Abstract
This study describes a modified Hargreaves’ method for assessing paw withdrawal threshold temperatures for heat (PWT-H) nociception in the rat hind paws. The method utilizes applications of radiant heat to maintain controlled lamp temperatures (CLT) on a glass floor beneath the rat hind paw. An ascending series of CLTs were each applied for 10-s, with 5–10 min intervals between applications, until a characteristic withdrawal behavior was observed, or a cutoff CLT was reached. Average plantar epicutaneous temperatures measured from anesthetized rats corresponding to CLTs and withdrawal latencies were used for determining PWT-H. The mean PWT-H in 2-months old (mo) naïve Sprague-Dawley rats (n=38) was 47.6±0.2°C, and is comparable to the noxious threshold temperature for human glabrous skin (46.5±0.5°C). The PWT-H is consistent between trials and daily assessments over four consecutive days. No significant differences were observed between the PWT-H in 2 mo, 6–8 mo, and >24 mo F344 rats, but the PWT-H in 1-mo rats was significantly decreased. Three hours following plantar incision, the PWT-H decreased to 37.5±0.2°C, which correlates with previous observations of C-fiber afferents from incised glabrous skin firing at 36.7±3.6°C. Parallel testing with the current method and an electronic von Frey device illustrated similar degrees of incision-induced hyperalgesia, improvement of hyperalgesia over time, and reversals induced by morphine and gabapentin. In conclusion, the present method allows us to compare PWT-H with electrophysiological and human psychophysical studies involving thermosensation and, as a behavioral assay identical to von Frey testing in measuring threshold for nociception.
Keywords: Threshold temperature, radiant heat, rat, nociception, morphine, and gabapentin
1. INTRODUCTION
Psychophysical studies have demonstrated that humans experience pain at cutaneous temperatures exceeding 43°C (LaMotte and Campbell, 1978). Neurophysiological evidence has supported this finding by showing cutaneous A-δ and C-fiber nociceptor activation within the range of 42–43°C (Raja et al., 1999). In 1996, Cesare and McNaugton first demonstrated that noxious temperatures open a set of ion channels on these afferent endings, leading directly to their activation (Cesare and McNaughton, 1996). Molecular characterization of these channels has been revealed through cloning of the capsaicin receptor, TrpV1 (Caterina et al., 1997), which are activated at temperatures ≥43.0°C.
Despite such dramatic progress in understanding thermosensation, our knowledge of the phenotypic changes in nociceptive heat thresholds within animal models remains deficient. In contrast to von Frey mechanosensitivity testing, which measures noxious threshold forces, most of our current behavioral paradigms for evaluating heat sensitivity generally rely on response latencies to either radiant or conductive heat stimuli. On the other hand, neurophysiologic studies routinely conduct experiments with feedback-controlled devices to measure the exact cutaneous temperatures required for primary afferent activation (Banik and Brennan, 2004; Du et al., 2001; Koltzenburg et al., 1997; Reeh, 1986), and human psychophysical studies measure threshold temperatures for pain perception (LaMotte and Campbell, 1978; Tillman et al., 1995). This latency vs. threshold dichotomy has hindered our ability to compare and correlate data between neurophysiologic and behavioral experiments involving thermosensation.
To this end, previous studies have characterized an ‘increasing temperature hot plate test,’ and ‘increasing temperature water bath test’ for measuring threshold temperatures for heat nociception in rats (Almasi et al., 2003; Furedi et al., 2009). Limitations of the increasing temperature hot plate test include the inability to individually evaluate the ipsi/contralateral hind paws, and the potential to inadvertently stimulate other bodily surfaces in contact with the hot plate. The increasing temperature water bath requires significant animal handling, and was unable to detect heat hyperalgesia in a well-characterized inflammation model (Furedi et al., 2009).
The current study aimed to modify the most widely utilized behavioral assay for evaluating heat nociception, Hargreaves method, (Hargreaves et al., 1988) to allow for determination of the specific threshold temperatures required for nociceptive behaviors. The unique advantages of Hargreaves method that carry over to the present technique include: minimal animal handling, animal acclimation prior to testing, and individual hind paw testing ipsilateral or contralateral to the experimental injury. Through incorporation of a feedback-controlled radiant heat stimulator, we were able to deliver reproducible energy profiles to focused areas of an unrestrained rat’s hind paw and, using pre-characterized epicutaneous thermocouple temperature (ETT) values, determine paw withdrawal temperatures for heat nociception (PWT-H). The methodology involves applying an ascending series of 10-s CLTs until a characteristic withdrawal behavior is observed, or a cutoff CLT is reached. Average hind paw plantar ETTs measured from anesthetized rats correspond to each applied CLT and withdrawal latencies are considered the PWT-H. The study evaluated the PWT-H in normal rats and assessed potential variables, such as animal age and repeated testing. The ability of this method was compared to that of von Frey testing in evaluating degrees of hyperalgesia at different time points relative to incision and the attenuating effects of analgesics. A preliminary report has been published in the abstract form (Banik and Kabadi, 2010).
2. METHODS
2.1. Feedback-controlled heat stimulator
In collaboration with the Bioengineering department at the University of Iowa (attn: Roger Anderson), a feedback-controlled heat stimulator was designed. The stimulator utilizes a proportional-integral-derivative (PID) feedback-control mechanism with a radiant heat source to produce and maintain user-defined, controlled lamp temperatures (CLTs) (Fig. 1). The stimulator consists of 1) a power source and controller unit, 2) an assembly unit with a keypad and digital display, 3) a T-type thermocouple (IT-18, Physitemp, NJ, USA) connected to 4) a transmitter (TX1502A-T, Omega Engineering Inc, CT, USA) for temperature feedback, and 5) a 150W halogen projector lamp (EKE 150W 21V; Ushio, Tokyo, Japan) housed within an aluminum cup with a circular aperture (Ac=34.2-mm2) for radiant heat to pass through. The parameters of temperature and stimulus duration are entered using the keypad and display on the assembly (Fig. 2). Once triggered, the lamp projects radiant heat onto the tip of the feedback thermocouple sensor (Fig. 1, Fig. 2), providing continuous temperature input for the main PID controller unit (Fig. 1). The controller unit, in turn, continuously modulates the current to the lamp to immediately correct any error between the measured and user-defined temperatures. If the trigger button is pressed mid-stimulation, the lamp immediately shuts off and the elapsed time and input temperature are displayed.
Figure 1.
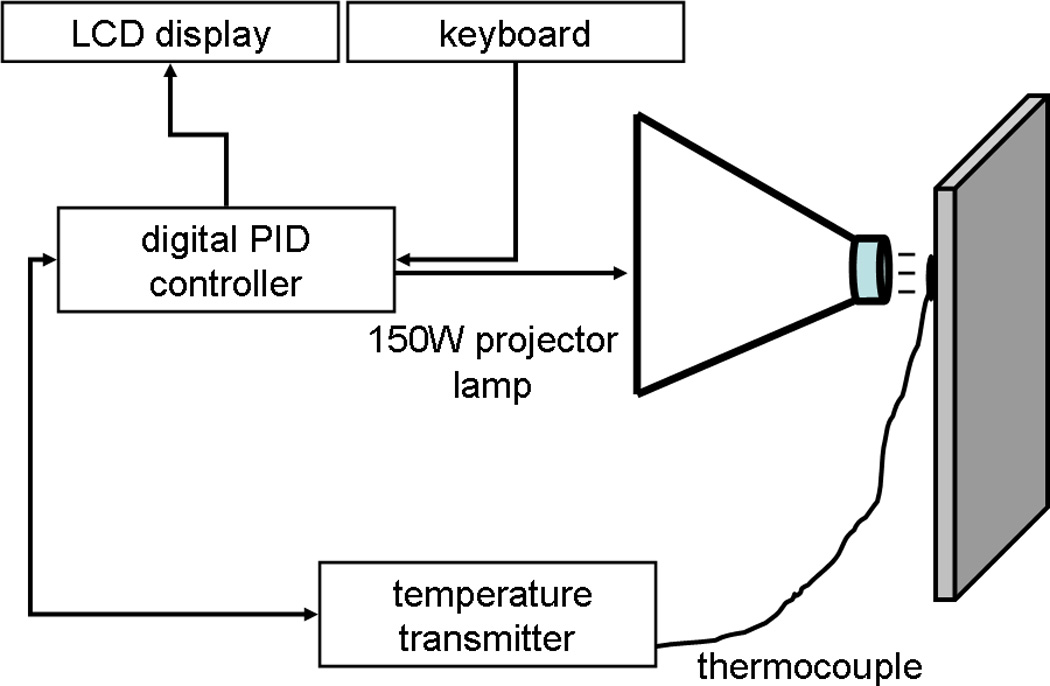
A closed feedback-controlled system maintains a fixed lamp temperature on the underside of a glass floor beneath the hind paw of an unrestrained rat. The parameters of temperature and stimulus duration are entered using a keypad and display. Once triggered, the proportional-integral-derivative controller unit of the stimulator signals the lamp to generate radiant heat onto a thermocouple sensor. The sensor provides real-time temperature input for the controller unit, which in turn, continuously modulates the current to the lamp to correct any error between the measured and user-defined temperatures.
Figure 2.
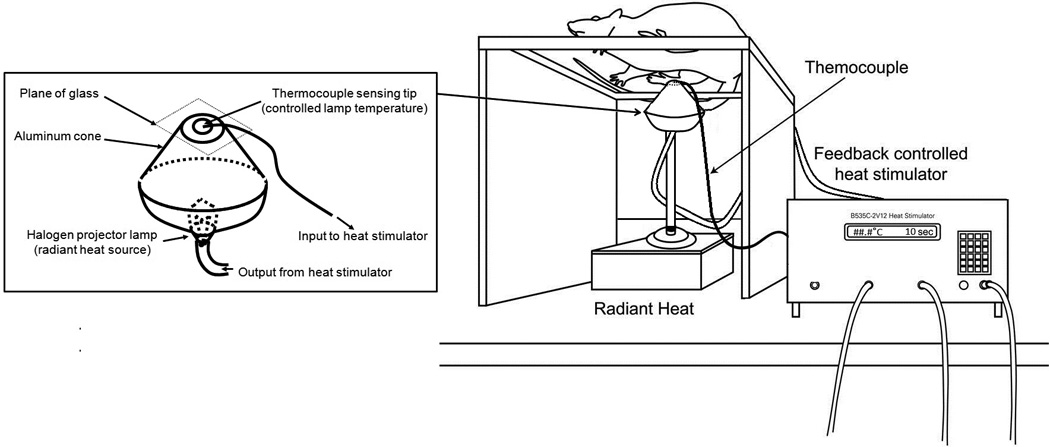
Schematic diagram of the experimental set-up used for assessing paw withdrawal threshold temperatures for heat nociception (PWT-H) in unrestrained rats. The set up consists of a stimulator unit, thermocouple sensor connected to a transmitter unit for temperature feedback, and a 150W halogen projector lamp inside an aluminum cup.
2.2. Characterization of epicutaneous thermocouple temperature (ETT) of the plantar hindpaw
Throughout testing, the temperature of the glass floor was maintained 29.0±1.0°C through manual applications of convective heat from a space heater (Lasko Products, West Chester, PA, Model #754200). To determine temperatures at the plantar epicutaneous surface, anesthetized (sodium pentobarbital 50-mg/kg i.p.) rats (n=6) were placed in normal weight-bearing positions on a glass surface with a thermocouple (IT-18) sensor placed between the hind paw plantar surface and glass floor. This thermocouple was connected to an analog thermometer (BAT-12, Physitemp, USA). The sensing tip of the heat stimulator’s input thermocouple was placed between the heat lamp and the underside of the glass floor, directly beneath the hind paw plantar surface. A data acquisition system (Micro 1401 MK II; CED, Cambridge, UK) simultaneously digitized and fed the analog outputs into a personal computer. With data acquisition and analysis software (Spike2; CED, Cambridge, UK), real-time temperature changes within both thermocouples were recorded as corresponding pair of waveform traces.
Calibrating the analog output (mill volts) to degrees Celsius was performed using known water temperatures (22, 30, 40, 55, 60 and 65°C) in a circulating, feedbackcontrolled water bath. The thermocouple signals were linear (r2 = 0.99, see supplementary Fig. S1).
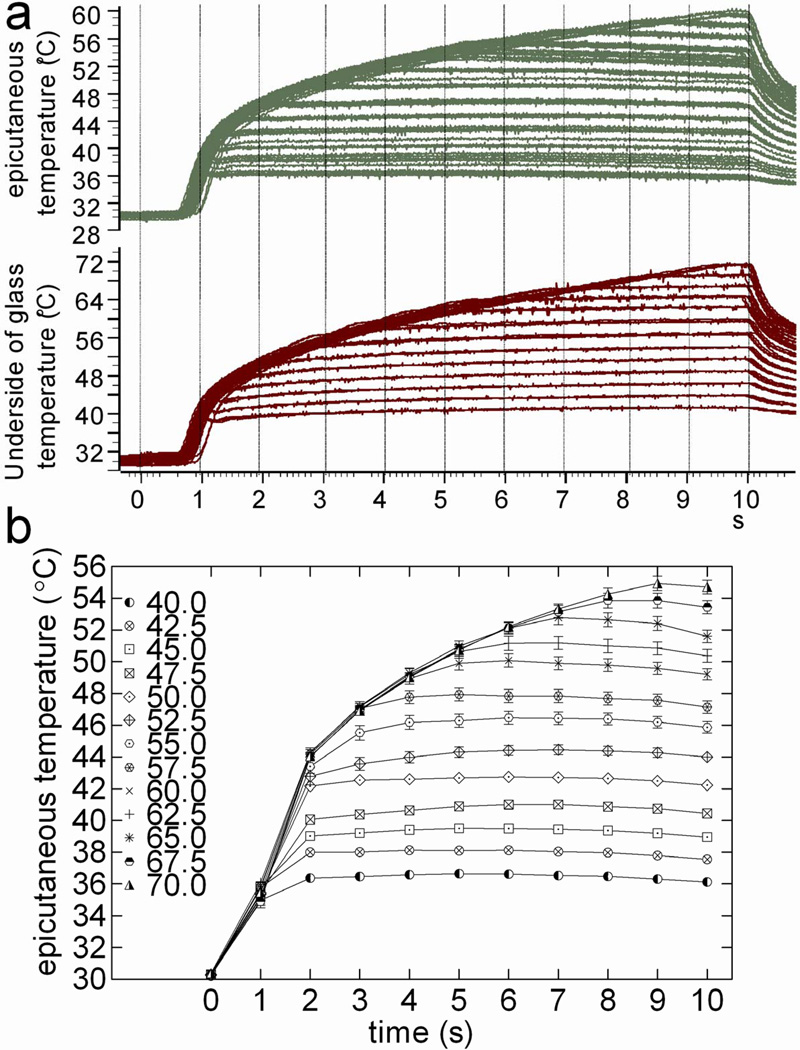
Using the setup described above, each anesthetized animal received a series of 10-s CLT applications from 40.0 to 70.0°C in 2.5°C increments (Fig. 3a). Each temperature was applied four times to each animal. Five to 10 min intervals were maintained between applications so that the lamp temperature and ETT could be restored to 29.0±1.0°C. Following this procedure with each of 6 rats generated 24 corresponding pairs of traces (CLT and ETT) at each temperature setting. The ETT at each second of each 10-s heat application was measured from the digital traces, and the values from all 24 traces averaged. In total, 130 averaged second-by-second ETT values were obtained from 10-s heat applications at each of the 13 input temperatures (40.0–70.0°C, step=2.5°C). These data were used as reference values to determine PWT-H based on 1) the stimulator input temperature, and 2) the latency to paw withdrawal (Fig. 3b).
Figure 3.
Controlled lamp temperature (CLT) on the underside of glass floor produces reproducible epicutaneous thermocouple temperatures (ETT) on plantar hind paw of anesthetized rats. Representative digital oscilloscope traces (a) illustrate CLT (lower panel) and ETT (upper panel). The stimulator was set to 40.0°C for the first stimulation and increased in 2.5°C steps to a cut-off temperature of 70.0°C. Each temperature was applied 4 times per animal and 24 traces were obtained from 6 animals. Second-by-second ETT values were averaged from the 24 traces, and are shown in b. Each data point represents the average ETT ± SEM.
2.3 Animals and ethics
The studies adhered to the proposals of the Committee for Research and Ethical Issues of the IASP and were approved by the Seton Hall University Animal Care and Use Committee. Male Sprague-Dawley (SD) rats from Harlan (Somerville, NJ) weighing 250–300 grams were used in this study, though not in the aging studies, which used Fisher-344 rats from Taconic (Germantown, NY). Two to three rats were housed together in one 43×21.5×25.5 cm Plexiglas cage and kept on a 12 hr light/dark cycle. Food and water were available ad libitum.
2.4. Plantar skin and muscle incision
Anesthesia was induced by placing each animal within a Plexiglas chamber containing room air with 5% isoflurane. Once loss of righting reflex was observed, each animal was moved to the operating stage where 2–3% isoflurane with room air was maintained through a tightly fitting nose cone.
As previously described by Brennan et al. (Brennan et al., 1996), a 1-cm longitudinal incision was made through the skin and fascia of the plantar surface of the hind paw. The plantaris muscle was then elevated, stressed, and incised longitudinally with the muscle origin and insertion remaining intact, after which the skin was closed with two mattress 5-0 silk sutures. The animals were then housed upon soft bedding and allowed to recover.
2.5. Evaluating paw withdrawal threshold for heat (PWT-H) nociception
Animals were placed within individual Plexiglas compartments (12×20×17-cm) on a shared, elevated glass floor. The temperature above the glass floor was monitored by an affixed thermocouple connected to an analog thermometer (see above), and maintained at 29.0±1.0°C with convective heat from a space heater (see above). Animals were allowed approximately 15 minutes for acclimation. Testing commenced once the animals became largely inactive, demonstrating only occasional grooming behaviors. The input temperature was set using a numeric keypad and display located on the stimulator assembly.
Testing began at 40.0°C, and increased in steps of 2.5°C until paw withdrawal behaviors were observed, or a cut-off temperature of 70.0°C was reached. Intervals of 5 to 10 minutes were observed between stimulations. Once the temperature was set, the feedback thermocouple sensing tip was affixed above the aperture of the heat lamp and placed directly against the underside of the glass floor beneath the animal hind paw (Fig. 2). A trigger button on the keypad was then pressed to initiate a 10s heat stimulation. If a nociceptive withdrawal response was noted during stimulation, the trigger button was immediately pressed again to discontinue the stimulation. Then the input temperature and withdrawal latency were recorded. These values were used to determine corresponding average ETT values (see 2.1.2, Table 1), which were considered the PWT-H. Three trials were conducted and the PWT-H values averaged. The baseline CLT and glass temperatures were restored to 29.0±1.0°C between trials. Two consecutive heat stimulations were not applied to the same area of the glass floor. If an animal did not move between trials, locomotion was induced by gently stroking the glass floor. In addition, the lamp and metallic cone were cooled with compressed air between applications to avoid an elevated thermocouple temperature (input to heat stimulator), which could decrease the amount of radiant heat generated by the lamp due to feedback control (Fig. 1). However, if the thermocouple temperature dropped below 29.0±1.0°C, the lamp was activated for a sufficient period to elevate the temperature to within the baseline range. Moreover, thermocouple contact to the glass floor was confirmed from displayed temperature. If this temperature was lower than 29.0±1.0°C, the thermocouple was repositioned until it sensed glass floor temperature.
Table 1.
Characterization of the plantar epicutaneous thermcouple temperature during feedback-controlled application of radiant heat on underside of glass floor
| Set temp (°C) |
Peak CLT (°C) on underside of glass floor |
Peak ETT (°C) |
Latency to stable (peak±0.2°C) ETT (s) |
Peak Δ ETT (0–3s) (°C/s) |
Peak Δ ETT (4–10s) (°C/s) |
|---|---|---|---|---|---|
| 40.0 | 41.4 (0.0) | 36.7 (0.2) | 1.2 (0.0) | 4.8 (0.3) | 0.1 (0.0) |
| 42.5 | 43.7 (0.1) | 38.1 (0.2) | 1.3 (0.0) | 5.6 (0.3) | 0.1 (0.1) |
| 45.0 | 46.4 (0.1) | 39.5 (0.2) | 1.7 (0.2) | 5.5 (0.4) | 0.2 (0.1) |
| 47.5 | 49.2 (0.1) | 41.0 (0.3) | 2.2 (0.3) | 5.2 (0.4) | 0.3 (0.1) |
| 50.0 | 51.6 (0.1) | 42.7 (0.3) | 2.0 (0.1) | 6.7 (0.3) | 0.1 (0.0) |
| 52.5 | 54.2 (0.1) | 44.4 (0.3) | 3.2 (0.4) | 7.4 (0.4) | 0.4 (0.2) |
| 55.0 | 56.9 (0.1) | 46.5 (0.4) | 3.8 (0.3) | 8.2 (0.5) | 0.6 (0.2) |
| 57.5 | 59.5 (0.0) | 47.9 (0.4) | 3.7 (0.1) | 8.3 (0.2) | 0.7 (0.2) |
| 60.0 | 62.4 (0.0) | 50.1 (0.4) | 5.0 (0.1) | 8.9 (0.5) | 2.0 (0.1) |
| 62.5 | 64.7 (0.0) | 51.2 (0.4) | 5.4 (0.2) | 8.2 (0.2) | 2.0 (0.0) |
| 65.0 | 66.9 (0.1) | 52.8 (0.5) | 6.5 (0.2) | 8.5 (0.3) | 2.1 (0.0) |
| 67.5 | 69.1 (0.1) | 53.9 (0.5) | 7.6 (0.2) | 9.1 (0.3) | 2.1 (0.0) |
| 70.0 | 71.4 (0.1) | 54.9 (0.4) | 8.8 (0.3) | 8.6 (0.5) | 2.1 (0.1) |
CLT, controlled lamp temperature; ETT, epicutaneous thermocouple temperature. Glass and epicutaneous temperature were maintained at 29.0±1.0°C prior to applying heat. Feedback controlled radiant heat was applied on the underside of glass floor beneath the hindpaw plantar surface of an anesthetized rat. Each heat application was repeated for 4 times and 24 digitized traces were obtained from 6 rats to measure average temperature change at each second of 10-s stimulation. Data are presented mean (±S.E.M.).
2.4. Mechanosensitivity testing with electronic von Frey esthesiometer
The electronic von Frey esthesiometer consists of a handheld force transducer with a series of rigidity-graded, attachable 0.8-mm polypropylene tips (IITC Life Science, Woodland Hills, CA). Unrestrained rats were situated within individual Plexiglas (12×20× 17-cm) compartments on a shared metal mesh floor. Starting with the least rigid tip, force was transversely applied to the mid-plantar surface of the ipsilateral hind paw (adjacent to the site of surgical injury). The criterion for the stimulation end point was a rapid paw withdrawal response. The results were expressed as the mean threshold force (in grams) for nociceptive behaviors from 3–5 trials for each animal.
2.5. Experimental protocol
A total of 116 rats were used. Prior to behavioral testing, all animals were acclimated to the laboratory facilities for at least 1 week. Unless otherwise stated, experimental conditions such as animal age, gender, room temperature, time of day (for behavioral testing), drug preparation and injection, and animal handling were consistent. On each day of testing, heat sensitivity was evaluated before mechanosensitivity.
2.5.1. Study 1: Characterization of PWT-H in naive rats
Eighteen SD rats received a three trial testing session for baseline PWT-H and PWT-M prior to receiving an incision for the time course study (Study 2). Eight SD rats were tested for PWT-H on each of 4 consecutive days. Twelve additional SD rats received single PWT-H evaluation to supplement baseline PWT-H data. Two to three trials were conducted for each testing session, with 10–15 minute intervals. To evaluate the effects of animal age, the PWT-H were evaluated in groups of naïve 1-mo (n=6), 2-mo (n=7), 6 to 8-mo (n=10), 16 to 18-mo (n=6), and 25 to 28-mo (n=10) Fisher-344 rats.
2.5.2. Study 2: Ability of the current method to evaluate degree and time course of incision-induced hyperalgesia
Eighteen rats were tested in two groups. Six rats underwent a sham operation (anesthesia only), while 12 rats received hind paw plantar incision surgery. They were then tested at 3-h, 1, 2, 3, 4, 7, and 9-d post-incision. On each day of testing, the PWT-H and then the PWT-M were evaluated. To determine if PWT-H measurements depict degrees of hyperalgesia comparably to PWT-M measurements, correlation analysis was performed between PWT-M and PWT-H measurements evaluated from the same rats at various time points relative to plantar incision.
2.5.3. Study 3: Ability of PWT-H to evaluate attenuation of incision-induced hyperalgesia following administration of analgesics
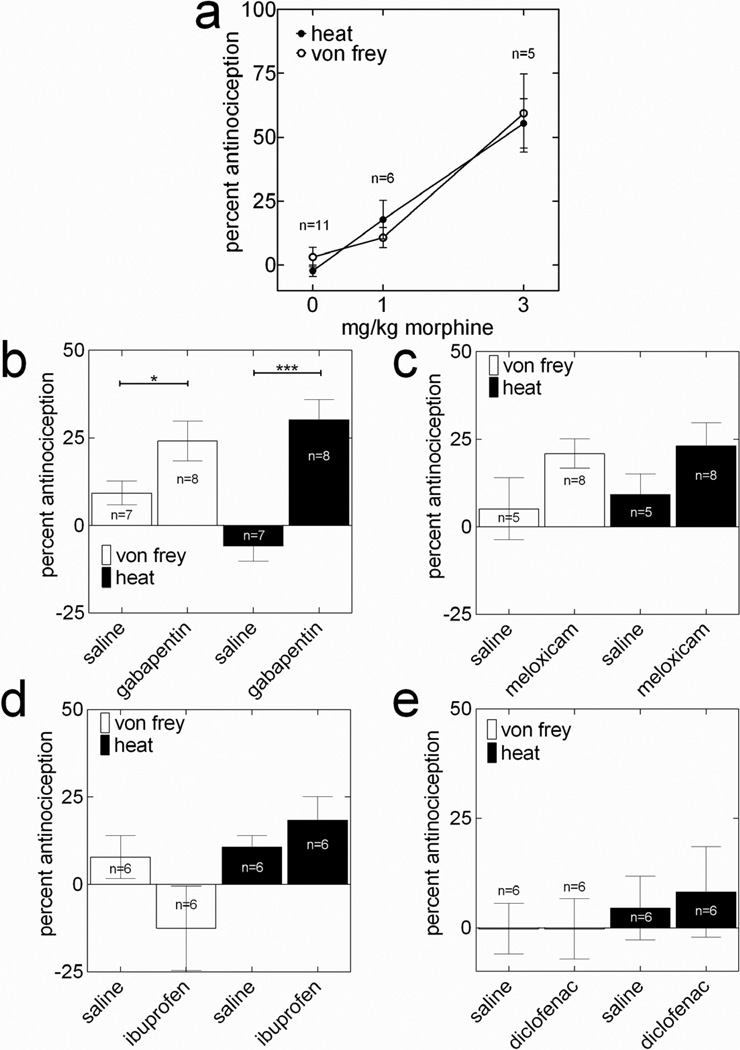
Thirty-three rats were randomly tested in 8 groups of four or five rats each. At 3-h, 1, 2, 3, and 4-d after plantar incision, the PWT-H and PWT-M were evaluated. Each rat randomly received an injection of saline or morphine 1 or 3 mg/kg s.c. (2-h post-incision), gabapentin 50 mg/kg i.p. [1st postoperative day (POD)], meloxicam 10 mg/kg i.p. (2nd POD), ibuprofen 20 mg/kg i.p. (3rd POD), or diclofenac 12 mg/kg i.p. (4th POD). Twenty minutes following drug administration, the animals were tested for PWT-H and PWT-M by an experimenter blinded to treatment assignments (drug vs. saline). Always, at first PWT-H and then PWT-M were evaluated. Testing was completed within 2-h of drug administration. Stock solutions of the drugs were refrigerated, warmed, and diluted to their final concentrations with saline (0.9%) prior to injection.
2.6. Data analysis and statistics
The PWT-H data range from 35.0 to 54.9°C with an average step size of 0.15°C, and have been considered continuous. PWT-M data obtained with the electronic von Frey device also constitute continuous data as they represent exact noxious threshold force values. Both PWT-H and PWT-M data are presented as the mean±standard error and were analyzed using parametric tests following a Kolmogorov-Smirnov test. A one-way ANOVA followed by a Newman-Keuls multiple comparison test was used to determine if there were significant differences between mean PWT-H of different age groups, and whether the PWT-H in normal animals differed between repeated measurements. Two- way repeated measures ANOVA (group vs. time) were conducted on PWT measurements obtained before and after the incision/sham operations. Bonferroni post-hoc comparisons followed these ANOVAs to identify significant differences between individual time points. A Pearson correlation analysis was conducted between PWT-H and PWT-M measurements to determine the relationship between these PWT data. The presence of inter-trial variance within PWT testing sessions was evaluated using a one-way ANOVA followed by a Newman-Keuls test. PWT data were normalized by calculating the percentage of the maximum possible effect (%-MPE) of each administered drug or saline on heat and mechanical thresholds to evaluate each drug’s effects. The %-MPE values were calculated with the equation: %-MPE=100 x (PWTpost-drug - PWTpre-drug)/(PWTsham-operated - PWTpre-drug). The %-MPE data are continuous and are presented as mean±standard error. The %-MPE values between groups were compared using unpaired t-tests. Additionally, paired t-tests were conducted between the PWTpre-drug and PWTpost-drug to compare the sensitivities of the PWT-M/H to analgesics and saline. To determine the interaction between the PWT-M/H and various doses of morphine, a one-way ANOVA followed by Dunnett’s multiple comparison test was used. All analyses were performed with Prism 5.0 (GraphPad Software, San Diego, CA). P<0.05 was considered statistically significant.
3. RESULTS
3. 1. Feedback-controlled lamp temperature under glass floor produces reproducible plantar epicutaneous temperatures
At stimulator settings of 40.0, 50.0, 60.0, and 70.0°C, the peak CLTs beneath the glass floor were 41.4±0.0, 51.6±0.1, 62.4±0.0 and 71.4±0.1°C, respectively (mean±SEM, Table 1). To achieve stable (peak±0.2°C) CLTs at these settings, the stimulator required 1.1±0.0, 2.0±0.0, 5.0±0.1, and 8.8±0.3-s, respectively (mean±SEM). Minute variations in the lamp temperature prior to stimulation (29.0±1.0°C) were considered negligible as the stimulator continuously adjusted the intensity of the projector lamp to dynamically maintain the user-defined temperature.
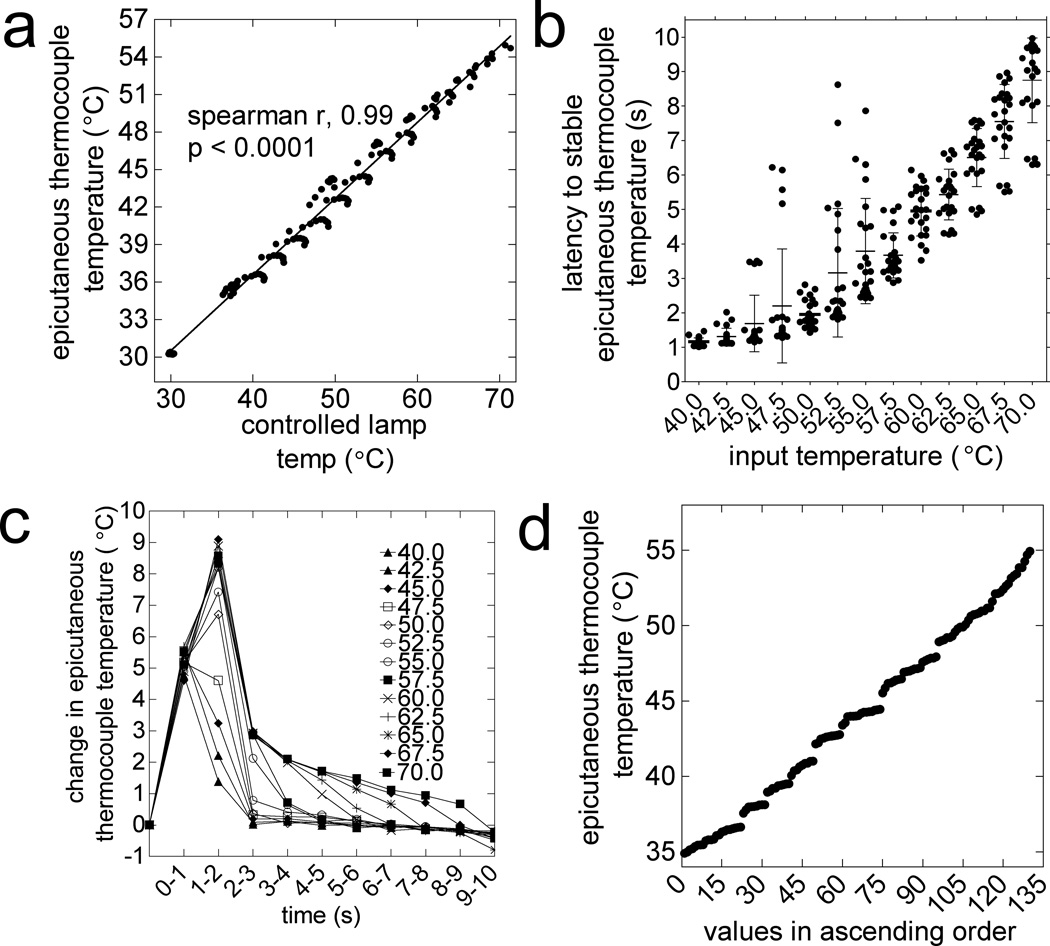
The ETTs above the glass floor (thickness = 1.91-mm) was considerably lower than the CLTs below the glass floor (Table 1, Fig. 3b). A linear relationship was observed between the changes in ETTs and CLTs at each second of 10-s heat applications (Fig. 4a; R2=0.986, P<0.0001). Heat applications at stimulator settings of 40.0, 50.0, 60.0, and 70.0°C corresponded to peak ETTs of 36.7±0.2, 42.7±0.3, 50.1±0.4, and 54.9±0.4°C (mean±SEM; Table 1). To achieve stable (peak±0.2°C) ETTs at these settings, the stimulator required 1.2±0.0, 2.0±0.1, 5.0±0.1, and 8.8±0.3-s, respectively (mean±SEM, Table 1, Fig. 4b). The use of a thicker (thickness = 4.69-mm) or unheated glass floor (23–25°C) significantly attenuated the ETTs (data not shown).
Figure 4.
Characteristics of heat stimulus used for measuring paw withdrawal threshold for heat nociception (PWT-H). a) Correlation of the rates of changes in ETT and CLT in each second of 10-s heat applications (spearman r = 0.99, P<0.0001). (b) Latency to stable (peak±0.2°C) ETT (elapsed time from onset to peak temperature) varies with the set temperature. (c) The rate of ETT change is different between applications, but is consistently highest over the first 2-s of 10-s heat application. (d) One hundred thirty temperature values were obtained from 10-s stimulations at 13 set temperatures (13 × 10, 1-s bin). The PWT-H data ranges from 35.0°C to 54.9°C, with an average step size of 0.15°C.
The rate of ETT change during stimulation varied with the stimulator’s temperature setting, but was consistently greatest over the first 2-s (Fig 4c). At settings of 40.0, 50.0, 60.0, and 70.0°C, the maximum rates of ETT change were 4.8±0.3, 6.7±0.3, 8.9±0.5, and 8.6±0.5°C/s, respectively (Fig. 4c). After the initial 2-s, the highest rates of ETT change, at the same settings were 0.1±0.0, 0.1±0.0, 2.0±0.0, and 2.1±0.1°C/s, respectively.
In total, 130 ETT values were obtained from all the applied 10s heat applications (40.0–70.0°C, step = 2.5°C). The average step size between these data points was 0.15°C, and data were considered continuous (Fig. 4d).
3. 2. Behavioral threshold of heat nociception in the normal rats
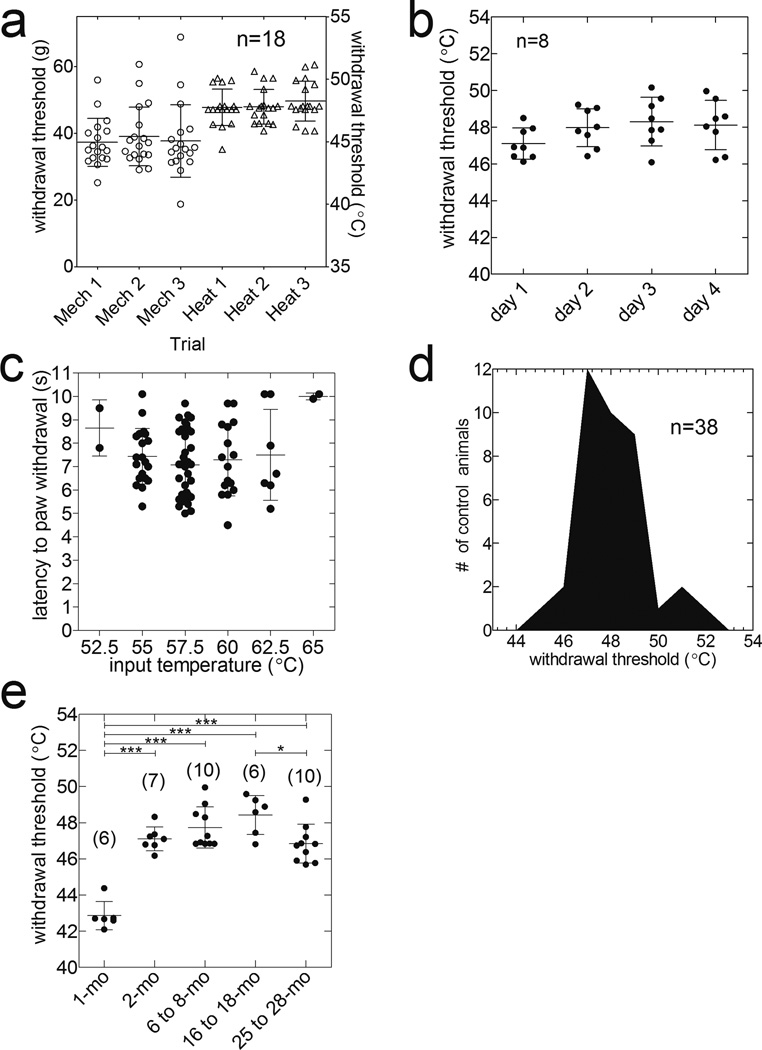
Figure 5a displays consistency between trials of PWT-H measurements comparable to PWT-M measurements with an electronic von Frey esthesiometer. No significant inter-trial differences were seen within sets of three trials evaluating the PWT-H and PWT-M (baseline/pre-incision data from time course studies, Fig. 5a). Moreover, daily measurements over 4 consecutive days did not significantly alter the PWT-H in rats (n=8, P>0.05, ANOVA followed by Newman-Keuls multiple comparison test; Fig. 5b). The PWT-H for naïve animals did not occur within the first 4-s of any 10-s CLT application, when the rate of temperature change was highest (4.8–9.1°C/s, varying with the set temperature (Fig. 5c)). The mean PWT-H in naïve Sprague-Dawley rats was 47.6±0.2°C (n=38) and the data were normally distributed (Kolmogorov-Smirnov test, P>0.1; Fig. 5d).
Figure 5.
Characteristics of PWT-H in normal rats. (a) Three trial testing sessions of paw withdrawal thresholds for heat (PWT-H) and mechanical nociception from pre-incision testing (n=18) (P>0.05, ANOVA followed by Newman-Keuls multiple comparison test) are shown. PWT-H measurements were consistent between multiple trials within the same testing session (a) or daily testing across four consecutive days (b) (n=8, P>0.05, ANOVA followed by Newman-Keuls test). PWT-H was observed in the static but not dynamic phase of ETT change (c, n=38 also see Fig. 4c). (d) Histogram of PWT-H distribution within naïve Sprague-Dawley rats (n=38). These data are normally distributed (P>0.1, Kolmogorov-Smirnov test). (e) PWT-H from F-344 rats of different age groups (*P<0.05, *** P<0.001, ANOVA followed by Newman-Keuls test). mo, months old.
The PWT-H for 1-mo (n=6), 2-mo (n=7), 6 to 8-mo (n=10), 16 to 18-mo (n=6) and 25 to 28-mo (n=10) rats were, respectively, 42.9±0.3, 47.1±0.3, 47.7±0.4, 48.4±0.4, and 46.8±0.3°C (F4,34=29.85, ANOVA, P<0.001). Juvenile (1-mo) rats had significantly lower PWT-H than the other groups (P<0.05, ANOVA followed by Newman-Keuls test; Fig. 5e). Additionally, the PWT-H for the 25 to 28-mo group was significantly (P<0.05) lower than that of the 18-mo group. The remaining groups did not differ from each other significantly (P>0.05, Newman-Keuls multiple comparison test)
3.3. Ability of current method to evaluate degree of hyperalgesia after plantar incision
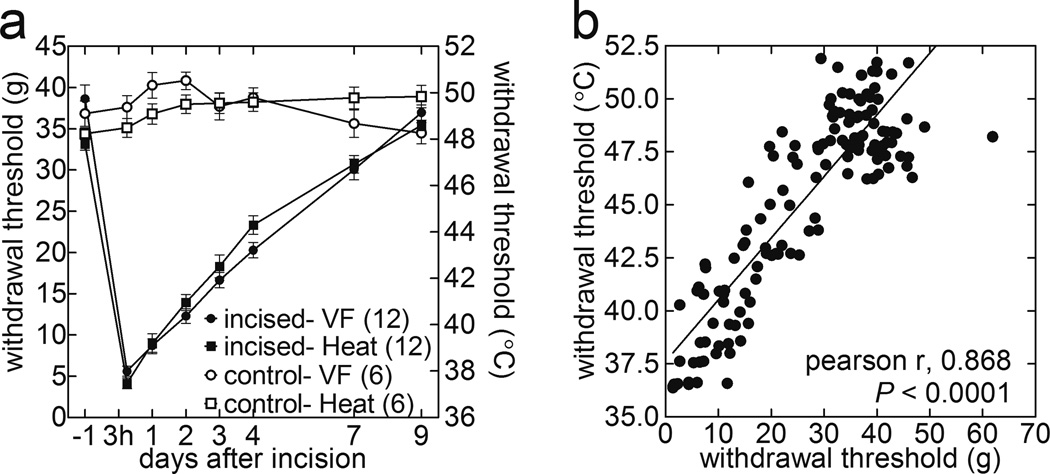
The ability of the current method to assess hyperalgesia was evaluated using the plantar incision model. The PWT-H decreased from 47.8±0.3°C to 37.5±0.2°C as evaluated 3-h following surgery. The time courses of the PWT-H and PWT-M evaluated at baseline, 3-h, 1, 2, 3, 4, 7, and 9-d relative to the incision are displayed in Figure 6a. The PWT-H was lowest 3-h post-incision, and exhibited gradual recovery on each subsequent day beginning at 1-d post-incision. Compared to the sham-operated rats, the PWT-H was significantly decreased at 3-h, 1, 2, 3, 4, and 7-d post-incision (F1, 16=72.0, P<0.0001, two-way ANOVA followed by Bonferroni’s post hoc test). The PWT-M, measured via electronic von Frey esthesiometer, from the same animals followed a similar time course (Fig. 6a). The PWT-M was significantly decreased in the incised group compared to the sham-operated rats at 3-h, 1, 2, 3, 4, and 7-d post-incision (F1,16=73.5, P<0.0001, two-way ANOVA followed by Bonferroni’s post hoc test). The time courses of hyperalgesia depicted by PWT-H and PWT-M measurements were significantly correlated with each other (Fig. 6b; Pearson r = 0.832; P<0.0001).
Figure 6.
PWT-H measure is comparable with PWT-M in detection of incision-induced hyperalgesia, and its recovery over time. Baseline PWT data used in Figure 5a. (a) Time courses of the mean PWT-H and PWT-M evaluated at baseline, 3-h, 1, 2, 3, 4, 7, and 9-d post-incision. PWT-H: sham vs. incision, F1,16 =72.0, P<0.0001 at 3-h, 1, 2, 3, 4, and 7-d; PWT-M: sham vs. incision, F1,16 =73.5, P<0.0001 at 3-h, 1, 2, 3, 4, and 7-d (2-way ANOVA followed by Bonferroni’s post-hoc tests). (b) Correlation between PWT-M and PWT-H throughout post-incision recovery period. The degree of hyperalgesia increases from left to right along the regression line. (Pearson r = 0.87; P<0.0001).
3.4. Ability of current method to evaluate attenuation of incision-induced hyperalgesia by analgesics
The ability of the current method to evaluate analgesic-induced reversals in heat and mechanical hyperalgesia is illustrated in Figure 7. At 3-h post-incision, compared to the %-MPE of saline, the %-MPEs of morphine (s.c.) at doses of 1 mg/kg [n=6] and 3 mg/kg [n=5] on PWT-H were significant (Fig 7a; F2,19=26.26, n=26, ANOVA followed by Dunnett’s test), while only the %-MPE of morphine 3 mg/kg on PWT-M was significant (Fig 7a; F2,19=15.99, n=22, ANOVA followed by Dunnett’s test). Two-way ANOVA analysis did not discern any notable differences between the dose-response curves of the PWT-H and PWT-M.
Figure 7.
PWT-H and PWT-M measures are identical in illustrating effects of analgesic compounds on recovery of incision-induced hyperalgesia. Two hours after incision, and on the 1st, 2nd, 3rd, and 4th postoperative days, rats were tested for baseline, and 30 min following blinded administrations of saline or morphine 1 or 3-mg/kg (a, PWT-H; F2,19=26.26 and PWT-M; F2,19=15.99, 1-way ANOVA followed by Dunnett’s test), gabapentin (b, 50 mg/kg i.p.; *P<0.05, ***P<0.001, unpaired t-test; n=8), meloxicam (c, 10 mg/kg; i.p.; n=8), ibuprofen (d, 20 mg/kg i.p.; n=6), or diclofenac (d, 12 mg/kg; i.p.; n=6), on each respective day. Data are presented as mean±SEM of the percentage of maximum possible effect (%-MPE). See materials and methods for calculation of %-MPE values.
The ability of the current method to evaluate the blockade of hyperalgesia by gabapentin, meloxicam (selective COX-2 inhibitor), ibuprofen (nonselective COX inhibitor), and diclofenac (nonselective COX inhibitor) was tested on the 1st, 2 3rd and 4th postoperative days in the same group of rats, respectively, following plantar incision. On the 1st POD, both the PWT-H (P<0.0005) and PWT-M (P<0.05) displayed significantly greater mean %-MPE of gabapentin (50-mg/kg, i.p.) than saline (Fig 7b; unpaired t-test). On the 2nd, 3rd and 4th POD, the PWT-H and PWT-M measurements both failed to detect any appreciable %-MPE of meloxicam (10 mg/kg, i.p.), ibuprofen (20-mg/kg, i.p.) or diclofenac (12 mg/kg, i.p.) compared to that of saline (Fig. 7c,d,e).
4. DISCUSSION
The study describes a modified Hargreaves’ method based on stepwise feedback-controlled applications of radiant heat, for the assessment of threshold temperatures for heat nociception within unrestrained rats. This technique allows us to compare between behavioral and electrophysiological or human psychophysical studies, and detects hyperalgesia comparably to electronic von Frey testing. These conclusions are based on the following: 1) The PWT-H in naïve adult rats is 47.6±0.2°C, which is similar to the noxious heat threshold in human glabrous skin of 46.5±0.5°C (Tillman et al., 1995). 2) One day after plantar incision, the PWT-H dropped to 37.5±0.2°C, which corresponds with a previous observation that rat C-fiber primary afferent endings from incised glabrous skin initiate firing at 36.7±3.6°C (Banik and Brennan, 2004). 3) A highly significant (>0.8) correlation was observed between the PWT-M and PWT-H at different time points after plantar incision. 4) The %-MPE of morphine dose-response curves for PWT-H and PWT-M measurements were identical in rats at 3-h post-incision.
4.1. Characteristics of heat stimulus
Despite significant heat dissipation across the glass floor, the rates of change of the CLT and ETT are nearly identical (Fig. 3) with both attained peak values almost simultaneously. This suggests that temperature changes at the epicutaneous thermocouple are predominantly due to the transfer of radiant heat.
Each of the 13 (Fig. 3) temperature slopes used in this study contains a rising (first 3-s,Δ4.8–9.1°C/s) and a stable (after 3- s,Δ0.1–2.1°C/s) phase. The nociceptive behaviors predominantly occurred in the rising phase (Fig 4c, Fig 5c). Previously, Yeomans and Proudfit showed a higher rate of temperature increase (6.5°C/s) to preferentially activate A5 nociceptors, while lower rates (0.9°C/s) preferentially activated C-fiber nociceptors (Yeomans et al., 1996). Therefore, it may be hypothesized that PWT-H measured in this study is primarily due to the activity of C-fibers. However, in this study, the PWT-H is much higher than the heat threshold of C-fibers innervating glabrous skin studied in vivo (Andrew and Greenspan, 1999) and in vitro (Banik and Brennan, 2004). On the other hand, at 3-h post-incision, PWT-H and the heat activation threshold of C-fibers are similar (37.5±0.2°C vs. 36.7±3.6°C) (Banik and Brennan, 2004; Banik et al., 2005). It has been shown that heat nociception mechanistically differs between normal and pathological states; the heat gated receptor, TrpV1, is not required for normal behavioral responses to noxious heat, but is essential in pathological states (Davis et al., 2000; Woodbury et al., 2004). Further, both peripheral and central mechanisms shape behavioral thresholds for heat nociception (Kauppila et al., 1998; Sung et al., 1998), and can be expected to differ from the response thresholds of individual nociceptors.
The absence of nociceptive behaviors during the rapid phase of epicutaneous temperature increase could be related to the absence of cutaneous type 2 Aδ nociceptors in primate glabrous skin. These nociceptors respond with very short latencies (tenths of milliseconds), have lower heat activation thresholds for heat, and are believed to signal ‘first pain’ in response to heat stimuli (Raja et al., 1999). Recordings from monkey glabrous skin afferents did not find these nociceptor responses during heat stimulation, and human subjects did not identify the first pain sensation when heat stimuli were applied to the palmar surface of the hand (Campbell and LaMotte, 1983; Meyer et al., 2006; Treede et al., 1995). In contrast, Iannetti and colleagues have recently found evidence of these nociceptors within human glabrous skin (Iannetti et al., 2006).
The rate of temperature change and the latency to peak temperature varied between stimulator temperature settings (Fig. 4a, c). Whether or not such differences have any effect on the PWT-H remains unclear. In previous studies on humans, Pertovaara (Pertovaara et al., 1996) and Nielsen and Arendt-Nielsen (Nielsen and Arendt-Nielsen, 1998) found no interaction between the rates of temperature change (1–16°C/s) and pain ratings, whereas Tillman and colleagues (Tillman et al., 1995) reported decreased heat threshold in human subjects when the rate of temperature change was greater. Moreover, it has been shown that the peak rates of discharge from C-fiber nociceptors increase with higher rates of temperature increase in humans (Yarnitsky et al., 1992) and monkeys (Tillman et al., 1995).
4.2. Threshold temperature for heat nociception in unrestrained normal rats
The PWT-H is stable between multiple trials in the same testing session and in daily measurements over 4 consecutive days (Fig. 4a,b). The consistency in PWT-H measurements is comparable to that of PWT-M measurements by an electronic von Frey device. A previous study has shown that repeated measurements significantly affect paw withdrawal latencies in the Hargreaves test (Kocevski and Tvrdeic, 2008). However, another study acclimated mice to the testing environment for three consecutive days and observed consistent paw withdrawal latencies with repeated measurements (Banik et al., 2006).
For consistent applications of heat, the temperature above the glass was maintained 29.0±1.0°C throughout the experiment. Previously, Dirig and colleagues (Dirig et al., 1997) demonstrated that a glass floor at room temperatures 23–25°C acts as a heat sink. Additionally, the thermocouple providing input to the heat stimulator was maintained 29.0±1.0°C, as it has been shown previously that the baseline temperature significantly affects the quantity of radiant heat produced and, consequently, alters the threshold temperature for heat nociception (Dyck et al., 1996; Pertovaara et al., 1996; Wu et al., 2001). Following each heat application, residual heat from the lamp was dissipated with compressed cold air to reduce the thermocouple temperature, or, if the lamp temperature was depressed, the lamp was activated to sufficiently increase the thermocouple temperature.
This study found the PWT-H in unrestrained young rats (47.6±0.2°C; 2 mo, SD) to be higher than reported in previous studies using either an increasing temperature hot plate (45.3±0.3°C) (Almasi et al., 2003) or water bath (43.5±0.4°C) (Furedi et al., 2009). This discrepancy may be attributed to inter-study differences in techniques. Only a small area of a single hind paw was in contact with noxious heat in the current method, whereas, the other two methods apply heat to either the glabrous tissue of all four paws or to both the hairy and glabrous tissue of both hind paws. The average noxious threshold temperature in humans (46.5±0.5°C) on a small area of the hand and the average PWT-H (47.6±0.2°C) in the current study are comparable (Tillman et al., 1995).
Senescent rats (25 to 28-mo) had PWT-H similar to those evaluated within 2-mo and 6 to 8-mo rats. Using the tail flick test, previous studies have shown that withdrawal latencies increase (4/15 studies), decrease (2/15 studies) or do not change (9/15 studies) with increasing age (Gagliese and Melzack, 2000). Three previous studies using the hot plate test found increased withdrawal latencies (decreased heat sensitivity) in rats with aging (Gagliese and Melzack, 2000). Similarly, Wang and colleagues found significantly increased withdrawal latencies to noxious heat in aged mice (Wang et al., 2006). In contrast, Taguchi et al. (Taguchi et al., 2010) recently found significantly lower withdrawal latencies in the older rats using the Hargreaves method. These authors used calorie-restricted rats of different ages (29 to 32-mo) and strains (SD). SD and Fisher 344 rats have previously been reported to differ in behavioral measures (Shir et al., 2001).
The PWT-H measure demonstrated heat hypersensitivity in juvenile (1-mo) rats compared to all older groups, which is supported by the findings of earlier studies (Falcon et al., 1996; Fitzgerald and Jennings, 1999). Relatively thinner skin in juvenile rats may allow heat to more readily permeate the glabrous tissue and activate afferent terminals. Supporting this, Benoist et al. found a tendency for the noxious threshold temperature to decrease distally along the tail, which is consistent with decreasing epidermal thickness (Benoist et al., 2008). Alternately, as shown by Fitzgerald and colleagues, juvenile rats may have hypersensitive dorsal horn neurons which amplify discharges from peripheral stimulation (Fitzgerald, 1985).
4.3. Ability of the current method to detect incision-induced hyperalgesia and its attenuation by analgesics
Previous studies have used the conventional Hargreaves method to show that paw withdrawal latencies profoundly decrease when evaluated 2 to 4-h post-incision, and then gradually returned to baseline values within 7-d post-incision (Banik et al., 2005; Zahn and Brennan, 1999). The PWT-H measured by the current technique followed an identical time course (Fig. 6a). Moreover, the PWT-M and the PWT-H were correlated throughout the time courses of incision-induced hyperalgesia (Fig. 6b; Pearson r = 0.83, P<0.0001). Finally, the F values (ratio of variances between groups and variance within groups) for both methods were similar (see results), when data from sham and incised rats were compared using a two-way ANOVA test. Taken together, it may be reasonable to state that the current method and the electronic von Frey method have similar abilities to detect degrees of incision-induced hyperalgesia and recovery over time.
Additional evidence for the current method’s ability stems from a comparison of the analgesic-induced attenuation of heat and mechanical hyperalgesia. The percentage of the maximum possible effect (%-MPE) of morphine doses on both measurements produced similar dose-response curves at 3-h post-incision (Fig. 7a). However, the F-value obtained using PWT-H data was higher than that from PWT-M data (26.3 vs. 16.0). Moreover, in both methods, gabapentin (50-mg/kg) was efficacious in significantly reversing hyperalgesia at 1-d post-incision (Fig 7b), while meloxicam (10-mg/kg), ibuprofen (20-mg/kg) and diclofenac (12-mg/kg) all failed to significantly attenuate hyperalgesia, respectively, at 2, 3 and 4-d post-incision (Fig 7c, d, e). However, it should be noted that all NSAIDs were given at 2nd, 3rd, and 4th days post-incision. As shown by others (Whiteside et al., 2004), they can be efficacious if were given immediately post-incision.
4.4. Advantages and limitations
The current paradigm is conceptually similar to conventional von Frey testing and may reduce incongruities between latency vs. threshold measurements. PWT-H measurements from different laboratories may be compared and correlated without the contextual information required for interpretation of latency data (cut-off values, lamp amperage, or intensity of radiant light). Lastly, PWT-H data from behavioral studies may be correlated with neurophysiological observations and human psychophysical studies.
A limitation of this method is that the ETT values are obtained from a preliminary characterization. Inter-experimental variation and environmental conditioning has potential to interact with and offset the measured PWT-H as this has been shown in calibrated von Frey hairs (Andrews, 1993; Moller et al., 1998).
Supplementary Material
Highlights.
We describe a method to measure threshold temperatures for heat nociception in rats.
The method relies on characterizing average plantar epicutaneous temperatures.
We show similar degrees of incision-induced allodynia and reversals by analgesics.
The method allows us to compare animal data with human psychophysical studies. Basic neuroscience
Acknowledgement
This work is supported in part by the American Federation for Aging Research and the National Institutes of Health, Bethesda, Maryland grant AG030352 and AG030352-02S2 to RKB. We thank Timothy J. Brennan, MD, PhD and Alberto Subieta, BS for technical assistance, and Francois Kouya, PhD for contributing to the pilot studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no personal financial interest in the method or equipment discussed in this article. The authors did not receive any benefit, financial or otherwise, by referring to the Bioengineering Department in the University of Iowa for development of the equipment discussed.
Summary statement: The study describes a behavioral assay based on stepwise applications of feedback-controlled radiant heat for determining threshold temperatures for heat nociception in an unrestrained rat’s hind paw.
REFERENCES
- Almasi R, Petho G, Bolcskei K, Szolcsanyi J. Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br J Pharmacol. 2003;139:49–58. doi: 10.1038/sj.bjp.0705234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Andrews K. The effect of changes in temperature and humidity on the accuracy of von Frey hairs. Journal of neuroscience methods. 1993;50:91–93. doi: 10.1016/0165-0270(93)90059-z. [DOI] [PubMed] [Google Scholar]
- Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112:204–213. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Banik RK, Kabadi RA. 13th World Congress of Pain. Montreal, Canada: International Association for the study of Pain; 2010. a modified hargreaves’ method for measuring approximate threshold temperature for thermonociception. [Google Scholar]
- Banik RK, Subieta AR, Wu CR, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- Benoist JM, Pincede I, Ballantyne K, Plaghki L, Le Bars D. Peripheral and central determinants of a nociceptive reaction: an approach to psychophysics in the rat. PLoS One. 2008;3:e3125. doi: 10.1371/journal.pone.0003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res. 1983;266:203–208. doi: 10.1016/0006-8993(83)90650-9. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–1549. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. Journal of neuroscience methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;89:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Zimmerman IR, Johnson DM, Gillen D, Hokanson JL, Karnes JL, Gruener G, O'Brien PC. A standard test of heat-pain responses using CASE IV. J Neurol Sci. 1996;136:54–63. doi: 10.1016/0022-510x(95)00277-9. [DOI] [PubMed] [Google Scholar]
- Falcon M, Guendellman D, Stolberg A, Frenk H, Urca G. Development of thermal nociception in rats. Pain. 1996;67:203–208. doi: 10.1016/0304-3959(96)03070-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. In: Neuronal growth and plasticity. Kuno M, editor. Vol. 23. Tokyo: Japan Scientific Societies Press, VNU Science Press; 1985. p. 211. Utrecht, 1984, DM142. Pain. [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci U S A. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furedi R, Bolcskei K, Szolcsanyi J, Petho G. Effects of analgesics on the plantar incision-induced drop of the noxious heat threshold measured with an increasing-temperature water bath in the rat. Eur J Pharmacol. 2009;605:63–67. doi: 10.1016/j.ejphar.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24:843–854. doi: 10.1016/s0149-7634(00)00041-5. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Tracey I. Similar nociceptive afferents mediate psychophysical and electrophysiological responses to heat stimulation of glabrous and hairy skin in humans. J Physiol. 2006;577:235–248. doi: 10.1113/jphysiol.2006.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila T, Kontinen VK, Pertovaara A. Influence of spinalization on spinal withdrawal reflex responses varies depending on the submodality of the test stimulus and the experimental pathophysiological condition in the rat. Brain Res. 1998;797:234–242. doi: 10.1016/s0006-8993(98)00379-5. [DOI] [PubMed] [Google Scholar]
- Kocevski D, Tvrdeic A. The effect of repeated daily measurements on paw withdrawal latencies in Hargreaves test. Coll Antropol. 2008;1(32 Suppl):93–97. [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Meyer R, Ringkamp M, Campbell BA, Raja S. In: Peripheral mechanisms of cutaneous nociception. McMahon SB, Koltzenburg M, editors. Elsevier: Textvook of Pain; 2006. [Google Scholar]
- Moller KA, Johansson B, Berge OG. Assessing mechanical allodynia in the rat paw with a new electronic algometer. Journal of neuroscience methods. 1998;84:41–47. doi: 10.1016/s0165-0270(98)00083-1. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Arendt-Nielsen L. The influence of rate of temperature change and peak stimulus duration on pain intensity and quality. Somatosens Mot Res. 1998;15:220–229. doi: 10.1080/08990229870781. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Kauppila T, Hamalainen MM. Influence of skin temperature on heat pain threshold in humans. Exp Brain Res. 1996;107:497–503. doi: 10.1007/BF00230429. [DOI] [PubMed] [Google Scholar]
- Raja S, Meyer R, Ringkamp M, Campbell J. Peripheral mechanisms of nociception. 4th ed. Churchill Livingstone; 1999. [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Shir Y, Zeltser R, Vatine JJ, Carmi G, Belfer I, Zangen A, Overstreet D, Raber P, Seltzer Z. Correlation of intact sensibility and neuropathic pain-related behaviors in eight inbred and outbred rat strains and selection lines. Pain. 2001;90:75–82. doi: 10.1016/s0304-3959(00)00388-2. [DOI] [PubMed] [Google Scholar]
- Sung B, Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Supraspinal involvement in the production of mechanical allodynia by spinal nerve injury in rats. Neurosci Lett. 1998;246:117–119. doi: 10.1016/s0304-3940(98)00235-3. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Ota H, Matsuda T, Murase S, Mizumura K. Cutaneous C-fiber nociceptor responses and nociceptive behaviors in aged Sprague-Dawley rats. Pain. 2010;151:771–782. doi: 10.1016/j.pain.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: correlation with pain threshold in humans. J Physiol. 1995;485(Pt 3):767–774. doi: 10.1113/jphysiol.1995.sp020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483(Pt 3):747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004;141:85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Campbell JN, Meyer RA. Effects of baseline skin temperature on pain ratings to suprathreshold temperature-controlled stimuli. Pain. 2001;90:151–156. doi: 10.1016/s0304-3959(00)00399-7. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Simone DA, Dotson RM, Cline MA, Ochoa JL. Single C nociceptor responses and psychophysical parameters of evoked pain: effect of rate of rise of heat stimuli in humans. J Physiol. 1992;450:581–592. doi: 10.1113/jphysiol.1992.sp019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.