Abstract
Background
Depression is a well-recognised problem in the elderly. The aim of this study was to determine the factors associated with predictors of change in depressive symptoms, both in subjects with and without baseline significant depressive symptoms.
Methods
Longitudinal study of community-dwelling elderly people (>60 years or older), baseline evaluations, and two additional evaluations were reported. Depressive symptoms were measured using a 30-item Geriatric Depression Scale, and a score of 11 was used as cutoff point for significant depressive symptoms in order to stratify the analyses in two groups: with significant depressive symptoms and without significant depressive symptoms. Sociodemographic data, social support, anxiety, cognition, positive affect, control locus, activities of daily living, recent traumatic life events, physical activity, comorbidities, and quality of life were evaluated. Multi-level generalised estimating equation model was used to assess the impact on the trajectory of depressive symptoms.
Results
7,882 subjects were assessed, with 29.42% attrition. At baseline assessment, mean age was 70.96 years, 61.15% were women. Trajectories of depressive symptoms had a decreasing trend. Stronger associations in those with significant depressive symptoms, were social support (OR .971, p<.001), chronic pain (OR 2.277, p<.001) and higher locus of control (OR .581, p<.001). In contrast for those without baseline significant depressive symptoms anxiety and a higher locus of control were the strongest associations.
Conclusions
New insights into late-life depression are provided, with special emphasis in differentiated factors influencing the trajectory when stratifying regarding basal status of significant depressive symptoms.
Limitations
The study has not included clinical evaluations and nutritional assessments
Keywords: late-life depression, depression trajectories, depressive symptoms, geriatric syndromes, geriatric depression scale
INTRODUCTION
Depression in the elderly is a major public health problem, that causes suffering for many, who often go undiagnosed, and untreated, and it burdens families, and institutions that provide care for the elderly by disabling those people who might otherwise be able-bodied (Wagner et al., 2012). A World Health Organization (WHO) report attributed 32% of all years lived with disabilities to neuropsychiatric conditions: the major contributor to this proportion was unipolar depression (11.8%) (Mathers and Loncar, 2006). Depression also predicts the onset, and progression of both physical, and social disabilities, particularly in the elderly (Bruce et al., 1994, Penninx et al., 1998), and it results in higher health service utilisation rates (García-Peña et al., 2008). In Mexico, the prevalence of depressive symptoms in the elderly was estimated at approximately 20% (García-Peña et al., 2008). In addition, depressive symptoms were reported to be strongly associated with the same adverse outcomes observed in subjects with clinical depression (Penninx et al., 1998). Moreover, prognostic implications were reported in already depressed subjects (Bruce et al., 1994). In contrast, in a recent report, a depression intervention in elderly Mexican subjects, which was aimed to be cost-effective, decreased disability at a low cost (Salomon et al., 2012), a major achievement in a limited-resource setting.
One problem that makes depressive symptoms in the elderly so insidious is that often neither the person nor the health care provider recognises the symptoms in the context of the multiple physical problems, and comorbidities; moreover, health care professionals often conclude that depressive symptoms are a normal consequence of these problems, an attitude that is often shared by the patients themselves (stigma)(Conner et al., 2010, Perez-Zepeda et al., 2012). These factors conspire to make depressive symptoms go underdiagnosed, and more importantly, undertreated (Nyunt et al., 2009). Other barriers to receiving care for depressive symptoms can be imputed to negative life events, with a trend among health care professionals towards attributing affective problems to these events, and not treating them as a clinical problem (Kraaij et al., 2002).
Some reports have analysed the differences in presentation, and clinical outcomes among depressed elderly patients (Cole, 2002, Murphy, 1983, Solomon et al., 1997). However, more knowledge about how these symptoms change over time among community dwelling elderly people is needed. These changes in affect have been hypothesised to interact closely with health, and social factors (i.e., high morbidity, lower functioning, less social participation, more severe depression) (Mirowsky and Ross, 1992). Different approaches have been used to study depressive symptom trajectories in the elderly. One of the more widely used is that of latent class trajectories to compare groups classified by their depressive symptom trajectories. A number of different reports have agreed that, in general, four categories of depressive symptoms exist, with the majority of elder subjects classified with “low depressive symptomatology” (48.6%-76.6%), and three other categories of elderly subjects with significant depressive symptomatology (Byers et al., 2012, Cui et al., 2008, Huang et al., 2011, Kuchibhatla et al., 2012, Lincoln and Takeuchi, 2010). Depressive symptoms depend on a wide range of factors, and not only depression alone. Self-esteem, social support, and comorbidity, among other factors, can produce modifications in self-perception, and reporting of depressive symptoms over time (Bryant et al., 2012). To represent those trajectories, and to identify other factors (biological, psychological, and social), the aim of this study was to determine the differential impacts of multiple factors on the trajectories of depressive symptoms.
METHODS
Sample, and design
The data reported here are from the “Integrated Study of Depression Among the Elderly,” a population-based, longitudinal study of risk factors for depression in Mexicans aged 60 years, and older, who are beneficiaries of the largest social security system in Mexico (Instituto Mexicano del Seguro Social-IMSS). The preliminary cross-sectional results, methods, and sampling processes were described elsewhere (García-Peña et al., 2008).
In this baseline assessment 7,449 subjects were interviewed, of whom 21.7% had significant depressive symptoms. To perform a stratified analysis of factors associated with depressive symptom trajectories between those subjects with significant depressive symptoms, and those without these symptoms, an identical number of subjects without significant depressive symptoms at baseline was randomly selected, and was followed up, along with the other group.
Sample weights were estimated using probabilities defined through the stratified sampling, and the probability of being screened as positive for depressive symptoms. The expansion factor was then calculated as the inverse of the sample weights. A thorough explanation of the sampling and weighting procedures are presented in additional material.
Procedures
Trained and standardised interviewers conducted face-to-face interviews at the participants’ home during every stage of the study. All of the measurements were assessed at the baseline visit, and at the two follow-up visits (12-month apart). Attempts were made to reach the subjects at each stage; three causes of loss to follow-up were recorded: death, rejection, and not found.
Measurements
Depressive symptoms were assessed using the 30-item Geriatric Depression Scale (GDS 30) (Fernández-San Martín et al., 2002, Yesavage et al., 1982). The GDS 30 was developed for older persons, and it has been widely used in different settings (Boult et al., 2001, Rapp et al., 1988, Sharp and Lipsky, 2002), including the Mexican population (Sánchez-García et al., 2008). A score of 11 points or higher was considered as significant depressive symptoms (sensitivity 92%-specificity 89%, compared to depression)(García-Peña et al., 2008, Lyness et al., 1997, Montorio and Izal, 1996); accordingly the stratification was: with significant depressive symptoms (GDS 30 score of 11 or greater), and without significant depressive symptoms (GDS 30 score of 10 or less). The internal consistency (Cronbach’s alpha) of the GDS 30 was 0.916 at the basal assessment, and it was 0.927, and 0.918, respectively, for the two subsequent stages. Variables were classified in four categories: social (age, scholarship, marital status, living alone, number of friends and relatives, religion, and social support); lifestyle (remunerated activity, exercise, activities, smoking, alcohol drinking, and use of illicit drugs or psychotropic medicines); health (health care use, cognition, anxiety, activities of daily living, comorbidities, quality of life, and use of antidepressants); and psychological (negative life events, positive affect, and locus of control). A validated Spanish version of the Social Support Scale was used, which included 19 items that explore the frequency of receiving social support in different domains; it scores each item from zero (never) to four points (always), and the total score is the sum of each item, with zero the lowest score, and 76 the highest (best social support) (Revilla-Ahumada et al., 2005, Sherbourne and Stewart, 1991). A single question was asked to explore whether the subjects engaged in remunerated activities or exercised regularly. In addition, an average of daily activities (24-activity list) was obtained by asking the number of days per week the subjects performed the activity, and the approximate number of hours per day devoted to them. Current smoking status was assessed by a single question. Alcohol drinking was defined as the self-reported average ingestion of more than one glass of an alcoholic beverage per day (Dawson et al., 2005). The use of different substances was recorded with a question of whether the subject had ever used illicit drugs (amphetamines, marijuana, cocaine, heroin, hallucinogens, and inhalants) or psychotropic medicines (opioids, benzodiazepines, barbiturates).
Health care utilisation was assessed using an incremental questionnaire, ranging from family practice consultation (number of visits) to third-level hospital usage (number of times using any service) in the last year. Comorbidities were assessed by self-reporting, a variable was created to indicate whether the subject had more than two. Regarding depression treatment, the use of antidepressants was asked. Anxiety was assessed by means of the Short Anxiety Screening Test (SAST), a scale developed to standardise the detection of anxiety in the elderly, including somatic symptoms, consisting of 10 items, scored from one to four points, with a highest possible score of 40 (highest anxiety level) (Sinoff et al., 1999). Cognitive domain was assessed using a validated version of the Mini Mental State Examination (MMSE), a 30-item scale that examines different cognitive domains (de Beaman et al., 2004, Franco-Marina et al., 2010), with higher scores meaning better performance. Activities of daily living were evaluated with a scale that included 30 questions, which were scored as zero if no difficulty in performing the activity was present, and six if the activity could not be performed; a total score for the scale was generated, with zero as the lowest score possible (completely functional).
Health-Related Quality of Life (HRQoL) was measured with a validated version of the 36-item Short Form Health Survey (SF-36) (Ware and Sherbourne, 1992, Zúniga et al., 1999). Scoring was performed using the RAND algorithm (Hays and Shapiro, 1992), which results in an overall SF-36 score, as well as scores for eight subscales (physical functioning, role limitations due to physical health or to emotional problems, vitality, emotional well-being, social functioning, bodily pain, and general health). Scores range from 0 to 100, with higher scores indicating better HRQoL.
Questions were asked about negative life events during the previous year (serious illness or injury, increased trouble with daily activities, vision and hearing impairment, retirement, financial problems, trouble with neighbours, marital separation, serious illness of someone close, death of someone close, moving out, and losing a pet) (Kendler et al., 1999). The sum of the negative life events in each stage was recorded. Locus of control has been defined as the personal belief that someone has regarding the potential to influence important life events. Seven dichotomous questions were asked, modified from the Mexican Health, and Aging Study, and the total score was integrated with the addition of individual question scores, with zero as the lowest locus of control, and seven as the highest (Angel et al., 2009). Regarding positive affect, four questions were asked from the Centre for Epidemiologic Studies Depression Scale, with a score of 16 indicating the best positive affect (Miller et al., 1997).
Data analysis
Sampling weights were calculated, and reported. A flow chart of the subjects regarding their significant depressive symptoms status was created. Missing values were replaced by stochastic regression multiple imputation. A descriptive analysis, with means or proportions, and 95% confidence intervals, was performed for all variables by stage. Trajectories of GDS 30 scores, stratified by baseline significant depressive symptom status during the three stages, were graphed in both empirical (box plots with medians, quartiles, and outliers), and fitted (along with 95% confidence intervals [CIs]). To identify those variables associated with different trajectories, a multi-level longitudinal model was used in each of the strata. Marginal modelling was employed through generalised estimating equation (GEE) with exchangeable correlation matrices, and Gaussian family (to assess changes in the continuous scores on the GDS 30). The dependent variable was the GDS 30 score (continuous), and all of the variables were introduced into the model, with the exception of the eight domains of the SF36; a separate model was used for these variables because of potential collinearity. A reduction of the model was performed by hand, according to the p-values of individual variables, until all of the variables reached significance (p<0.05); to maximise those factors with the highest values, odds ratios are reported along with 95% confidence intervals.
RESULTS
A total of 7,882 subjects were assessed, representing 248,811 subjects from the population. An attrition rate of 29.42% occurred in the cohort, mainly due to mortality (327 deaths). The mean GDS 30 score (intercept) for those subjects with significant depressive symptoms was 17.21points (95% CI=16.79–17.63), while for those without significant depressive symptoms, it was 5.24 points (95% CI=4.89–5.6). Table 1 presents the descriptive data for the total studied population. The mean age at baseline was 70.96 years (95% CI=70.31–71.61). Female sex had a proportion of 61.15% (95% CI=57.85–64.4), the mean number of years of school attendance was 5.19 years (95% CI=3.86–6.53), the proportion practicing any religion was 97.84% (95% CI=96.12–99.55), and the mean number of friends was 0.84 (95% CI=0.67–1.01); no significant differences were found among stages regarding these variables (data not shown). A total of 60.29% were married at the beginning of the study (95% CI 55.99–64.59), a proportion that diminished over the years; similarly, the mean number of relatives was 6.04 (95%) CI 4.11–7.97), which also decreased in the subsequent stages. The proportion of participants living alone increased from 5.57% in the first stage to 7.31% in the last, with a significant difference. The social support survey score underwent a significant increase among stages, from a mean score in the first stage of 62.76 to 68.72 in the third stage (Table 1).
Table 1.
General characteristics (weighted)
| Stage 1 (n=2,949; weighted n=96,863) (95% CI) |
Stage 2 (n=2,581; weighted n=79,001) (95% CI) |
Stage 3 (n=2,352; weighted n=72,947) (95% CI) |
||||
|---|---|---|---|---|---|---|
|
SOCIAL | ||||||
| Age in years; mean | 70.96 | (70.31–71.61) | 72.53 | (71.75– 73.32) |
73.54 | (72.92– 74.15) |
| Sex; proportion | ||||||
| Male | 38.8 | (35.5–42.1) | 38.93 | (34.68– 43.17) |
37.11 | (33.37– 40.85) |
| Female | 61.15 | (57.85–64.4) | 61.06 | (56.82– 65.31) |
62.88 | (59.14– 66.62) |
| Education in years; mean | 5.19 | (3.86–6.53) | 5.09 | (3.79– 6.39) |
5.31 | (3.84– 6.78) |
| Married; proportion | 60.29 | (55.99–64.59) | 56.8 | (52.4– 61.2) |
54.32 | (50– 58.58) |
| Living alone; proportion | 5.57 | (4.32–6.83) | 5.73 | (4.07– 7.38) |
7.31 | (5.86– 8.77) |
| Number of relatives; mean | 6.04 | (4.11–7.97) | 4.84 | (4.47– 5.21) |
5.01 | (4.22– 5.79) |
| Number of friends; mean | 0.84 | (0.67–1.01) | 1.04 | (0.79– 1.29) |
1.13 | (0.66– 1.6) |
| Social support score; mean | 62.76 | (60.6–64.91) | 65.84 | (63.21– 68.47) |
68.72 | (65.81– 71.64) |
| Any religion; proportion | 97.84 | (96.12–99.55) | 98.48 | (97.55– 99.41) |
97.68 | (95.77– 99.6) |
|
LIFESTYLE | ||||||
| Current remunerated activity; proportion | 36.23 | (32.85–39.61) | 45.55 | (40.8– 50.3) |
49.51 | (45.73– 53.29) |
| Exercises regularly; proportion | 20.61 | (15.3–25.92) | 22.22 | (13.86– 30.65) |
23.29 | (17.75– 28.83) |
| Average daily activity hours; mean | 3.93 | (3.37–4.48) | 3.54 | (3.31– 3.77) |
4.42 | (4.09– 4.76) |
| Current smoking; proportion | 13.00 | (11.71–14.3) | 13.48 | (10.04– 16.92) |
12.1 | (10.7– 13.51) |
| Alcohol drinking; proportion | 30.19 | (22.68–37.71) | 30.26 | (24.15– 36.37) |
29.33 | (22.59– 36.07) |
| Use of psychotropic drugs; proportion | 21.6 | (18.33–24.87) | 23.88 | (20.75– 27.02) |
21.04 | (16.79– 25.29) |
| Use of illicit drugs; proportion | 2.64 | (1.67–3.61) | 3.03 | (1.31– 4.74) |
2.41 | (1.44– 3.38) |
|
HEALTH | ||||||
| Number of family physician visits; mean | 3.85 | (3.46–4.24) | 3.69 | (3.29– 4.08) |
3.86 | (3.44– 4.28) |
|
Number of visits to the general hospital; mean |
0.75 | (0.55–0.94) | 0.71 | (0.4– 1.01) |
0.53 | (0.39– 0.66) |
| GDS score; mean | 7.72 | (7.27–8.18) | 8.77 | (8.17– 9.37) |
8.78 | (8.15– 9.42) |
| MMSE score; mean | 25.7 | (25.4–26.1) | 26.02 | (25.72– 25.32) |
26.12 | (25.69– 26.56) |
| SAST score; mean | 17.2 | (16.6–17.7) | 17.19 | (16.87– 17.51) |
17.73 | (17.25– 18.21) |
| Activities of daily living score; mean | 24.53 | (23.8–25.27) | 22.71 | (21.66– 23.76) |
23.37 | (22.18– 24.55) |
| Diabetes; proportion | 27 | (23.8–30.2) | 28 | (24.8– 31.2) |
29.1 | (25.9– 32.3) |
| Hypertension; proportion | 47.1 | (41.2–52.8) | 52.7 | (48.5– 56.8) |
51.9 | (48.7– 55.1) |
| Falls; proportion | 6 | (4.9–7.2) | 10 | (7.8– 12.2) |
7.7 | (5.2– 10.2) |
| Arthropathy; proportion | 11.4 | (9.1–13.7) | 14.1 | (10.1– 18.1) |
9.5 | (8.1– 10.8) |
| Cancer; proportion | 1.4 | (0.8–2.1) | 2.5 | (1.5– 3.5) |
2.7 | (1.5– 3.9) |
| Stroke; proportion | 1.8 | (1.1–2.5) | 1.8 | (0.9– 2.7) |
1.7 | (0.5– 2.9) |
| Cardiopathy; proportion | 10.7 | (9.2–12.2) | 15.6 | (13.02– 18.2) |
12.2 | (9.12– 15.35) |
| Pain; proportion | 73.1 | (67.7–78.6) | 70.9 | (64.3– 77.5) |
71.1 | (67.5– 74.7) |
| More than 2 comorbidities; proportion | 34 | (27.9–40.1) | 42 | (36.9– 47.1) |
36.9 | (34.4– 39.5) |
| Use of antidepressants; proportion | 3.8 | (2.8–4.7) | 3.9 | (2.4– 5.3) |
2.51 | (1.35– 3.67) |
| SF 36 | ||||||
| Physical functioning; mean | 63.47 | (60.55–66.38) | 59.01 | (57.16– 60.86) |
58.11 | (53.52– 62.7) |
| Role limitations due to physical health; mean |
57.37 | (52.96–61.78) | 49.21 | (45.04– 53.38) |
53.7 |
(47.13– 60.26) |
| Role limitations due to emotional problems; mean |
72.36 | (70.22–74.5) | 63.95 | (58.22– 69.68) |
64.36 |
(59.12– 69.6) |
| Vitality; mean | 62.11 | (59.5–64.73) | 60.44 | (58.08– 62.8) |
61.81 | (60.43– 63.18) |
| Social functioning; mean | 79.72 | (77.81–81.64) | 80.77 | (78.53– 83) |
79.82 | (74.76– 84.88) |
| Bodily pain; mean | 63.42 | (59.72–67.13) | 64.11 | (60.78– 67.44) |
63.91 | (60.14– 67.68) |
| General health; mean | 51 | (48.97–53.02) | 50.14 | (49.21– 51.08) |
52.41 | (50.63– 54.19) |
|
PSYCHOLOGICAL | ||||||
| Number of negative life events; mean | 1.59 | (1.26–1.92) | 1.73 | (1.64– 1.81) |
1.89 | (1.54– 2.25) |
| Positive affect score; mean | 10.58 | (9.87–11.29) | 9.7 | (8.62– 10.79) |
11.53 | (11.12– 11.94) |
| Locus of control score; mean | 4.44 | (4.64–4.25) | 4.56 | (4.73– 4.4) |
4.82 | (5.1– 4.55) |
CI=Confidence Interval; GDS=Geriatric Depression Scale; MMSE=Mini-Mental Status Examination; SAST=Short anxiety Screening Test; ADL=Activities of Daily Living; SF=Short Form
The most frequent disease reported was hypertension (47%), followed by diabetes (27%), cardiopathy (10.7%), and arthropathy (11.4%); the rest had frequencies of 10% or lower. Pain had a frequency of 73.1% (95% CI=67.7–78.6), and falls of 6% (95% CI=4.9–7.2). Usage of antidepressants was reported by 3.8% of the subjects. All of these health variables remained time-invariant. HRQoL domain scores showed a trend towards diminishing over the three stages, as shown in Table 1.
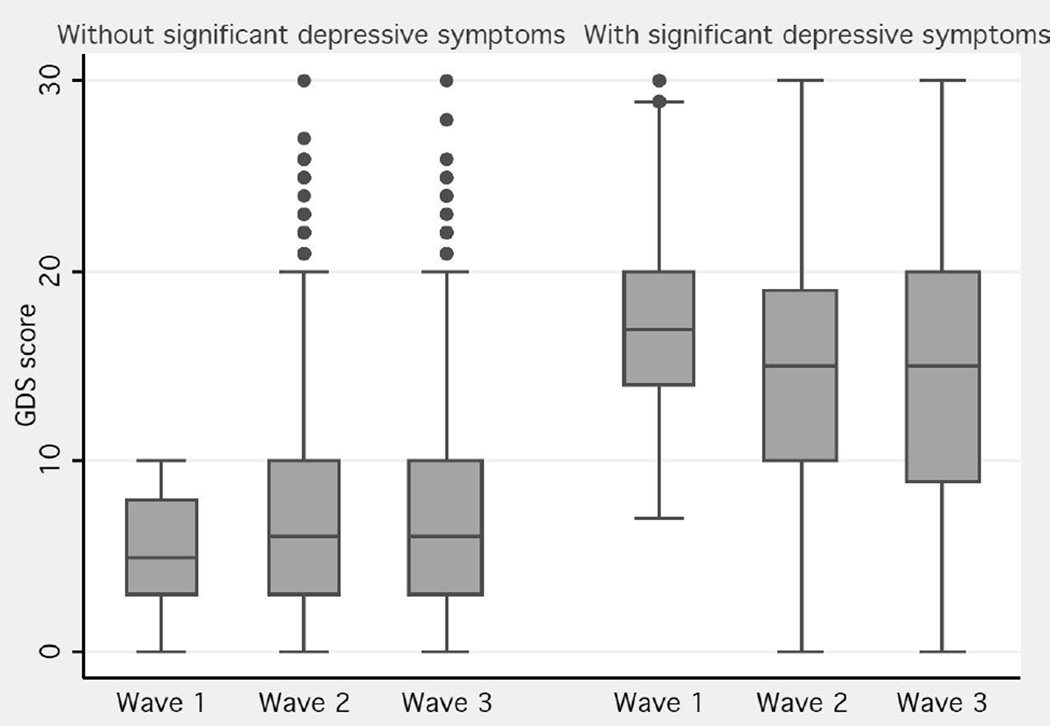
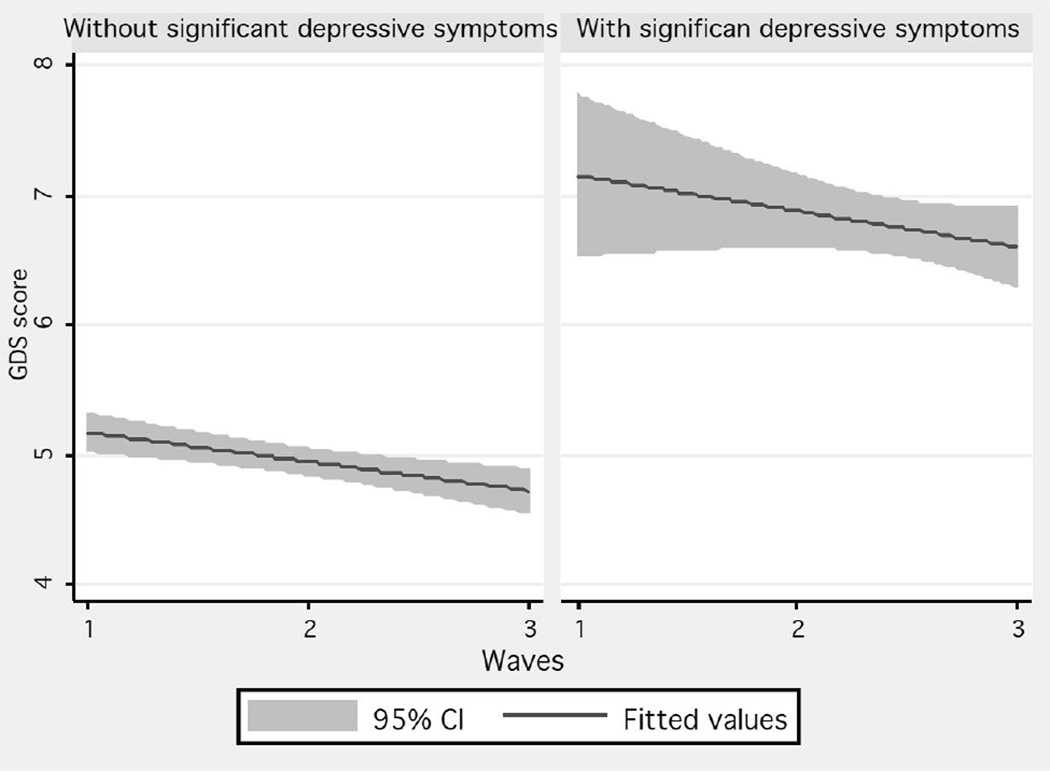
The mean GDS 30 score was 7.72 (95% CI=7.27–8.18), with a median of four in the first stage, and six in the last, as shown in the empirical data for those subjects without baseline significant depressive symptoms; in contrast, for those with significant depressive symptoms, the median GDS 30 score in the first stage was 18, and in the last stage 15 (Figure 1). A fitted trajectory showed a lowering of half a point of the GDS 30 score each year for those subjects without significant depressive symptoms; in contrast, a difference of one point was seen between the first and last stages for those with significant depressive symptoms (Figure 2, Table 2).
Figure 1.
GDS score means and 95% confidence intervals of the three stages stratified by baseline significant depressive symptom status (weighted)
Figure 2.
Fitted trajectories of GDS scores stratified by baseline significant depressive symptom status (weighted)
Table 2.
Generalised estimating equation of the GDS score trajectory and explanatory variables, including a Gaussian model with exchangeable correlation; stratified by baseline significant depressive symptoms (weighted).
| With baseline significant depressive symptoms |
Without baseline significant depressive symptoms |
|||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
|
Model 1 (Fully adjusted) | ||||||
| Age in years | 0.986 | (0.963–1.01) | 0.274 | 1.04 | (1.021–1.06) | <0.001 |
| Sex | 1.061 | (0.69–1.63) | 0.787 | 0.891 | (0.651–1.219) | 0.472 |
| Education in years | 0.942 | (0.906–0.98) | 0.003 | 0.919 | (0.895–0.945) | <0.001 |
| Married | 0.843 | (0.595–1.193) | 0.336 | 0.928 | (0.696–1.236) | 0.612 |
| Living alone | 1.42 | (0.813–2.5) | 0.215 | 0.807 | (0.505–1.29) | 0.372 |
| Number of relatives | 0.97 | (0.949–0.993) | 0.011 | 0.964 | (0.947–0.981) | <0.001 |
| Number of friends | 0.911 | (0.86–.965) | 0.002 | 0.992 | (0.965–1.019) | 0.579 |
| Social support score | 0.971 | (0.964–0.979) | <0.001 | 0.999 | (0.99–1.008) | 0.896 |
| Any religion | 2.14 | (0.727–6.344) | 0.167 | 1.468 | (0.584–3.688) | 0.414 |
| Current remunerated activity | 0.492 | (0.347–0.697) | <0.001 | 1.253 | (0.96–1.637) | 0.097 |
| Exercises regularly | 0.66 | (0.428–1.019) | 0.061 | 1.01 | (0.87–1.19) | 0.576 |
| Average hours of daily activity | 0.948 | (0.882–1.02) | 0.155 | 0.927 | (0.887–0.968) | 0.001 |
| Current smoking | 1.1 | (0.67–1.814) | 0.699 | 1.062 | (0.747–1.51) | 0.736 |
| Alcohol drinking | 0.736 | (0.52–1.041) | 0.084 | 1.082 | (0.861–1.36) | 0.498 |
| Use of psychotropic drugs | 1.09 | (0.789–1.528) | 0.575 | 1.06 | (0.792–1.419) | 0.693 |
| Use of illicit drugs | 1.978 | (1.014–3.858) | 0.045 | 1.2 | (0.607–2.378) | 0.596 |
| Number of family physician visits | 1.005 | (0.964–1.048) | 0.789 | 0.974 | (0.948–1) | 0.057 |
| Number of visits to the general hospital | 0.987 | (0.947–1.027) | 0.532 | 0.973 | (0.903–1.048) | 0.478 |
| MMSE score | 0.945 | (0.901–0.991) | 0.02 | 0.979 | (0.942–1.016) | 0.277 |
| SAST score | 1.615 | (1.564–1.667) | <0.001 | 1.79 | (1.714–1.87) | <0.001 |
| Activities of daily living score | 0.987 | (0.97–1.005) | 0.175 | 0.958 | (0.939–0.978) | <0.001 |
| Diabetes | 1.21 | (0.835–1.763) | 0.309 | 1.331 | (0.958–1.85) | 0.088 |
| Hypertension | 1.013 | (0.453–0.953) | 0.023 | 1.081 | (0.814–1.435) | 0.54 |
| Falls | 0.711 | (0.447–1.13) | 0.150 | 1.272 | (0.818–1.978) | 0.284 |
| Arthropathy | 0.983 | (0.622–1.552) | 0.942 | 0.761 | (0.52–1.113) | 0.159 |
| Cancer | 1.049 | (0.41–2.683) | 0.919 | 1.01 | (0.394–2.591) | 0.983 |
| Stroke | 1.213 | (0.562–2.62) | 0.622 | 3.215 | (1.052–9.83) | 0.04 |
| Cardiopathy | 1.228 | (0.827–1.823) | 0.308 | 1.326 | (0.874–2.012) | 0.184 |
| Pain | 2.224 | (1.451–3.409) | <0.001 | 1.24 | (0.989–1.555) | 0.061 |
| More than 2 comorbidities | 0.872 | (0.557–1.366) | 0.552 | 1.064 | (0.726–1.562) | 0.747 |
| Use of antidepressants | 1.611 | (0.907–2.863) | 0.104 | 1.885 | (0.948–3.747) | 0.070 |
| Number of traumatic life events | 1.35 | (1.234–1.489) | <0.001 | 1.369 | (1.261–1.312) | <0.001 |
| Positive affect score | 0.82 | (0.795–0.845) | <0.001 | 0.916 | (0.895–0.938) | <0.001 |
| Locus of control score | 0.595 | (0.431–0.625) | <0.001 | 0.641 | (0.592–0.687) | <0.001 |
|
Model 2 (Adjusted only for Health Related Quality of Life) | ||||||
| Physical functioning | 1.007 | (1.001–1.013) | 0.027 | 0.992 | (0.987–0.997) | 0.005 |
| Role limitations due to physical health | 0.999 | (0.995–1.004) | 0.891 | 0.997 | (0.994–1.001) | 0.254 |
| Role limitations due to emotional problems | 0.984 | (0.981–0.988) | <0.001 | 0.98 | (0.976–0.984) | <0.001 |
| Vitality | 0.964 | (0.954–0.974) | <0.001 | 0.982 | (0.973–0.99) | <0.001 |
| Emotional well-being | 0.91 | (0.901–0.919) | <0.001 | 0.929 | (0.92–0.938) | <0.001 |
| Social functioning | 0.99 | (0.901–0.919) | 0.001 | 0.987 | (0.98–0.994) | <0.001 |
| Bodily pain | 0.99 | (0.984–0.996) | 0.005 | 0.998 | (0.993–1.003) | 0.628 |
| General health | 0.946 | (0.937–0.955) | <0.001 | 0.961 | (0.954–0.969) | <0.001 |
CI=Confidence Interval; GDS=Geriatric Depression Scale; MMSE=Mini-Mental Status Examination; COPD=Chronic Obstructive Pulmonary Disease; ADL=Activities of Daily Living; SAST=Short Anxiety Screening Test; SF=Short Form
Fully adjusted model is presented in table 1. The significant variables that were inversely associated with depressive symptoms trajectory in the final model, for subjects with significant baseline depressive symptoms, were years of schooling, number of relatives, number of friends, social support score, current remunerated activity, regular exercise, alcohol drinking, MMSE score, hypertension, positive affect score, and locus of control score, with remunerated activity being the variable with the lowest OR (0.48 CI 95%=0.349–0.662). Same direction-associated significant variables were use of illicit drugs, SAST score, pain, and number of negative life events, with being in pain the variable with the highest OR (2.277 CI 95%=1.484–3.494). In contrast, in the group without significant depressive symptoms at baseline, the inversely associated significant variables were years of education, number of relatives, average hours of daily activity, number of family physician visits, positive affect score, and locus of control score (also the lowest OR, 0.635). Those variables in the same direction as the depressive symptom trajectory were age, current remunerated activity, alcohol drinking, SAST score (highest OR), diabetes, and number of negative life events (see Table 3).
Table 3.
Final model only with significant variables (excluding Health Related Quality of Life) for GDS trajectories stratified by baseline significant depressive symptoms (weighted).
| With baseline significant depressive symptoms |
Without baseline significant depressive symptoms |
|||||
|---|---|---|---|---|---|---|
| OR (95% CI) |
p-value | OR (95% CI) |
p-value | |||
| Age in years | - | - | 1.043 | (1.025–1.061) | <0.001 | |
| Education in years | 0.946 | (0.909–0.984) | 0.006 | 0.923 | (0.899–0.948) | <0.001 |
| Number of relatives | 0.968 | (0.946–0.991) | 0.007 | 0.962 | (0.946–0.98) | <0.001 |
| Number of friends | 0.912 | (0.861–0.966) | 0.002 | - | - | |
| Social support score | 0.971 | (0.963–0.978) | <0.001 | - | - | |
| Current remunerated activity | 0.48 | (0.349–0.662) | <0.001 | 1.337 | (1.064–1.68) | 0.013 |
| Exercises regularly | 0.66 | (0.398–0.952) | 0.029 | - | - | |
| Average hours of daily activity | - | - | 0.921 | (0.882–0.961) | <0.001 | |
| Alcohol drinking | 0.709 | (0.504–0.998) | 0.049 | 1.283 | (1.056–1.56) | 0.012 |
| Use of illicit drugs | 2.1 | (1.07–4.1) | 0.029 | - | - | |
| Number of family physician visits | - | - | 0.978 | (0.957–1) | 0.058 | |
| MMSE score | 0.943 | (0.901–0.987) | 0.013 | - | - | |
| SAST score | 1.614 | (1.564–1.666) | <0.001 | 1.806 | (1.732–1.883) | <0.001 |
| Activities of daily living score | - | - | 0.956 | (0.938–0.975) | <0.001 | |
| Diabetes | - | - | 1.361 | (1.029–1.801) | 0.031 | |
| Hypertension | 0.655 | (0.483–0.889) | 0.007 | - | - | |
| Pain | 2.277 | (1.484–3.494) | <0.001 | - | ||
| Number of negative life events | 1.36 | (1.239–1.493) | <0.001 | 1.395 | (1.284–1.515) | <0.001 |
| Positive affection score | 0.816 | (0.792–0.841) | <0.001 | 0.916 | (0.896–0.938) | <0.001 |
| Locus of control score | 0.581 | (0.533–0.629) | <0.001 | 0.635 | (0.591–0.683) | <0.001 |
CI=Confidence Interval; GDS=Geriatric Depression Scale; MMSE=Mini-Mental Status Examination; SAST=Short Anxiety Screening Test; ADL=Activities of Daily Living; SF=Short Form
Regarding the model in which only HRQoL was used, with the exception of role limitations due to physical health (not significant), all of the other categories of the HRQoL SF 36 were associated with depressive symptom trajectories in subjects with significant baseline depressive symptoms, and with the exception of physical functioning, all of the others were inversely associated. In the case of subjects without significant baseline symptoms, with the exception of role limitations due to physical health, and bodily pain, the remainder of the variables were significant, and inversely associated with depressive symptom trajectories.
DISCUSSION
This article presents information representing almost 100,000 elderly subjects from the main social, and health security system of Mexico regarding their depressive symptom trajectories. To our knowledge, this is the first report of elderly Mexican adults on this topic, showing the multifactorial implications of trajectories of significant depressive symptoms, which have already been reported in other populations (Kasen et al., 2003). Although there are already identified trajectories, and they seem to be highly variable, the design of our study permitted us to fix a baseline point (intercept) by stratifying the groups, regarding the presence of significant depressive symptoms at the baseline assessment (Byers et al., 2012, Chen et al., 2011), into groups already identified in other studies (Byers et al., 2012, Cui et al., 2008, Huang et al., 2011, Kuchibhatla et al., 2012, Lincoln and Takeuchi, 2010), thus allowing to analyse how different factors had different impacts on the groups. Moreover, a higher burden of depressive symptoms has been consistently associated with the persistence of higher scores for depression, which allowed us to observe this trend over a short period of time (Harris et al., 2006). In addition, the inclusion of previously selected subjects with significant depressive symptoms could aid in clinical settings in a number of ways, such as determining those factors associated with the worsening of depressive symptoms, preventing the appearance of overwhelming depression, and knowing which subjects will be refractory to treatment due to their characteristics associated with worsening depression or with constantly high trajectories of depressive symptoms.
In contrast to earlier findings, in which overall depressive symptom trajectories seemed to be U-shaped with greater peaks in older subjects (Mirowsky and Ross, 1992), our findings show how trajectories, instead of increasing, seem to decay, a finding that was also reported by Kuchibhattla et al. (Kuchibhatla et al., 2012). In addition, short term-trajectories (2 years of follow up) have been associated with changes in other studies, such as the findings reported by Harris et al. in elderly English subjects, in which a large set of associated factors was also shown to be related to onset or persistence of depression in a two-year follow-up (Harris et al., 2006). In addition, early detection of changes in affect could lead to interventions to diminish the effects shown in comorbidity, and frailty (Andrew and Rockwood, 2007, Judd and Akiskal, 2002). There are complex interactions between different factors in the function of depression trajectory. In addition to previously reported associated factors with depressive symptom trajectories, such as education, social support, comorbidity, cognitive status, functionality, negative life events, and locus of control, this study provides insights into new factors (Angel et al., 2009, Beekman et al., 2002, Kuchibhatla et al., 2012, Lynch and George, 2002, Mirowsky and Ross, 1992). As subjects grow older, a higher burden of depressive symptoms has been shown; nevertheless, results in elderly populations have been conflicting, demonstrating that attributing affect problems to age might be misleading because of other factors that are more frequently present in elderly (Mirowsky and Ross, 1992). Our results show that age is associated with an increasing trajectory of symptoms of depression only in those subjects without significant depressive symptoms at baseline; this finding merits further research to clarify the factors underlying this association. Contrary to other reports, female sex seemed not to be associated with the depressive symptom trajectory; however, in the report by Harris, female sex was not found to be associated with depressive symptomatology; among other similarities, this study was also adjusted for a large number of variables, including locus of control, which could explain our findings (Harris et al., 2006).
Level of education is consistently associated with depressive symptom trajectories, as has already been reported in other studies, such as in the reports of Yang, and Lynch; the higher the level of education is, the lower the probability that depressive symptom trajectories (in subjects with, and without baseline significant depressive symptoms) will increase (Lynch and George, 2002). This phenomenon has been attributed by some authors to a cohort effect, due to older generations not having been able to achieve higher education in their early years (Huang et al., 2011, Mirowsky and Ross, 1992). Social support is associated in the literature with a lower probability of progression to worse depressive symptomatology (Beekman et al., 2002, Chen et al., 2011, Harris et al., 2006, Kasen et al., 2003). However, in our report, we had the opportunity to observe some differences between subjects with significant baseline depressive symptoms, and those without symptoms, with an apparent increase in the need of social support in the subjects already having depressive symptomatology, and with an inverse association with number of relatives, and number of friends, as well as overall social support. In contrast, in subjects without depressive symptomatology, only the number of relatives was associated. Religion was not found to be associated, in contrast to some cross-sectional reports, which might have been due to the still homogenous practices of religion in our country, which are greatly bounded by tradition (Braam et al., 2001). Some reports have suggested that living alone is related to a higher burden in depressive symptomatology, something not corroborated in our results; this finding might have been due to an effect of the neighbourhood living already described in Mexican Americans, whereby elderly people living in these neighbourhoods had a lower probability of having depression, even in subjects who lived alone (Ostir et al., 2003). Regarding activity, those subjects with significant baseline depressive symptoms seemed to experience a beneficial effect from exercising regularly, as exercise is a well-established treatment for depression in the elderly (Blumenthal et al., 1999); nevertheless, exercise seems not to be associated with depressive symptomatology in subjects without symptoms. In contrast, more basic activities of daily living are associated with the trajectory of depressive symptomatology in subjects not engaging in activities at baseline, and subjects with lower amounts of physical activity or with difficulty in performing such activities are prone to developing worse depressive symptomatology, as already established association among elderly Mexican subjects (Gallegos-Carrillo et al., 2012). As shown by Gill et al, a higher burden of symptoms is associated with difficulty performing activities of daily living, but not in those subjects with low depressive symptomatology; however, the follow-up in that report was nine years (Barry et al., 2009).
A paradoxical result in alcohol consumption was found, with a lower probability of worse depressive symptoms among those subjects with baseline significant depressive symptoms who reported that they drank more than one glass of alcohol per day on average; one possible explanation for this finding is the modulatory effect that alcohol has on coping, in contrast to enhancement effects observed in those subjects who are more socially active; Cooper et al. found that when alcohol was used to enhance social encounters (among other situations), its use resulted more frequently in mental health problems (i.e., alcoholism) (Cooper et al., 1995); however, these findings merit more research. Substance abuse is a well-known risk factor for developing affective disorders (Abraham and Fava, 1999); however, the association in the elderly between any history using illegal substances, and the development of late-life depressive symptoms remains unclear; our results show an association with an increasing trend towards depressive symptomatology with any history of using illegal substances. This finding will be of increasing interest because newer generations of elderly people will have experienced greater availability of these drugs, and there are estimates that substance-related disorders, and their consequences (including over the long term) will increase in the near future. Drug use is increasing globally, and locally, as shown in the latest addiction survey in Mexico, which showed a slight trend towards higher illicit drug consumption (Blazer and Wu, 2009, Secretaria-de-Salud, 2012).
In contrast to other reports, a comorbid state was not found to be associated with worse depressive symptomatology; nevertheless, specific pathologies, such as hypertension, and diabetes, had impacts on depressive symptom trajectories. Hypertension was inversely associated with symptoms in subjects with significant depressive symptomatology at baseline, an effect that might have to due to the use of services. In contrast, diabetes was associated with worse depressive symptoms over time. This relationship has been widely reported, although the causal relationship is not clear. Demakakos et al showed that depressive symptoms were associated with a higher risk of type 2 diabetes over 45.8 months of follow-up (Demakakos et al., 2010).
As suggested by Powell, and colleagues, depressive symptoms, and depression in the elderly could have non-pervasive (non-permanent) natures, with periods of positive affect (happiness) (Lawton et al., 1996). Our results show that this finding held true, both in subjects with significant depressive symptoms, and without, and that positive affect was a factor that lessened symptomatology.
Locus of control seems to be a culturally defined construct, as shown by Angel et al. [39] in a recent report, in which differences between two populations were found, and an impact of this construct was found on health-related decisions. The strong association with depression trajectory should point to a thorough evaluation of beliefs, and ways of thinking to have better prognoses of elderly subjects with depressive symptoms. The perception that seeking help would not have an impact on the final result of the symptoms could be associated with a persistence of manifestations of depression due to a lack of mental care. These psychological findings open the door to the use of certain psychotherapy techniques, such as cognitive reframing, to change the mental structures of subjects regarding their beliefs about specific topics (in this case depressive symptoms); such interventions have proved useful in other mental health settings, such as in subjects with dementia (Hollon et al., 1991).
HRQoL was separated from the rest of the analysis because of the global health information it provides, which could be useful for stakeholders because it provides a general status of the subject, rather than an individual vision, which is more useful for clinicians. An earlier study by our group reported an association between low HRQoL that worsens, and a high comorbid burden (Gallegos-Carrillo et al., 2009). Although the study was conducted in a large population, our results only represent a specific group of subjects receiving social security. Regarding adjusting variables, there were no nutritional assessments, which would have provided useful information due to the strong association previously reported between anorexia, and depressed mood in the elderly (Kuo et al., 2011). Moreover, there is no registry of pharmacological or psychological treatment; only self-reporting of antidepressant use was available, which might not have reflected the impact of standard treatment, which also includes psychotherapy, and other non-pharmacological measures.
CONCLUSION
New insights into depressive symptom trajectories are provided by this report. Knowing some characteristics of subjects could improve the detection of elderly people with depressive symptoms, and result in earlier interventions in affective problems to prevent further complications that burden both the subject, and society (family, health systems). As shown by our results, the differentiation between subjects with recent histories of depressive symptoms, and those without could aid in making decisions regarding the status of the subject by the identification of the different factors that could either be associated with significant depressive symptom onset or with the persistence of symptoms. In addition, cross-cultural differences in these factors seem to play an important role, and there is an urgent need to conduct research in different populations using frameworks similar to that used in our report. Generally speaking, and accounting for our results, an elderly person with a recent history of depressive symptoms should be carefully assessed if he or she has a low level of education, has poor social support, does not work, does not exercise regularly, has a history of illicit drug use, has lower cognitive performance, is anxious, has uncontrolled pain, has had recent negative life events, has low positive affect or has a low locus of control. If an elderly person has no recent history of depressive symptoms, advanced age, less education, few relatives, does work, engages in few activities, and has difficulty with some of them, does not regularly visit a primary care physician, is anxious or diabetic, has had recent negative life events, has low positive affect, and has a low locus of control, a health professional should be alerted to perform a closer assessment for early detection of depression.
Supplementary Material
ACKNOWLEDGEMENTS
Financial Disclosure:
Funding sources: this project was supported by grants from CONACYT (México) 2002- CO1-6868, the Mexican Institute of Social Security (IMSS 2002-382), and NIH-FIRCA R03 TW005888. Dr. Wagner was funded through grant DA 17796-01 from NIDA, and P60-MD002217-01 from the NCMHHD.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Sponsor’s Role:
The sponsors were the National Council of Science, and Technology (Mexico), the Mexican Institute of Social Security (IMSS 2002-382), and NIH-FIRCA R03 TW005888. Dr. Wagner was funded through grant DA 17796-01 from NIDA, and P60-MD002217-01 from the NCMHHD. The funds were obtained through contests. CONACYT, and the other financing agencies had no role in the design methods, subjects, and analysis, other than providing funds for the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest: The authors have declared that no competing interests exist.
Author Contributions:
CGP conceived of the protocol, and the original idea, coordinated the research, obtained the funds, and reviewed, and approved the final version of the manuscript. FEW participated in the original protocol, proposed methodological issues, organised the analysis, and reviewed critically the manuscript
SSG, CEB, and TJC supported the conduction of research, coordinated, and supervised the field work, and organised the database, and data analysis
MPZ participated in the organisation, control, and analysis of the data, and prepared the second draft, and final version of the paper
VAL coordinated the process of integrating the epidemiological data, and participated in writing the second draft, and final version of the paper
FFM, and RRA were in charge of database management, control, and review, and analysis of the data.
JJG participated in the original protocol, proposed methodological issues, and organized the analysis. He also critically reviewed the final version of the manuscript, and obtained funding from one of the sources.
REFERENCES
- Abraham HD, Fava M. Order of onset of substance abuse and depression in a sample of depressed outpatients. Compr Psychiatry. 1999;40:44–50. doi: 10.1016/s0010-440x(99)90076-7. [DOI] [PubMed] [Google Scholar]
- Andrew MK, Rockwood K. Psychiatric illness in relation to frailty in community-dwelling elderly people without dementia: a report from the Canadian Study of Health and Aging. Can J Aging. 2007;26:33–38. doi: 10.3138/8774-758w-702q-2531. [DOI] [PubMed] [Google Scholar]
- Angel RJ, Angel JL, Hill TD. Subjective control and health among Mexican-originelders in Mexico and the United States: structural considerations in comparative research. The journals of gerontology. Series B, Psychological sciences and social sciences. 2009;64:390–401. doi: 10.1093/geronb/gbn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry LC, Allore HG, Bruce ML, Gill TM. Longitudinal association between depressive symptoms and disability burden among older persons. J Gerontol A Biol Sci Med Sci. 2009;64:1325–1332. doi: 10.1093/gerona/glp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman AT, Penninx BW, Deeg DJ, de Beurs E, Geerling SW, van Tilburg W. The impact of depression on the well-being, disability and use of services in older adults: a longitudinal perspective. Acta psychiatrica Scandinavica. 2002;105:20–27. doi: 10.1034/j.1600-0447.2002.10078.x. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Wu LT. The epidemiology of substance use and disorders among middle aged and elderly community adults: national survey on drug use and health. Am J Geriatr Psychiatry. 2009;17:237–245. doi: 10.1097/JGP.0b013e318190b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49:351–359. doi: 10.1046/j.1532-5415.2001.49076.x. [DOI] [PubMed] [Google Scholar]
- Braam AW, Van den Eeden P, Prince MJ, Beekman AT, Kivelä SL, Lawlor BA, Birkhofer A, Fuhrer R, Lobo A, Magnusson H, Mann AH, Meller I, Roelands M, Skoog I, Turrina C, Copeland JR. Religion as a cross-cultural determinant of depression in elderly Europeans: results from the EURODEP collaboration. Psychol Med. 2001;31:803–814. doi: 10.1017/s0033291701003956. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C, Bei B, Gilson K, Komiti A, Jackson H, Judd F. The relationship between attitudes to aging and physical and mental health in older adults. Int Psychogeriatr. 2012;24:1674–1683. doi: 10.1017/S1041610212000774. [DOI] [PubMed] [Google Scholar]
- Byers AL, Vittinghoff E, Lui LY, Hoang T, Blazer DG, Covinsky KE, Ensrud KE, Cauley JA, Hillier TA, Fredman L, Yaffe K. Twenty-year depressive trajectories among older women. Arch Gen Psychiatry. 2012;69:1073–1079. doi: 10.1001/archgenpsychiatry.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Mullan J, Griffiths D, Kreis IA, Lan TY, Chiu HC. Trajectories of depression and their relationship with health status and social service use. Arch Gerontol Geriatr. 2011;53:e118–e124. doi: 10.1016/j.archger.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Cole MG. Public health models of mental health care for elderly populations. Int Psychogeriatr. 2002;14:3–6. doi: 10.1017/s1041610202008220. [DOI] [PubMed] [Google Scholar]
- Conner KO, Copeland VC, Grote NK, Koeske G, Rosen D, Reynolds CF, Brown C. Mental health treatment seeking among older adults with depression: the impact of stigma and race. Am J Geriatr Psychiatry. 2010;18:531–543. doi: 10.1097/JGP.0b013e3181cc0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry. 2008;16:406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcoholism, clinical and experimental research. 2005;29:902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- de Beaman S, de Beaman P, Garcia-Peña C, Villasis M, Heres J, Córdova A, Jagger C. Validation of a modified version of the Mini-Mental State Examination (MMSE) in Spanish. Aging, Neuropsychology, and Cognition. 2004;11:1–11. [Google Scholar]
- Demakakos P, Pierce MB, Hardy R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults: the English longitudinal study of aging. Diabetes Care. 2010;33:792–797. doi: 10.2337/dc09-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-San Martín MI, Andrade-Rosa C, Andrade C, Molina JD, Molina J, Muñoz PE, Carretero B, Rodríguez M, Silva A. Validation of the Spanish version of the geriatric depression scale (GDS) in primary care. Int J Geriatr Psychiatry. 2002;17:279–287. doi: 10.1002/gps.588. [DOI] [PubMed] [Google Scholar]
- Franco-Marina F, García-González JJ, Wagner-Echeagaray F, Gallo J, Ugalde O, Sánchez-García S, Espinel-Bermúdez C, Juárez-Cedillo T, Rodríguez MA, García-Peña C. The Mini-mental State Examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int Psychogeriatr. 2010;22:72–81. doi: 10.1017/S1041610209990822. [DOI] [PubMed] [Google Scholar]
- Gallegos-Carrillo K, Flores YN, Denova-Gutiérrez E, Méndez-Hernández P, Dosamantes-Carrasco LD, Henao-Morán S, Borges G, Halley-Castillo E, Macias N, Salmerón J. Physical Activity and Reduced Risk of Depression: Results of a Longitudinal Study of Mexican Adults. Health Psychol. 2012 doi: 10.1037/a0029276. [DOI] [PubMed] [Google Scholar]
- Gallegos-Carrillo K, García-Peña C, Mudgal J, Romero X, Durán-Arenas L, Salmerón J. Role of depressive symptoms and comorbid chronic disease on health-related quality of life among community-dwelling older adults. J Psychosom Res. 2009;66:127–135. doi: 10.1016/j.jpsychores.2008.07.007. [DOI] [PubMed] [Google Scholar]
- García-Peña C, Wagner FA, Sánchez-Garcia S, Juárez-Cedillo T, Espinel-Bermúdez C, García-Gonzalez JJ, Gallegos-Carrillo K, Franco-Marina F, Gallo JJ. Depressive symptoms among older adults in Mexico City. J Gen Intern Med. 2008;23:1973–1980. doi: 10.1007/s11606-008-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Cook DG, Victor C, DeWilde S, Beighton C. Onset and persistence of depression in older people--results from a 2-year community follow-up study. Age Ageing. 2006;35:25–32. doi: 10.1093/ageing/afi216. [DOI] [PubMed] [Google Scholar]
- Hays RD, Shapiro MF. An overview of generic health-related quality of life measures for HIV research. Qual Life Res. 1992;1:91–97. doi: 10.1007/BF00439716. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Shelton RC, Loosen PT. Cognitive therapy and pharmacotherapy for depression. J Consult Clin Psychol. 1991;59:88–99. doi: 10.1037//0022-006x.59.1.88. [DOI] [PubMed] [Google Scholar]
- Huang JF, Wong RH, Chen CC, Mao IF, Huang CC, Chang WH, Wang L. Trajectory of depression symptoms and related factors in later life--a population based study. J Affect Disord. 2011;133:499–508. doi: 10.1016/j.jad.2011.04.048. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. Am J Geriatr Psychiatry. 2002;10:233–238. [PubMed] [Google Scholar]
- Kasen S, Cohen P, Chen H, Castille D. Depression in adult women: age changes and cohort effects. Am J Public Health. 2003;93:2061–2066. doi: 10.2105/ajph.93.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. The assessment of dependence in the study of stressful life events: validation using a twin design. Psychol Med. 1999;29:1455–1460. doi: 10.1017/s0033291798008198. [DOI] [PubMed] [Google Scholar]
- Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2002;57:P87–P94. doi: 10.1093/geronb/57.1.p87. [DOI] [PubMed] [Google Scholar]
- Kuchibhatla MN, Fillenbaum GG, Hybels CF, Blazer DG. Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatr Scand. 2012;125:492–501. doi: 10.1111/j.1600-0447.2011.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SY, Lin KM, Chen CY, Chuang YL, Chen WJ. Depression trajectories and obesity among the elderly in Taiwan. Psychol Med. 2011;41:1665–1676. doi: 10.1017/S0033291710002473. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Parmelee PA, Katz IR, Nesselroade J. Affective states in normal and depressed older people. J Gerontol B Psychol Sci Soc Sci. 1996;51:P309–P316. doi: 10.1093/geronb/51b.6.p309. [DOI] [PubMed] [Google Scholar]
- Lincoln KD, Takeuchi DT. Variation in the trajectories of depressive symptoms: results from the Americans' Changing Lives Study. Biodemography Soc Biol. 2010;56:24–41. doi: 10.1080/19485561003709180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, George LK. Interlocking trajectories of loss-related events and depressive symptoms among elders. J Gerontol B Psychol Sci Soc Sci. 2002;57:S117–S125. doi: 10.1093/geronb/57.2.s117. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–454. [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TQ, Markides KS, Black SA. The factor structure of the CES-D in two surveys of elderly Mexican Americans. The journals of gerontology. Series B, Psychological sciences and social sciences. 1997;52:S259–S269. doi: 10.1093/geronb/52b.5.s259. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Age and depression. J Health Soc Behav. 1992;33:187–205. discussion 206-12. [PubMed] [Google Scholar]
- Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–112. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- Murphy E. The prognosis of depression in old age. Br J Psychiatry. 1983;142:111–119. doi: 10.1192/bjp.142.2.111. [DOI] [PubMed] [Google Scholar]
- Nyunt MS, Ko SM, Kumar R, Fones CC, Ng TP. Improving treatment access and primary care referrals for depression in a national community-based outreach programme for the elderly. Int J Geriatr Psychiatry. 2009;24:1267–1276. doi: 10.1002/gps.2256. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Eschbach K, Markides KS, Goodwin JS. Neighbourhood composition and depressive symptoms among older Mexican Americans. J Epidemiol Community Health. 2003;57:987–992. doi: 10.1136/jech.57.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- Perez-Zepeda M, Arango-Lopera V, Garcia-Pena C. Help seeking pathways and associated factors in elderly with depressive symptoms. The Gerontologist. 2012;52:370–371. [Google Scholar]
- Rapp SR, Parisi SA, Walsh DA, Wallace CE. Detecting depression in elderly medical inpatients. J Consult Clin Psychol. 1988;56:509–513. doi: 10.1037//0022-006x.56.4.509. [DOI] [PubMed] [Google Scholar]
- Revilla-Ahumada L, Luna-Castillo J, Bailón-Muñoz E, Medina-Moruno I. Validación del cuestionario MOS de apoyo social en Atención Primaria. Medicina de Familia. 2005;6:10–18. [Google Scholar]
- Salomon JA, Carvalho N, Gutiérrez-Delgado C, Orozco R, Mancuso A, Hogan DR, Lee D, Murakami Y, Sridharan L, Medina-Mora ME, González-Pier E. Intervention strategies to reduce the burden of non-communicable diseases in Mexico: cost effectiveness analysis. BMJ. 2012;344:e355. doi: 10.1136/bmj.e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secretaria-de-Salud. Salud SdPyPdl., editor. [Encuesta Nacional de Adicciones 2011: Drogas] Reportes Nacionales. 2012 Secretaría de Salud: www.spps.gob.mx/spps-ena-2011.
- Sharp LK, Lipsky MS. Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–1008. [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social science & medicine. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Sinoff G, Ore L, Zlotogorsky D, Tamir A. Short Anxiety Screening Test--a brief instrument for detecting anxiety in the elderly. International journal of geriatric psychiatry. 1999;14:1062–1071. doi: 10.1002/(sici)1099-1166(199912)14:12<1062::aid-gps67>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Keller MB, Leon AC, Mueller TI, Shea MT, Warshaw M, Maser JD, Coryell W, Endicott J. Recovery from major depression. A 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry. 1997;54:1001–1006. doi: 10.1001/archpsyc.1997.01830230033005. [DOI] [PubMed] [Google Scholar]
- Sánchez-García S, Juárez-Cedillo T, García-González JJ, Espinel-Bermúdez C, Gallo JJ, Wagner FA, Vázquez-Estupiñán F, García-Peña C. Usefulness of two instruments in assessing depression among elderly Mexicans in population studies and for primary care. Salud Publica Mex. 2008;50:447–456. doi: 10.1590/s0036-36342008000600005. [DOI] [PubMed] [Google Scholar]
- Wagner F, González-Forteza C, Sánchez-García S, García-Peña C, Gallo J. Enfocando la depresión como problema de salud pública en México. Salud Mental. 2012;35:3–11. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zúniga MA, Carrillo-Jiménez GT, Fos PJ, Gandek B, Medina-Moreno MR. [Evaluation of health status using Survey SF-36: preliminary results in Mexico] Salud Publica Mex. 1999;41:110–118. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.