Abstract
Octreotide is a potent somatostatin analog therapeutically used to treat several conditions including hyper growth hormone secretion in patients with acromegaly. We infused octreotide into female Sprague Dawley rats every 12 h for 6 days at levels considerably greater than typical human therapeutic doses. Resulting circulating growth hormone profiles were characterized by ~25% reduction in plasma levels, including both pulse and interpulse components, but still contained in an otherwise female-like “continuous” secretory profile. The normally elevated feminine expression levels (protein and/or mRNA) of CYP2C12, CYP2A1, CYP2C7 and insulin-like growth factor-1 (IGF-1), all dependent on the continuous feminine growth hormone profile, were dramatically down-regulated. Octreotide suppression of the female-dependent levels of CYPs (cytochromes P450) and IGF-1 could not be explained by the apparently inconsequential alterations in the feminine circulating growth hormone profile. In this regard, somatostatin and its analogs are known to have a myriad of extra-pituitary actions effecting nearly all tissues in the body. Focusing our attention on CYP2C12, accounting for >40% of the total hepatic cytochrome P450 content in the female rat liver, we found an ~4-fold increase in hepatic ubiquitin-CYP2C12 levels in octreotide treated rats suggesting a possible contributing factor for the >60% suppression of CYP2C12 protein concentrations.
Keywords: cytochrome P450, CYP2A1, CYP2C12, CYP2C7, growth hormone, octreotide, ubiquitination
1. Introduction
Octreotide is a potent somatostatin analog most commonly used to reduce blood levels of growth hormone and insulin-like growth factor-1 (IGF-1), also known as somatomedin C, in acromegaly patients (Lamberts et al., 1993; Yang and Keating, 2010). Whereas a single dose of the drug to humans (Marbach et al., 1985) or rats (Turner and Tannenbaum, 1995) can profoundly reduce plasma growth hormone concentrations for many hours, the therapeutic goal, however, is to achieve normalization of growth hormone and IGF-1 levels in patients (Lamberts et al., 1993; Yang and Keating, 2010).
In addition to regulating IGF-1 expression, growth hormone regulates expression of both constituent and inducible cytochromes P450 (CYP) and its own growth hormone receptor in rat liver, as well as in every other species examined (Jansson et al., 1985; Shapiro et al., 1995). In fact, the sex differences in CYP expression exhibited in rats, mice and humans appear to be solely regulated by sex differences in secretory growth hormone profiles (Shapiro et al., 1995; Dhir et al., 2006). Whereas males and females secrete a similar daily amount of growth hormone, females secrete a so-called “continuous” growth hormone profile comprised of numerous daily pulses interrupted by short-lived interpulses of usually low or barely detectable hormone concentrations. In contrast, the “episodic” masculine growth hormone profile is characterized by significantly fewer but larger secretory bursts of the hormone separated by lengthy interpulses that are invariably devoid of growth hormone. In fact, it is the difference between the continuous (female) and episodic (male) circulating growth hormone profiles, and not plasma hormone levels per se that are responsible for phenotypic sexual dimorphisms ranging from growth patterns to expression levels of hepatic enzymes (Jansson et al., 1985; Shapiro et al., 1995). Moreover, the sex-dependent growth hormone profiles are composed of various “signaling elements” to which each hepatic CYP isoform is independently responsive.
These signals may be recognized by the hepatocyte in the frequencies, concentrations and/or durations of the pulse and interpulse periods. Alternatively, the hepatocyte can monitor the mean plasma concentration of the hormone (Pampori and Shapiro, 1996, 1999; Agrawal and Shapiro, 2000). Since the therapeutic intent of octreotide administration is to simply reduce elevated plasma concentrations of growth hormone to near normal without altering the characteristic profile, we measured expression levels of key CYP isoforms as well as IGF-1 and growth hormone receptor as indicators or markers of alterations, possibly subtle, but physiologically significant, in the feminine growth hormone profile resulting from octreotide infusion.
2. Materials and methods
2.1. Animals
Female Sprague-Dawley CD rats obtained from Charles River Laboratories (Wilmington, MA) were housed in the University of Pennsylvania Laboratory Animal Resources facility under the supervision of certified Laboratory Animal Medicine veterinarians, and were treated according to a research protocol approved by the University's Institutional Animal Care and Use Committee. Rats were housed under conditions of regulated temperature (20-23°C) and photoperiod (12 h light-dark cycle; lights on at 7:00AM).
2.2. Catheter implantation and octreotide treatment
Eight female rats at 10 weeks of age were implanted with our patented indwelling right atrial catheters as described previously (Pampori et al., 1991). After 4 to 5 days, serial blood samples were collected from each rat (40μl every 15 min for 8 h). Another 4 to 5 days later, the unrestrained and unstressed catheterized rats were infused, iv, with 25μg octreotide (Sandostatin)/kg body weight (Bioniche Pharma, Rosemont, IL) over 30 s and the catheter flushed with diluent. Five min later, serial blood samples were again collected from each rat. Twelve hours after the first injection of octreotide, a second 25μg/kg dose was infused. During the next 5 days rats were treated with octreotide at the same dose every 12 h. Five minutes following the 11th dose of octreotide, serial blood samples were again collected. Twelve hours after the previous dose, the rats received their final evening infusion of octreotide. On the following morning (10 h post infusion) the rats were quickly decapitated, the livers quickly excised, weighed, infused with ice cold physiologic saline and minced; a portion reserved for mRNA determinations was plunged into liquid nitrogen and subsequently stored at −80°C. The remaining minced liver was used for microsome preparation. In an additional experimental group, 7 females (10 weeks of age) were implanted with indwelling right atrial catheters and treated with an equivalent amount of diluent (95μl/kg body weight of physiological saline/infusion) in the same manner as octreotide for 12 infusions. These rats were decapitated 10 h after the last treatment to serve as controls for the biochemical analyses.
2.3. Growth hormone
With the exceptions of using plasma instead of whole blood and including plasma from long-term hypophysectomized female rats as controls, circulating growth hormone was measured by a sensitive sandwich ELISA using the same materials and methods as reported by Steyn et al., 2011 and modified by us (Das et. al., 2013).
2.4. RNA Isolation
Total RNA from liver tissue was isolated using Trizol® reagent (Life Technologies, Carlsbad, CA) purified with the Qiagen RNeasy mini kit and treated with DNase I in order to remove any trace of genomic DNA using RNase-Free DNase Set (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA concentrations and purity were determined by UV spectrophotometry (A260/280>1.8 & A260/230>1.7) and integrity was verified by the intensities of 28S and 18S rRNA bands on a denaturing agarose gel visualized on a FluorChem IS-8800 Imager (Alpha Innotech, San Leandro, CA).
2.5. Northern blots
Northern blots were probed with a [32P] labeled oligonucleotide probe for CYP2C11 and CYP2C12 mRNA (Waxman, 1991) using generally followed procedures including hybridization and high stringency washing conditions as we described previously (Pampori and Shapiro, 1996). The consistency of RNA loading between samples was confirmed by ethidium bromide staining of 18S and 28S ribosomal RNAs and were verified and normalized with an 18S oligonucleotide probe (Li et al., 2000). The hybridized mRNA signals were quantified by scanning the autoradiographs with a FluorChem IS-8800 Imager (Alpha Innotech).
2.6. Quantitative reverse transcription-PCR (qRT-PCR)
CYP2C12, CYP2C11, CYP2A1, CYP3A2, CYP2C6, CYP2C7 and rat growth hormone receptor gene expressions were determined by qRT-PCR using SYBR green on an Applied Biosystem 7500 Fast Real-Time PCR System (Life Technologies). RNA isolation, concentration and purity determination were performed as mentioned above. cDNA synthesis was completed using the High Capacity RNA-to-cDNA kit (Life Technologies) as per instructions with appropriate no-reverse transcription (-RT) and non-template controls. PCR primers for CYP2C12 and CYP2C11 (Ahluwalia et al., 2004), CYP3A2 (Kisanga et al., 2005), CYP2A1 (He et al., 2007), CYP2C6 (F:5′-CAGCAGGAAAACGGATGTG-3′, R:5′-AATCGTGGTCAGGAATAAAAATAACTC-3′), CYP2C7 (Choi et al., 2011) and β-actin (F:5′-CACGGCATTGTCACCAACTG-3′, R:5′-CTGGGTCATCTTTTCACGGT-3′) were synthesized by Integrated DNA Technologies (Coralville, IA) whereas primers for growth hormone receptor (F:5′-TGATGCGGATGAGAAGACTG-3′, R:5′-AAGTACCATCGCACATGTCA-3′) were synthesized by Real Time Primers (Elkins Park, PA). To analyze IGF-1 mRNA expression, an IGF-1 TaqMan® assay (Rn00710306_m1) was performed using β-actin (Rn00667869_m1) as the housekeeping gene on an Applied Biosystem step-one plus q-PCR instrument as per the manufacturer's recommended protocol (Life Technologies).
2.7. Western blotting
As previously described, hepatic microsomes were prepared from freshly isolated rat livers (Shapiro et al., 1989) and assayed for individual CYP isoforms by western blotting (Pampori et al., 1995). The blots were probed with monoclonal anti-rat CYP2C11 and anti-rat CYP3A2 (Detroit R & D, Inc., Franklin, MI), anti-rat CYP2C12 (a gift from Dr. Marika Rönnholm, Huddinge University Hospital, Huddinge, Sweden), monoclonal anti-rat CYP2C6 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti- growth hormone receptor (a gift from Dr. G. Peter Frick, University of Massachusetts Medical School, Worchester, MA) and detected with an enhanced chemiluminescence kit (Amersham Biosciences Corp, Piscataway, NJ). Signals were normalized to a control sample repeatedly run on all blots and/or to the expression of β-actin (Sigma Chemical Co., St. Louis, MO). The protein signals were scanned and the densitometric units were obtained as integrated density values quantitated by using FluorChem IS-8800 Imager (Alpha Innotech) software supplied with the gel documentation system.
2.8. Immunoprecipitation
As previously described (Wang et al., 2011), hepatic whole cell lysate protein (500μg) or microsomal protein (100μg) were incubated overnight at 4°C with the highly specific anti-CYP2C12 (MacGeoch et al., 1984) in 100μl of modified immunoprecipitation buffer containing 20 mM HEPES [pH 7.9], 40 mM KCl, 1 mM MgCl2, 0.1 mM EGTA, 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.1 mM NaF, 0.1 mM Na3VO4, 0.5% NP-40, and 3 mg/ ml BSA and 0.2mM phenylmethylsulfonyl fluoride. Immunoprecipitates were then bound to protein A/G PLUS-Agarose (Santa Cruz Biotechnology) for 2 h on a rotary mixer at 4°C. Next, samples were centrifuged for 2 min at 3000 rpm at 4°C and washed with immunoprecipitation buffer 3 times. Non-specific IgG control was also used as a pull-down control to confirm the binding specificity. Proteins were eluted from the gel by boiling for 10 min in 30μl of loading buffer, electrophoresed and immunoblotted with mouse anti-Ub IgG (Santa Cruz Biotechnology).
2.9. Catalytic activity
Of the 4 female-dependent CYPs measured at the protein and/or mRNA level, CYP2C12, CYP2C6, CYP2C7 and CYP2A1, only the latter has been shown to exhibit a specific catalytic activity, i.e., CYP2A1-dependent testosterone 7α-hydroxylase (Waxman, 1991) which we measured by our previously reported method (Agrawal et al., 1995a).
2.10. Statistics
The ultradian patterns in plasma growth hormone concentrations were analyzed with the aid of the Cluster analysis computer program (Veldhuis and Johnson, 1986), as we have reported previously (Agrawal et al., 1995b; Dhir and Shapiro, 2003). All data, including that obtained from the Cluster analysis program, were subjected to analysis of variance, and differences were determined using t statistics and the Bonferroni procedure for multiple comparisons.
3. Results
3.1. Circulating growth hormone profiles
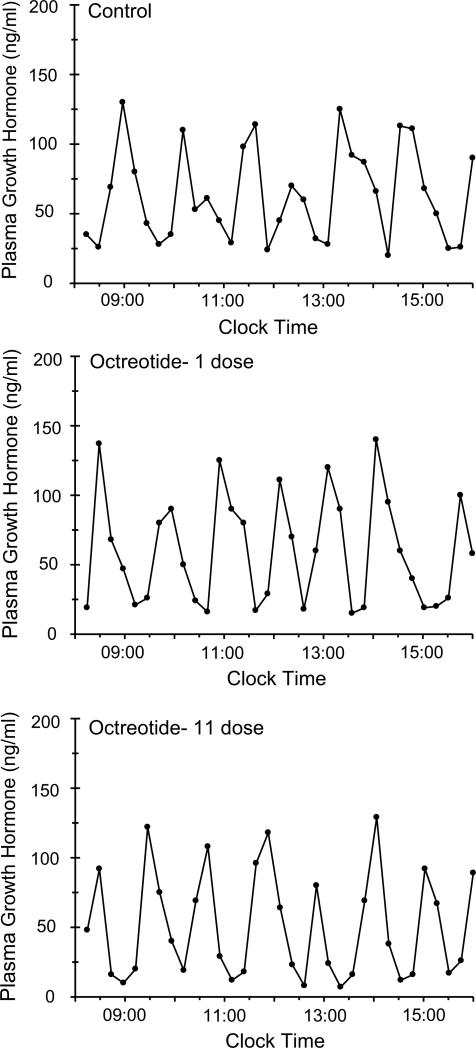
Plasma growth hormone profiles are presented as schematic representations of the actual circulating profiles (Fig. 1) as well as mathematical analyses of the characteristic components in the profile (Table 1). As controls, the rats secreted the typical feminine growth hormone profile (Agrawal et al., 1995b) characterized by frequent low amplitude pulses (compared to males) resulting in peaks of ~125ng/ml, followed by short-lived ~30 min troughs that were always measurable and averaged ~25ng/ml. Exposure to a single dose of octreotide reduced nadir concentrations, but otherwise had no other significant effect on the growth hormone profile. When the control rats were infused with an additional 10 doses of octreotide for a total of 6 days, the basic feminine growth hormone profile was still apparent. However, there was a significant decrease in the durations (width) and resulting contents (area) of the growth hormone pulses as well as reduced nadir concentrations, all responsible for the reduction in both peak intervals and the mean concentration of growth hormone in the secretory profile.
Fig. 1.
Plasma levels of circulating growth hormone obtained from individual undisturbed catheterized octreotide-infused female rats. Every rat was fitted was a chronic indwelling right atrial catheter for serial blood sampling (Pampori et al., 1991). Four to 5 days following catheter placement, serial blood samples were collected over 8 consecutive h at 15 min intervals (Controls). Four to 5 days later, every rat was injected, iv, with 25μg octreotide/kg body weight and 5 min later, serial blood samples were again collected. Next, the rats were injected, iv, every 12 h with the same dose of octreotide for a total of 11 doses. Five min following the last injection, serial blood collections were again obtained for 8 h. Similar findings were obtained from 6 additional animals.
Table 1.
Analysis of circulating growth hormone profiles in female rats serving as their own controls and subsequently treated with one dose of octreotide followed by ten additional doses.
| Number of Doses | Mean Concentration (ng/ml) | Peak Interval (min) | Pulse |
Valley |
||||
|---|---|---|---|---|---|---|---|---|
| Group | Width (min) | Height (ng/ml) | Area (μg.min.ml−1) | Width (min) | Nadir (ng/ml) | |||
| Control | - | 54.8± 7.0 | 81.1± 10.5 | 58.9± 7.5 | 125.7± 36.2 | 3.74± 0.62 | 28.0± 4.6 | 27.6± 5.7 |
| Octreotide | 1 | 48.1± 8.1 | 76.2± 9.4 | 49.3± 9.1 | 131.0± 29.7 | 3.13± 0.58 | 32.0± 2.8 | 19.9± 5.4* |
| Octreotide | 11 | 41.2± 4.6** | 69.2± 7.1* | 45.2± 4.8** | 118.8± 22.4 | 2.73± 0.45** | 33.1± 2.6* | 17.0± 4.4** |
Values are means ± S.D. with n=7.
P<0.05
P<0.01 compared to Control treatment.
Every rat was fitted with a chronic indwelling right atrial catheter for serial blood sampling (Pampori et al., 1991). Four to 5 days following catheter placement, serial blood samples were collected over 8 consecutive hours at 15 min intervals. Four to 5 days later, every rat was injected, iv, with 25μg octreotide/kg body weight and 5 min later, serial blood samples were again collected. Next, the rats were injected, iv, every 12 hr with the same dose of octreotide for a total of 11 doses. Five min following the last injection, serial blood collections were again obtained for 8 hr. Data were analyzed with the aid of the Cluster analysis program for hormonal pulse detection (Veldhuis and Johnson, 1986) according to peak interval, time period between peaks; width, duration of growth hormone pulses or interpulse valleys; height, amplitude of hormone peaks; area, integrated area under growth hormone pulses (concentration × duration); nadir, mean baseline growth hormone concentration. Mean concentration was calculated for the entire 8 hr collection period.
3.2. CYPs, IGF-1 and growth hormone receptor expression
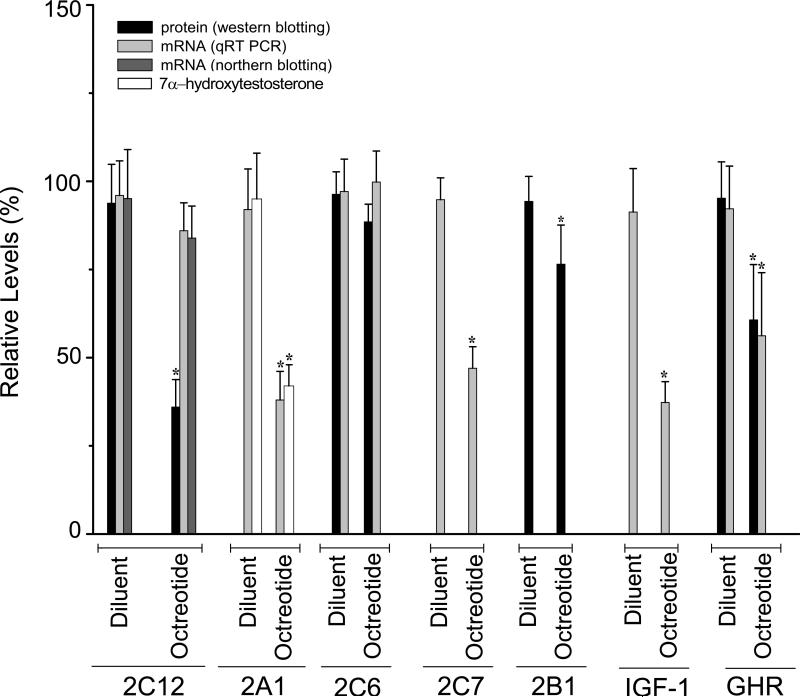
As expected (Pampori and Shapiro, 1996; 1999), the secretion of a feminized continuous growth hormone profile in both diluent and octreotide-treated rats completely blocked expression of male-specific CYP2C11 and CYP3A2 (not presented). In contrast, exposure to the normal feminine growth hormone profile of diluent-treated control rats was characterized by robust expression levels of female-specific CYP2C12, female-predominant CYP2A1, CYP2C6 and CYP2C7 as well as IGF-1 and growth hormone receptor (Fig. 2). Six days of octreotide treatment reduced mRNA levels of CYP2A1 (and its specific testosterone 7α-hydroxylase activity), CYP2C7 and IGF-1 by 55 to 60% and growth hormone receptor mRNA concentrations by 40%. In contrast, mRNA levels of CYP2C12 and CYP2C6 were statistically unchanged by the somatostatin analog. In the case of growth hormone receptor, CYP2C6 and CYP2C12 for which we had specific antibodies for western blotting, protein concentrations for the first two were in agreement with their mRNA levels (Fig. 2). However, octreotide treatment reduced CYP2C12 protein levels by >60%, whereas mRNA levels of the isoform were unchanged by the analog. This seeming contradiction was supported at the mRNA level by our observation (Fig. 2) of similar normal-like concentrations of the CYP2C12 transcript when measured by both qRT-PCR and northern blotting.
Fig. 2.
Relative protein, catalytic activities and/or mRNA levels of CYP2C12, CYP2A1, CYP2C6, CYP2C7, CYP2B1, IGF-1 and growth hormone receptor in livers from female rats treated with either diluent (controls) or octreotide. Rats fitted with our chronic indwelling atrial catheters (Pampori et al., 1991) were injected with either octreotide (25μg octreotide/kg body weight) or an equivalent volume of physiologic saline every 12 h for a total of 12 infusions. Relative levels were calculated by comparing all values to the control level with the highest isoform concentration (i.e., 100%). Values are means ± S.D. with a N=7. *P<0.01 compared to diluent-treated controls. Comparisons of expression levels between isoforms are not possible. In this regards, whereas CYP2C12 concentrations represent >40% of the total CYP in normal female rat liver, CYP2B1 is an inducible isoform expressed constitutively at only nominal levels (Ryan and Levin, 1993).
3.3. CYP2C12 ubiquitination
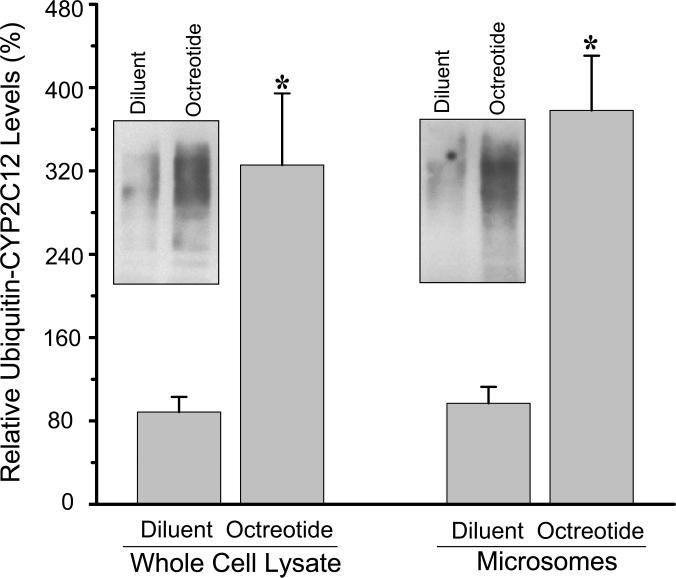
In an attempt to understand the dissimilar response of CYP2C12 mRNA and protein to octreotide, we examined one likely step in CYP2C12 protein degradation, i.e., ubiquitination. Accordingly, we found a ~4-fold increase in ubiquintin-CYP2C12 levels in hepatic whole cell lysates as well as the previously identified microsomal site of CYP ubiquitination (Wang et al., 2011) from female rats administered octreotide (Fig. 3) suggesting a possible contributing factor for the selective reduction in protein levels of the isoform in treated animals.
Fig. 3.
Immunoprecipitated hepatic whole cell lysate and microsomal ubiquitinated CYP2C12 protein from diluent or octreotide female rats. Rats fitted with our chronic indwelling atrial catheter (Pampori et al., 1991) were injected with either octreotide (25μg octreotide/kg body weight) or an equivalent volume of physiologic saline every 12 h for a total of 12 infusions. Hepatic whole cell lysate and microsomes were immunoprecipitated with antibodies against CYP2C12 and subsequently probed with mouse anti-ubiquitin IgG. Relative levels were calculated by comparing all values to the control liver with the highest concentration of ubiquitinated CYP2C12 (i.e., 100%). A representative immunoblot of CYP2C12 ubiquitin is presented in the figure. Values are means ± S.D. with a N=7. *P<0.01 compared to diluent-treated controls of the same cell fraction.
4. Discussion
We found that iv infusion of octreotide (25μg b.i.d./kg body weight) for 6 days reduced growth hormone secretion by ~25%, which includes both pulse and interpulse components, in adult female rats. In contrast, a considerably smaller single 50μg dose of octreotide to healthy volunteer men caused a rapid fall in plasma growth hormone to near zero concentrations that took almost 9 h to completely recover (Marbach et al., 1985). The possibility that the rat is less sensitive to the growth hormone inhibitory effects of the somatostatin analog seems unlikely as a dose of octreotide as small as 2μg (Perez-Romero et al., 1999) or 15μg/kg body weight (Pless et al., 1986) has been reported to substantially (~50%) reduce growth hormone secretion in male rats. In general, however, animal studies (near exclusively conducted in males) investigating the effects of octreotide on growth hormone secretion tend to examine the inhibitory effects of just a single in vivo dose of the analog (Marbach et al., 1985; Pless et al., 1986; Turner and Tannenbaum, 1995) making it difficult to judge the relevance of these findings to our longer term study.
Although multiple octreotide infusions did not produce the expected dramatic decline in plasma growth hormone reported in acromegaly patients (Lamberts et al., 1993; Yang and Keating, 2010), it clearly had a major effect on hepatic function. All of the hepatic functions examined in the present study have been shown to be regulated to varying degrees by the feminine circulating growth hormone profile, and in some cases, by specific signaling elements in the profile. Feminine expression levels of CYP2C12, CYP2A1, CYP2C6, CYP2C7, IGF-1 and growth hormone receptor are dependent on exposure to the constant growth hormone profile secreted in females (Waxman et al., 1991; Pampori and Shapiro, 1996). In the growth hormone ablated rat (e.g. hypophysectomy), expression of these proteins and their mRNAs are either completely (e.g. CYP2C12), profoundly (e.g. CYP2C7), moderately (eg. CYP2A1) or nominally (growth hormone receptor) suppressed (Pampori and Shapiro, 1996). While we observed a dramatic (>50%) decline in the expression of most of the growth hormone - dependent proteins, plasma levels of growth hormone were not ablated. In fact, growth hormone continued to be secreted in its characteristic feminine profile with only minor alterations in its constituent components. Although there appears to be no reports examining the in vitro effects of octreotide on the expression of hepatic isoforms of CYPs, exposure of rat hepatocyte cultures to the somatostatin analog suppressed both IGF-1 expression as well as the activation and nuclear translocation of signal transduction molecules mediating growth hormone induction of CYP2C11 (Murray et al., 2004).
Previously, we investigated the selective sensitivities of various growth hormone-dependent hepatic genes to the feminine growth hormone profile by manipulating the profile in female rats (Pampori and Shapiro 1996; 1999). The characteristic feminine growth hormone profile was infused into hypophysectomized female rats to restore circulating hormone levels (i.e., total mean, pulse and interpulse concentrations) from normal to just 3% of physiologic. In this regard, in order to reduce expression levels of CYP2C12, CYP2A1, CYP2C7, IGF-1 and growth hormone receptor to that observed in the octreotide-treated rats, the feminine secretory growth hormone profile would have had to be reduced to <3 to 12% of normal; far lower than actually occurred.
Clearly, octreotide suppression of the female-dependent levels of CYPs, IGF-1 and growth hormone receptor cannot be explained by the minor alterations in an otherwise feminine circulating growth hormone profile. To investigate this seeming contradiction, we focused our attention on CYP2C12, the primary isoform in the female representing >40% of the total CYP in the female rat liver (MacGeoch et al., 1985). The dissimilar effects of the somatostatin analog on CYP2C12 mRNA (near normal) and protein (reduced by two-thirds) suggested a posttranslational effect. In this regard, we chose to examine endoplasmic reticulum-associated degradation, a prevalent ubiquitin-dependent mechanism for CYP3A and CYP2E1 protein degradation (Wang et al., 2011). We found a ~4-fold increase in hepatic microsomal ubiquitin-CYP2C12 levels in octreotide-infused female rats suggesting a possible contributing factor for the selective reductions of CYP2C12 protein concentrations.
It should be noted, however, that somatostatin and its analogs are known to be involved in a myriad of extra-pituitary functions [some of which have recently been associated with the suppression of male-dependent CYP isoforms (Das et al., 2012)] including the activation of the immune response, inhibition of cellular proliferation, regulation of growth factors, and more specifically, blocking the secretion of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, pancreatin polypeptide, glucagon and insulin explaining their therapeutic use in treating carcinoid tumors and vasoactive intestinal peptide tumors (Marbach et al., 1985; Lamberts et al., 1993; Murray et al., 2004; Yang and Keating, 2010). Moreover, somatostatin action is generally mediated by 6 different specific somatostatin receptors present throughout all tissues in the body (Taniyama et al., 2005).
In conclusion, our data indicates that octreotide treatment of female rats can suppress major hepatic growth hormone-dependent CYP expression with unsubstantial effects on growth hormone secretory profiles suggesting the activation of growth hormone independent mechanisms involved in the regulation of female dependent CYPs as well as IGF1. Whereas the extra growth hormone actions of octreotide are numerous, the actual mechanism(s) by which the analog suppresses hepatic CYP, IGF-1 and growth hormone receptor expression will now require further studies.
Acknowledgements
We acknowledge Drs. Ravindra N. Dhir and Chellappagounder Thangavel for their generous help and valuable suggestions. This work was supported by the National Institute of Health Eunice Kennedy Shriver National Institute of Health and Human Development [Grant HD-061285].
Abbreviations
- CYP
cytochrome P450
- IGF-1
insulin-like growth factor-1
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was previously presented, in part, at the 18th North American ISSX Meeting, Dallas, TX October 14-18, 2012.
Conflict of Interest Statement
All of the authors declare no conflict of interest.
References
- Agrawal AK, Pampori NA, Shapiro BH. Thin-layer chromatographic separation of regioselective and stereospecific androgen metabolites. Anal. Biochem. 1995a;224:455–457. doi: 10.1006/abio.1995.1071. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Pampori NA, Shapiro BH. Neonatal phenobarbital-induced defects in age- and sex-specific growth hormone profiles regulating monooxygenases. Am. J. Physiol. Endocrinol. Metab. 1995b;268:E439–E445. doi: 10.1152/ajpendo.1995.268.3.E439. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J. Pharmacol. Exp. Ther. 2000;292:228–237. [PubMed] [Google Scholar]
- Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol. Endocrinol. 2004;18:747–760. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- Choi SY, Fisher L, Yang K, Chung H, Jeong H. Isoform-specific regulation of cytochrome P450 expression and activity by estradiol in female rats. Biochem. Pharmacol. 2011;81:777–782. doi: 10.1016/j.bcp.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Banerjee S, Shapiro BH. Noncanonical suppression of growth hormone – dependent isoforms of cytochrome P450 by the somatostatin analog octreotide. J. Endocrinol. 2013;216:87–97. doi: 10.1530/JOE-12-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir RN, Dworakowski W, Thangavel C, Shapiro BH. Sexually dimorphic regulation of hepatic isoforms of human cytochrome P450 by growth hormone. J. Pharmacol. Exp. Ther. 2006;316:87–94. doi: 10.1124/jpet.105.093773. [DOI] [PubMed] [Google Scholar]
- Dhir RN, Shapiro BH. Interpulse growth hormone secretion in the episodic plasma profile caused the sex reversal of cytochrome P450s in senescent male rats. Proc. Nat. Acad. Sci. USA. 2003;100:15224–15228. doi: 10.1073/pnas.2434273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Yamauchi H, Suzuki K, Ueno M, Nakayama H, Doi K. Gene expression profiles of drug-metabolizing enzymes (DMEs) in rat liver during pregnancy and lactation. Exp. Mol. Path. 2007;83:428–434. doi: 10.1016/j.yexmp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Edén S, Isaksson O. Sexual dimorphism in the control of growth hormones secretion. Endocrine. Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- Kisanga ER, Moi LLH, Gjerde J, Mellgren G, Lien EA. Induction of hepatic drug-metabolising enzymes and tamoxifen metabolite profile in relation to administration route during low-dose treatment in nude rats. J. Steroid. Biochem. Mol. Biol. 2005;94:489–498. doi: 10.1016/j.jsbmb.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lambert SWJ, Hofland LJ, de Herder WW, Kwekkeboom DJ, Reubi JC, Krenning EP. Octreotide and related somatostatin analogs in the diagnosis and treatment of pituitary disease and somatostatin receptor scintigraphy. Frontiers Neuroendocrinol. 1993;14:27–55. doi: 10.1006/frne.1993.1002. [DOI] [PubMed] [Google Scholar]
- MacGeoch C, Morgan ET, Halpert J, Gustafsson JÅ. Purification, characterization and pituitary regulation of the sex-specific cytochrome P-45015β-hydroxylase from liver microsomes of untreated female rats. J. Biol. Chem. 1984;259:15433–15439. [PubMed] [Google Scholar]
- Li YQ, Prentice DA, Howard ML, Mashford ML, Wilson JS, Desmond PV. Alcohol up-regulates UDP-glucuronosyltransferase mRNA expression in rat liver and in primary rat hepatocyte culture. Life Sci. 2000;66:574–584. doi: 10.1016/s0024-3205(99)00630-x. [DOI] [PubMed] [Google Scholar]
- MacGeoch C, Morgan ET, Gustafsson JÅ. Hypothalamic-pituitary regulation of cytochrome P45015β apoprotein levels in rat liver. Endocrinology. 1985;117:2085–2092. doi: 10.1210/endo-117-5-2085. [DOI] [PubMed] [Google Scholar]
- Marbach P, Neufeld M, Pless J. Clinical applications of somatostatin analogs. Adv. Exp. Med. Biol. 1985;188:339–353. doi: 10.1007/978-1-4615-7886-4_19. [DOI] [PubMed] [Google Scholar]
- Murray RD, Kim K, Ren SG, Chelly M, Umehara Y, Melmed S. Central and peripheral actions of somatostatin on the growth hormone-IGF-1 axis. J. Clin. Invest. 2004;114:349–356. doi: 10.1172/JCI19933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampori NA, Agrawal AK, Shapiro BH. Renaturalizing the sexually dimorphic profiles of circulating growth hormone in hypophysectomized rats. Acta. Endocrinology. 1991;124:283–289. doi: 10.1530/acta.0.1240283. [DOI] [PubMed] [Google Scholar]
- Pampori NA, Pampori MK, Shapiro BH. Dilution of the chemiluminescence reagents reduces the background noise on western blots. Biotechniq. 1995;18:588–590. [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile. Mol. Pharmacol. 1996;50:1148–1156. [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinol. 1999;140:1245–1254. doi: 10.1210/endo.140.3.6545. [DOI] [PubMed] [Google Scholar]
- Pérez-Romero A, Rol de Lama MAR, Ariznavarreta C, Tresguerres JAF. Effect of long-term GHRH and somatostatin administration on GH release and body weight in prepubertal female rats. J. Physiol. Biochem. 1999;55:315–324. [PubMed] [Google Scholar]
- Pless J, Bauer W, Briner U, Doepfner W, Marbach P, Maurer R, Petcher TJ, Reubi JC, Vonderscher J. Chemistry and pharmacology of SMS 201-995, a long-acting octapeptide analogue of somatostatin. Scand. J. Gastroenterol. 1986;21(suppl.119):54–64. doi: 10.3109/00365528609087432. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int. J. Biochem. Cell. Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, MacLeod JN, Pampori NA, Morrissey JJ, Lapenson DP, Waxman DJ. Signalling elements in the ultradian rhythm of circulating growth hormone regulating expression of sex-dependent forms of hepatic cytochrome P450. Endocrinology. 1989;125:2935–2944. doi: 10.1210/endo-125-6-2935. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152:3165–3171. doi: 10.1210/en.2011-0253. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: A immunohistochemical study. Endocrine, J. 2005;52:605–611. doi: 10.1507/endocrj.52.605. [DOI] [PubMed] [Google Scholar]
- Turner JP, Tannenbaum GS. In vivo evidence of a positive role for somatostatin to optimize pulsatile growth hormone secretion. Am. J. Physiol. Endocrinol. Metab. 1995;269:E683–E690. doi: 10.1152/ajpendo.1995.269.4.E683. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am. J. Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Guan S, Acharya P, Koop DR, Liu Y, Liao M, Burlingame AL, Correia MA. Ubiquitin-dependent proteasomal degradation of human liver cytochrome P450 2EI. Identification of sites targeted for phosphorylation and ubiquitination. J. Biol. Chem. 2011;286:9443–9456. doi: 10.1074/jbc.M110.176685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ. Rat hepatic P450IIA and P450IIC subfamily expression using catalytic, immunochemical, and molecular probes. Meth. Enzymol. 1991;206:249–267. doi: 10.1016/0076-6879(91)06095-k. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc. Nat. Acad. Sci. USA. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LPH, Keating GM. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs. 2010;70:1745–1769. doi: 10.2165/11204510-000000000-00000. [DOI] [PubMed] [Google Scholar]