Abstract
Introduction
Preclinical studies have demonstrated that tumor reactive T cells expressing the IL-15 transgene had enhanced activity. Gene therapy strategies utilizing IL-15 should include a safety mechanism in anticipation of possible adverse effects because IL-15 overexpression has been implicated in autoimmune disorders and may be involved in the pathogenesis of some leukemias. We developed a retroviral vector carrying both IL-15 and the HSV-TK suicide gene and characterized its application in the transduction of human T lymphocytes.
Methods
A retroviral vector carrying IL-15 and HSV-TK genes was optimized for the transduction of human T lymphocytes. IL-15 production was measured by ELISA. Thymidine incorporation and cell viability assays were utilized to assess the efficacy of the HSV-TK suicide gene. Genetically modified tumor infiltrating lymphocytes (TILs) were assayed for survival after withdrawal from exogenous IL-2. The activity and specificity of retrovirally transduced TILs was assessed utilizing tumor coculture assays.
Results
Human T cells transduced with the IL-15/HSV-TK vector exhibited thymidine uptake in the absence of exogenous cytokine support and survived in culture for up to 80 days without IL-2. IL-15/HSV-TK transduced T cells were efficiently killed by ganciclovir at concentrations as low as 0.1 uM. TILs transduced with the IL-15/HSV-TK vector retained specific recognition of HLA-A2+, MART1+ melanomas, even after withdrawal of IL-2.
Conclusions
Human T lymphocytes genetically modified with the IL-15/HSV-TK retroviral vector retained the ability to recognize tumor antigen while gaining the ability to secrete IL-15 and prolong their own survival. IL-15/HSV-TK transduced T cells expressed HSV-TK and could be efficiently eliminated by ganciclovir.
Keywords: Human, T Cells, Retroviral Vector, Gene Therapy, Cytokine, Cancer Immunotherapy, Suicide Gene
Introduction
Adoptive immunotherapy for cancer involves the generation of large numbers of autologous, tumor-specific lymphocytes in vitro and subsequent transfer to a tumorbearing host. While this strategy has resulted in objective responses in patients with lymphoma and melanoma, the factors critical to mediating a clinical response continue to be investigated.1–3 Current T cell-based therapies for melanoma are dependent on the administration of IL-2 to support the growth and function of transferred cells. Though IL-2 has an established record of antitumor activity against renal cell cancer and melanoma, recent evidence suggests that the main in vivo function of IL-2 relates to the production of CD4+CD25+ T regulatory cells and the maintenance of peripheral tolerance.4 This revelation has stimulated interest in evaluating alternative common γ- chain cytokines for cancer immunotherapy.
It has been suggested that IL-15 may be a rational substitute IL-2 in cancer immunotherapy regimens.5 Both cytokines signal through shared β- and γ-chain receptor subunits and both are able to stimulate the proliferation of T cells in vitro, suggesting redundancy in their function. However, animal studies have revealed distinct and divergent actions of IL-2 and IL-15 in vivo.6 In contrast to IL-2, IL-15 opposes activation induced cell death and is critical for the development and function of NK cells and CD8+ memory T cells. The differences between IL-2 and IL-15 have been exploited in murine cancer immunotherapy models in which IL-15 was shown to be superior to IL- 2 for the in vitro generation of antitumor T cells.7, 8 IL-15 was also superior to IL-2 for the in vivo maintenance of adoptively transferred antitumor memory CTLs.9 In a murine leukemia model, the function of tolerant antitumor CD8+ T cells could be restored after exposure to IL-15.10 Furthermore, treatment of established tumor in a murine melanoma model was markedly enhanced when tumor reactive T cells bearing an IL-15 transgene were used.8 These findings suggest that IL-15 may be useful in immune based cancer therapies.
Considering the promising preclinical studies, we hypothesized that retroviral transduction of tumor reactive human T lymphocytes with the IL-15 gene could enhance their activity and/or survival. However, we also had to consider the potential toxicities of IL-15. IL-15 has been associated with a variety of autoimmune phenomena and is possibly leukemogenic.11, 12 To address these safety concerns, we developed a bicistronic retroviral vector carrying both the IL-15 gene and the suicide gene herpes simplex virus-thymidine kinase (HSV-TK). In this report, we demonstrated that human T cells transduced with the IL-15/HSV-TK vector exhibited IL-2 independent proliferation and prolonged survival in the absence of exogenous cytokine support. The transduced cells could be completely eradicated with ganciclovir administration. Potential gene therapy applications of this vector are discussed.
Materials and Methods
Vector construction
The MSGV1 retroviral vector contains a murine stem cell virus long-terminal repeat and RNA processing signals similar to the MFG class of retroviral vectors. Construction of this vector was previously described.13 Codon optimization of IL-15 was performed by Blue Heron Biotechnology (Bothell, WA). Cloning of MSGV1 wild type (WT) IL-15 and MSGV1 codon optimized (CO) IL-15 was reported previously; in a direct comparison of these vectors, codon optimization resulted in augmented IL-15 production by retrovirally transduced NIH-3T3 cells.14 pORF-HSV TK, containing WT HSV-TK, was obtained from InvivoGen (San Diego, CA). A mutant version of HSV-TK, lacking the cryptic splice sites described by Chalmers, et al.15 and incorporating amino acid changes previously described in clone SR39 by Black, et al.,16 was expression optimized and synthesized by Blue Heron Biotechnology. This modified HSV-TK was designated CO HSV-TK. IRES and PGK were subcloned from pBS cloning plasmids. Vector components were subcloned into the MSGV1 multiple cloning site. When fragments were amplified by PCR for cloning, DNA sequencing was used to confirm the fidelity of the amplified sequence.
Combinations of the 6 components (WT IL-15 vs. CO IL-15, IRES vs. PGK, and WT HSV-TK vs. CO HSV-TK) were subcloned into the MSGV1 retroviral vector backbone. 16 configurations were possible. With anticipation that efficient IL-15 expression may be limiting, we cloned the majority of the vectors with IL-15 preceding the HSV-TK. In T cell receptor bicistronic vectors used to transduce T lymphocytes, IRES has been comparable to PGK in driving expression of the second gene, thus our strategy has been to make constructs containing either IRES or PGK and compare their function head to head.13, 18 A total of 8 retroviral vector plasmids were cloned and their configurations are depicted in Figure 1.
Figure 1.
Configuration of retroviral vector plasmids. The MSGV1 retroviral vector is driven by the MSCV LTR. Each vector contains either wild type (WT) or codon optimized (CO) IL-15, the internal ribosomal entry site (IRES) or phosphoglycerine kinase (PGK) internal promoter, and the wild type (WT) or codon optimized (CO) HSVTK.
Cell lines
The cell lines used include the human ecotropic packaging cell line Phoenix Eco (kindly provided by G. Nolan, Stanford University, Stanford, CA), the mouse fibroblast line NIH/3T3 (ATCC CRL-1658), the PG13 gibbon ape leukemia virus-packaging cell line (ATCC CRL-10686), and 3 melanoma lines: 526mel, 624mel, and 888mel (generated at the Surgery Branch from resected tumors). Culture conditions for these lines has been described.13
RNA dot blot titering of retroviral preparations
2-fold serial dilutions of each retrovirus preparation were made using fresh media. 200 µl of undiluted retrovirus and serially diluted samples were then transferred to a nylon membrane (Ambion, Austin, TX) by a vacuum manifold technique and UV crosslinked.17 The blot was then probed with a 32P-labeled DNA probe prepared from a gel purified Pml I/Spe I fragment of the retroviral backbone pMSGV1. The blot was developed, imaged, and quantitated using a FLA-5100 imaging system (Fujifilm Medical Systems USA, Stamford, CT).
Assessment of vector function in transduced NIH/3T3 cells
Phoenix Eco was transfected with 2 µg of each retroviral vector plasmid using GenePorter2 (Gene Therapy Systems, San Diego, CA). The resulting retroviral supernatants were used to transduce NIH/3T3 cells as described previously.14 For these experiments, 2 ml of retroviral supernatant was used in each transduction well and the cells were transduced twice, on successive days. Transduced cells were plated in 24 well tissue culture plates at a density of 8 × 104/well for experiments. 24 h IL-15 production by transduced cells was assayed by ELISA (R&D Systems, Minneapolis, MN). Sensitivity to ganciclovir was assessed by adding serially diluted ganciclovir (Sigma-Aldrich, St. Louis, MO), at the specified concentrations, to transduced cell cultures. Viability of these cells was assessed by trypan exclusion 5 days later.
Transduction of human T lymphocytes
High titer PG13 packaging cell clones were isolated for the transduction of human lymphocytes as described previously.14 The murine monoclonal antibody OKT3 (anti-CD3, Ortho Biotech) binds to the T cell receptor and was used to activate peripheral blood lymphocytes (PBLs) and tumor infiltrating lymphocytes (TILs); culture conditions for these cells was previously reported.2, 14 The cultures were initiated in lymphocyte culture medium containing IL-2 and transduced with 2 to 4 exposures to retrovirus coated plates. PBLs were transduced on culture days 2 and 3 and TILs were transduced on culture days 3 and 4. T cell assays were performed on culture day 7 for PBLs and culture day 14 for TILs. IL-15 production from transduced cells was measured in the presence or absence of plate-bound OKT3 by ELISA (R&D Systems); the details of this assay were previously described in detail.14
Thymidine incorporation
Cells were washed extensively and plated in triplicate at 1 × 105 cells/well in a 96-well microplate in 0.2 ml of lymphocyte culture medium, with or without IL-2 (300 IU/ml). Wells were also exposed to indicated concentrations of ganciclovir. The cells were cultured for a total of 72 h and in the final 16 hours of culture, 1 µCi/well [methyl-3H] thymidine (PerkinElmer Life Sciences, Boston, MA) was added to each well. Cellular DNA was harvested and quantitated by liquid scintillation counting. Error bars represent ± SEM.
Cytokine withdrawal assay
On culture day 7, untransduced and transduced PBLs were washed extensively and resuspended in 20 ml of lymphocyte culture medium without added IL-2. 6 × 106 lymphocytes were cultured in each T25 flask. Every 4–7 days, the cells were enumerated and half of the spent medium was replaced with fresh media without added IL-2.
Tumor Recognition Assay
The activity and specificity of TILs was assessed through tumor coculture assays. Briefly, TILs used in this study are reactive against the HLA-A2 restricted MART1 antigen while the melanoma lines used were either HLA-A2+ (526mel and 624 mel) or HLA-A2- (888 mel). 1 × 105 TILs were co-cultured with 1 × 105 tumor targets in a final volume of 0.2 ml in each well of a round-bottom 96-well plate. 24 hours later, cell culture supernatants were harvested and assayed for IFN-g content by ELISA (R&D Systems).
Results
Screening of bicistronic IL-15/HSV-TK vectors
In earlier studies of IL-15 retroviral vectors, we found no differences in gene expression when cell lines were transfected with either the wild type or codon-optimized IL-15 retroviral vector plasmids. However, we found that cells retrovirally transduced with the CO IL-15 vector produced 2.5-fold more IL-15 than cell transduced with the WT IL-15 vector, controlling for viral titer and transduction efficiency.14 Thus, we used used a similar strategy to test bicistronic vectors for expression of IL-15 and HSV-TK.
Phoenix Eco retroviral packaging cells were transfected with retroviral vector plasmids and the physical titer of the retroviral supernatants was quantitated by RNA dot blot (Figure 2a). All of the retroviral preparations resulting from transfection with bicistronic vectors had comparable titers. Retrovirus generated by transfection with single gene IL- 15 vector plasmids (WT IL-15 and CO IL-15) had slightly higher titers, but this difference was less than 2-fold. Transfection with vectors containing 2 codon optimized genes (pMSGV CO IL-15-IRES-CO HSV-TK and pMSGV CO IL-15-PGK-CO HSV-TK) resulted in slightly lower titers, but again this difference was less than 2-fold.
Figure 2.
Evaluation of IL-15 and HSV-TK expression in transduced NIH/3T3 cells. Phoenix Eco packaging cells were transfected with the MSGIN retroviral vector plasmid, which encodes for GFP, and the other retroviral vector plasmids depicted in figure 1. (a) The physical titer of the retroviral supernatants was measured using the RNA dot blot technique. (b) Retroviral vector supernatants were used to transduce NIH/3T3 cells. Two days post-transduction, cells were washed and plated at 8 × 104/well in 24-well tissue culture plates. After 24 h, cell culture media was assayed for IL-15 content by ELISA. (c) Concurrently, the transduced cells were cultured in the presence of the indicated concentrations of ganciclovir and viable cells were enumerated 5 days later.
The Phoenix Eco retroviral supernatants were used to transduce NIH/3T3 cells. Two days post-transduction, the plates were washed to eliminate any trace of IL-15 that could be carried over from the retroviral vector supernatant. The culture media was replaced and subsequently assayed for IL-15 content 24 hours later (Figure 2b). Cells transduced with GFP (MSGIN) did not produce IL-15. CO IL-15 transduced cells produced approximately 2.8-fold more IL-15 than WT IL-15 transduced cells (3603 and 1266 pg/ml, respectively), consistent with our previous report.14 CO IL-15-IRES-WT HSVTK cells exhibited IL-15 production comparable to WT IL-15 transduced cells (1343 and 1266 pg/ml, respectively). Surprisingly, transduction with the CO IL-15-IRES-CO HSV-TK vector resulted in substantially lower IL-15 production (311 pg/ml). Transduction with the remaining vectors also resulted in poor IL-15 production. Transduction with vectors utilizing IRES consistently resulted in higher IL-15 production than matched vectors utilizing PGK. The two vectors in which WT HSV-TK preceded the CO IL-15 resulted in the lowest IL-15 production from transduced cells.
Two days post-transduction, transduced NIH/3T3 cells were tested for HSV-TK expression by exposing the cultures to serial dilutions of ganciclovir and assessing cell viability 5 days later (Figure 2c). HSV-TK converts the pro-drug ganciclovir to its monophosphate intermediate derivative. Cellular kinases phosphorylate it to a triphosphate compound which is incorporated into DNA, resulting in inhibition of DNA chain elongation and cell death. Cells that were not transduced with HSV-TK were unaffected by ganciclovir concentrations up to 10 µM. All of the cells that had been transduced with a bicistronic retroviral vector containing HSV-TK were killed with comparable efficiency. At ganciclovir concentrations of 0.1 µM, more than half of the cells were dead. At concentrations of 1 µM, few viable cells were present in cultures transduced with vectors containing HSV-TK.
Based on these results we generated PG13 packaging cell clones, transduced with pMSGV CO IL-15-IRES-CO HSV-TK, pMSGV CO IL-15-PGK-CO HSV-TK, pMSGV CO IL-15-IRES-WT HSV-TK. High titer clones were selected based on packaging cell IL-15 secretion. When these clones were used to transduce human lymphocytes, only packaging cells transduced with pMSGV CO IL-15-IRES-WT HSV-TK produced virus capable of transducing human lymphocytes. The best clone was selected on the basis of IL-15 production by transduced human lymphocytes. A Southern blot was performed, probing for HSV-TK; this revealed that the clone contained 7 copies of the vector and that the vector was intact (data not shown). This packaging cell clone was used to transduce human lymphocytes in subsequent experiments. For comparison, lymphocytes were also transduced with retrovirus produced by the previously published packaging cell clone carrying the CO IL-15 vector, designated pMSGV CO IL-15.14
We evaluated the physical titer of retroviral supernatants produced by packaging cell clones using the RNA dot blot technique and found that the single gene IL-15 vector and the bicistronic pMSGV CO IL-15-IRES-WT HSV-TK vector had nearly identical titers (data not shown).
T lymphocytes transduced with the pMSGV CO IL-15-IRES-WT HSV-TK bicistronic vector proliferate in the absence of exogenous cytokine support and exhibit sensitivity to ganciclovir
OKT3-stimulated PBLs were transduced with both the pMSGV CO IL-15 and pMSGV CO IL-15-IRES-WT HSV-TK vectors under conditions similar to those used for current gene therapy protocols under way in the Surgery Branch. Specifically, high volumes of unconcentrated retroviral vector were applied to recombinant fibronectin plates and the cells were exposed to retrovirus-coated plates 2–4 times during a period of rapid proliferation.
IL-15 production by 7 day cultured transduced PBLs was measured (Table 1). Untransduced cells did not produce IL-15. pMSGV CO IL-15 and pMSGV CO IL-15-IRES-WT HSV-TK transduced cells exhibited easily detected quantities of IL-15 which was increased upon stimulation with OKT3, reflecting stimulation of the retroviral long terminal repeat.14 Cells transduced with the pMSGV CO IL-15 vector produced 10–17 times more IL-15 than cells transduced with the pMSGV CO IL-15-IRES-WT HSV-TK vector. For the pMSGV CO IL-15 vector, going from 2 to 4 transductions resulted in a modest increase in IL-15 production. In contrast, IL-15 production was nearly doubled when PBLs were transduced 4 times with the pMSGV CO IL-15-IRES-WT HSV-TK vector.
Table 1.
IL-15 Production by Transduced PBLs
| IL-15 (pg/ml) | |||||
|---|---|---|---|---|---|
| Vector | UT | IL-15 | IL-15 | IL- 15/HSVTK |
IL-15/HSVTK |
| # Transductions | n/a | 2 | 4 | 2 | 4 |
| − OKT3 | 0 | 1401 | 1871 | 80 | 170 |
| + OKT3 | 2 | 6242 | 7423 | 394 | 770 |
A thymidine incorporation assay was performed, measuring the proliferation of lymphocytes in the presence or absence of IL-2 over 3 days (Figure 3a). Untransduced cells proliferated minimally in the absence of exogenous IL-2. The pMSGV CO IL-15 and pMSGV CO IL-15-IRES-WT HSV-TK transduced cells exhibited comparable proliferation after withdrawal from IL-2. Furthermore, proliferation of transduced cells withdrawn from IL-2 nearly matched that of transduced cells maintained in media containing IL-2. In the same assay, the cells were also exposed to serial dilutions of ganciclovir. We found that the proliferation of pMSGV CO IL-15 transduced cells was not affected by any concentration of ganciclovir, while proliferation of pMSGV CO IL- 15-IRES-WT HSV-TK transduced cells was almost completely attenuated at ganciclovir concentrations of 0.1 µM (Figure 3b).
Figure 3.
Proliferation of pMSGV CO IL-15 (IL15) and pMSGV CO IL15-IRES-WT HSV-TK (IL15/TK) transduced PBLs. For each assay, 1 × 105 cells were plated in triplicate in 96-well plates. Cells were cultured for a total of 72 h. [3H]-thymidine was added to cultures 18 hours prior to scintillation counting. (a) In order to evaluate IL-2 independent lymphocyte proliferation, untransduced (UT) and IL-15 or IL-15/TK transduced cells (transduced either 2 or 4 times) were cultured in the presence or absence of IL-2 (300 IU/ml). (b) To assess lymphocyte sensitivity to ganciclovir, UT, IL-15, or IL-15/TK transduced cells were cultured without IL-2 and in the presence of the indicated concentrations of ganciclovir. Results are shown as mean cpm ± SEM.
In order to examine the long-term in vitro survival of the cells in the absence of exogenous cytokine support, 7 day cultured lymphocytes were washed extensively and returned to culture without exogenous cytokine support. In addition, a fraction of the transduced cells were immediately exposed to 10 µM ganciclovir upon initiation of the experiment. Viable cells were enumerated every 4–7 days at which time half of the media was replaced. No additional ganciclovir was added to the cultures after the initial dose. Untransduced cells proliferated briefly and then declined in number, surviving approximately 20 days. pMSGV CO IL-15 transduced cells proliferated for approximately 40 days and survived in culture beyond 80 days. pMSGV CO IL-15 transduced cells were not sensitive to ganciclovir. pMSGV CO IL-15-IRES-WT HSV-TK transduced cells proliferated in a similar fashion and survived for up to 80 days in the absence of exogenous IL-2. In contrast to pMSGV CO IL-15 transduced cells, pMSGV CO IL-15-IRES-WT HSV-TK transduced cells were exquisitely sensitive to ganciclovir, disappearing completely from culture after less than 10 days (Figure 4).
Figure 4.
pMSGV CO IL-15 and pMSGV CO IL15-IRES-WT transduced PBLs demonstrated expansion and prolonged viability after IL-2 withdrawal. PBLs were activated with OKT3 and IL-2. Cells were transduced 2 to 4 times with retroviral supernatants on culture days 2 and 3. Control cells were not transduced (UT). On day 7 of the culture, the PBLs were washed extensively and 6 × 106 cells from each culture were plated in fresh media, in the absence of added IL-2. The transduced cells were cultured in the presence or absence of 10 µM ganciclovir, which was added to cultures as a single dose, immediately after IL-2 withdrawal. Viable cells were enumerated every 4–7 days by trypan blue exclusion; concurrently, the cell culture media was refreshed by replacing half of the spent media with fresh media.
Tumor infiltrating lymphocytes transduced with pMSGV CO IL-15-IRES-WT HSV-TK retain anti-tumor activity after withdrawal from IL-2
Enhanced proliferation and survival of PBLs transduced with pMSGV CO IL-15 or pMSGV CO IL-15-IRES-WT HSV-TK prompted us to evaluate the vectors in the transduction of tumor infiltrating lymphocytes, which are highly dependent on IL-2 support for maintaining their function.19 TILs with known reactivity to the HLA-A2 restricted MART1 antigen were rapidly expanded with allogeneic feeder cells, OKT3, and IL-2. The cells were retrovirally transduced on the 3rd and 4th days of culture. pMSGV CO IL-15 and pMSGV CO IL-15-IRES-WT HSV-TK transduced TILs were tested for IL-15 production after 2 weeks in culture (Table 2). TILs receiving 2 transductions with the pMSGV CO IL-15-IRES-WT HSV-TK vector produced small amounts of IL-15. In comparison, IL-15 production was markedly increased after 4 transductions with pMSGV CO IL-15-IRES-WT HSV-TK. As was the case with the PBLs, transduction with the pMSGV CO IL-15 vector (2 transductions) resulted in greater IL-15 production than transduction with the pMSGV CO IL-15-IRES-WT HSVTK vector. In contrast to the PBL studies, pMSGV CO IL-15-IRES-WT HSV-TK transduced TILs exhibited IL-2 independent proliferation and prolonged survival in culture only after 4 transductions. TILs that were transduced twice with pMSGV CO IL- 15-IRES-WT HSV-TK did not proliferate or persist in vitro in the absence of exogenously supplied IL-2 (data not shown).
Table 2.
IL-15 Production by Transduced TILs
| IL-15 (pg/ml) | ||||
|---|---|---|---|---|
| Vector | UT | IL-15 | IL-15/HSVTK | IL-15/HSVTK |
| # Transductions | n/a | 2 | 2 | 4 |
| − OKT3 | 10 | 163 | 16 | 393 |
| + OKT3 | 10 | 1202 | 141 | 629 |
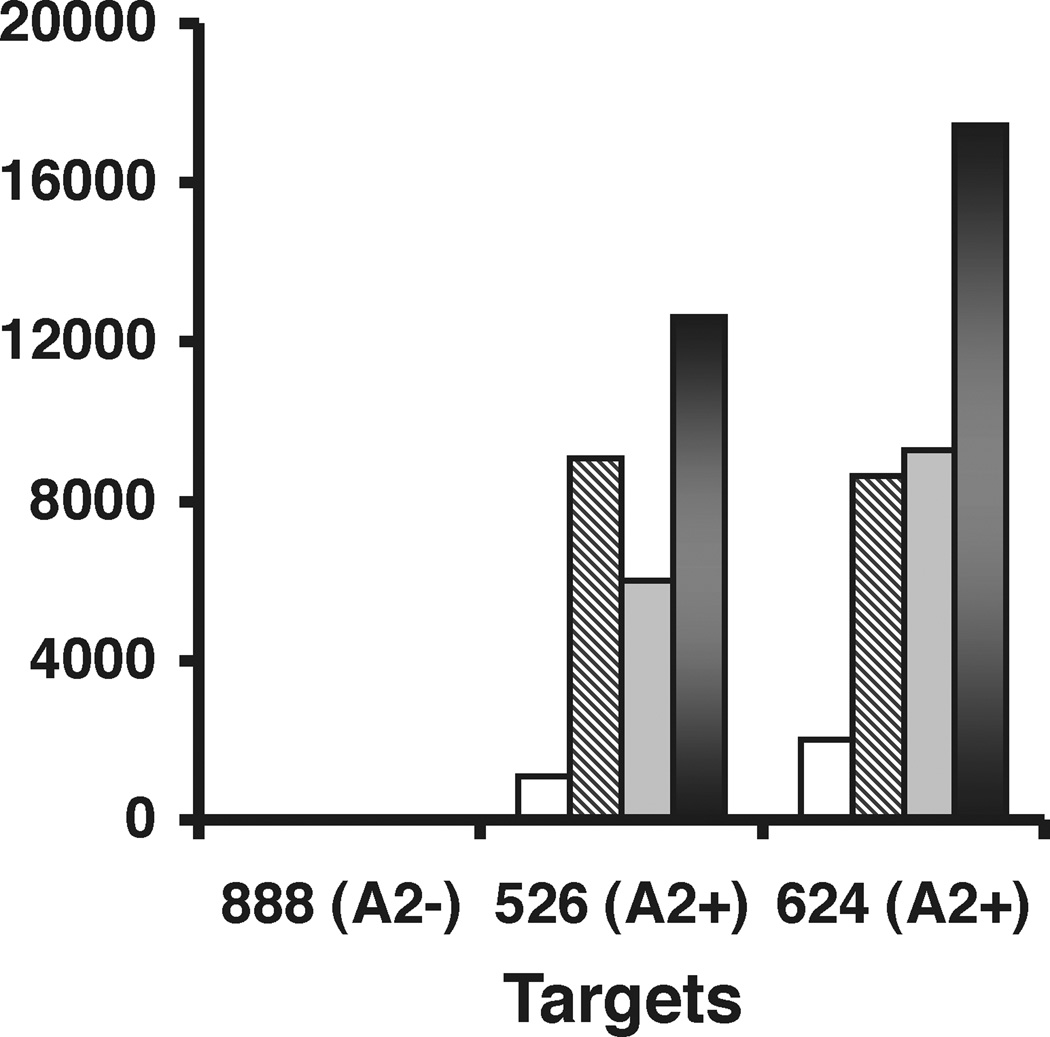
2 week cultured TILs were cocultured with melanoma lines that were HLA-A2+ (526mel and 624 mel) and a melanoma line that was HLA-A2- (888mel). Untransduced, pMSGV CO IL-15 transduced (2 transductions), and pMSGV CO IL-15-IRES-WT HSV-TK transduced (4 transductions) TILs exhibited specific reactivity, secreting IFN-γ upon encountering HLA-A2+ melanoma lines (Figure 5a). In order to evaluate TIL function in the absence of exogenously supplied IL-2, 2 week cultured TILs were washed extensively and re-tested in a tumor coculture assay after 3 days in the absence of added cytokine (Figure 5b). After withdrawal from IL-2, antigen-specific IFN-γ secretion decreased 80–90% in untransduced TILs when compared to untransduced TILs maintained continuously in media containing IL-2. pMSGV CO IL-15 and pMSGV CO IL-15-IRES-WT HSV-TK transduced TILs exhibited minimal loss of antigen-specific IFN-γ after withdrawal from IL-2.
Figure 5.
Antigen-specific tumor reactivity is maintained in pMSGV CO IL-15 (IL15) and pMSGV CO IL15-IRES-WT (IL15/TK) transduced TILs after withdrawal from IL-2. MART1 reactive TIL cultures were generated and subsequently untransduced (UT), transduced with the IL-15 vector, or transduced with IL-15/TK. (a) On culture day 14, these lymphocytes were tested for tumor reactivity by coculturing them with HLA-A2 positive (526 and 624) and negative (888) melanoma lines. The cell culture supernatants were harvested and assayed for IFN-γ content 24 h after the cocultures were initiated. (b) To evaluate for tumor reactivity after cytokine withdrawal, untransduced and transduced TIL cultures were withdrawn from IL-2 for 3 days, prior to tumor coculture on day 17. Untransduced TILs were also maintained in media containing IL-2 (UT + IL-2) prior to coculture as a positive control for untransduced withdrawn from IL-2 (UT).
Discussion
For over 20 years, researchers have been engaged in efforts to improve the survival and function of T lymphocytes used in adoptive cell transfer cancer therapies. With advances in gene transfer technologies, it is possible to investigate the clinical application of T cells engineered to express potentially desirable genes, such as highly avid T cell receptors, co-stimulatory molecules, adhesion molecules, and growth factors.20 This report details the development of a bicistronic retroviral vector encoding IL-15 and the suicide gene, HSV-TK.
In the treatment of established B16 melanomas in mice, adoptively transferred, tumorreactive CD8+ T cells derived from IL-15 transgenic mice treated tumors better than tumor-reactive CD8+ T cells that did not secrete IL-15.8 This influenced the development of an IL-15 retroviral vector that could be used to efficiently transduce human lymphocytes. In vitro, IL-15 transduced lymphocytes demonstrated superior survival and function after IL-2 withdrawal than untransduced cells.14 Unexpectedly, 1 of 23 cultures of IL-15 transduced cells exhibited logarithmic, clonal expansion in excess of 365 days.21 This raised concerns that transduction of human lymphocytes with IL-15 would come with some risk of leukemogenesis. Previously, it has been suggested that IL-15 may be leukemogenic and it has been established that IL-15 transgenic mice are prone to the development of T cell leukemias.12, 22 In addition, aberrant IL-15 expression has been associated with autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and sarcoidosis.11 Given the potential toxicities of administration of IL-15 transduced cells, we desired a reliable safeguard to control the transduced cells should adverse effects arise in patients.
We utilized the suicide gene herpes simplex virus-thymidine kinase (HSV-TK) because of its proven track record in controlling graft-versus-host disease in patients receiving allogeneic donor lymphocytes engineered with HSV-TK.23 In these studies, cells modified with HSV-TK retained their native function and could efficiently be eliminated with ganciclovir administration. No toxicities directly related to gene transfer have been reported. Furthermore, patients treated with HSV-TK transduced lymphocytes have been followed for up to 9 years and demonstrated no evidence of clonal selection or insertional oncogenesis.24
Cloning of bicistronic vectors for this study was done empirically as there is much inherent ambiguity in the variables which determine viral titer, vector integration, and ultimately transgene expression. These factors are intimately related, thus our strategy for screening the vectors was to produce retrovirus and perform cell line transductions under identical conditions. We assessed the different vectors by evaluating transgene expression and found that HSV-TK activity was nearly identical in all of the vectors tested. This demonstrated that HSV-TK was active in all of the constructs tested, however, the sensitivity of the assay is limited by the contribution of bystander killing to the observed effect. We did find important differences in IL-15 expression in the cloned vectors. Surprisingly, vectors in which CO IL-15 was paired with CO HSV-TK, resulted in lower IL-15 production than when CO IL-15 was paired with WT HSV-TK. This may relate to the process of codon optimization, which minimizes the potential for nucleic acid folding. Ultimately, we generated 3 high-titer PG13 packaging cell clones derived from the vectors that produced the highest IL-15 concentrations in transduced NIH/3T3 cells (pMSGV CO IL15-IRES-WT HSV-TK, pMSGV CO IL15-IRES-CO HSV-TK, pMSGV CO IL15-PGK-WT HSV-TK). Only virus derived from packaging cells transduced with pMSGV CO IL15-IRES-WT HSV-TK were able to transduce human lymphocytes, which was correctly predicted to be the best of the bicistronic vectors based on IL-15 production in transduced NIH/3T3 cells.
T cells transduced with the bicistronic pMSGV CO IL15-IRES-WT HSV-TK vector produced detectable levels of IL-15. Transduction with the bicistronic vector resulted in considerably lower levels of IL-15 production than when the single gene IL-15 vector was used. Nevertheless, production of IL-15 by pMSGV CO IL15-IRES-WT HSV-TK transduced lymphocytes had a significant biological impact, resulting in IL-2- independent proliferation as well as prolonged in vitro growth and viability in the absence of exogenous cytokine. This may be related to a unique feature of IL-15 biology; the IL-15Rα stably binds IL-15 with high affinity. IL-15Rα-bound IL-15 can be recycled through endosomes and subsequently presented in trans.6 pMSGV CO IL15- IRES-WT HSV-TK transduced TILs exhibited no perturbation of antigen specificity and maintained tumor reactivity after withdrawal from IL-2. Importantly, pMSGV CO IL15-IRES-WT HSV-TK transduced lymphocytes could be eliminated in vitro with the administration of ganciclovir at concentrations approximating levels that can be achieved in the serum at therapeutic doses.25 pMSGV CO IL15-IRES-WT HSV-TK transduced lymphocytes exposed to ganciclovir died more quickly than untransduced cells withdrawn from IL-2, implying that a significant fraction of the lymphocytes were transduced.
One limitation of the described vector system is the lack of a selectable marker. Consequently, we are unable to enrich for transduced cells nor are we able to quantify transduction efficiency on a per cell basis. Conversely, there are advantages. We are able to transduce T cells with enough efficiency to result in a measurable biological impact without extensive ex vivo manipulation, thus the process of gene transfer can be achieved within the paradigm of rapidly generating large numbers of tumor reactive cells for adoptive cell transfer.26 As described in this report, TILs with tumor reactivity could be transduced with the pMSGV CO IL15-IRES-WT HSV-TK vector while being expanded for therapeutic administration. It may also be possible to transduce lymphocytes activated from peripheral blood concurrently with tumor antigen-specific T cell receptor genes and the pMSGV CO IL15-IRES-WT HSV-TK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 5.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7(1):23–35. doi: 10.1080/14653240510018037. [DOI] [PubMed] [Google Scholar]
- 6.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roychowdhury S, May KF, Jr, Tzou KS, Lin T, Bhatt D, Freud AG, et al. Failed adoptive immunotherapy with tumor-specific T cells: reversal with low-dose interleukin-15 but not low-dose interleukin 2. Cancer Res. 2004;64(21):8062–8067. doi: 10.1158/0008-5472.CAN-04-1860. [DOI] [PubMed] [Google Scholar]
- 10.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12(3):335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 11.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 12.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3(7):477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 13.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, et al. Transfer of a TCR Gene Derived from a Patient with a Marked Antitumor Response Conveys Highly Active T-Cell Effector Functions. Hum Gene Ther. 2005;16(4):457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175(11):7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalmers D, Ferrand C, Apperley JF, Melo JV, Ebeling S, Newton I, et al. Elimination of the truncated message from the herpes simplex virus thymidine kinase suicide gene. Mol Ther. 2001;4(2):146–148. doi: 10.1006/mthe.2001.0433. [DOI] [PubMed] [Google Scholar]
- 16.Black ME, Kokoris MS, Sabo P. Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve prodrug-mediated tumor cell killing. Cancer Res. 2001;61(7):3022–3026. [PubMed] [Google Scholar]
- 17.Onodera M, Yachie A, Nelson DM, Welchlin H, Morgan RA, Blaese RM. A simple and reliable method for screening retroviral producer clones without selectable markers. Hum Gene Ther. 1997;8(10):1189–1194. doi: 10.1089/hum.1997.8.10-1189. [DOI] [PubMed] [Google Scholar]
- 18.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171(6):3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 20.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5(12):928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 21.Hsu C, Jones SA, Cohen CJ, Zheng Z, Kerstann K, Zhou J, et al. Cytokine-independent growth and clonal expansion of a primary human CD8+ T-cell clone following retroviral transduction with the IL-15 gene. Blood. 2007;109(12):5168–5177. doi: 10.1182/blood-2006-06-029173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciceri F, Bonini C, Gallo-Stampino C, Bordignon C. Modulation of GvHD by suicide-gene transduced donor T lymphocytes: clinical applications in mismatched transplantation. Cytotherapy. 2005;7(2):144–149. doi: 10.1080/14653240510018136. [DOI] [PubMed] [Google Scholar]
- 24.Recchia A, Bonini C, Magnani Z, Urbinati F, Sartori D, Muraro S, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci U S A. 2006;103(5):1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crumpacker CS. Ganciclovir. N Engl J Med. 1996;335(10):721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]