Abstract
Central administration of neuropeptide Y (NPY) increases food intake in laboratory rats and mice, as well as food foraging and hoarding in Siberian hamsters. The NPY-Y1 and Y5 receptors (Rs) within the hypothalamus appear sufficient to account for these increases in ingestive behaviors. Stimulation of NPY-Y2Rs in the Arcuate nucleus (Arc) has an anorexigenic effect as shown by central or peripheral administration of its natural ligand peptide YY (3–36) and pharmacological NPY-Y2R antagonism by BIIE0246 increases food intake. Both effects on food intake by NPY-Y2R agonism and antagonism are relatively short-lived lasting ~4 h. The role of NPY-Y2Rs in appetitive ingestive behaviors (food foraging/hoarding) is untested, however. Therefore, Siberians hamsters, a natural food hoarder, were housed in a semi-natural burrow/foraging system that had a) foraging requirement (10 revolutions/ pellet), no free food (true foraging group), b) no running wheel access, free food (general malaise control) or c) running wheel access, free food (exercise control). We microinjected BIIE0246 (antagonist) and PYY(3–36) (agonist) into the Arc to test the role of NPY-Y2Rs there on ingestive behaviors. Food foraging, hoarding, and intake were not affected by Arc BIIE0246 microinjection in fed hamsters 1, 2, 4, and 24 h post injection. Stimulation of NPY-Y2Rs by PYY(3–36) inhibited food intake at 0–1 and 1–2 h and food hoarding at 1–2 h without causing general malaise or affecting foraging. Collectively, these results implicate a sufficiency, but not necessity, of the Arc NPY-Y2R in the inhibition of food intake and food hoarding by Siberian hamsters.
Keywords: appetitive behavior, Siberian hamster, Peptide YY, BIIE0246, food foraging, food deprivation
INTRODUCTION
In modern industrialized nations, the incidence of obesity has increased markedly over the last few decades and has led to a rise in severe secondary health consequences. Given that most animals forage for food, including humans [for reviews see:7,31], we postulated recently that a largely ignored set of related factors leads to sizeable food hoards and has helped propel the obesity crisis: a) size of refrigerators, freezers and pantries, b) processes that extend the shelf lives of food well beyond that of 25–50 years ago, and c) ample and inexpensive calorically dense food stuffs7. Therefore, a deepened understanding of food foraging and hoarding may lead to behavioral and/or pharmacological treatments for overweight/obese humans, as we have suggested previously5,7,31.
Using Wallace Craig’s14 division of animal behavior into appetitive (behavior leading to the goal) and consummatory (realization of the goal) phases, ingestive behavior is dichotomized as food foraging/hoarding (appetitive phase) and food intake (consummatory phase). We know considerably more about consummatory ingestive behaviors than appetitive behaviors because the most commonly studied animals in ingestive behavior research are laboratory rats and mice. They are not natural hoarders [for review:7] and are typically housed in standard cages that do not permit a significant effort to obtain food. We are able to measure food foraging, hoarding, and intake using our simulated burrow system17 and Siberian hamsters (Phodopus sungorus), as they hoard food in nature49 and in the laboratory (for review see:7,31)
Unlike laboratory rats and mice that overeat after a fast [e.g.,27,53], food deprived Siberian hamsters do not overeat, nor do humans, once access to food is restored but instead ‘overhoard’, as do humans [for review see:7]. Therefore, we reasoned that other stimuli that increase food intake by laboratory rats and mice may trigger increases in food hoarding by these hamsters. Indeed, we launched several studies of the peptidergic control of food hoarding guided by this premise. Some of these studies focused on the Arcuate nucleus (Arc) and the neuropeptide Y (NPY) and agouti-related protein (AgRP) neurons found therein15,16,19,20,28,29. As in laboratory rats41,42,44, and mice8, NPY and AgRP are nearly exclusively co-localized in neurons within the medial portions of the Arc in Siberian hamsters and Arc NPY and AgRP synthesis is stimulated by food deprivation in Siberian hamsters22,25,34 making them a possible mediator of food deprivation-induced increases in foraging/hoarding.
NPY is a powerful orexigenic peptide when applied centrally in laboratory rats [e.g.,33,43 and other species [for review see:6]. Moreover, NPY is not only a powerful orexigenic peptide in Siberian hamsters10,15, but also is a powerful short-term (1–4 h, but up to 24 h) stimulator of food hoarding15,16,20,28,29. NPY has several receptor (R) sub-types (NPY-Y1-5) that are broadly distributed and their stimulation results in a diverse range of functions [for review see:48]. The NPY Y1- and Y5-R have been implicated in the control of food intake in laboratory rats and mice [for review see:21]. Microinjections of a Y1-R agonist into the PVH or PFA triggers a dose-dependent increase in food intake in laboratory rats45 and, conversely, prior or co-injection of a NPY Y1-R antagonist into the PVH blocks the ability of PVH NPY injections to increase food intake50,51. NPY Y1-R agonism primarily increases food hoarding, whereas NPY Y5-R agonism primarily increases food intake in our foraging/hoarding model using Siberian hamsters20,29.
Another NPY receptor subtype that has been strongly implicated in food intake, the NPY Y2-R, is located presynaptically and found in a number of CNS sites, including the Arc and appears to function as an autoreceptor on NPY/AgRP neurons to inhibit their activity and thereby inhibit food intake11. A naturally-occurring ligand for the NPY Y2-R is peptide tyrosine-tyrosine (PYY), a gut-derived hormone released from L cells in the intestine after a meal primarily in the form of PYY(3–36) 2. PYY(3–36) is a selective agonist for the NPY-Y2R resulting in inhibition of food intake, both endogenously and exogenously1,9. Consistent with these effects, antagonism of the NPY-Y2R using the Y2-R selective antagonist BIIE0246, increases food intake, adding further support for a role of NPY-Y2R in the cessation of food intake1. Therefore, the purpose of the present experiments was to test the role of the NPY Y2-R in food foraging, food hoarding, and food intake in Siberian hamsters. To do so we asked two questions: 1) Does antagonism of NPY Y2-R using BIIE0246 increase ingestive behaviors in fed animals and 2) Does agonism of NPY Y2-R using the naturally-occurring PYY(3–36) inhibit the food deprivation-induced increases in ingestive behaviors?
MATERIALS AND METHODS
Animals and Housing
Two separate cohorts of 40 male Siberian hamsters 2.5–3 months of age and weighing 35–45 g were selected from our breeding colony. After weaning animals were group housed according to sex and raised in a long day photoperiod (16L:8D, light offset: 1900) with ad libitum access to rodent chow (LabDiet® 5001, Purina, St. Louis, MO) and tap water unless otherwise indicated. Room temperature was maintained at 21 ±2 °C. Each cohort was treated identically. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with Public Health Service and United States Department of Agriculture guidelines.
Foraging and Hoarding Apparatus
Animals were transferred to the foraging and hoarding room where they were singly housed in shoebox cages 290 × 180 × 130 mm (length × width × height), maintained in a 16L:8D photoperiod (light offset: 1330), and with ad libitum access to the pelleted test diet (DPPs, Purified 75 mg pellets; Bio-Serve, Frenchtown, NJ) and water. After two weeks to acclimate to the new light offset, animals were placed into the foraging and hoarding apparatus modified from Perrigio and Bronson39 and previously described19. Briefly, a bottom, “burrow”, cage 290 × 180 × 130 mm (length × width × height) containing Alpha-Dri bedding (Specialty Papers, Kalamazoo, MI) and one cotton nestlet (Anacare, Belmore, NY). The bottom cage was opaque and covered to simulate the darkness of a burrow. The top, “foraging”, cage 456 × 234 × 200 mm (length × width × height) was equipped with a pellet dispenser, running wheel (525 cm circumference), and ad libitum access to water. The two cages were connected via convoluted polyvinyl chloride tubing (38.1 mm inner diameter and ~1.52 m long). Wheel revolutions were counted using a magnetic detection system with monitoring by a hardware/software computer interface (Med Associates, Georgia, VT). Hamsters were acclimated/trained to this apparatus for one week prior to and after cannulation (see below).
We used an acclimation/training regimen that minimizes changes in body mass and food intake that can occur when initially housed in the foraging and hoarding apparatus. Specifically, hamsters were given free access to food pellets and were able to earn a food pellet for every 10 wheel revolutions. After the first two days the free access to food was removed and all food had to be earned (1 pellet/10 wheel revolutions) for 5 d, during which body mass, wheel revolutions, pellets earned (food foraging), food intake, and food hoarding were measured daily. After the 7 d acclimation/training period, animals were placed temporarily back into the shoebox cages before cannula implantation (see below).
Foraging Groups and Measurement of Foraging, Food Hoarding, and Food Intake
Three foraging groups were used as in our first of many reports of these groups17. When foraging effort is required beyond traversing the tubing, then completion of a programmed number of wheel revolutions triggers food pellet delivery, usually 10, as >10 inhibits hoarding due to decreased payoff – this is the 10 revolution per pellet group (10REVS). Two non-foraging conditions critical to interpreting the 10REVS foraging results were included. In the Free Wheel (FW) condition, food (300 pellets) was presented in the cage non-contingently and independent of wheel running, but wheel running was allowed (controlling for non-specific locomotor stimulation/inhibition thereby providing insight into earned food by the 10REVS group). In the Blocked Wheel (BW) condition, food (300 pellets) also was presented noncontingently, but the wheel was blocked (controlling for locomotor activity-induced changes – i.e., sedentary controls).
Foraging (pellets earned) was defined as the number of pellets earned (10REV) and food hoarding was defined as the number of pellets found in the bottom cage plus those removed from the cheek pouches. For the BW and FW groups where food was given non-contingently, food intake was defined as the number of pellets supplied (300 pellets/day) minus the total pellets hoarded or left in the top cage (surplus pellets). In the 10REV group, food intake was defined as the number of pellets earned minus the total pellets hoarded or left in the top cage (surplus pellets). The electronic scale used to weigh the food pellets was set to “parts” measurement, resulting in one 75 mg food pellet = 1 with fractions of pellets computed by the scale.
Cannula Implantation, Injections, and Verification
Cannulae were stereotaxically implanted aimed unilaterally at the ventromedial aspect of the Arc (posterior to bregma: −1.4 mm, lateral to midline: 0.3 mm, and ventral to skull: −8.0 mm), because this region shows the densest NPY-Y2R expression in rats37 and mice23 under isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL) inhalation anesthesia as previously described19. In brief, each animal had hair removed from the top of their head, skull exposed, and were placed into a stereotaxic surgical apparatus (David Kopf Instruments, Tujunga, CA). A guide cannula (26 gauge stainless steel; Plastics One, Roanoke, VA) was lowered into place and secured to the skull using cyanoacrylate ester gel, 3/16 mm jeweler’s screws, and dental acrylic. The opening in the guide cannula was sealed using a removable obturator throughout the experiment except during parenchymal injections. Hamsters received buprenorphine (0.2 mg/kg body mass, s.c.) to minimize discomfort and apple slices to facilitate food and water intake immediately following surgery and for the following 2 d. Animals were housed in shoebox cages for 2 wks following surgery before being returned to the foraging and hoarding apparatus.
Each animal was “mock-injected” daily in the week before a test day, where the obturator was removed and the animal was lightly restrained for 1 min to acclimate the animal to the injection procedure. On test days, an inner cannula (33 gauge stainless steel, Plastics One, Roanoke, VA) was connected to a Hamilton syringe via PE-20 tubing and inserted into the guide cannula, extending 0.5 mm below the guide cannula tip. All injections were given at light offset (1330 EST). Each injection (200 nl) of neurochemical or vehicle was delivered over 30 s and the injection needle remained in place for ~30 s before removal, as done previously [e.g.,15,19].
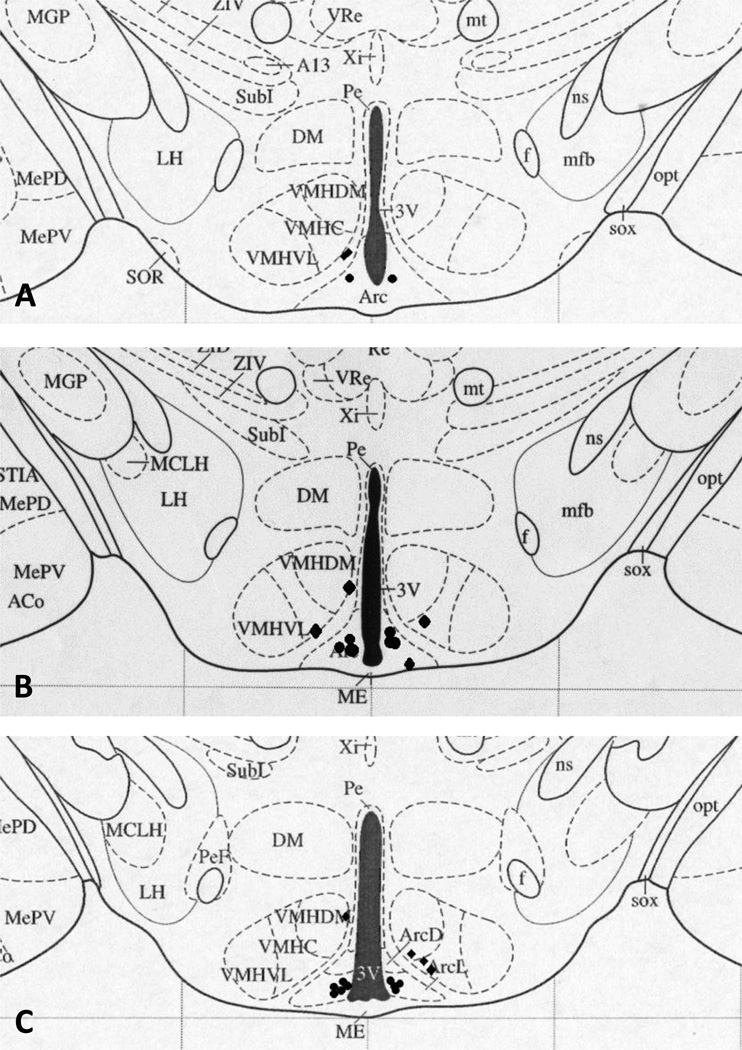
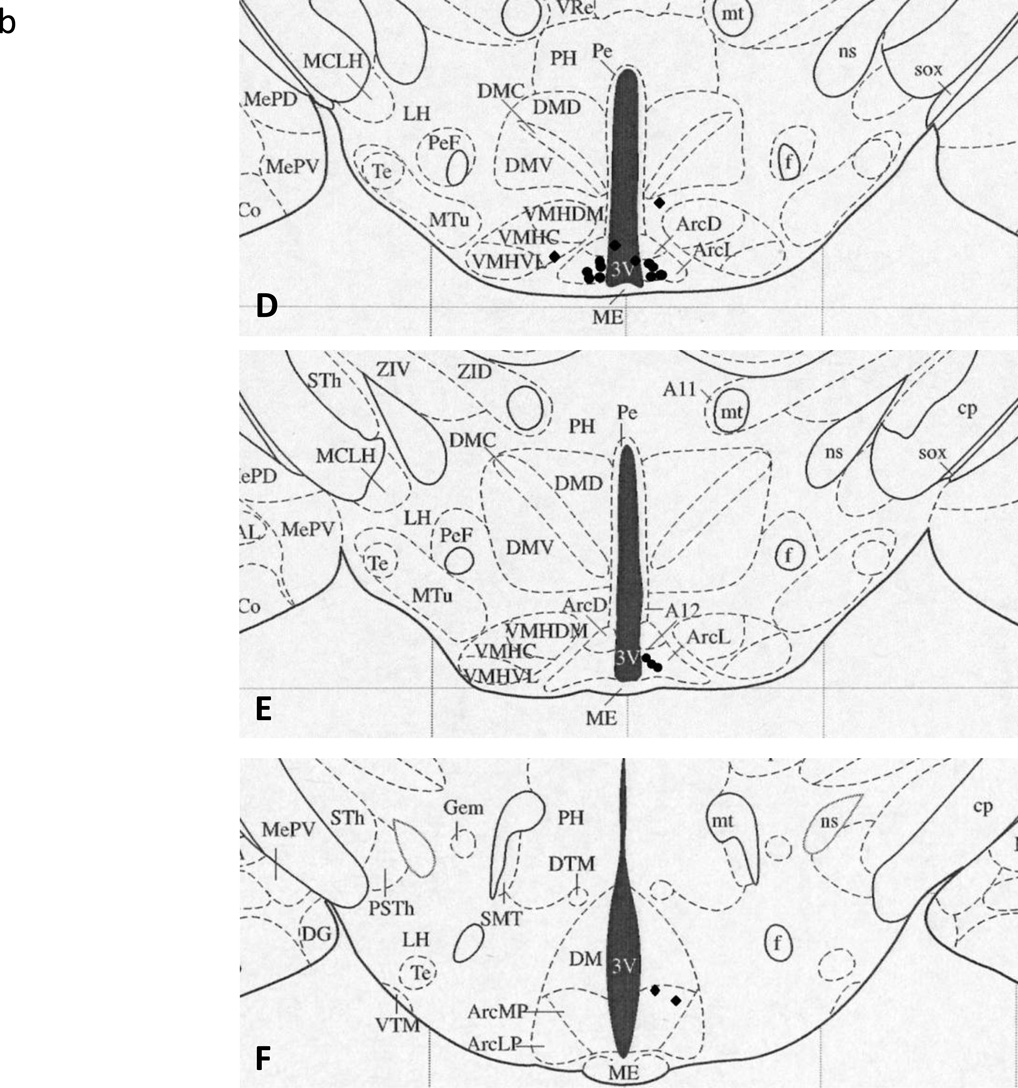
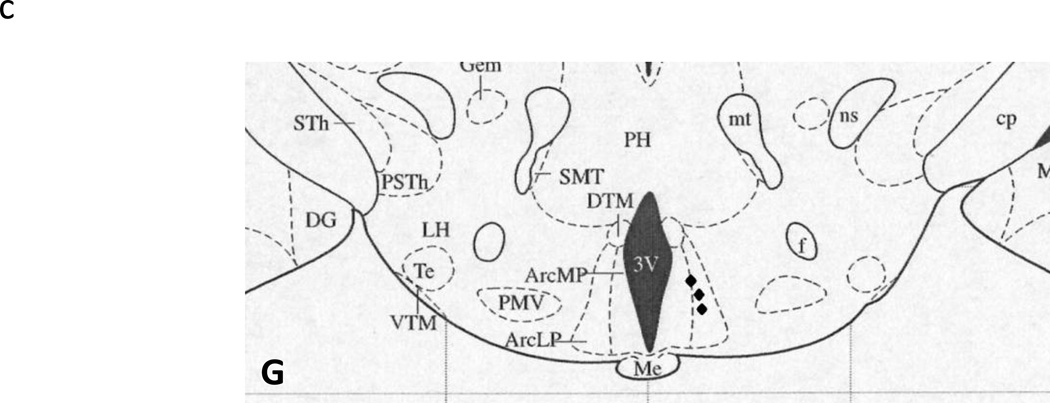
Following the final test day, animals were injected with 300 nl bromophenol blue dye to mark the location of the cannula tip and animals were then given an overdose of pentobarbital sodium (100 mg/kg), transcardially perfused with 100 ml of heparinized saline followed by 125 ml of 4% paraformaldehyde in phosphate buffered saline, pH=7.4. The brains were then removed and post fixed in a 4% paraformaldehyde solution for 2 d, followed by a 30% sucrose solution until sectioning, replacing the sucrose solution after 24 h. Brains were sectioned at 80 µm for cannula location verification using light microscopy. Cannulae were considered an Arc hit if the blue dye was visible in the ventromedial aspect of the Arc and only these animals were included in the analyses (n = 75, see Figure 1 for cannula locations).
Figure 1.
Cannula location plotted onto schematic brain section diagrams (taken from Paxinos and Watson38) with filled circles (•) representing the tip of a correctly placed cannula and filled diamonds (♦) representing misplaced cannula. A. Plate 43, B. Plate 44, C. Plate 45, D. Plate 46, E. Plate 47, F. Plate 49, and G. Plate 50. Some circles represent more than one cannula. 3V, third ventricle; Arc, Arcuate nucleus; ArcD, dorsal Arcuate nucleus; ArcL, lateral Arcuate nucleus; ArcLP, lateroposterior Arcuate nucleus; ArcMP, medioposterior Arcuate nucleus; DM, dorsomedial hypothalamus; ME, median eminence; VMHC, central ventromedial hypothalamus; VMHDM, dorsomedial ventromedial hypothalamus; VMHVL, ventrolateral ventromedial hypothalamus.
Baseline Data and Foraging Groups
At the conclusion of the acclimation/training period animals were separated into one of the three foraging groups (10REV, FW, BW) described above. Animals were separated into the groups matched for body mass, food intake, and food hoarding and were allowed 2 wks to acclimate to their foraging treatment group.
Experiment 1: NPY-Y2R Antagonism by BIIE0246 in Fed Animals
Arc injections consisted of one of three doses of BIIE0246 (0.1, 1.0, 5.0 nmol in 200 nl) or vehicle (5% DMSO), with vehicle choice and doses based on effective Arc delivered drug in laboratory rats1. Each animal received all injections in a counterbalanced-within subjects design. A washout period of 1 wk separated individual injections to ensure all measures had returned to baseline values similar to our previous work29. On injection days, animals were provided with a clean burrow cage and access to food was prevented by blocking access to the top cage 2 h before injections. Animals were injected at light offset and access to food was returned. Wheel revolutions, food foraging, food hoarding, and food intake were measured at 1, 2, 4, 24 h and each day post-injection until the next test day (final group sizes BW: n=21, FW: n=22, and 10REV: n=26).
Experiment 2: NPY-Y2R Agonism by PYY(3–36) in Food-Deprived Animals
After the week-long washout period following the final BIIE0246 test day, animals were maintained in their foraging treatment group for an additional week and then assigned to an injection treatment group (see below) balanced for body weight, food intake, and food hoarding as above. The animals were then food deprived for 56 h (IACUC approved), a time length previously shown to maximize food hoarding4,18. Before access to food was returned at light offset, half of the animals received an injection of PYY(3–36) (0.1 nmol in 200 nl), the active form of the peptide for satiation52, into the Arc and the other half received the saline vehicle. Wheel revolutions, food foraging, food intake, and food hoarding were measured at 1, 2, 4, 24 h and each day post-injection until all animals returned to pre-injection levels. After the animals returned to behavioral baseline, brain tissue was collected to verify cannula location (Fig. 1; 69 hits and 11 misses or removed their cannula; final group sizes: PYY(3–36): BW: n=12, FW: n=11, and 10REV: n=13 and vehicle: BW: n=9, FW: n=11, and 10REV: n=13).
Statistics
Raw data from Experiment 1 were transformed for each individual into percent change from vehicle before statistical analyses using the formula: [((X-Vehicle)/Vehicle)*100], where “X” equals the value measured in response to the dose of BIIE0246 and “Vehicle” equals the value measured for that individual after vehicle injection. No statistical comparisons were made among the time intervals because the intervals were of unequal duration. No statistical comparisons are reported across test days in this counterbalanced-within subject design, as repeated measures two-way ANOVA (Foraging Treatment × Arc-Injection) showed no effect of injection order. The data were analyzed using a two-way ANOVA (Foraging Treatment × Arc-Injection; 3 × 4). For Experiment 2, data were not transformed into percent change from vehicle, because animals only were food deprived once and therefore could not serve as their own control, and the absolute values were analyzed using a two-way ANOVA (Foraging Treatment × Arc-Injection; 3 × 2) within each individual time point for the same reason as above. All statistical analyses were performed using NCSS (version 2007, Kaysville, UT). Exact probabilities and test values were omitted for simplicity and clarity of presentation. Differences were considered statistically significant if P<0.05. Tukey-Kramer Multiple Comparison Tests were used for post hoc tests when appropriate. Misplaced cannulae were not included in the final statistical comparisons.
RESULTS
Experiment 1: NPY-Y2R Antagonism with BIIE0246 in Fed Animals
Wheel Running
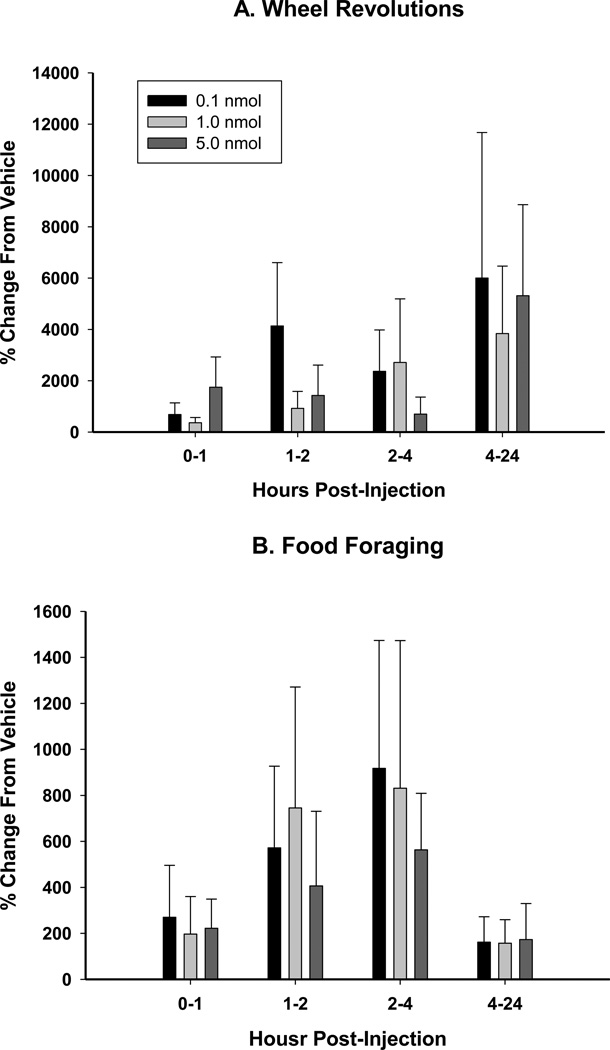
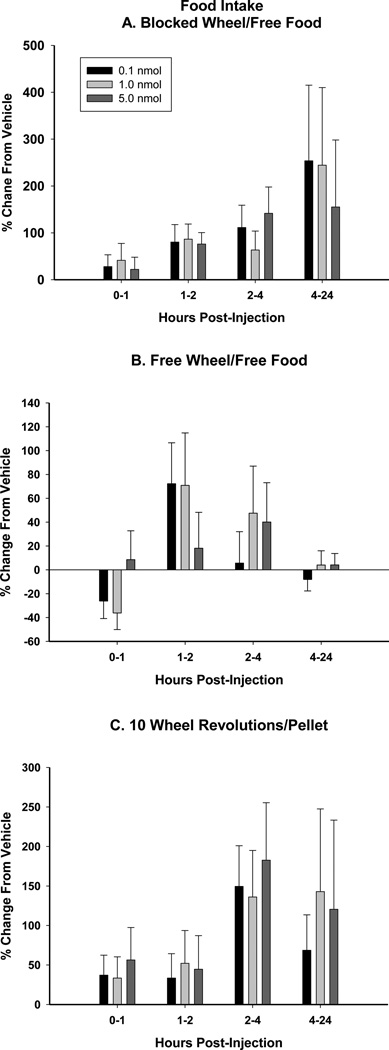
At each time interval, Arc injection of BIIE0246 did not significantly stimulate wheel running activity compared to vehicle injection at any of the three doses tested (0.1, 1.0, and 5.0 nmol; Fig. 2A). The lack of wheel running increase in the FW group, where food delivery was not contingent upon wheel running, suggests that there was not non-specific stimulation of locomotor activity.
Figure 2.
Mean ± SEM percent change from vehicle (5% DMSO) in response to BIIE0246 (0.1, 1.0, and 5.0 nmol) of A. wheel revolutions in hamsters with no foraging requirement and a functional running wheel [Free Wheel (FW)] and B. Foraging [pellets earned; in hamsters with a 10 wheel revolutions per pellet foraging requirement (10REV)].
Food Foraging
Arc injection of BIIE0246 did not significantly increase food foraging (wheel running contingent food delivery) at any time point examined (Fig. 2B) above vehicle-treated animals.
Food Intake
Food intake was not stimulated above vehicle at any time interval examined (0–1, 1–2, 2–4, 4–24 h) by Arc injection of BIIE0246 for all three foraging treatments (10REV, FW, and BW; Fig. 3A–C).
Figure 3.
Mean ± SEM percent change from vehicle (5% DMSO) in response to BIIE0246 (0.1, 1.0, and 5.0 nmol) of food intake with A. no foraging requirement and a stationary running wheel [Blocked Wheel (BW)], B. no foraging requirement and a functional running wheel [Free Wheel (FW)], and C. a 10 wheel revolutions per pellet foraging requirement [10 revolutions/pellet (10REV)].
Food Hoarding
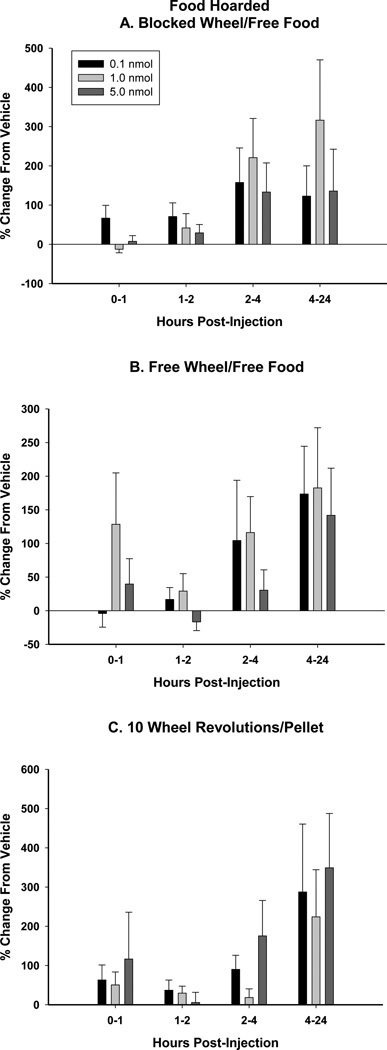
BIIE0246 injection into the Arc did not stimulate food hoarding compared to vehicle injection at any time point examined for each foraging treatment (10REV, FW, and BW; Fig. 4A–C). During the 2–4 h interval after the 5.0 nmol BIIE0246 injection, hoarding in the 10REV group approached significance (p=0.059) when compared with vehicle injection.
Figure 4.
Mean ± SEM percent change from vehicle (5% DMSO) in response to BIIE0246 (0.1, 1.0, and 5.0 nmol) of food hoarded with A. no foraging requirement and a stationary running wheel [Blocked Wheel (BW)], B. no foraging requirement and a functional running wheel [Free Wheel (FW)], and C. a 10 wheel revolutions per pellet foraging requirement [10 revolutions/pellet (10REV)].
Experiment 2: NPY-Y2R Agonism using PYY(3–36) in Food-Deprived Animals
Wheel Running
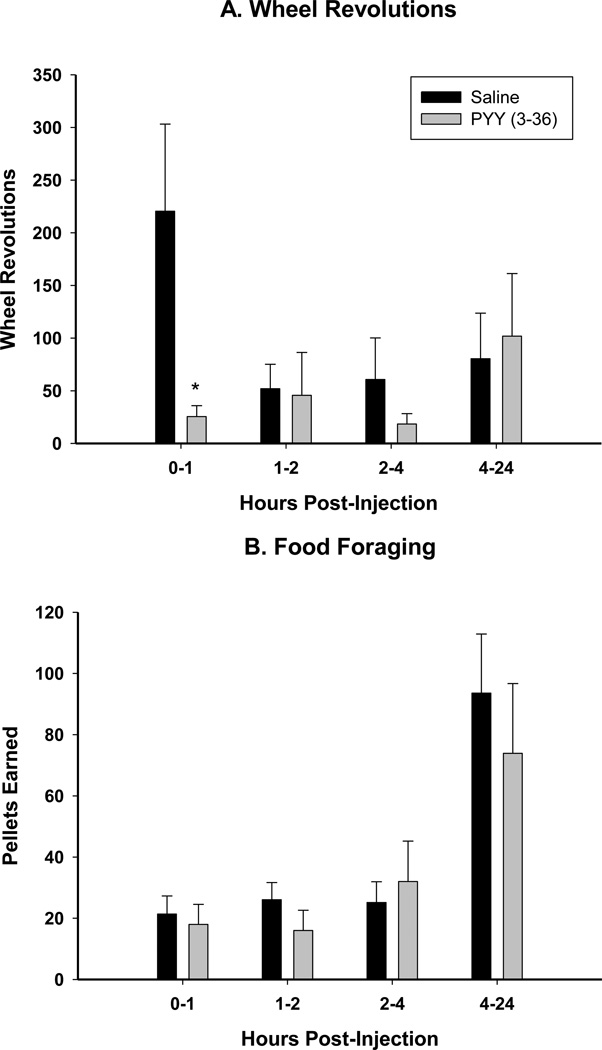
PYY(3–36) treatment inhibited the food deprivation-induced increases in wheel running during the 0–1 h interval (Fig. 5A). The inhibition of wheel running is not indicative of malaise caused by PYY(3–36), because the inhibition also was not seen in the 10REV group (see below).
Figure 5.
Mean ± SEM of Arc injection of PYY (3–36) (0.1 nmol) or Saline post-food deprivation of A. wheel revolutions in hamsters with no foraging requirements and a functional running wheel [Free Wheel (FW)] and B. foraging (pellets earned) in hamsters with a 10 wheel revolutions per pellet foraging requirement [10 revolutions/pellet (10REV)]. *= P<0.05 vs Saline.
Food Foraging
Arc injection of PYY(3–36) did not result in a significant inhibition of food foraging compared to saline injection at any time point measured (0–1, 1–2, 2–4, 4–24 h as well as during the next 6 d; Fig. 5B).
Food Intake
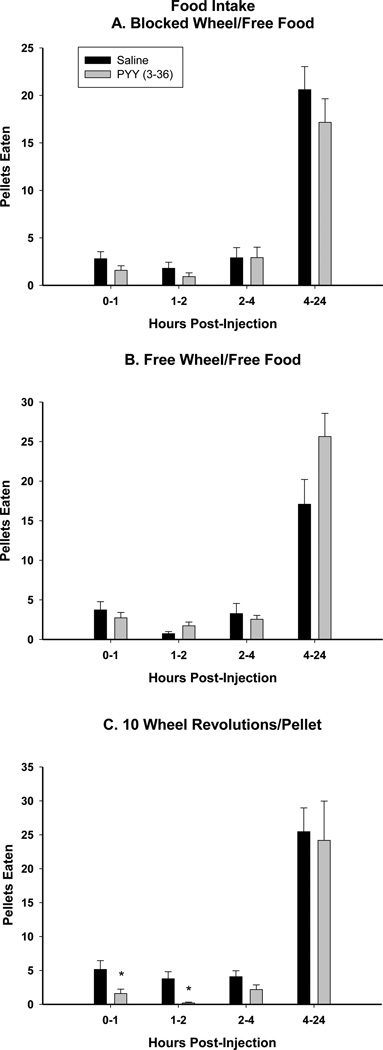
Arc injection of PYY(3–36) attenuated food intake after food deprivation compared with Arc saline injection at 0–1 and 1–2 h for the 10REV foraging treatment (Fig. 6C), but not in the BW or FW group (Fig. 6A and B); no significant differences were present after the first two h of refeeding for any group.
Figure 6.
Mean ± SEM of Arc injection of PYY (3–36) (0.1 nmol) or Saline post-food deprivation of food intake with A. no foraging requirement and a stationary running wheel [Blocked Wheel (BW)], B. no foraging requirement and a functional running wheel [Free Wheel (FW)], and C. a 10 wheel revolutions per pellet foraging requirement [10 revolutions/pellet (10REV)]. *= P<0.05 vs Saline.
Food Hoarding
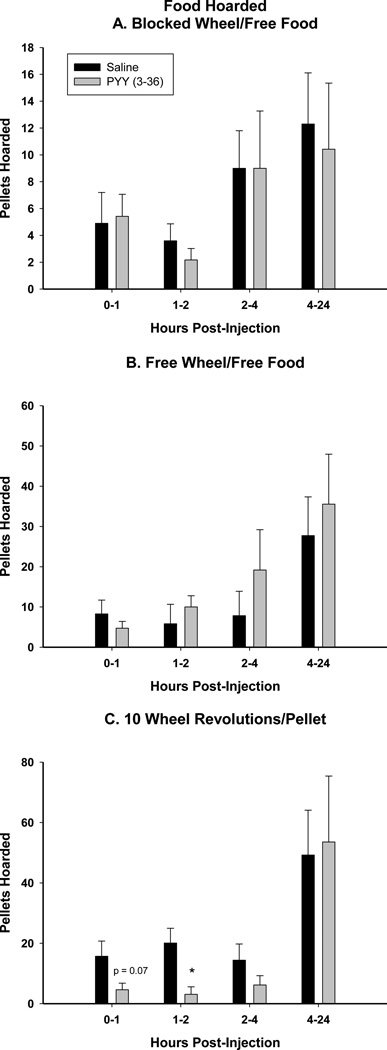
In the 10REV group, agonism of the NPY-Y2R in the Arc using PYY(3–36) inhibited food hoarding upon refeeding compared with saline injection at 1–2 h and approached significance at 0–1 h (p=0.07; Fig. 7C). Significant differences were not seen at any other time point or foraging treatment (Fig. 7A and B).
Figure 7.
Mean ± SEM of Arc injection of PYY (3–36) (0.1 nmol) or Saline post-food deprivation of food hoarded with A. no foraging requirement and a stationary running wheel [Blocked Wheel (BW)], B. no foraging requirement and a functional running wheel [Free Wheel (FW)], and C. a 10 wheel revolutions per pellet foraging requirement [10 revolutions/pellet (10REV)]. *= P<0.05 vs Saline.
DISCUSSION
Controlling ingestive behavior is a vital aspect of preventing/treating obesity and accordingly a concerted effort has been made to describe the mechanisms involved in food intake. NPY is the most potent central orexigenic neurochemical in laboratory rats13,33,43 with marked increases in food hoarding and intake occurring with stimulation of Y1-R and Y5-R, respectively in Siberian hamsters20. The NPY-Y2R agonism/antagonism had not been tested for its role in the appetitive ingestive behaviors of food hoarding or foraging14 in any species before the present study. Here we found for the first time that agonism of the Y2-R by PYY(3–36) inhibited food intake and hoarding early (first few hours) after refeeding following food deprivation and that antagonism of the Y2-R by BIIE0246 did not affect appetitive or consummatory ingestive behaviors in fed hamsters. These results suggest a possible inhibitory role of PYY(3–36) in food hoarding.
Antagonism of the Y2-R in the Arc using BIIE0246 causes short term increases in food intake by laboratory rats1, effects similar to those of NPY Y1-R and Y5-R agonism [e.g.,24,45]. NPY-Rs have a dual role in the control of food intake, where Y2-R and Y4-R agonism is anorexigenic and Y1-R and Y5-R agonism is orexigenic in other rodents3,21,32. This dualism only partially extended to Siberian hamsters here as PYY(3–36) microinjections into the Arc inhibited food intake and especially food hoarding, but the NPY-Y2R antagonist BIE0246 did not stimulate food foraging, intake, or hoarding. This lack of effect of BIIE0246 on baseline food intake also has been reported for laboratory rats40. It is possible that our BIIE0246 dose was insufficient to block endogenous Y2 signaling, although this seems somewhat unlikely because we used a dose 5-times greater than a dose effective in rats1. In two pilot studies, we injected the highest dose of BIIE0246 (5.0 nmol) used here into the Arc followed 2–3 minutes later by PYY(3–36) peripherally (7.5 nmol/kg) or into the Arc (0.1 nmol). In both studies, BIIE0246 co-administered with PYY(3–36) resulted in no significant change in ingestive behaviors when compared to saline-treated Siberian hamsters. These data suggest that our dose BIIE0246 is able to prevent the inhibition of ingestive behaviors caused by Y2 agonism. The present data suggests that there is not a chronic stimulation of Y2 signaling in non-energetically challenged (i.e., ad libitum-fed) Siberian hamsters similar to laboratory rats40, which is unlike the apparent underlying inhibition of ingestive behaviors by leptin30 and cholecystokinin46 in Siberian hamsters.
It is worth noting the large standard error values found in most of the variables measured after Arc administration of the Y2 antagonist. The animals exhibited a dichotomous split into high and low levels of food foraging, intake, and hoarding, but hamsters showing high or low levels of one behavior did not necessarily predict high or low levels of the other behaviors as seems apparent for food hoarding by Syrian hamsters12. In addition, the exact location of the cannula within the Arc also was not associated with a particular ingestive behavioral response or the magnitude of the response. Large variations in food hoarding both within and between animals from day-to-day are common, quite unlike that of food intake studies in this species in our experience. The cause of the variations in spontaneous food hoarding by Siberian hamsters remains a mystery presently and is not due to differences in body fat (for example: fat hamsters hoarding less than lean animals because they possess greater internal energy stores).
The second experiment was designed to test the inhibitory role of the Y2-R signaling using the naturally occurring NPY Y2-R agonist PYY(3–36). PYY(3–36) has potent anorexigenic effects whether administered peripherally and centrally in laboratory rats and mice [for review:35], with few exceptions47. The anorexic effects of PYY(3–36) are diminished when animals are stressed26, which may account for the studies where PYY(3–36) was unable to inhibit food intake47. Because of the possible effects of stress interacting with the PYY(3–36) treatment, our animals were habituated to the injection protocol. As noted above, when food deprived Siberian hamsters are refed, large increases in food hoarding and foraging occur persisting for ~7 d, whereas food intake does not increase beyond the first few h18. Arc injected PYY(3–36) inhibited food intake and food hoarding in the true foraging group (10REV) during the first few hours of refeeding, a timeframe of effectiveness similar to that of 24 h food-deprived-refed laboratory rats after PYY(3–36) treatment for food intake9. The finding that the effect of PYY(3–36) only was seen in the hamsters ‘earning’ their food via foraging (wheel running; i.e., 10REV) is consistent with our findings with other anorexigenic peptides that exhibit their greatest inhibition in the 10REV group including the NPY Y1-R antagonist 1229U9129, leptin30, melanocortin 4-R agonism [melanotan II28] as well as triggering the greatest increases in food hoarding for orexigenic peptides administered centrally [NPY15,20, AgRP19]. The reason that ‘earned’ food elicits both larger decreases and increases in food hoarding is not clear. It is not that these animals are in any greater increase in negative energy balance due to the wheel running because the FW group runs approximately the same number of wheel revolutions as the 10REV group, although food is not contingent on the wheel running. Thus, some factor(s) associated with foraged (‘earned’) food rather than freely available food seems in play in the present and our previous studies that certainly warrants further study.
Collectively, the present data indicate that NPY Y2-R agonism inhibits food intake and hoarding, albeit in the short term (0–2 h) with refeeding after food deprivation. In addition, there does not appear to be underlying NPY Y2-R signaling inhibiting ingestive behaviors in this species because the antagonism of NPY Y2-R signaling does not increase appetitive or consummatory ingestive behaviors in ad libitum-fed hamsters. The short term nature of PYY(3–36) is not unique to this study, and as such seems to be limited in its ability to decrease foraging/hoarding in Siberian hamsters. Longer lasting NPY Y2-R agonists are being developed36, however, some of which may have the potential for therapeutic use to curtail food intake and hoarding in humans.
Arcuate antagonism of NPY-Y2Rs, with BIIE0246, did not stimulate ingestive behavior
Arcuate agonism of NPY-Y2Rs, with PYY(3–36), inhibited food intake and hoarding
NPY-Y2R signaling is sufficient, but not necessary, to inhibit ingestive behavior
ACKNOWLEDGMENTS
The authors thank the Department of Animal Resources at Georgia State University for expert animal care and Dr. Cheryl H. Vaughan, Danni Liu, Alex Thomas, Daniel Vizcaino, Dominiq Okoduwa, Melissa Chaney, and Shasmine Kelly for assistance in data collection.
This work was supported by the NIH R01 DK078358 to TJB and NIH F32 DK091984 to BJWT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3‖36) on food intake. Brain Res. 2005 May;1043(1–2):139–144. doi: 10.1016/j.brainres.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 2.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985 Nov;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniam A, Joshi R, Su C, Friend LA, James JH. Neuropeptide Y (NPY) Y2 receptor-selective agonist inhibits food intake and promotes fat metabolism in mice: combined anorectic effects of Y2 and Y4 receptor-selective agonists. Peptides. 2007 Feb;28(2):235–240. doi: 10.1016/j.peptides.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Progress in Psychobiology and Physiological Psychology. 2003;18:69–100. [Google Scholar]
- 6.Bartness TJ, Demas GE. Comparative studies of food intake: Lessons from nontraditionally studied species. In: Stricker EM, Woods SC, editors. Food and Fluid Intake. New York: Plenum; 2004. pp. 423–467. [Google Scholar]
- 7.Bartness TJ, Keen-Rhinehart E, Dailey MJ, Teubner BJ. Neural and hormonal control of food hoarding. Am J Physiol. 2011;301:R641–R655. doi: 10.1152/ajpregu.00137.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC, Palmiter RD, Schwartz MW. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 1998;47:538–543. doi: 10.2337/diabetes.47.4.538. [DOI] [PubMed] [Google Scholar]
- 9.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002 Aug;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 10.Boss-Williams KA, Bartness TJ. NPY stimulation of food intake in Siberian hamsters is not photoperiod dependent. Physiol Behav. 1996;59:157–164. doi: 10.1016/0031-9384(95)02037-3. [DOI] [PubMed] [Google Scholar]
- 11.Broberger C, Landry M, Wong H, Walsh JN, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortinand neuropeptide-Y-containing neurons of the rat hypothalamic Arcuate nucleus. Neuroendocrinology. 1997 Dec;66(6):393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 12.Buckley CA, Schneider JE. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2003 Nov;285(5):R1021–R1029. doi: 10.1152/ajpregu.00488.2002. [DOI] [PubMed] [Google Scholar]
- 13.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 14.Craig W. Appetites and aversions as constituents of instincts. Biol Bull. 1918;34:91–107. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dailey MJ, Bartness TJ. Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R877–R892. doi: 10.1152/ajpregu.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dailey MJ, Bartness TJ. Arcuate nucleus destruction does not block food deprivation-induced increases in food foraging and hoarding. Brain Res. 2010 Apr;1323:94–108. doi: 10.1016/j.brainres.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool. 2001;289:162–171. [PubMed] [Google Scholar]
- 18.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav. 2003;78:655–668. doi: 10.1016/s0031-9384(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 19.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol. 2004;286:R38–R45. doi: 10.1152/ajpregu.00284.2003. [DOI] [PubMed] [Google Scholar]
- 20.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005 Jul;289(1):R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 21.Duhault J, Boulanger M, Chamorro S, Boutin JA, Della ZO, Douillet E, Fauchere JL, Feletou M, Germain M, Husson B, Vega AM, Renard P, Tisserand F. Food intake regulation in rodents: Y5 or Y1 NPY receptors or both? Can J Physiol Pharmacol. 2000 Feb;78(2):173–185. [PubMed] [Google Scholar]
- 22.Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999 Oct;48(10):2028–2033. doi: 10.2337/diabetes.48.10.2028. [DOI] [PubMed] [Google Scholar]
- 23.Fetissov SO, Byrne LC, Hassani H, Emfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol. 2004;470:256–265. doi: 10.1002/cne.11047. [DOI] [PubMed] [Google Scholar]
- 24.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996 Jul;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 25.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998 Aug;1(4):271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 26.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004 Jun;145(6):2585–2590. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 27.Hill JO, Fried SK, DiGirolamo M. Effects of fasting and restricted refeeding on utilization of ingested energy in rats. Am J Physiol. 1984;247:R318–R327. doi: 10.1152/ajpregu.1984.247.2.R318. [DOI] [PubMed] [Google Scholar]
- 28.Keen-Rhinehart E, Bartness TJ. MTII attenuates ghrelin- and food deprivationinduced increases in food hoarding and food intake. Horm Behav. 2007;52:612–620. doi: 10.1016/j.yhbeh.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol. 2007 Apr;292(4):R1728–R1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keen-Rhinehart E, Bartness TJ. Leptin inhibits food-deprivation-induced increases in food intake and food hoarding. Am J Physiol Regul Integr Comp Physiol. 2008 Dec;295(6):R1737–R1746. doi: 10.1152/ajpregu.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keen-Rhinehart E, Dailey MJ, Bartness TJ. Physiological mechanisms for foodhoarding motivation in animals. Philos Trans R Soc Lond B Biol Sci. 2010 Mar;365(1542):961–975. doi: 10.1098/rstb.2009.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee NJ, Enriquez RF, Boey D, Lin S, Slack K, Baldock PA, Herzog H, Sainsbury A. Synergistic attenuation of obesity by Y2- and Y4-receptor double knockout in ob/ob mice. Nutrition. 2008 Sep;24(9):892–899. doi: 10.1016/j.nut.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Levine AS, Morley JE. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999 Feb;140(2):814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011 Feb;18(1):56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz AA, Milardo LF, DeCarr LB, Buckholz TM, Mays MR, Claus TH, Livingston JN, Mahle CD, Lumb KJ. A novel long-acting selective neuropeptide Y2 receptor polyethylene glycol-conjugated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents. J Pharmacol Exp Ther. 2007 Nov;323(2):692–700. doi: 10.1124/jpet.107.125211. [DOI] [PubMed] [Google Scholar]
- 37.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999 Apr;11(4):1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. New York: Academic Press; 2007. [Google Scholar]
- 39.Perrigo G, Bronson FH. Foraging effort, food intake, fat deposition, and puberty in female mice. Biol Reprod. 1983;29:455–463. doi: 10.1095/biolreprod29.2.455. [DOI] [PubMed] [Google Scholar]
- 40.Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3–36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol. 2005 Jul;17(7):452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 41.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: Evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 42.Stanley BG, Daniel DR, Chin AS, Leibowitz SF. Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides. 1985;6:1205–1211. doi: 10.1016/0196-9781(85)90452-8. [DOI] [PubMed] [Google Scholar]
- 43.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984 Dec;35(26):2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 44.Stanley BG, Leibowitz SF. Neuropeptide Y: Stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 45.Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides. 1992;13:581–587. doi: 10.1016/0196-9781(92)90093-i. [DOI] [PubMed] [Google Scholar]
- 46.Teubner BJ, Bartness TJ. Cholecystokinin-33 acutely attenuates food foraging, hoarding and intake in Siberian hamsters. Peptides. 2009 Dec; doi: 10.1016/j.peptides.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-Sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature. 2004 Jul;430(6996):1. doi: 10.1038/nature02665. [DOI] [PubMed] [Google Scholar]
- 48.Walther C, Morl K, Beck-Sickinger AG. Neuropeptide Y receptors: ligand binding and trafficking suggest novel approaches in drug development. J Pept Sci. 2011 Apr;17(4):233–246. doi: 10.1002/psc.1357. [DOI] [PubMed] [Google Scholar]
- 49.Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp zool Soc Lond. 1987;57:167–187. [Google Scholar]
- 50.Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol. 1998 Oct;125(3):549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokosuka M, Kalra PS, Kalra SP. Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology. 1999 Oct;140(10):4494–4500. doi: 10.1210/endo.140.10.7058. [DOI] [PubMed] [Google Scholar]
- 52.Zac-Varghese S, De SA, Bloom SR. Translational studies on PYY as a novel target in obesity. Curr Opin Pharmacol. 2011 Oct; doi: 10.1016/j.coph.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Zhao ZJ, Cao J. Plasticity in energy budget and behavior in Swiss mice and striped hamsters under stochastic food deprivation and refeeding. Comp Biochem Physiol A Mol Integr Physiol. 2009 Sep;154(1):84–91. doi: 10.1016/j.cbpa.2009.05.004. [DOI] [PubMed] [Google Scholar]