Abstract
Exhaled nitric oxide (eNO) is increasingly used as a non-invasive measure of airway inflammation. Despite this, little information exists regarding the potential effects of indoor microbial components on eNO. We determined the influence of microbial contaminants in house dust and other indoor environmental characteristics on eNO levels in seven-year-olds with and without a physician- diagnosis of asthma. The study included 158 children recruited from a birth cohort study, and 32 were physician-diagnosed as asthmatic. The relationship between eNO levels and exposures to home dust streptomycetes, endotoxin, and molds was investigated. Streptomycetes and endotoxin were analyzed both as loads and concentrations in separate models. Dog, cat, and dust mite allergens also were evaluated. In the multivariate exposure models high streptomycetes loads and concentrations were significantly associated with a decrease in eNO levels in asthmatic (p <0.001) but not in healthy children. The presence of dog allergen, however, was associated with increased levels of eNO (p = 0.001). Dust endotoxin was not significant. The relationship between eNO and indoor exposure to common outdoor molds was u-shaped. In non-asthmatic children, none of the exposure variables were significantly associated with eNO levels. To our knowledge, this is the first study demonstrating a significant association between microbial components in the indoor environment and eNO levels in asthmatic children. This study demonstrates the importance of simultaneously assessing multiple home exposures of asthmatic children to better understand opposing effects. Common components of the indoor Streptomyces community may beneficially influence airway inflammation.
Keywords: streptomycetes, mold, allergens, asthma, exhaled nitric oxide, children
1. INTRODUCTION
Exhaled nitric oxide (eNO) is a non-invasive marker of eosinophilic airway inflammation, and is increasingly used in the diagnosis and management of childhood asthma (Pijnenburg and De Jongste, 2008). Apart from respiratory disease, other predictors of eNO are not well understood. A few studies have demonstrated associations between indoor environmental exposures and both reductions and increases in eNO levels. Kovesi and Dales (2009) demonstrated in children an association between lower eNO levels and the presence of dogs, but did not find a significant association with either environmental tobacco smoke exposure or visible mold. In a cross-sectional analysis of 170 asthmatic children, Spanier et al. (2006) found that higher eNO values were significantly associated with the lack of carpeting, not owning a cat, higher dust mite exposure, and allergen sensitization.
Microbial components in indoor environments are known to be associated with adverse respiratory health, such as wheezing, rhinitis, and asthma. Timing and dose appear to influence the effects. Both epidemiological and experimental studies have implicated spores and microbial cell wall components such as endotoxin and (1-3)-β-D-glucan as risk factors for respiratory disease (Sahakian et al., 2008), but other studies have also demonstrated a protective effect in accordance with the so called hygiene hypothesis (Douwes et al., 2006; Ryan et al., 2009; Sordillo et al., 2010; Ege et al., 2011). Although most previous studies have focused on endotoxin, which is representative of Gram-negative bacteria, Gram-positive bacteria are also relevant to indoor environments (van Strien et al., 2004; Zhao et al., 2008). Streptomyces is a large genus of spore forming soil bacteria, and streptomycetes are among the Gram-positive bacteria most commonly isolated from moisture-damaged buildings (Anderson et al., 1997). Some Streptomyces strains are potent inducers of inflammatory reactions in mouse and human macrophage cells in vitro (Huttunen et al., 2003). Moreover, intratracheal exposure of mice to S. californicus spores resulted in recruitment of neutrophils, macrophages, and lymphocytes in the airways (Jussila et al., 2003).
Since indoor microbial components are known to be associated with asthma and rhinitis, it was our hypothesis that these exposures will also influence eNO levels. However, there are inconsistent findings among the few studies that have explored the association between eNO levels and indoor microbial contamination. Experimental studies with adults have demonstrated elevated eNO levels after respiratory challenge with endotoxin (Kitz et al., 2006) and Aspergillus fumigatus (Stark et al., 2005). In contrast, Purokivi et al. (2002) measured eNO levels in employees working in moisture damaged buildings versus reference buildings and detected no association. In a study of 115 asthmatic children in Hong Kong (Leung et al., 2010), endotoxin levels in house dust were associated with wheezing frequency but not with eNO. To assist in clarification of these varying findings, the current study was designed to improve understanding of the impact of simultaneous exposures to several indoor microbial contaminants on eNO levels in asthmatic and non-asthmatic children. Streptomycetes, endotoxin, and mold content in floor dust were used as proxy measures of these contaminants in indoor air. Other environmental factors, including levels of common household allergens and exposure to tobacco smoke, were included as potential effect modifiers or confounders.
2. MATERIALS AND METHODS
2.1. Study subjects
The children in this study were recruited from a birth cohort, the Cincinnati Childhood Asthma and Air Pollution Study (CCAAPS). A detailed description of subject recruitment for the CCAAPS study has been published (LeMasters et al., 2006). Briefly, newborns were identified from birth records, and parents were recruited when the infant was approximately six months old. Inclusion required that at least one parent had allergy and/or asthma symptoms, and tested positive to at least one of 15 common aeroallergens. The children subsequently underwent similar skin prick testing (SPT) and a physical exam at ages one through four and seven years. For the present study, children were recruited from a cohort of 577 CCAAPS if their dwellings had had a home assessment completed at age one (Reponen et al., 2011) and they also completed an age seven clinical evaluation (n = 176). The study was approved by the University of Cincinnati Institutional Review Board, and a written informed consent was obtained.
2.2. Asthma diagnosis
At the age seven clinical evaluation the children had a skin prick test for 15 aeroallergens (LeMasters et al., 2006), spirometry, measures of airway hyperreactivity, and exhaled nitric oxide measurement. In addition, a questionnaire covering respiratory health symptoms, home exposures, and demographics was administered to the child’s caregiver. Spirometry was performed in a clinical office by trained health professionals according to American Thoracic Society criteria. Children with reported asthma symptoms, or an exhaled nitric oxide (eNO) concentration greater than 20 ppb, or a predicted forced expiratory volume in one second (FEV1) less than 90% and/or FEV1 ratio to forced vital capacity (FVC) less than the lower limit of normal (LLN) were administered 2.5 mg of Xoponex® by nebulizer. Spirometry was subsequently repeated, and children with equal or less than 12% increase in FEV1 following Xoponex® (levalbuterol) treatment were administered a methacholine challenge test (MCCT) at a follow-up visit. Children were defined as asthmatic if they fulfilled the following two criteria: 1) caregiver report of asthma symptoms and 2) demonstration of airway reversibility (defined as ≥12% increase in FEV1 following bronchodilator) or positive MCCT (defined as ≥20% drop in baseline FEV1 at cumulative inhaled methacholine concentration of ≤4 mg/ml).
2.3. eNO measurements
eNO was measured by the online single breath method using NIOX Flex (Aerocrine Inc.). The procedure was performed in a physician’s office by experienced health professionals according to the joint guidelines of the American Thoracic Society and the European Respiratory Society (ATS/ERS, 2005). Each child was seated, breathed quietly for five minutes to acclimate, before inhalation to total lung capacity and exhalation at a constant flow rate of 50 ml/second +10%. eNO was recorded during the last two seconds of up to six exhalations with at least 30 seconds rest between each, and calculated as the mean of three accepted exhalations. Children refrained from strenuous exercise, eating, and drinking one hour prior to testing. Children with current prescriptions for asthma medications were not excluded
2.4. Home visits and dust collection
When the child was seven years old a trained two-person team visited the home to administer a questionnaire and perform dust sampling. The questionnaire included questions about housing conditions, demographics, presence of pets, and smoking habits. Dust was collected from the child’s primary activity room as described (Cho et al., 2006, Johansson et al., 2010). Briefly, floor dust was collected in filter bags by vacuuming a 2-m2 area for carpeted floors, or the entire room for hardwood floors. Large dust particles were removed by sieving through a 355-μm sieve, and the dust was stored at -20°C. Dust load was defined as mg collected dust per m2 vacuumed area.
2.5. Quantification of streptomycetes in house dust
DNA was purified from sieved dust using DNA-EZ kit from GeneRite. Five mg dust was placed in a sterile 2-ml tube containing 0.3 g acid washed glass beads (#G1277, Sigma-Aldrich). To the tube was added 0.35 ml Lysis buffer and 10 μl of a 2 × 108 conidia/ml reference suspension of Geotrichum candidum (#7863, University of Alberta Microfungus Collection and Herbarium) which was included as an internal positive control. The tubes were shaken for 1 min in a Mini Bead-Beater (Biospec Products), and DNA was isolated according to manufacturer’s instructions.
Real-time PCR primers and probe targeted towards the ribosomal 23S gene and specific for the Streptomyces genus were from a previous study (Rintala and Nevalainen, 2006). The real-time PCR assay of streptomycetes has been described (Johansson et al., 2010). Briefly, separate reactions were performed for Streptomyces cells and G. candidum cells, and each reaction contained 1 × Taqman® Universal PCR Master Mix (Applied Biosystems), 1 μM of each primer, 80 nM of the probe, 0.01 mg/ml BSA, and 5 μl purified DNA in a total reaction volume of 25 μl. Reactions were performed using the Roche LightCycler® 480 System (Roche Applied Science). Standard curves were used to calculate the number of streptomycetes cells corresponding to the amount of DNA in each reaction sample. Streptomycetes concentration in dust was expressed as cells per mg of sieved dust, and streptomycetes load was expressed as cells per m2 of floor area. Streptomyces load was calculated by multiplying concentration with total amount of dust collected and dividing with floor area vacuumed.
2.6. Determination of endotoxin in dust
Determination of endotoxin concentrations in dust was performed according to the Limulus amebocyte lysate method using the Pyrochrome® Endotoxin Detection Reagent Kit from Associates of Cape Cod, Inc. as described earlier (Johansson et al., 2010). Endotoxin concentration in dust was expressed as endotoxin units (EU) per mg of sieved dust. Endotoxin load was expressed as EU per m2 of floor area and was derived from concentration as described above for streptomycetes.
2.7. Quantification of dust-borne mold
To obtain a measure of the levels of indoor mold, the Environmental Relative Moldiness Index (ERMI) was used (Vesper et al., 2007). ERMI is based on the real-time PCR analysis of 26 mold strains characteristic of moisture-damaged buildings (Group 1 molds) and 10 strains mostly found outdoors that are characteristic of undamaged buildings (Group 2 molds) (Haugland et al., 2004). ERMI is calculated by subtracting the sum of log-transformed Group 2 mold concentrations (SLG2) from the sum of log-transformed Group 1 concentrations (SLG1). Purification of DNA from dust was performed as described above for real-time PCR quantification of streptomycetes.
2.8. Determination of allergen levels
Measurements of dog (Can f 1), cat (Fel d 1), and dust mite (Der f 1) allergens were performed as described previously (Cho et al., 2006). Allergens were extracted from 50 mg dust in 1 ml PBS-Tween (phosphate-buffered saline + 0.05% Tween 20, pH 7.4) on a shaker at 4°C overnight. The supernatant was analyzed using a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) (Indoor Biotechnologies, Inc.). The limit of detection (LOD) for Can f 1, Fel d 1, and Der f 1 was 0.005 μg/g, 0.006 μg/g, and 0.007 μg/g, respectively.
2.9. Statistical methods
Statistical analyses were performed to assess associations between eNO and independent variables for environmental exposures and demographic/ clinical characteristics. Histograms and scatterplots were generated to assess the distributions of eNO and continuous independent variables. Because of right-skewed distributions, the loge transformation was applied to eNO, streptomycetes load and concentration, endotoxin load and concentration, and dust load. As there are known differences between eNO levels of asthmatics and non-asthmatics (Pijnenburg and De Jongste, 2008), differences were evaluated by unpaired t-tests for continuous variables, and chi square or Fisher’s Exact tests for count data. Values of eNO were left-censored at 2 ppb, the limit of detection of the NIOX Flex.
The Tobit model, which allows censoring, was used for the regression analyses. Univariate Tobit regression analyses were performed to assess individual associations between loge-transformed eNO and each independent variable. For continuous exposure variables, associations were also assessed by curves that showed the shape of their relation with eNO. Linear and non-linear components of these curves were tested for a significant association with eNO. Spline fit analyses showed that slopes of eNO as a function of SLG2 were different for SLG2 < 15.5 and SLG2 ≥ 15.5. Therefore, the relationship between eNO and SLG2 was modeled by two linear functions. The univariate analyses were repeated when asthma status, the independent variable, and the interaction of the independent variable with asthma status were modeled.
Since our analyses showed that asthma modified the effect of streptomycetes on eNO, further analyses were stratified by asthma status. Independent variables significantly related to eNO at p < 0.20, either linearly or non-linearly, were included in an asthma-specific Tobit multiple regression model. This level is often used in building models using stepwise forward regression. A smaller level such as 0.05 can fail to identify variables known to be important. For asthmatics, these variables included Can f 1 ≥ LOD, streptomycetes load, streptomycetes concentration, endotoxin load, SLG2 in the range ≥ 15.5, SLG2 in the range < 15.5, and dust load. For non-asthmatics the variables included in the multiple regression model were Der f 1 ≥ LOD, and Can f 1 ≥ LOD, being African-American, and being SPT positive. The Tobit multiple regression models were reduced by stepwise regression. Final models included independent variables that were significant at p < 0.05. Because of the relatively high percentage of dust samples with allergen levels below LOD, the values of Fel d 1, Can f 1, and Der f 1 were dichotomized, as < LOD versus ≥ LOD.
Graphical assessments of predicted values versus residuals of final streptomycetes load and concentration models for asthmatics were generated to assess model fit. Owing to the relatively small number of asthmatics (n=30), sensitivity analyses were undertaken to help ensure that no single subject had undue influence on the results. Accordingly, final multivariate streptomycetes load and concentration models were analyzed after removal of a different randomly chosen subject, and parameter estimates were compared to those of the full data set. Analyses were performed using SAS for Windows version 9.2 (Statistical Analysis System). A p-value < 0.05 was considered significant unless stated otherwise.
3. RESULTS
Exhaled nitric oxide measurements were obtained for 161 of the 176 enrolled children. Of these, three did not complete the asthma clinical examination. Streptomycetes measurements were unavailable for five homes due to low dust amounts.
The distributions of demographic, clinical, and exposure variables are shown in Table 1. The asthma incidence for the 158 children who completed the asthma clinical examination was 20%. Although not significant, geometric means of eNO in asthmatic versus non-asthmatic children were 5.6 ppb and 4.7 ppb, respectively. There was, however, a significant difference between eNO in those with a positive SPT compared to those with a negative SPT (5.8 ppb vs. 4.0 ppb, p = 0.008). Those children (n=23) who were both asthmatic and had a positive SPT had the highest eNO at 6.6 ppb (data not shown). Other factors that were associated with asthma were being African American (44%) having lower household incomes (59%), having a positive SPT (72%), and living in the same household as a current smoker (34%) (Table 1). For the asthmatics, being prescribed inhaled corticosteroids (n=8) did not have a significant effect on eNO levels (data not shown), and therefore, children prescribed inhaled corticosteroids were not excluded from the study.
Table 1.
Distributions of variables included in the study for asthmatic and non-asthmatic children
|
All subjects (n = 158) |
Non-asthma (n = 126) |
Asthma (n = 32) | |
|---|---|---|---|
| Demographics | |||
| Gender (male)a | 88 (56%) | 69 (55%) | 19 (59%) |
| Race (African-American)a | 42 (27%) | 28 (22%) | 14 (44%)* |
| Income (< $40,000)a | 46 (29%) | 30 (24%) | 19 (59%)* |
| Clinical parameters | |||
| SPT (positive)a | 83 (52%) | 61 (48%) | 23 (72%)* |
| Pollen sensitization (yes)a,b | 62 (39%) | 45 (36%) | 17 (53%) |
| eNO (ppb)c | 4.8 (2.6) | 4.7 (2.5) | 5.6 (2.9) |
| Environmental exposures | |||
| Streptomycetes load (cells/m2)c, d |
7.06 ×105 (4.8) | 6.87 × 105 (4.6) | 7.89 × 105 (5.9) |
| Streptomycetes concentration (cells/mg)c, d |
1711 (2.3) | 1775 (2.2) | 1473 (2.7) |
| Endotoxin load (EU/m2)c | 6.27 × 104 (5.1) | 6.50 × 104 (5.1) | 5.29 × 104 (5.5) |
| Endotoxin concentration (EU/mg)c |
155 (2.8) | 168 (2.8) | 112 (2.8) |
| ERMIe | 2.3 (6.4) | 2.5 (6.5) | 1.6 (6.2) |
| SLG2e | 18.1 (3.7) | 18.2 (3.5) | 17.9 (4.2) |
| Dust load (mg/m2)c | 403 (3.5) | 391 (3.3) | 457 (4.3) |
| Living in the same household as a current smoker (yes)a |
32 (20%) | 21 (17%) | 11 (34%)* |
| Fel d 1 (≥ LOD)a | 92 (58%) | 74 (59%) | 18 (56%) |
| Can f 1(≥ LOD)a | 118 (75%) | 94 (75%) | 24 (75%) |
| Der f 1 (≥ LOD)a | 53 (34%) | 43 (34%) | 10 (31%) |
ERMI = Environmental Relative Moldiness Index; SLG2 = sum of log-transformed Group 2 mold concentrations; SPT = skin prick test. LOD = limit of detection.
The distribution was significantly different in asthma subjects compared to healthy subjects at a significance level of p < 0.05.
Categorical variable. Counts given, with percentage within parenthesis.
Sensitization to meadow fescue, timothy, white oak, maple mix, American elm, red cedar, or short ragweed.
Continuous variable. Geometric mean given, with geometric standard deviation within parenthesis.
Streptomycetes analysis was not performed for the homes of three non-asthmatic and two asthmatic children because of low amount of available dust.
Continuous variable. Arithmetic mean given, with arithmetic standard deviation within parenthesis
3.1. Candidate predictors of eNO
Results from univariate regression analyses are shown in Table 2. For asthmatic children, independent variables positively associated with eNO at p < 0.20 included high levels of outdoor mold found in the home (SLG2 ≥ 15.5) and detectable Can f 1. Streptomycetes load, streptomycetes concentration, endotoxin load, low levels of outdoor mold in the home (SLG2 < 15.5), and dust load were inversely associated with eNO (Table 2). These variables were included in the multivariate analyses. Gender, race, income, being SPT positive, sensitization to pollen (meadow fescue, timothy, white oak, maple mix, American elm, red cedar, or short ragweed), endotoxin concentration, ERMI, living in the same household as a current smoker, detectable Can f 1 and detectable Der f 1 were not associated with eNO at p < 0.20. Having a positive SPT was not significant for asthmatic likely because 72% of the asthmatics were positive.
Table 2.
Univariate analyses of relations between independent variables and eNO
| Non-asthma | Asthma | |||
|---|---|---|---|---|
|
|
||||
|
Parameter estimate
(standard error) |
p-value |
Parameter estimate
(standard error) |
p-value | |
| Gender (male) | 0.035 (0.19) | 0.86 | 0.13 (0.43) | 0.77 |
| Race (African-American) | 0.33 (0.23) | 0.14 | −0.14 (0.43) | 0.74 |
| Income | 0.31 | 0.67 | ||
| < $40,000 | −0.35 (0.24) | 0.14 | −0.18 (0.67) | 0.78 |
| $40,000 - $89,999 | −0.034 (0.20) | 0.86 | −0.13 (0.73) | 0.86 |
| ≥ 90,000 | ref | - | ref | - |
| SPT (positive) | 0.33 (0.19) | 0.08 | 0.41 (0.46) | 0.37 |
| Pollen sensitization (yes) | 0.085 (0.20) | 0.67 | 0.055 (0.43) | 0.90 |
| Streptomycetes load (cells/m2)a |
−0.050 (0.063) | 0.43 | −0.40 (0.11) | <0.001 |
| Streptomycetes concentration (cells/mg)a |
−0.15 (0.12) | 0.20 | −0.65 (0.21) | 0.002 |
| Endotoxin load (EU/m2)a | 0.005 (0.060) | 0.94 | −0.24 (0.13) | 0.07 |
| Endotoxin concentration (EU/mg)a |
0.027 (0.094) | 0.77 | −0.02 (0.23) | 0.93 |
| ERMIa | −0.011 (0.015) | 0.45 | −0.016 (0.036) | 0.65 |
| SLG2 < 15.5a | −0.005 (0.067) | 0.94 | −0.52 (0.26) | 0.048 |
| SLG2 ≥ 15.5a | 0.005 (0.046) | 0.91 | 0.12 (0.06) | 0.06 |
| Dust load (mg/m2)a | 0.066 (0.078) | 0.39 | −0.30 (0.17) | 0.09 |
| Living in the same household as a current smoker (yes) |
0.17 (0.25) | 0.50 | −0.38 (0.45) | 0.40 |
| Fel d 1 ≥ LOD | 0.067 (0.19) | 0.73 | 0.41 (0.43) | 0.33 |
| Can f 1 ≥ LOD | −0.40 (0.21) | 0.06 | 0.93 (0.47) | 0.046 |
| Der f 1 ≥ LOD | 0.27 (0.20) | 0.18 | 0.030 (0.46) | 0.95 |
ERMI = Environmental Relative Moldiness Index; SLG2 = sum of log-transformed Group 2 mold concentrations; SPT = skin prick test. LOD = limit of detection.
Parameter estimates represent the change in log-transformed eNO corresponding to a 1 unit increase in the log-transformed independent variable, except for ERMI and SLG2, which were not log-transformed.
As shown in Table 2, in the non-asthmatic children, being African-American, being SPT positive (48%) and detectable Der f 1 were positively associated with eNO, whereas Can f 1 was negatively associated with eNO at p < 0.20. eNO was not associated (p ≥ 0.20) with gender, income, sensitization to pollen, streptomycetes load, streptomycetes concentration, endotoxin load, endotoxin concentration, ERMI, SLG2 when SLG2 values were < 15.5 and ≥ 15.5, dust load, living in the same household as a current smoker, or Fel d 1.
3.2. Multivariate models for prediction of eNO
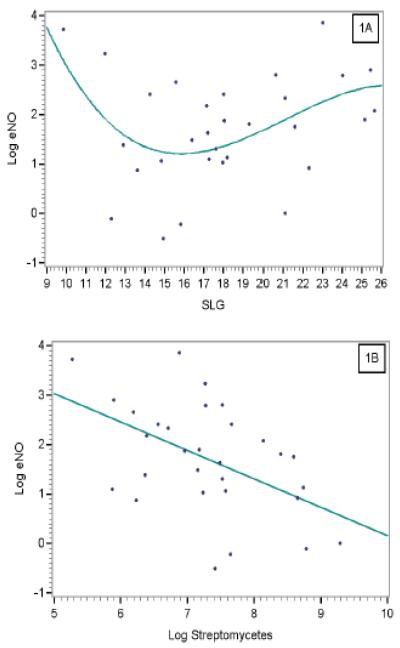
The multivariate models for predicting eNO in asthmatic children are shown in Table 3. Increasing streptomycetes load was the most significant factor correlated with lower eNO for asthmatic children. A biphasic pattern was observed between eNO and outdoor mold; at low SLG2 (SLG2 values < 15.5), increase in SLG2 was associated with lower eNO, whereas at high SLG2 (SLG2 values ≥ 15.5), increase was associated with higher eNO (Figure 1A). Finally, amounts of Can f 1 above LOD were associated with higher eNO.
Table 3.
Multivariate Tobit regression models describing the association between eNO and each independent variable, controlling for the other variables, in asthmatic children (n = 30)a
| Parameter estimate | Standard error | p value | |
|---|---|---|---|
|
Multivariate model for asthmatic children, streptomycetes expressed as load |
|||
| Streptomycetes load (cells/m2), log transformedb | −0.42 | 0.08 | < 0.001 |
| SLG2 < 15.5b | −0.29 | 0.11 | 0.01 |
| SLG2 ≥ 15.5b | 0.45 | 0.14 | 0.001 |
| Can f 1 < LOD (0.005 μg/g) | ref | - | - |
| Can f 1 ≥ LOD | 1.06 | 0.33 | 0.001 |
|
| |||
|
Multivariate model for asthmatic children, streptomycetes expressed as concentration |
|||
| Streptomycetes concentration (cells/mg), log transformedb |
−0.73 | 0.17 | < 0.001 |
| SLG2 < 15.5b | −0.14 | 0.13 | 0.27 |
| SLG2 ≥ 15.5b | 0.33 | 0.16 | 0.04 |
| Can f 1 < LOD (0.005 μg/g) | Ref | - | - |
| Can f 1 ≥ LOD | 1.03 | 0.36 | 0.004 |
SLG2 = sum of log-transformed Group 2 mold concentrations. LOD = limit of detection.
Streptomycetes analysis was not performed for the homes of two asthmatic children because of low amount of available dust.
Parameter estimates of continuous variables represent the change in log-transformed eNO corresponding to a 1 unit increase in the log-transformed independent variable, or to a 1 unit increase on the normal scale for SLG2.
Figure 1.
Predicted value of log eNO for asthmatic children obtained from the multivariate model including log streptomycetes concentration (A) as a function of log streptomycetes and (B) as a function of SLG2.
When concentrations were substituted for loads, high streptomycetes levels remained the most significant factor associated with lower eNO (Figure 1B). High SLG2 remained significantly associated with higher eNO, whereas there was no significant association between eNO and low SLG2. Detectable Can f 1 remained associated with higher eNO. Plots of predicted values versus residuals showed no systematic relationship for the analyses of asthmatic children. Sensitivity analysis showed essentially no change in any variable parameter estimate for either model when randomly chosen individuals were removed. Interestingly, for non-asthmatic children, no factor was significantly associated with eNO levels in the multivariate analysis.
4. DISCUSSION
The multivariate analyses in our study consistently showed inverse associations between eNO levels and streptomycetes exposure in asthmatic children but not in non-asthmatic children. There are several possible explanations for this unique finding. Streptomycetes are important producers of a variety of secondary metabolites; while some are potent toxins, others have anti-inflammatory activities (Deshpande et al., 1988), and anti-inflammatory effects of some streptomycetes could possibly result in lower airway inflammation. It is also possible that the effect of streptomycetes is non-specific. In the context of the hygiene hypothesis, the most studied microbial component is the Gram-negative cell-wall component endotoxin. Peptidoglycan is a cell wall component that is present at substantially higher levels in Gram-positive than in Gram-negative bacteria. It is therefore considered a marker of Gram-positive bacteria, including streptomycetes. Although far less is known about the respiratory health effects of peptidoglycan than that of endotoxin, a few studies have indicated that muramic acid, one of the monomers of peptidoglycan, may be associated with decreased wheezing (van Stren et al., 2004; Zhao et al., 2008). In another study, both peptidoglycan and the endotoxin component lipid A were shown to decrease the ovalbumin-induced pulmonary recruitment of eosinophils and airway hyperresponsiveness in mice (Velasco et al., 2005). These studies suggest that cell-wall components in Gram-positive bacteria such as streptomycetes may affect respiratory health outcomes.
In an earlier study, we showed that the presence of two or more dogs in the home was significantly associated with decreased wheezing (Campo et al., 2006). The presence of two or more dogs is also a strong determinant of streptomycetes levels in house dust (Johansson et al., 2010). Our study used Can f 1 levels in dust as a marker for exposure to dog allergens. The association between eNO and streptomycetes levels, however, remained unaltered whether the Can f 1 variable was included in the model or not, suggesting that the presence of dogs in the home was not a confounder in this study. Several other potential confounders previously shown to be associated with streptomycetes levels (Johansson et al., 2010) were explored, including endotoxin load and outdoor molds (SLG2). None of these, however, significantly altered the parameter estimates for streptomycetes in the multivariate analyses. High ERMI has been shown to be associated with adverse respiratory health outcomes in children (Reponen et al., 2011), and was therefore included among the independent variables. No correlation, however, was found between ERMI and eNO.
The relationship between eNO levels and SLG2, the metric for 10 indicator mold strains, demonstrated a U-shaped pattern. A biphasic response pattern for environmental exposures to allergens and microbial components has previously been reported in several studies (Platt-Mills et al. 2001; Tovey et al., 2002; Campo et al., 2006; Iossifova et al., 2007), and has been explained by the reaction of maturing immune system in children. However, the pattern in above-quoted studies was an inverted U-shaped association. In the current study, this association was only significant when streptomycetes were expressed as load, and therefore may be a chance association. The positive association between eNO and SLG2 when SLG2 values ≥ 15.5, however, supports a study by Leuppi et al. (2002), in which sensitization to the common outdoor mold Cladosporium was associated with increased eNO levels.
Streptomycetes levels were correlated with eNO in asthmatics, but not in non-asthmatics. The modifying effect of streptomycetes and other environmental contaminants by asthma status may be the result of the pathway for nitric oxide production in the airways. Nitric oxide is produced by three isoforms of nitric oxide synthase (NOS), and inducible NOS (iNOS) is expressed in response to proinflammatory cytokines as well as environmental factors (Ricciardolo et al., 2004). Although low levels of iNOS are expressed in healthy airways, the increased levels of eNO observed in asthma patients may be the result of up-regulation of iNOS. In clinical studies using selective iNOS inhibitors, eNO was principally lowered in asthmatic compared to healthy subjects (Hansel et al., 2003; Brindicci et al., 2007), which suggests that most of the eNO in asthmatics is produced by iNOS. Similarly, it may be hypothesized that inhaled streptomycetes selectively affect iNOS, which could help explain why streptomycetes reduce eNO levels in asthmatics but not in healthy children.
Our eNO findings are in part similar to those of Frank at al. (1998), who tested children aged 5 to 15 and found that the geometric mean of eNO was = 12.5 (95% CI = 8.3, 18.8) for children with allergic asthma and eNO = 3.8 (95% CI = 2.7, 5.5) for children with non-allergic asthma. Jackson et al. (2009) found that six-year old children with allergic asthma had eNO = 10.3 (95% CI = 6.8, 26.2), and those with non-allergic asthma had lower eNO at 8.6 (95% CI = 6.7, 14.3). Kukkonen et al. (2011) found that young children age five who ever had asthma had eNO = 6.2 (95% CI = 4.9, 10.9), while others have reported higher values for children seven and under.
The absolute levels of eNO in asthmatic children found in our study were at the lower end of the range of values found by other investigators. Study differences on eNO levels for asthmatic children may be related to ethnic status, use of medication at the time of testing, season when the test was administered, technical experience, type of instrument, the use of standardized procedures, and location of testing such as physician’s office versus other locations. In our study, all testing was conducted in a physician’s office, across all seasons, and over 80% were Caucasian. The asthmatic children included newly diagnosed cases that may have had less airway inflammation, and less inflammation may have resulted in lower eNO compared with asthmatics with more established inflammation. We did not detect any effect on eNO levels in asthmatic children prescribed inhaled corticosteroids, possibly because of the small sample number. Furthermore, no information was available on when children prescribed inhaled corticosteroids had last used their medication, and therefore, any possible effect on eNO levels by these short-acting drugs may have been obscured.
The difference between eNO in those with a positive SPT compared to those with a negative SPT found in this study support previous studies in finding a significant difference in eNO levels between sensitized and nonsensitized children (Pijnenburg and De Jongste 2008).
The CCAAPS cohort was recruited from atopic families, and the geographic area of recruitment was limited to the Greater Cincinnati/Northern Kentucky area. Therefore, the results and conclusions of this study may not be applicable to other regions or types of cohorts.
The study was also limited by a relatively small number of asthma cases (n=32), which may have decreased study power to detect significant associations between eNO and independent variables. The strong associations observed between eNO and levels of microbial contaminants even with this relatively limited number of asthma cases, however, is noteworthy. Future studies will be able to confirm or refute the results in larger cohorts, and explore the causal nature of the relationship between eNO and dust-borne microbial contaminants in homes, especially streptomycetes. The influence on eNO levels of other Gram-positive taxa, and Gram-positive markers such as peptidoglycan, is also worth exploring.
Highlights for manuscript Johansson et al.
We assessed association between indoor microbial contaminants and eNO in children.
We demonstrated a protective effect of Streptomycetes in house dust.
The association between outdoor molds and eNO followed biphasic pattern.
eNO was associated with microbial exposures only in asthmatic children.
ACKNOWLEDGEMENTS
This study was supported by NIEHS grants ES10957 and ES11170. The home assessment was supported by the US Department of Housing and Urban Development grant #OHLHH0162-07. We thank all the CCAAPS families who allowed us into their homes for sampling and participated in clinical assessments. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- eNO
exhaled nitric oxide
- ERMI
Environmental Relative Moldiness Index
- LOD
limit of detection
- SLG2
sum of log-transformed Group 2 mold concentrations
- SPT
skin prick test
Footnotes
NOTICE The ERMI qPCR technology was patented (#6,387,652) by the U.S. Environmental Protection Agency (EPA) and commercial applications can result in royalties paid to the EPA. The EPA, through its Office of Research and Development, partially funded and collaborated in the research described here. It has been subjected to the Agency’s peer review and has been approved as an EPA publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson MA, Nikulin M, Köljalg U, Andersson MC, Rainey F, Reijula K, Hintikka EL, Salkinoja-Salonen M. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 1997;63:387–393. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS and ERS ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- Brindicci C, Ito K, Barnes PJ, Kharitonov SA. Effect of an inducible nitric oxide synthase inhibitor on differential flow-exhaled nitric oxide in asthmatic patients and healthy volunteers. Chest. 2007;132:581–588. doi: 10.1378/chest.06-3046. [DOI] [PubMed] [Google Scholar]
- Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus ZL, Cho SH, Khurana Hershey GK, Lockey J, Villareal M, Stanforth S, Lemasters G, Bernstein DI. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J. Allergy Clin. Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, Wilson K, Lemasters G. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci. Total Environ. 2006;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande BS, Ambedkar SS, Shewale JG. Biologically active secondary metabolites from Streptomyces. Enzyme Microb. Technol. 1988;10:455–473. [Google Scholar]
- Douwes J, van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, Postma D, de Jongste J, Travier N, Brunekreef B. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J. Allergy Clin. Immunol. 2006;117:1067–1073. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- Frank TL, Adisesh A, Pickering AC, Morrison JF, Wright T, Francis H, Fletcher A, Frank P.I. Hannaford P. Relationship between exhaled nitric oxide and childhood asthma. Am. J. Respir. Crit. Care Med. 1998;158:1032–1036. doi: 10.1164/ajrccm.158.4.9707143. [DOI] [PubMed] [Google Scholar]
- Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–1300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- Haugland RA, Varma M, Wymer LJ, Vesper SJ. Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Syst. Appl. Microbiol. 2004;27:198–210. doi: 10.1078/072320204322881826. [DOI] [PubMed] [Google Scholar]
- Huttunen K, Hyvärinen A, Nevalainen A, Komulainen H, Hirvonen MR. Production of proinflammatory mediators by indoor air bacteria and fungal spores in mouse and human cell lines. Environ. Health Perspect. 2003;111:85–92. doi: 10.1289/ehp.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, Villareal M, Lockey J, Hershey GK, LeMasters G. House dust (1-3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–513. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DJ, Virnig CM, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Burton RM, Salazar LP, DaSilva DF, Shanovich KM, Tisler CJ, Gern JE, Lemanske RF., Jr. Fractional exhaled nitric oxide measurements are most closely associated with allergic sensitization in school-age children. J. Allergy Clin. Immunol. 2009;124:949–953. doi: 10.1016/j.jaci.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E, Vesper S, Levin L, LeMasters G, Grinshpun S, Reponen T. Streptomycetes in house dust: associations with housing characteristics and endotoxin. Indoor Air. 2010;21:300–310. doi: 10.1111/j.1600-0668.2010.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila J, Pelkonen J, Kosma VM, Mäki-Paakkanen J, Komulainen H, Hirvonen MR. Systemic immunoresponses in mice after repeated exposure of lungs to spores of Streptomyces californicus. Clin. Diagn. Lab. Immunol. 2003;10:30–37. doi: 10.1128/CDLI.10.1.30-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitz R, Rose MA, Borgmann A, Schubert R, Zielen S. Systemic and bronchial inflammation following LPS inhalation in asthmatic and healthy subjects. J Endotoxin Res. 2006;12:367–74. doi: 10.1179/096805106X153934. [DOI] [PubMed] [Google Scholar]
- Kovesi TA, Dales RE. Effects of the indoor environment on the fraction of exhaled nitric oxide in school-aged children. Can. Respir. J. 2009;16:e18–e23. doi: 10.1155/2009/954382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen AK, Kuitunen M, Savilahti E, Pelkonen A, Malmberg P, Mäkelä M. Airway inflammation in probiotic-treated children at 5 years. Pediatr. Allergy Immunol. 2011;22:249–251. doi: 10.1111/j.1399-3038.2010.01079.x. [DOI] [PubMed] [Google Scholar]
- LeMasters GK, Wilson K, Levin L, Biagini J, Ryan PH, Lockey JE, Stanforth S, Maier S, Yang J, Burkle J, Villareal M, Khurana Hershey GK, Bernstein DI. High prevalence of aeroallergen sensitization among infants of atopic parents. J. Pediatr. 2006;149:505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung TF, Wong YS, Chan IH, Yung E, Wong CK, Lam CW, Wong GW. Indoor determinants of endotoxin and dust mite exposures in Hong Kong homes with asthmatic children. Int. Arch. Allergy Immunol. 2010;152:279–287. doi: 10.1159/000283039. [DOI] [PubMed] [Google Scholar]
- Leuppi JD, Downs SH, Downie SR, Marks GB, Salome CM. Exhaled nitric oxide levels in atopic children: relation to specific allergic sensitisation, AHR, and respiratory symptoms. Thorax. 2002;57:518–523. doi: 10.1136/thorax.57.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg MWH, De Jongste JC. Exhaled nitric oxide in childhood asthma: a review. Clin. Exp. Allegy. 2008;38:246–259. doi: 10.1111/j.1365-2222.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- Purokivi M, Hirvonen MR, Randell J, Roponen M, Tukiainen H. Nitric oxide alone is an insufficient biomarker of exposure to microbes in a moisture-damaged building. Inhal. Toxicol. 2002;14:1279–1290. doi: 10.1080/08958370290084917. [DOI] [PubMed] [Google Scholar]
- Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, Grinspun SA, Zheng S, Bernstein DI, Lockey J, Villareal M, Khurana Hershey GK, LeMasters G. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Ann. Allergy, Asthma & Immunol. 2011;107:120–126. doi: 10.1016/j.anai.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- Rintala H, Nevalainen A. Quantitative measurement of streptomycetes using real-time PCR. J. Environ. Monit. 2006;8:745–749. doi: 10.1039/b602485h. [DOI] [PubMed] [Google Scholar]
- Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, Villareal M, Hershey GK, Burkle J, LeMasters G. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am. J. Respir. Crit. Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian NM, Park JH, Cox-Ganser JM. Dampness and mold in the indoor environment: implications for asthma. Immunol. Allergy Clin. North Am. 2008;28:485–505. doi: 10.1016/j.iac.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Sordillo JE, Hoffman EB, Celedon JC, Litonjua AA, Milton DK, Gold DR. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clin. Exp. Allergy. 2010;40:902–910. doi: 10.1111/j.1365-2222.2010.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier AJ, Hornung R, Lierl M, Lanphear BP. Environmental exposures and exhaled nitric oxide in children with asthma. J. Pediatr. 2006;149:220–226. doi: 10.1016/j.jpeds.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Randell JT, Hirvonen MR, Purokivi MK, Roponen MH, Tukiainen HO. The effects of Aspergillus fumigatus challenge on exhaled and nasal NO levels. Eur Respir J. 2005;26:887–93. doi: 10.1183/09031936.05.00061405. [DOI] [PubMed] [Google Scholar]
- Tovey ER, Almqvis t C., Li Q, Crisafulli D, Marks GB. Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J. Allergy Clin. Immunol. 2008;22:114–118. doi: 10.1016/j.jaci.2008.05.010. [DOI] [PubMed] [Google Scholar]
- van Strien RT, Engel R, Holst O, Bufe A, Eder W, Waser M, Braun-Fahrländer C, Riedler J, Nowak D, von Mutius E. Microbial exposure of rural school children, as assessed by levels of N-acetyl-muramic acid in mattress dust, and its association with respiratory health. J. Allergy Clin. Immunol. 2004;113:860–867. doi: 10.1016/j.jaci.2004.01.783. [DOI] [PubMed] [Google Scholar]
- Velasco G, Campo M, Manrique OJ, Bellou A, He H, Arestides RS, Schaub B, Perkins DL, Finn PW. Toll-like receptor 4 or 2 agonists decrease allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2005;32:218–224. doi: 10.1165/rcmb.2003-0435OC. [DOI] [PubMed] [Google Scholar]
- Vesper S, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P, Cox D, Dewalt G, Friedman W. Development of an environmental relative moldiness index for US homes. J. Occup. Environ. Med. 2007;49:829–33. doi: 10.1097/JOM.0b013e3181255e98. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Sebastian A, Larsson L, Wang Z, Zhang Z, Norback D. Asthmatic symptoms among pupils in relation to microbial dust exposure in schools in Taiyuan, China. Pediatr. Allergy Immunol. 2008;19:455–65. doi: 10.1111/j.1399-3038.2007.00664.x. [DOI] [PubMed] [Google Scholar]