006-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croker BA, Krebs DL, Zhang JG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]