Abstract
A fully functional immune system is essential to protect the body against pathogens and other diseases, including cancer. Vesicular trafficking provides the correct localization of proteins within all cell types, but this process is most exquisitely controlled and coordinated in immune cells because of their specialized organelles and their requirement to respond to selected stimuli. More than 60 Rab GTPases play important roles in protein trafficking, but only five Rab-encoding genes have been associated with inherited human disorders, and only one of these (Rab27a) causes an immune defect. Mutations in RAB27A cause Griscelli Syndrome type 2 (GS2), an autosomal recessive disorder of pigmentation and severe immune deficiency. In lymphocytes, Munc13-4 is an effector of Rab27a, and mutations in the gene encoding this protein (UNC13D) cause Familial Hemophagocytic Lymphohistiocytosis Type 3 (FHL3). The immunological features of GS2 and FHL3 include neutropenia, thrombocytopenia, and immunodeficiency due to impaired function of cytotoxic lymphocytes. The small number of disorders caused by mutations in genes encoding Rabs could be due to their essential functions, where defects in these genes could be lethal. However, with the increasing use of next generation sequencing technologies, more mutations in genes encoding Rabs may be identified in the near future.
Keywords: Rab GTPases, Immunodeficiencies, Griscelli Syndrome Type 2, Rab27a, Familial Hemophagocytic Lymphohistiocytosis Type 3, Muc13-4
Introduction
A fully functional immune system is essential to defend the organism against infections and other diseases such as cancer. To function properly, an immune system must detect a wide variety of agents, from viruses and bacteria to parasitic worms, and distinguish them from the organism’s own healthy tissue. Disorders of the immune system can result in autoimmune diseases, inflammatory diseases and cancer. Immunodeficiency occurs when the immune system is less active than normal, resulting in recurrent, life-threatening infections. In humans, immunodeficiency can be the result of either a genetic disease such as severe combined immunodeficiency, congenital neutropenias, Griscelli Syndrome (GS), Familial Hemophagocytic Lymphohistiocytosis (FHL), acquired conditions such as HIV/AIDS, or the use of immunosuppressive medications.
Intracellular protein trafficking is a fundamental process in all eukaryotic cells, and even more so in immune cells. These cells secrete different molecules (e.g. cytokines, chemokines, and lysosomal enzymes), which regulate, cell migration, lytic activity, and other functions. These essential functions of immune cells, especially regulated secretion, require proper protein traffic to mediate the killing of target cells and the process of inflammation. Numerous protein families are required for the correct trafficking of proteins, including SNAREs, adaptor complex proteins, motor proteins (myosins, kinesins and dyneins), and Rab GTPases.
Rabs are Ras-like small GTPases that interact with compartments of the endocytic and exocytotic pathways through a geranyl-geranyl group [1]. Rabs regulate vesicle movement, sorting and secretion in a very specific manner, allowing precise trafficking of proteins and membranes throughout the cell. Like all small Ras-like GTPases, Rab proteins function as a molecular switch by cycling between a GTP-bound active form and a GDP-bound inactive form [2]. A delicate regulation of the conversion between these two forms is required to achieve successful trafficking, and this is accomplished by two families of proteins. Conversion from the GDP- to the GTP-bound form is caused by nucleotide exchange, catalysed by a guanine nucleotide exchange factor (GEF). Conversion from the GTP- to the GDP-bound form occurs by GTP hydrolysis, facilitated by a GTPase Activating Protein (GAP). The GTP-bound form recruits specific effector proteins, which usually links the vesicle and target membranes to the cytoskeleton, whereas the GDP-bound form interacts with Rab escort proteins (REPs) and GDP dissociation inhibitors (GDIs) [3, 4].
In this review we describe the various Rab GTPases implicated in the correct functioning of the immune system, giving specific examples of human genetic immune disorders in which Rab proteins are affected. We also discuss the role of Rab proteins in the immune system fighting microbial infections.
Protein trafficking in immune cells
The majority of cells of the immune system are specialized secretory cells, whose function depends on regulated exocytosis [5]. This is mediated by vesicular transport involving the sorting of specialized cargo into secretory granules, thereby generating transport vesicles. These vesicles are carried along microtubules and, upon stimulation, are transferred to the actin cytoskeleton where they eventually fuse with the plasma membrane [6]. Each of these trafficking steps is tightly controlled by mechanisms that involve the recognition of specific sorting signals found in the protein cargo to be transported to its correct location. In addition to exocytosis, fusion of secretory granules with the plasma membrane leads to the membrane incorporation of proteins, such as Fas ligand (FasL) that causes death of Fas receptor expressing target cells [7]. Cognate adaptor proteins, post-translationally modified cargo proteins, GTPases and SNARE proteins all work together to achieve these trafficking steps.
Lysosomes are membrane-bound cytoplasmic organelles that serve as the major degradative compartments in eukaryotic cells [8]. In addition to lysosomes, certain specialised cell types contain Lysosome Related Organelles (LROs) that share features with lysosomes but may have distinct morphologies, compositions, and functions. Such organelles include melanosomes in melanocytes, lytic granules in lymphocytes, delta granules in platelets, Weibel–Palade bodies in endothelial cells, and lamellar bodies in lung type 2 epithelial cells. LROs are produced through the interaction of ubiquitous trafficking mechanisms with cell-specific machinery that targets cargo to a particular compartment [9, 10]. LROs maintain their structure, composition, and function by means of a continuous flow of proteins and membranes from the biosynthetic and endosomal protein trafficking routes.
The biosynthetic route deals with the trafficking of newly synthesised (de novo) proteins, made in cytosolic ribosomes, to their final required destination, either within or outside of the cell. The endocytic pathway takes up proteins from the plasma membrane and molecules from outside of the cell, and internalizes them into peripheral early sorting endosomes, where proteins delivered by the biosynthetic and endosomal pathways merge [11]. The early endosome is a tubulovesicular network with a pH of ~6.0 that contains distinct resident proteins, including Rab5 and its effector, i.e. early endosome–associated protein (EEA-1). Material is sorted away from the early endosome to the cell surface via Rab4- or Rab11-positive recycling endosomes, or to late endosomes and in turn to lysosomes or LROs [9, 11, 12]. Components destined for lysosomes are sorted into Rab7-positive late endosomes, which have an acidic pH (5.0–6.0), and contain membranes and vesicles, including multivesicular bodies. Late endosomes morph into lysosomes, which lack multivesicular structures and have a pH of 5.0– 5.5. Components destined for specific LROs possess unique cell-type and LRO-specific sorting and trafficking pathways that divert them away from lysosomal delivery [9].
In eukaryotic cells, the default protein trafficking pathway is constitutive secretion, which leads to the exocytosis of proteins and incorporation of proteins and lipids in the plasma membrane. In addition to this pathway, specialized secretory cells, such as neurons, endocrine and exocrine cells, and cells of the immune system exhibit regulated secretion in which secretory granules are prevented from fusion with the plasma membrane unless a signal is provided by an external ligand or stimulus. Biogenesis of secretory granules begins at the TGN with the formation of a nascent granule that subsequently matures to generate mature secretory granules that are ready for release [13]. Unlike the biogenesis of secretory granules, which involves direct TGN to secretory granule protein trafficking, the biogenesis of secretory vesicles is tightly coupled with the endocytic system. Secretory vesicles have a unique collection of membrane proteins, indicating that these proteins must contain specific signals for proper targeting to their final destination. In some cells (e.g. mast cells and cytotoxic T cells) an intimate connection exists between the secretory system and the endocytic system; secretory lysosomes, a special subtype of regulated secretory granules whose biogenesis is also tightly linked to the endocytic system, are LROs.
Following the signaling events that trigger exocytosis, two processes take place: (i) a microtubule-dependent transport of secretory granules toward the plasma membrane, and; (ii) secretory granule docking and fusion resulting in the release of granule contents into the external space. While SNAREs have been implicated in mediating the docking/fusion events, the Rab GTPases have emerged as important regulators of exocytosis in numerous secretory cells, including immune cells.
Immune Disorders
More than 60 different Rab GTPases have been identified in humans, but only five genes encoding Rabs have been associated with monogenic human disorders. These include Warburg Micro Syndrome (Rab18) [14], Charcot-Marie-Tooth Type 2B (Rab7a) [15], Carpenter Syndrome (Rab23) [16], Griscelli Syndrome Type 2 (Rab27a) [17] and X-linked mental retardation (Rab39b) [18]. Furthermore, after a thorough literature search we could identify only one gene encoding a Rab protein that had immune deficiency (Griscelli Syndrome Type 2) as a clinical feature. However, there are several Rabs that have been implicated in human disorders based on RNA or protein expression changes, genome-wide association study (GWAS) data, or functional studies. Furthermore, there are a significant number of accessory proteins modulating each Rab’s function within cells, such as RabGEFs, RabGAPs and effectors, which could cause certain inherited human disorders.
Griscelli Syndrome Type 2
One of the best-evidenced examples for Rab-dependent traffic in immune disorders is the involvement of Rab27a in Griscelli Syndrome Type 2 (GS2). Three different types of Griscelli Syndrome (GS) exist, resulting from defects in 3 different genes: MYO5A (GS1), RAB27A (GS2), and MLPH (GS3), whose protein products interact in a tripartite complex in melanocytes. GS was first described in 1978 [19, 20], and is a rare autosomal recessive disorder of pigmentation that can be accompanied by the occurrence of either primary neurological impairment (GS1) or a severe immune disorder (GS2). The immunological features of GS2 include neutropenia, thrombocytopenia, and immunodeficiency due to impaired function of cytotoxic lymphocytes (NK and cytotoxic T cells) [17]. The mouse models of GS1, GS2, and GS3 (dilute, ashen, and leaden mice, respectively) have been extensively studied for the underlying pathology of GS, and helped elucidate the role of Myosin-Va in neurons, mechanisms of melanosome transfer to keratinocytes, and the role of Rab27a in the immune system [21–25].
Rab27a is a 25-kDa protein that has very strong expression in melanocytes and lymphocytes, but no expression in the brain. Conversely Rab27b, which shares 71% amino acid sequence homology with Rab27a, is not expressed in melanocytes but has very high expression in the brain. Therefore, clinical features of GS2 include oculocutaneous albinism, infections, and lymphohistiocytosis, but primary central nervous system defects have not been observed [17]. However, infiltration of leukocytes in the brain can cause secondary neurological impairment.
Interestingly, unlike in melanocytes, the role of Rab27a in cytotoxic lymphocytes is not dependent on myosin Va or melanophilin. While the Rab27a-deficient ashen mice have impaired cytotoxic lymphocyte responses, the myosin Va-deficient dilute mice, and the melanophilin-deficient leaden mice have normal function of cytotoxic lymphocytes [22, 23, 26]. In fact, cytotoxic lymphocytes do not express melanophilin and myosin Va, at least in mice [23]. Rather than affecting granule transport, Rab27a is involved in granule docking and secretion at the cell-cell interface in hematopoietic cells.
Both in humans and mice, mutations in RAB27A result in severely impaired degranulation of cytotoxic lymphocytes without affecting the granule polarization to the cell-cell contact site [17, 22, 26, 27] (Figure 1). The defects in secretory granule docking and release are most likely mediated by the lack of association between Rab27a and its binding partners. Mutations in the GTP-binding motif of Rab27a, observed in GS2 patients, interfere with direct interaction between Rab27a and Munc13-4 [17, 28]. The importance of the Rab27a-Munc13-4 complex formation for the lytic granule exocytosis is discussed below under the Familial Hemophagocytic Lymphohistiocytosis Type 3 section. In addition, Rab27a has been demonstrated to recruit a member of synaptotagmin-like protein family, Slp2a, to vesicular structures in T cells; the formation of the Rab27a-Slp2a complex is required for lytic granule exocytosis [29].
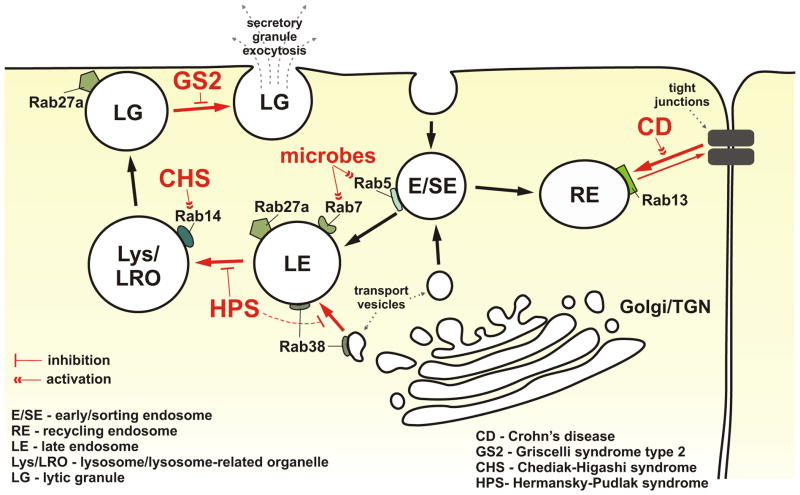
Figure 1. Rab GTPases in immune disorders.
Rab GTPases are involved in the trafficking of multiple cargos throughout the endo-lysosomal system. The endocytosed material or newly synthesized proteins are routed to early and sorting endosomes (E/SE), and then directed to different endosomal and lysosomal compartments. The cell surface levels of many proteins are kept in balance due to their routing to and from the plasma membrane through the recycling endosomes (RE). Dysregulation of the function of Rab proteins associated with REs affects the trafficking of proteins to and from the cell surface; defects in Rab13-mediated trafficking of proteins involved in the tight junction formation and maintenance could contribute to the development of Crohn’s disease (CD). Impaired function of Rab38 and the related defects in trafficking of cargos during lysosome-related organelle biogenesis, and/or maturation, could possibly be a mechanism involved in the pathogenesis of Hermansky-Pudlak syndrome (HPS). The lack of proper regulation of Rab14 activity could promote uncontrolled homotypic fusion between lysosomes or lysosome-related organelles (LRO), and be important in the pathogenesis of Chediak-Higashi syndrome (CHS). Mutations in Rab27a, causing Griscelli syndrome type 2 (GS2), prevent the proper docking of lysosomes or lysosome-related lytic granules (LG) at the plasma membrane, leading to defects in granule exocytosis. Mutations of Rab27a binding partner, Munc13-4, lead to Familial Hemophagocytic Lymphohistiocytosis type 3 (not shown in the diagram), a disease with immunological features similar to GS2. In addition, several intracellular microbial pathogens are known to affect Rab5 or Rab7 activities to avoid lysosomal degradation and promote pathogen survival. The schematic illustrates the location of several Rab proteins that are known to be involved in the pathogenesis of different disorders. Only relevant molecules are shown. The trafficking pathways affected in the indicated disorders are shown in red. For the more detailed description, please refer to the text.
Remarkably, Rab27a is not constitutively associated with the lytic granules in human cytotoxic lymphocytes, but is recruited to them in response to NK or T cell stimulation [27, 30–32]. This pattern of association most likely reflects the lytic granule maturation process, where Munc13-4-positive recycling vesicles and Rab27a-positive late endosomes fuse to generate the cytotoxic secretory granules/lysosomes [31, 32].
While the function of Rab27a in cytotoxic lymphocytes appears to be mainly related to vesicle docking at the plasma membrane, the GTPase is also involved in regulation of vesicle movement at the plasma membrane. RNAi-mediated loss of Rab27a results in increased motility of granules in the cytoplasm of NK cells, and fewer and less mobile lytic granules reaching the plasma membrane, indicating that Rab27a is required for retention and cytoskeleton-dependent directional movement of lytic granules at the plasma membrane [33]. The exact role of Rab27a in lytic granule motility is not well defined; however, a recent report has demonstrated that in cytotoxic T cells active Rab27a forms a complex with Slp3 and kinesin-1 to regulate the terminal steps of the lytic granule transport and release at the cell-cell contact site [34]. Furthermore, in NK cells Rab27a recruitment to the lytic granules depends on myosin IIA, a motor protein essential for the exocytosis of polarized lytic granules [27, 35].
Familial Hemophagocytic Lymphohistiocytosis Type 3
Familial Hemophagocytic Lymphohistiocytosis (FHL) is a genetically heterogeneous immune disorder of autosomal recessive inheritance characterized by uncontrolled proliferation and activation of polyclonal T cells, NK lymphocytes and macrophages that infiltrate multiple organs including the liver, spleen, and CNS [36]. Disease-causing mutations in the genes encoding perforin (PRF1; FHL2) [37], Munc13-4 (UNC13D; FHL3) [38], syntaxin-11 (STX11; FHL4) [39], and Munc18-2 (STXBP2; FHL5) [40] have been identified in FHL. All these genes encode proteins involved in the cytotoxic activity of lymphocytes.
As mentioned above, Munc13-4 is an effector of Rab27a in lymphocytes, so it is not surprising that the immunological features of GS2 and FHL3 are similar. Defective degranulation of NK and T cells occurs in both GS2 and FHL3, highlighting the importance of the Rab27a-Munc13-4 complex formation for the lytic granule exocytosis [38, 41–43]. Indeed, the Rab27a-Munc13-4 complex is required for efficient docking and fusion of granules at the plasma membrane [44].
Together with several other diseases (e.g. FHL2, 4 and 5), GS2 and FHL3 patients exhibit increased susceptibility to infections, severe primary immunodeficiency, and the “accelerated phase”, or hemophagocytic lymphohistiocytosis (HLH), a syndrome of inappropriate immune activation. The accelerated phase is often triggered by infection, mostly viral, and is characterized by lymphocyte/histiocyte infiltration of almost all organs, leading to a sepsis-like presentation with pyrexia, hepatosplenomegaly and cytopenias. GS2 and FHL display similarities in terms of their clinical manifestations and immunologic mechanisms, e.g. decreased or absent lytic activity of cytotoxic lymphocytes, leading to the inability to deliver granzymes into the target cell cytosol [45]. It has been proposed that the decreased cytolytic function could be responsible for the lack of elimination of infected or activated cells, and hence contribute to the persistent inflammation and uncontrolled cell proliferation observed in hemophagocytic syndromes [46]. Thus, the defective exocytosis of the lytic granules caused by Rab27a (or Munc13-4) mutations could lead to the impaired ability to clear the activated lymphocytes or histiocytes. Consequently, the sustained activation of those cell types would result in increased production of pro-inflammatory cytokines, and further activation of immune cells. A positive feedback loop of pro-inflammatory conditions would be generated, and the inflammatory response and cell proliferation of activated cells would continue because of the inability of cytotoxic lymphocytes cells to kill the activated cells, and stop the amplification of the immune response in GS2 and FHL.
If not treated, HLH in GS2 and FHL patients is eventually fatal in early childhood. Removal of the HLH trigger through chemotherapy or immune therapy may only suppress the accelerated phase, or achieve a transient remission. The most effective treatment and the only possible cure for GS2 patients is with hematopoietic stem cell transplant (HSCT). Successful bone marrow transplantation in a patient with GS2 was first reported in 1990 [47]. HSCT should be performed urgently after diagnosis, prior to the infant’s entering the accelerated phase of the disease, due to the risk of the HLH development with its devastating course.
Hermansky-Pudlak syndrome
Hermansky-Pudlak Syndrome (HPS) is a group of related monogenic inherited disorders with abnormalities in the biosynthesis and/or function of lysosomes and LROs, i.e. melanosomes and platelet-dense granules [48]. The disease is characterized by skin hypopigmentation (oculocutaneous albinism), bleeding diathesis and ceroid pigment deposition in lysosomes, with some patients developing pulmonary fibrosis, colitis and immunodeficiency [49, 50].
A naturally occurring mouse mutant, gunmetal, is one of many mouse models of HPS. The gunmetal phenotype is caused by a mutation in the gene encoding the alpha subunit of Rab geranyl-geranyl transferase (RabGGT), and results in severely reduced activity of RabGGT [51]. As a consequence, the prenylation of Rab proteins (e.g. Rab4, Rab11, Rab27a) is deficient in several cell types, including platelets, melanocytes, and T cells [22, 52], leading to the development of symptoms similar to HPS. In addition to hypopigmentation and prolonged bleeding times, gunmetal mice have defective function of cytotoxic lymphocytes, due to impaired transport of lytic granules to the cell-cell contact site formed between the cytotoxic T cell and a target cell [22, 53].
In addition to RabGGT, Rab38 has been associated with HPS. Rab38 is highly expressed in lung epithelia, alveolar pneumocytes, and skin melanocytes, and alteration of its function is likely to affect LROs and result in hypopigmentation and predisposition to lung diseases. Indeed, Rab38 has been demonstrated to control the size of lysosome-related lamellar bodies in alveolar pneumocytes (ATII cells) in rats, most likely by regulating the fusion of transporting vesicles with the lamellar bodies [54]. Lamellar bodies store surfactant, a mixture of lipids and proteins that plays an important role in the lung’s immune defense and lung expansion [55]. In Rab38-deficient mice or rats, the lung surfactant components (e.g. surfactant protein B) are decreased in broncho-alveolar lavages, but increased in lamellar bodies [56, 57]. Consequently, Rab38-deficient chocolate mice develop emphysematous lung disease [58].
While the murine models provide an important insight into the role of different Rabs or Rab-interacting molecules, no mutation of RABGGTA or RAB38 genes has been identified in HPS patients to date [48]. Other evidence, however, indicates a possible involvement of Rab38 in HPS pathogenesis. BLOC-3, a complex comprised of the HPS1 and HPS4 proteins and deficient in Hermansky-Pudlak Syndrome types 1 and 4, acts as a Rab-GEF for Rab38 and its homolog, Rab32, in human cell lines, and recruits Rab32/38 to premelanosomal membranes required for melanosome biogenesis [59]. Furthermore, active GTP-bound forms of Rab38 and Rab32 directly interact with other protein complexes involved in the development of Hermansky-Pudlak syndromes, namely BLOC-2 and AP-3, in a human melanocyte line [60]. Rab38 co-localizes with BLOC-2 and AP-3 on endosomal membranes, and silencing of BLOC-2 or AP-3 significantly destabilizes the association of Rab38 with the membranes. Melanocytes or megakaryocytes deficient in Rab38 (or Rab32) have altered trafficking of Tyrp-1 to melanosomes and LAMP2 to dense granules, respectively [60, 61], indicating that Rab38 functions in concert with AP-3 and BLOC-2 in trafficking cargos during lysosome-related organelle biogenesis and/or maturation (Figure 1).
HPS-2 patients, who have defects in the beta sub-unit of the AP-3 complex, have a severe neutropenia that is generally responsive to rGCSF [62]. The AP-3 complex along with the BLOC-2 and the BLOC-3 complexes have been shown to interact with Rab32 and Rab38 [60]. However, only the HPS-2 subtype of HPS has an immune disorder, therefore, further research is needed to tease out the mechanisms and roles that each of these protein complexes plays in the generation of LROs.
Crohn’s disease
An intriguing possibility is the involvement of Rab13 in Crohn’s disease, a chronic inflammatory bowel disease with deregulated immunological response in the gastrointestinal tract. Although the exact etiology of Crohn’s disease is unknown, it has been hypothesized that defective regulation of tight junctions, and consequent increased permeability of the intestine could contribute to altered immune responses and persistent inflammation [63]. Rab13 and its effector, JRAB are important for the formation of tight junctions by the virtue of regulating the transport to and from the plasma membrane and recycling of proteins essential for tight junction assembly (e.g. occludin, claudin-1, ZO-1, E-cadherin), as well as affecting actin remodeling through regulation of PKA-dependent phosphorylation and recruitment of VASP to tight junctions [64–66]. Importantly, Rab13, VASP and ZO-1 have been reported to dislocate from the apical tight junctions to the basolateral side of intestinal epithelial cells in patients with inactive Crohn’s disease [67]. Thus, abnormalities in the formation and maintenance of tight junctions caused by Rab13 mutations could contribute to the pathogenesis of Crohn’s disease (Figure 1).
Chediak-Higashi syndrome
Mutations in LYST cause Chediak-Higashi syndrome (CHS), a rare lysosomal storage disorder characterized by oculocutaneous albinism, a bleeding diathesis, and immune deficiency caused by impaired function of cytotoxic lymphocytes; one of the hallmarks of CHS is the presence of giant lysosomes [68]. A recent report has demonstrated that in Dictyostelium overexpression of activate Rab14 results in formation of giant lysosomes, reminiscent of those caused by mutation in LsvB, an ortholog of human LYST [69]. Furthermore, overexpression of a dominant negative form of Rab14 rescues the LsvB phenotype, restoring the size and morphology of the post-lysosomal compartment in Dictyostelium cells. Since Rab14 promotes homotypic fusion between the lysosomes in Dictyostelium, the authors propose that LsvB regulates the size and maturation of the lysosomal compartment by controlling the function of Rab14 [69]. Whether the same occurs in human cells remains to be examined, but the existence of a functional link between LYST and Rab GTPases is a very interesting notion that warrants further examination.
Infections
Rab proteins are critical for regulation of many trafficking pathways in the cell, including parasitic- or bacterial-containing cargos. Engulfed microorganisms are transported in phagosomes to lysosomes for degradation, a process requiring several Rab proteins, such as Rab5 and Rab7 [70]. Not surprisingly, several pathogens developed strategies to interfere with Rab function to promote their survival (Figure 1). For example, Salmonella dublin avoids lysosomal degradation by using its SopE protein, which has Rab5 GEF activity, to stabilize Rab5-GTP on the surface of the phagosome, thus interfering with phagosome maturation [71]. Listeria monocytogenes uses its p40 protein to block interactions between Rab5 and the Rab5 GEF, Vps9, preventing Rab5 extraction from the membrane, and thereby inhibiting phagosome maturation and pathogen degradation [72, 73]. Helicobacter pylori sequesters active Rab7 and Rab-interacting lysosomal protein RILP in large vacuoles, due to action of bacterial VacA protein, and blocks the delivery of bactericidal proteases (e.g. cathepsin D) to bacterium-containing vacuoles/phagosomes [74].
Furthermore, some viruses utilize Rab proteins for their replication. The measles virus, as well as hemorrhagic fever viruses, Marburg and Ebola, require functional Rab9 for their replication; similarly, HIV replication is dependent on Rab9 and Rab11, since silencing of these Rabs inhibits virus reproduction [75]. How the Rab GTPases are involved in HIV propagation has not been fully elucidated, but it has been postulated that HIV assembly starts in late endosomes, and endosomal membranes are required for viral budding from the cell, or for the formation of virus-containing exosomes [76, 77]. In this regard, the involvement of Rab GTPases (Rab9 and Rab11) required for transport between trans-Golgi and late endosomes could promote transport of viral proteins, virus budding into multivesicular bodies in endosomes, or creation of virus containing exosomes [75].
Conclusions and The Future
Despite over 60 Rab GTPases being identified in humans to date, only five have been shown to be mutated in human disorders. Of these five human diseases, only one has an immune defect as a cardinal feature. A possible explanation for the relative rarity of Rab deficiencies in immunological disorders could be that mutations in genes encoding Rabs are not compatible with life, due to the essential role of these GTPases in various trafficking steps in many cells types throughout the body. Furthermore, there may be some functional redundancy among several Rabs, so that defects in one particular Rab may appear silent. Nonetheless, defects in cargo trafficking caused by mutations in RAB27A and UNC13D genes, encoding Rab27a and its effector Munc13-4, cause severe immune deficiencies in humans, namely Griscelli Syndrome type 2 and Familial Hemophagocytic Lymphohistiocytosis Type 3, which have similar clinical and immunological features.
With the ever-increasing use of next generation sequencing technologies to identify new human disease causing genes, the number of mutations in genes encoding Rab GTPases could increase significantly. However, it is possible that the original hypothesis that mutations in Rabs are often lethal, remains true, and no more genes will be identified. In contrast, it may be possible that changes in the expression level of Rabs may cause or contribute to the pathology of human diseases. For example, over-expression of certain Rabs is often associated with an increased risk of cancer or metastases. Next generation sequencing, genome-wide association studies, and further functional characterization of Rabs in different cell types may increase the number of associations of Rabs with human disease in the future.
Highlights.
Precise protein trafficking is required for the immune system to function properly.
Rab5, Rab7, Rabl3, Rabl4, Rab27a, Rab38 are associated with immune disorders.
Mutations in RAB27A cause Griscelli Syndrome Type 2, a pigment and immune disorder.
UNC13D, encoding Muncl3-4, is mutated in FHL Type 3 and is an effector of Rab27a.
Acknowledgments
KK and ARC’s work is supported by the intramural programs of the National Institute of Allergy and Infectious Diseases and the National Human Genome Research Institute respectively. The authors wish to thank William A. Gahl (NHGRI, NIH) for his helpful discussion and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gomes AQ, Ali BR, Ramalho JS, Godfrey RF, Barral DC, Hume AN, Seabra MC. Membrane targeting of Rab GTPases is influenced by the prenylation motif. Mol Biol Cell. 2003;14:1882–1899. doi: 10.1091/mbc.E02-10-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 4.Haas AK, Fuchs E, Kopajtich R, Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- 5.Benado A, Nasagi-Atiya Y, Sagi-Eisenberg R. Protein trafficking in immune cells. Immunobiology. 2009;214:507–525. doi: 10.1016/j.imbio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Pfeiffer JR, Zhang J, Martinez AM, Griffiths GM, Wilson BS. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic. 2003;4:302–312. doi: 10.1034/j.1600-0854.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 7.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nature medicine. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 8.Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- 9.Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 12.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 13.Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bem D, Yoshimura S, Nunes-Bastos R, Bond FC, Kurian MA, Rahman F, Handley MT, Hadzhiev Y, Masood I, Straatman-Iwanowska AA, Cullinane AR, McNeill A, Pasha SS, Kirby GA, Foster K, Ahmed Z, Morton JE, Williams D, Graham JM, Dobyns WB, Burglen L, Ainsworth JR, Gissen P, Muller F, Maher ER, Barr FA, Aligianis IA. Loss-of-function mutations in RAB18 cause Warburg micro syndrome. Am J Hum Genet. 2011;88:499–507. doi: 10.1016/j.ajhg.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, Van Gerwen V, Wagner K, Hartung HP, Timmerman V. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Mathijssen IM, Morton JE, Orstavik KH, Sweeney E, Wall SA, Marsh JL, Nurnberg P, Passos-Bueno MR, Wilkie AO. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menasche G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F, de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 18.Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D’Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, Ropers HH, Tzschach A, Kalscheuer V, Oehl-Jaschkowitz B, Skinner C, Schwartz CE, Gecz J, Van Esch H, Raynaud M, Chelly J, de Brouwer AP, Toniolo D, D’Adamo P. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet. 2010;86:185–195. doi: 10.1016/j.ajhg.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 20.Griscelli C, Prunieras M. Pigment dilution and immunodeficiency: a new syndrome. Int J Dermatol. 1978;17:788–791. doi: 10.1111/j.1365-4362.1978.tb05980.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA., 3rd Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 22.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume AN, Collinson LM, Hopkins CR, Strom M, Barral DC, Bossi G, Griffiths GM, Seabra MC. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 2002;3:193–202. doi: 10.1034/j.1600-0854.2002.030305.x. [DOI] [PubMed] [Google Scholar]
- 24.Pachlopnik Schmid J, Ho CH, Diana J, Pivert G, Lehuen A, Geissmann F, Fischer A, de Saint Basile G. A Griscelli syndrome type 2 murine model of hemophagocytic lymphohistiocytosis (HLH) Eur J Immunol. 2008;38:3219–3225. doi: 10.1002/eji.200838488. [DOI] [PubMed] [Google Scholar]
- 25.Miyata M, Kishimoto Y, Tanaka M, Hashimoto K, Hirashima N, Murata Y, Kano M, Takagishi Y. A role for myosin Va in cerebellar plasticity and motor learning: a possible mechanism underlying neurological disorder in myosin Va disease. J Neurosci. 2011;31:6067–6078. doi: 10.1523/JNEUROSCI.5651-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad EK, Wu X, Hammer JA, 3rd, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. The Journal of cell biology. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, Horiuchi H, Rosthoj S, Rutynowska O, Winiarski J, Stow JL, Nordenskjold M, Henter JI, Ljunggren HG, Bryceson YT. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 28.Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, Heck AJ, Brose N, Kleijmeer M, van der Sluijs P. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Molecular biology of the cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menasche G, Menager MM, Lefebvre JM, Deutsch E, Athman R, Lambert N, Mahlaoui N, Court M, Garin J, Fischer A, de Saint Basile G. A newly identified isoform of Slp2a associates with Rab27a in cytotoxic T cells and participates to cytotoxic granule secretion. Blood. 2008;112:5052–5062. doi: 10.1182/blood-2008-02-141069. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Ruiz Y, Valitutti S, Dupre L. Stepwise maturation of lytic granules during differentiation and activation of human CD8+ T lymphocytes. PLoS One. 2011;6:e27057. doi: 10.1371/journal.pone.0027057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nature reviews Immunology. 2010;10:568–579. doi: 10.1038/nri2803. [DOI] [PubMed] [Google Scholar]
- 32.Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Meckel T, Long EO. Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells. PLoS One. 2010;5:e12870. doi: 10.1371/journal.pone.0012870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint Basile G, Menasche G. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–3889. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983;140:221–230. doi: 10.1007/BF00443367. [DOI] [PubMed] [Google Scholar]
- 37.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 38.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, de Saint Basile G. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 39.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nurnberg P, Janka G, Hennies HC. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–834. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 40.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nurnberg G, Becker C, Maul-Pavicic A, Beutel K, Janka G, Griffiths G, Ehl S, Hennies HC. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–2323. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 42.Rohr J, Beutel K, Maul-Pavicic A, Vraetz T, Thiel J, Warnatz K, Bondzio I, Gross-Wieltsch U, Schundeln M, Schutz B, Woessmann W, Groll AH, Strahm B, Pagel J, Speckmann C, Janka G, Griffiths G, Schwarz K, zur Stadt U, Ehl S. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica. 2010;95:2080–2087. doi: 10.3324/haematol.2010.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeths M, Chiang SC, Wood SM, Entesarian M, Schlums H, Bang B, Nordenskjold E, Bjorklund C, Jakovljevic G, Jazbec J, Hasle H, Holmqvist BM, Rajic L, Pfeifer S, Rosthoj S, Sabel M, Salmi TT, Stokland T, Winiarski J, Ljunggren HG, Fadeel B, Nordenskjold M, Henter JI, Bryceson YT. Familial hemophagocytic lymphohistiocytosis type 3 (FHL3) caused by deep intronic mutation and inversion in UNC13D. Blood. 2011;118:5783–5793. doi: 10.1182/blood-2011-07-369090. [DOI] [PubMed] [Google Scholar]
- 44.Elstak ED, Neeft M, Nehme NT, Voortman J, Cheung M, Goodarzifard M, Gerritsen HC, van Bergen En Henegouwen PM, Callebaut I, de Saint Basile G, van der Sluijs P. The munc13-4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118:1570–1578. doi: 10.1182/blood-2011-02-339523. [DOI] [PubMed] [Google Scholar]
- 45.Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol. 2012;3:335. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider LC, Berman RS, Shea CR, Perez-Atayde AR, Weinstein H, Geha RS. Bone marrow transplantation (BMT) for the syndrome of pigmentary dilution and lymphohistiocytosis (Griscelli’s syndrome) J Clin Immunol. 1990;10:146–153. doi: 10.1007/BF00917914. [DOI] [PubMed] [Google Scholar]
- 48.Wei AH, Li W. Hermansky-Pudlak syndrome: pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013;26:176–192. doi: 10.1111/pcmr.12051. [DOI] [PubMed] [Google Scholar]
- 49.Huizing M, Gahl WA. Disorders of vesicles of lysosomal lineage: the Hermansky-Pudlak syndromes. Curr Mol Med. 2002;2:451–467. doi: 10.2174/1566524023362357. [DOI] [PubMed] [Google Scholar]
- 50.Gochuico BR, Huizing M, Golas GA, Scher CD, Tsokos M, Denver SD, Frei-Jones MJ, Gahl WA. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Detter JC, Zhang Q, Mules EH, Novak EK, Mishra VS, Li W, McMurtrie EB, Tchernev VT, Wallace MR, Seabra MC, Swank RT, Kingsmore SF. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4144–4149. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, Zhen L, Li W, Novak EK, Collinson LM, Jang EK, Haslam RJ, Elliott RW, Swank RT. Cell-specific abnormal prenylation of Rab proteins in platelets and melanocytes of the gunmetal mouse. Br J Haematol. 2002;117:414–423. doi: 10.1046/j.1365-2141.2002.03444.x. [DOI] [PubMed] [Google Scholar]
- 53.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;301:L461–477. doi: 10.1152/ajplung.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright JR. Immunoregulatory functions of surfactant proteins. Nature reviews Immunology. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 56.Osanai K, Oikawa R, Higuchi J, Kobayashi M, Tsuchihara K, Iguchi M, Jongsu H, Toga H, Voelker DR. A mutation in Rab38 small GTPase causes abnormal lung surfactant homeostasis and aberrant alveolar structure in mice. The American journal of pathology. 2008;173:1265–1274. doi: 10.2353/ajpath.2008.080056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol. 2010;298:L243–251. doi: 10.1152/ajplung.00242.2009. [DOI] [PubMed] [Google Scholar]
- 58.Osanai K, Voelker DR. Analysis and expression of Rab38 in oculocutaneous lung disease. Methods in enzymology. 2008;438:203–215. doi: 10.1016/S0076-6879(07)38014-2. [DOI] [PubMed] [Google Scholar]
- 59.Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Current biology : CB. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. The Journal of biological chemistry. 2012;287:19550–19563. doi: 10.1074/jbc.M112.351908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambrosio AL, Boyle JA, Di Pietro SM. Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood. 2012;120:4072–4081. doi: 10.1182/blood-2012-04-420745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 63.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. The American journal of pathology. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marzesco AM, Dunia I, Pandjaitan R, Recouvreur M, Dauzonne D, Benedetti EL, Louvard D, Zahraoui A. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Molecular biology of the cell. 2002;13:1819–1831. doi: 10.1091/mbc.02-02-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. The Journal of cell biology. 2004;165:175–180. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Molecular biology of the cell. 2008;19:971–983. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohira M, Oshitani N, Hosomi S, Watanabe K, Yamagami H, Tominaga K, Watanabe T, Fujiwara Y, Maeda K, Hirakawa K, Arakawa T. Dislocation of Rab13 and vasodilator-stimulated phosphoprotein in inactive colon epithelium in patients with Crohn’s disease. Int J Mol Med. 2009;24:829–835. doi: 10.3892/ijmm_00000300. [DOI] [PubMed] [Google Scholar]
- 68.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 69.Kypri E, Falkenstein K, Lozanne AD. Antagonistic control of lysosomal fusion by Rab14 and the Lyst-related protein LvsB. Traffic. 2013 doi: 10.1111/tra.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nature reviews Molecular cell biology. 2008;9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. The Journal of biological chemistry. 2001;276:23607–23615. doi: 10.1074/jbc.M101034200. [DOI] [PubMed] [Google Scholar]
- 72.Prada-Delgado A, Carrasco-Marin E, Pena-Macarro C, Del Cerro-Vadillo E, Fresno-Escudero M, Leyva-Cobian F, Alvarez-Dominguez C. Inhibition of Rab5a exchange activity is a key step for Listeria monocytogenes survival. Traffic. 2005;6:252–265. doi: 10.1111/j.1600-0854.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 73.Alvarez-Dominguez C, Madrazo-Toca F, Fernandez-Prieto L, Vandekerckhove J, Pareja E, Tobes R, Gomez-Lopez MT, Del Cerro-Vadillo E, Fresno M, Leyva-Cobian F, Carrasco-Marin E. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic. 2008;9:325–337. doi: 10.1111/j.1600-0854.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 74.Terebiznik MR, Vazquez CL, Torbicki K, Banks D, Wang T, Hong W, Blanke SR, Colombo MI, Jones NL. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun. 2006;74:6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray JL, Mavrakis M, McDonald NJ, Yilla M, Sheng J, Bellini WJ, Zhao L, Le Doux JM, Shaw MW, Luo CC, Lippincott-Schwartz J, Sanchez A, Rubin DH, Hodge TW. Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J Virol. 2005;79:11742–11751. doi: 10.1128/JVI.79.18.11742-11751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nydegger S, Foti M, Derdowski A, Spearman P, Thali M. HIV-1 egress is gated through late endosomal membranes. Traffic. 2003;4:902–910. doi: 10.1046/j.1600-0854.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 77.Ono A, Freed EO. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J Virol. 2004;78:1552–1563. doi: 10.1128/JVI.78.3.1552-1563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]