Abstract
Cross-sectional neuroimaging studies are an important first step in examining developmental differences in brain function between adults and youth with bipolar disorder (BD). Impaired response flexibility may contribute to reduced ability to modify goal-directed behavior in BD appropriately. We compared neural circuitry mediating this process in child (CBD) vs. adult BD (ABD) and age-matched healthy subjects. fMRI data from 15 CBD, 23 ABD, 20 healthy children, 27 healthy adults were acquired during a response flexibility paradigm, a task where subjects inhibit a prepotent response and execute an alternative response. When successfully executing an alternate response, CBD showed frontal, parietal, and temporal hyperactivation relative to healthy children and ABD, while ABD hypoactivated these regions relative to healthy adults. Previous studies of response flexibility in healthy volunteers revealed frontal, temporal, and parietal cortex hyperactivation in children and hypoactivation in adults. Relative to age-matched healthy subjects, we found hyperactivation in these regions in CBD and hypoactivation in ABD. This suggests that our findings in patients may represent the extreme extension of the age-related response flexibility activation differences found in healthy subjects. Future studies should use longitudinal fMRI to examine the developmental trajectory of the neural circuitry mediating response flexibility in BD.
Keywords: bipolar disorder, neuroimaging, response flexibility, cross-sectional, stop-change task
1. Introduction
Developmental studies in bipolar disorder (BD) can inform future treatment and prevention efforts (National Institute of Mental Health Strategic Plan, 2007). Specifically, cross-sectional comparisons of neural activity in youth vs. adults with and without BD can help determine the extent of shared pathophysiology in early- and later-onset BD. Examining the pathophysiological differences between child and adult BD adds to existing literature showing smaller amygdala volume in early- vs. late-onset illness (Blumberg et al., 2003; Chang et al., 2005), and may help explain developmental differences in clinical course, with earlier age of onset associated with higher rates of comorbid disorders and number of recurrences, and shorter periods of euthymia (Perlis, 2004; Birmaher, 2007). A previous functional magnetic resonance (fMRI) study found evidence of age- and BD-related frontal dysfunction during unsuccessful motor inhibition. Compared with age-matched comparison subjects, children with BD (CBD) showed anterior cingulate cortex (ACC) hypoactivation, while adults with BD (ABD) showed ACC hyperactivation (Weathers et al., 2012). Response flexibility is a cognitive function closely related to motor inhibition, since successful response flexibility depends, in part, on the ability to inhibit prepotent motor responses in the presence of behaviorally salient cues. Studies of the neural mechanisms mediating response flexibility are particularly relevant in BD because BD patients show reduced ability to modify their behavior in response to environmental cues i.e., andedonic, depressed subjects who do not pursue rewarding goals, or manic patients who pursue unrealistic goals. However, no study has compared neural activity in adults and youth with BD during response flexibility. The goal of this study was to use a response flexibility task to compare brain activation in CBD, ABD, and age-matched healthy subjects, when subjects were confronted with changing behavioral demands.
Response flexibility is an executive function that resembles simple motor inhibition in that both depend on sustained attention and the inhibition of prepotent responses (Swann et al., 2003; Pavuluri et al., 2010). However, response flexibility differs from motor inhibition in that only the former requires subjects to execute an alternative response when the appropriate cue appears. Behavioral data on a response flexibility task indicate that BD youth are slower than healthy subjects at substituting a prepotent response (“go”) with an alternate response (“change”) (McClure et al., 2005), and thus have impaired response flexibility. While no study has used a response flexibility task in ABD, data indicate that BD adults are impaired in psychological domains related to response flexibility, such as attention shifting (Iverson et al., 2009) and motor inhibition (Bora et al., 2009).
In healthy subjects, executing the alternate response successfully during response flexibility engages brain regions mediating inhibition, cognitive control, sustained attention, and signal detection (Bunge et al., 2002; Rubia, et al., 2007b; Thomas et al., 2011). Studies suggest that response flexibility improves with age, with healthy adults showing faster response times to change signals than healthy children (Thomas et al., 2011). Further, regions mediating processes involved in response flexibility show more widespread cortical engagement in healthy youth than adults, including 1) inferior frontal cortex (IFC) during motor inhibition; 2) insula cortex during cognitive control 3) precuneus and inferior parietal cortex during sustained attention; and 4) middle frontal gyrus and temporal cortex during signal detection (Bunge et al., 2002; Rubia et al., 2007b; Thomas et al., 2011; Carp et al., 2012).
Neuroimaging studies have revealed abnormal brain activation in BD children vs. healthy children, and BD adults relative to healthy adults, during response flexibility and related tasks (Passarotti et al., 2010; Singh et al., 2010). Some of these studies report that, relative to healthy subjects, patients show hyperactivation, while other studies report hypoactivation in patients. This disparity in findings may be due to the differences in tasks across studies. For instance, in a fMRI study using the response flexibility task, CBD compared to child healthy subjects showed middle frontal gyrus, insula, and precuneus hyperactivation during successful change vs. go trials (Nelson et al., 2007). While no fMRI study has tested response flexibility in ABD, middle temporal gyrus, precuneus, and inferior frontal gyrus hypoactivation occur during response inhibition in ABD vs. healthy subjects (Strakowski et al., 2008; Mazzola-Pomietto et al., 2009). Furthermore, during unsuccessful response inhibition, CBD showed increased ACC activation, whereas adult ABD showed decreased activation, relative to healthy subjects (Weathers et al., 2012). Since inhibition of prepotent responses is a core component of response flexibility (Kenner et al., 2010), these data suggest that ABD may also exhibit neural dysfunction during response flexibility.
Using event-related fMRI, we compared adults and youth with BD and age-matched healthy subjects on neural function during successful and unsuccessful change trials. As noted above, existing literature shows 1) improved response flexibility in adult vs. child healthy subjects (Thomas et al., 2011); 2) increased precuneus, middle frontal gyrus, insula cortex and IFC activation in healthy adults vs. healthy children during successful response flexibility and cognitive control (Bunge et al., 2002; Thomas et al., 2011); 3) middle frontal gyrus, insula, and precuneus hyperactivation in CBD vs. child healthy subjects during successful change trials (Nelson et al., 2007); and 4) IFC hypoactivation in ABD vs. healthy adults during response inhibition (Mazzola-Pomietto et al., 2009). Finally, clinical studies have shown that earlier age of onset of BD is associated with higher rates of comorbid illness, more recurrences, and shorter periods of euthymia (Perlis, 2004; Birmaher, 2007). Based on these findings, we hypothesize that, compared to age-matched healthy subjects, BD patients will show hyperactivation of middle frontal, precuneus, insula, and inferior frontal regions during successful response flexibility, and that this dysfunction will be present in more cortical regions in BD youth than in BD adults.
2. Methods
2.1 Participants
Participants were part of an ongoing IRB approved study at the National Institute of Mental Health. Adult subjects and parents/guardians of child subjects provided informed consent; children provided informed assent.
We recruited BD patients via advertisements to support groups and clinicians. Patients in all mood states (euthymic, depressed, and hypo/manic), as well as both medicated and unmedicated BD patients were included in the study. Twenty healthy children and 27 healthy adults were recruited from the community through advertisements. They had no lifetime psychiatric diagnoses or first-degree relatives with a mood or anxiety disorder. Exclusion criteria in all groups were: I.Q.<70, substance abuse within the past three months, major medical illnesses, neurological damage/disorder, comorbid attention deficit hyperactivity disorder, and pervasive developmental disorders. No participants were related.
Children were assessed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (Kaufman et al., 1997) by masters or doctoral level clinicians with excellent interrater reliability (κ>0.9 for all diagnoses). Child BD participants (N=15) met criteria for “narrow phenotype” BD i.e., having experienced at least one hypomanic (≥ 4 days) or manic (≥ 7 days) episode characterized by abnormally elevated mood or grandiosity, and at least three criterion “B” mania symptoms (Leibenluft et al., 2003). Pediatric BD included both BD-I (N=12; 80%) and BD-II (N=3; 20%) (Table 2). Inclusion criteria for adult patients was a diagnosis of BD-I or BD-II using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (First, et al., 2002) or the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). The ABD group (N=23) consisted of BD-I (N=14; 60.9%) and BD-II (N=9; 39.1%) patients (Table 2). Age at onset of BD illness was computed as the chronological age at the time of first manic or hypomanic episode.
Table 2.
Patient Characteristics
| Characteristic | Child BD (N = 15) |
Adult BD (N = 23) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Number of medications | 1.69± 1.65 | 2.48± 1.53 |
| YMRSa | 7.20± 5.76 | 3.90± 4.90 |
| CDRSb | 24.73± 5.61 | NA |
| SIGH-SADc | NA | 16.62± 11.40 |
| *Age at onset: Maniad | 11.69 ±3.79 | 21.82 ± 10.66 |
| N (%) | N (%) | |
| Mood State | ||
| Euthymice | 12 (80.0) | 11 (52.3) |
| *Depressed | 0 (0.0) | 8 (38.1) |
| Hypomanic | 3 (20.0) | 1 (4.8) |
| Mixed State | 0 (0.0) | 1 (4.8) |
| Bipolar Diagnosis | ||
| Bipolar If | 12 (80.0) | 14 (60.9) |
| Bipolar II | 3 (20.0) | 9 (39.1) |
| Comorbid Diagnosis | ||
| One or more comorbid diagnosis | 12 (80.0) | 15 (60.0) |
| Anxiety | 7 (46.7) | 6 (26.1) |
| *Oppositional Defiant Disorder | 4 (26.7) | 0 (0.0) |
| Conduct Disorder | 1 (6.7) | 0 (0.0) |
| Substance Abuse/Dependence | 0 (0.0) | 2 (8.7) |
| Medication Use/Type | ||
| *Unmedicated | 6 (40.0) | 1 (4.3) |
| Atypical Antipsychotic | 4 (26.7) | 10 (43.8) |
| Lithium | 3 (20.0) | 5 (21.7) |
| Antiepileptic | 6 (40.0) | 15 (65.2) |
| Antidepressant | 5 (33.3) | 9 (39.1) |
| Stimulants | 2 (13.3) | 1 (4.3) |
| Benzodiazepines | 1 (6.7) | 3 (13.0) |
Young Mania Rating Score; Missing data from 2 adults with BD.
Children's Depression Rating Scale.
Structured Clinical Interview Guide for the Hamilton Rating Scale, Seasonal Affective Disorders Version. Missing data from 2 adult BD.
Data missing from 2 children with BD and 1 adult with BD.
Note, trend difference between adult BD and child BD (x2 =2.89, P=0.089).
Note, proportion of Bipolar I versus Bipolar II was no different between child and adult patients (P=0.23).
P<0.01.
Within 48 hours of scanning, mood was assessed for pediatric patients using the Children’s Depression Rating Scale (CDRS) (Poznanski et al., 1984) and the Young Mania Rating Scale (YMRS) (Young et al., 1978), and for adult patients using the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD) (Williams, 1988) and YMRS (data missing for two adult patients).
Of the 153 individuals scanned, data were excluded for 68 (44.4%): 19.6% (30/153) for excessive movement (>3 mm in any direction) [12 CBD, 9 child healthy subjects, 3 adult healthy subjects, 6 ABD], 15.7% (24/153) for poor performance (go trial accuracy <65%) [10 CBD; 6 child healthy subjects; 4 adult healthy subjects; 4 ABD], 7.1% (11/153) for equipment failure [3 CBD; 5 child healthy subjects, 2 adult healthy subjects, 1 ABD], and 2.0% (3/153) for abnormal clinical findings [2 child healthy subjects; 1 adult healthy subject]. Included vs. excluded patients did not differ in YMRS, CDRS, or SIGH-SAD scores, or proportion in euthymic, hypomanic, depressed, or mixed states (all P’s > 0.05). The final sample (N=85) includes 15 CBD, 20 child healthy subjects, and 27 adult healthy subjects who have been reported previously (Nelson et al., 2007; Thomas et al., 2011). None of the data on this task from the N=23 ABD have been reported in previous studies.
2.2 Design and Procedure
The paradigm used in this study has been described in detail elsewhere (McClure et al., 2005; Nelson et al., 2007; Thomas et al., 2011), and is an adaptation of the stop-signal paradigm (Logan et al., 1997). Briefly, at the start of each trial a white fixation cross appeared at the screen center for 500 ms. This was replaced by an “X” or “O” “go-signal” for 1000 ms. Using a button-box, subjects were instructed to press “1” for “X” and “2” for “O”. Participants were told to respond within 1000 ms, unless the change signal appeared (i.e., background changed to blue) when they were instructed to press “3”.
On the first change trial, the change signal appeared 250 ms after the go-signal. Subsequent change-signal timing was adjusted on a trial-by-trial basis based on subject performance. If the subject changed successfully, the next change signal appeared 50 ms later than on the last change trial; if the subject failed, the signal appeared 50 ms earlier than on the last change trial leading to an approximate change accuracy rate of 50%. Participants did not receive feedback on their performance during the task. To examine brain activation associated with each trial, jitter was introduced by randomly inserted fixation trials lasting 750 ms.
Before scanning, subjects were trained to a mean reaction time (RT) less than 1000 ms on “go” trials. To ensure the prepotency of the go response, there were more go trials (N=176) than change trials (N=80) while scanning. Therefore, the “go” response is the prepotent response, and the less common “3” button press to the change stimulus reflects response flexibility. Eighty-eight fixation trials were presented to measure baseline neural functioning. During scanning, four 3.5 minute runs of randomly presented go (N=44), change (N=20), and fixation trials (N=22) were completed by each participant, for a total of 176 go trials, 80 change trials, and 88 baseline fixation trials.
2.3 Scanning acquisition
Scans were conducted in a General Electric Signal 3T magnet where participants viewed stimuli through Avotec Silent Vision Glasses (Stuart, FL) positioned in the head coil above the eyes. Following manual shim and sagittal localization procedures, gradient echo planar images (23 contiguous 5 mm axial slices/brain volume; parallel to anterior commissure posterior line; single shot gradient echo T2* weighting [matrix 64×64; TR=2000 ms; TE=40ms; FOV=240mm; voxels were 3.75 × 3.75 × 5mm]) were obtained. A high-resolution T1 weighted anatomical image, following standardized magnetization prepared gradient echo sequence, was collected for spatial registration (180 1 mm sagittal slices; FOV=256; NEX=1; TR=11.4 ms; TE=4.4 ms; matrix=256 × 256; TI=300 ms; bandwidth=130 Hz/pixel, 33kHz/256 pixels).
2.4 Analyses
2.4.1 Behavior
For each group, we computed means for accuracy on go and change trials; response time (RT) on go trials (GoRT); and, on change trials, the time between the go signal onset and the change signal onset, or ‘inhibit delay’. Change signal reaction time (CSRT) was calculated by subtracting mean GoRT minus mean inhibit delay at the point when the participant’s accuracy on change trials was 50% (if accuracy rate was not 50%, interpolation was performed by subtracting the mean inhibit delay from the RT at the Xth percentile of go trials, where X was the participant’s change trial accuracy) (Nelson et al., 2007).
Using a 2 (Age group: children and adult) × 2 (Diagnosis: BD and healthy) factorial univariate analysis of variance (ANOVA), differences in mean change and go accuracy, GoRT, inhibit delay, and CSRT were tested. Post-hoc contrasts between groups were done using the Tukey’s test.
2.4.2 Imaging
Functional imaging data was analyzed using SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm) and Matlab 7 (The MathWorks, Natick, MA). Preprocessing included slice timing correction, motion correction, spatial normalization to Montreal Neurological Institute (MNI) space, and smoothing (kernel FWHM=8). At the subject level, event-related response amplitudes were estimated using the General Linear Model (GLM). Event types included unsuccessful change (“unsuccessful change”), go (“go correct”; only correct go trials included) and successful change (“successful change”) trials. Consistent with prior work using this paradigm (Nelson et al., 2007; Thomas et al., 2011), two contrasts were examined in the primary whole-brain analysis: (1) successful change vs. go, and (2) successful change vs. unsuccessful change. These contrasts controlled for task demand and response accuracy, respectively. Individual contrast images were created using pair-wise comparisons of event-related response amplitudes, which were then entered into second-level random-effects group analyses. A high pass filter (0.0078 Hz) was used.
When we conducted a whole-brain analysis at P<0.05 corrected (FWE) for the primary contrasts, no between-group differences survived. We then conducted whole-brain analysis using a statistical threshold of P<0.005 uncorrected with a spatial extent of 20 contiguous voxels. This threshold criteria is consistent with (albeit slightly more conservative than) a threshold that was suggested previously to balance Type I vs. Type II errors in whole-brain analyses (Lieberman and Cunningham, 2009). We used a factorial design to test for a 2 (age group: children and adult) × 2 (diagnosis: BD and healthy) interaction across the whole-brain. For each contrast, clusters of significant activation were identified using SPM8 and spatially located by converting their MNI coordinates to Talairach space (Talairach & Tournoux, 1988). In our post-hoc analyses designed to clarify the between-group differences in activation identified in the primary analyses, we used MarsBaR (Marseille boîte à région d’intérêt; Brett et al., 2002) to extract the average percent signal change across significant clusters identified by the primary analysis. These data were extracted for each participant, entered into SPSS (SPSS, Inc., Chicago, Ill.), and analyzed using a univariate ANOVA with Tukey’s post-hoc tests. Importantly, our age groups differed in mean GoRT (Table 2). Therefore, to test whether any differences in brain activation between children and adults during the successful change vs. go contrast were due to differences in GoRT performance, as a post-hoc analysis, we repeated our primary 2 (age group: children and adult) × 2 (diagnosis: BD and healthy subjects) ANOVA on this extracted data, this time including GoRT as a covariate.
The interactions represented by the two primary contrasts are complex. However, we decomposed them using univariate post-hoc ANOVAs that compare percent signal change associated with individual trial types vs. baseline fixation (e.g., successful change vs. fixation). Using the regions found in the primary analysis, we performed post-hoc analyses to examine group differences in successful change vs. fixation, go vs. fixation, and unsuccessful change vs. fixation in the regions that showed significant between-group differences on the a priori primary contrasts (successful change vs. go; successful change vs. unsuccessful change).
Finally, given differences between our child and adult patient groups on two clinical variables (i.e., percent currently depressed and comorbid oppositional defiant disorder (ODD) diagnosis), we conducted exploratory post-hoc analyses to examine the impact of these variables on our findings. To test whether primary effects remained significant when controlling for these differences, we repeated the 2 (age group: children and adult) × 2 (diagnosis: BD and healthy) ANOVA on extracted data when 1) only including euthymic patients vs. healthy subjects, and 2) when only including patients with no comorbid ODD vs. healthy subjects.
3. Results
3.1 Demographic and clinical characteristics
There were no between-group differences in IQ or gender across any group, or in age between healthy subjects or patients within the child or adult groups (Table 1). Differences emerged between patient groups in percentage depressed; more ABD than CBD were depressed (P<0.01). CBD were more likely than ABD to meet criteria for ODD, and more CBD than ABD were unmedicated (P<0.01) (Table 2).
Table 1.
Group Demographics and I.Q.
| Characteristic | Child BD (N = 15) |
ChildHealthy Subject (N = 20) |
Adult BD (N = 23) |
Adult Healthy Subject (N = 27) |
Between- Group test |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Child Agea | 14.74 ±2.23 | 13.88 ± 1.98 | P = 0.23 | ||
| Adult Age b | 40.85 ± 11.81 | 35.56 ±7.97 | P = 0.06 | ||
| WASI Full-scale I.Q.c,d | 109.13 ± 11.02 N (%) |
112.95± 13.44 N (%) |
114.81 ± 10.35 N (%) |
115.92± 9.49 N (%) |
P = 0.28 |
| Number of Malese | 8 (53.3) | 11 (55.0) | 7 (30.4) | 10 (37.0) | P = 0.30 |
Independent-samples t-test (two-tailed): t=1.21.
Independent-samples t-test (two-tailed): t=1.88.
Missing data from 2 adults with BD and 1 adult control.
ANOVA:F(3,81)=1.29.
Pearson Chi-Square (2-sided): x2=3.70
3.2 Behavioral findings
No age group × diagnosis interactions emerged on any measure (Table 3). There was a main effect of diagnosis on change accuracy and mean inhibit delay, with healthy subjects having higher accuracy (F(1,81)=8.43, P<0.005) and shorter delay (F(1,81)=7.12, P<0.01) There was also a main effect of age group on GoRT and mean inhibit delay, with adults having longer GoRT (F(1,81)=20.62, P<0.001) and mean inhibit delay (F(1,81)=10.56, P<0.005) than children. No diagnosis- or age-related group differences emerged for CSRT (Table 3).
Table 3.
Performance of subjects on the change signal task during scanning, presented by group.
| Child Healthy Subject N=20 |
Child Bipolar Disorder N=15 |
Adult Healthy Subject N=27 |
Adults with Bipolar Disorder N=23 |
Age by Diagnosis Factorial F statistic |
Planned post hoe's (Tukey's test) | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Interaction | Diagnosis | Age | ||
| Variable | ||||||||
| Accuracy Go (%) |
79.2 (12.7) | 77.8 (12.4) | 84.4 (11.2) | 75.5 (16.1) | 1.6 | 3.0 | 0.3 | |
| Accuracy Change (%) |
34.9 (9.6) | 30.0 (10.8) | 37.7 (13.2) | 26.1 (15.5) | 1.4 | 8.4** | 0.0 | HV > BD |
| Go ReactionTime (ms) |
696.8 (107.6) | 669.9 (79.8) | 761.8 (81.4) | 778.5 (73.7) | 1.3 | 0.1 | 20.6*** | Adult > Child |
| Inhibit Delaya (ms) |
457.0 (121.8) | 402.1 (81.8) | 537.0 (100.6) | 470.3 (99.1) | 0.1 | 7.1** | 10.6** | Adult > Child; HV > BD |
| Change signal reaction timeb (ms) |
183.3 (45.3) | 189.5 (80.2) | 186.2 (69.7) | 213.1 (78.5) | 0.5 | 1.2 | 0.7 | |
Age added as covanate for adult groups.
Inhibit Delay=interval between onset of go and onset of change signals.
See text for method of calculation.
P<0.05
P<0.0l
P<0.00l
3.3 Imaging Data
3.3.1 Successful Change vs. go
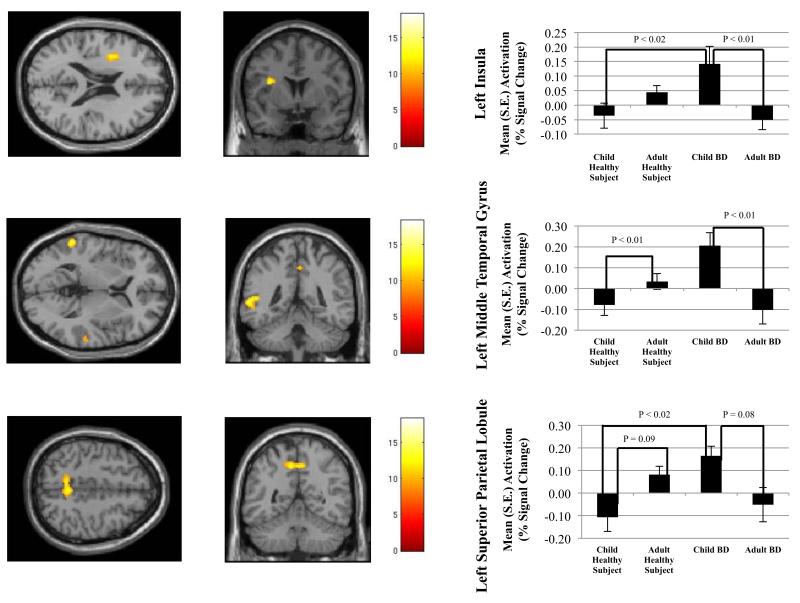
A whole-brain analysis revealed significant between-groups differences in a number of frontal, temporal, and parietal regions, with a similar pattern across clusters i.e., increased activation in CBD vs. child healthy subjects and ABD, and increased activation in adult healthy subjects vs. ABD (Figure 1A-C). Age × diagnosis interactions were detected in the left insula cortex (BA 13) (Figure 1 A), right precentral gyrus (BA 9), left middle temporal gyrus (BA 22) (Figure 1B), bilateral superior temporal gyri (BA 21 and 22), left superior parietal lobule (BA 7) (Figure 1C), left paracentral lobule (BA 5), and right postcentral gyrus (BA 4) (Table 4). There were more regions showing abnormal activation in children with BD than in adults with BD (Table 4).
Figure 1.
Mean activation in (A) left insula cortex, (B) left middle temporal gyrus, and (C) left superior parietal lobule on successful change versus go contrast. Between group differences in percent signal change are depicted in the accompanying bar graphs.
Table 4. Significant clusters of activation for age × diagnosis interaction on successful change versus go and successful change versus unsuccessful changecontrasts.
| Primary Contrast | Region | BA | # Voxels" | MNI coordinates | F | P< | Between group differences | ||
|---|---|---|---|---|---|---|---|---|---|
|
Successful Change versus
Go trials |
Subregion | X | Y | Z | |||||
| Frontal Cortex | |||||||||
| L Insula | 13 | 140 | −34 | 4 | 20 | 12.24 | 0.001 | Child BD>Adult BD***, Child Healthy Subject** |
|
| R PrecentralGyrus | 9 | 102 | 48 | 24 | 38 | 10.71 | 0.002 | Child BD> Child Healthy Subject***, Adult BD***, Adult Healthy Subject** |
|
| Parietal Cortex | |||||||||
|
L Precuneus
(Superior Parietal Lobule) |
7 | 210 | −8 | −52 | 48 | 12.48 | 0.001 | Child BD> Adult BD*, Child Healthy Subject**; Adult Healthy Subject> Child Healthy Subject* |
|
| L Paracentral Lobule | 5 | 35 | −8 | −28 | 54 | 10.99 | 0.001 | Child BD> Child Healthy Subject***, Adult BD* |
|
| R PostcentralGyrus | 4 | 32 | 18 | −26 | 62 | 12.56 | 0.001 | Child BD> Adult BD*; Adult Healthy Subject> Adult BD* |
|
| Temporal Cortex | |||||||||
| L Middle Temporal Gyrus | 22 | 159 | −60 | −44 | 4 | 13.73 | 0.000 | Child BD> Adult BD***, Child Healthy Subject*** |
|
| L Superior Temporal Gyrus | 21 | 45 | −64 | −24 | −2 | 17.64 | 0.000 | Child BD>Adult BD***; Adult Healthy Subject> Adult BD*** |
|
| R Superior Temporal Gyrus | 22 | 27 | 60 | −28 | 2 | 9.38 | 0.003 | Child BD> Adult BD* | |
|
Successful Change versus
Unsuccessful Change trials |
|||||||||
| Frontal Cortex | |||||||||
| R PrecentralGyrus | 9 | 352 | 48 | 22 | 38 | 15.73 | 0.000 | Child BD> Adult BD***, Child Healthy Subject**; Adult Healthy Subject> Adult BD* |
|
| L PrecentralGyrus | 6 | 21 | −36 | 2 | 22 | 11.09 | 0.001 | Child BD > Child Healthy Subject*;Child BD> Adult BD** |
|
| L Middle FrontalGyrus | 6 | 86 | −24 | 12 | 46 | 13.37 | 0.000 | Child BD> Child Healthy Subject**; Adult Healthy Subject> Child Healthy Subject** |
|
| L Inferior Frontal Gyrus | 45 | 114 | −50 | 22 | 8 | 11.48 | 0.001 | Child BD> Child Healthy Subject**; Adult Healthy Subject> Child Healthy Subject* |
|
| L Middle Frontal Gyrus | 6 | 76 | −42 | 6 | 46 | 12.89 | 0.001 | Child BD> Adult BD**, Child Healthy Subject* |
|
| R Medial Frontal Gyrus | 8 | 55 | 10 | 46 | 36 | 12.79 | 0.001 | Child BD> Adult BD**, Child Healthy Subject* |
|
| Parietal Cortex | |||||||||
|
R Precuneus
(Superior Parietal Lobule) |
7 | 333 | 6 | −50 | 48 | 18.27 | 0.000 | Child BD > Adult BD**, Child Healthy Subject** |
|
|
R Inferior Parietal Lobule
(SupramarginalGyrus) |
40 | 70 | 58 | −52 | 38 | 12.33 | 0.001 | Child BD> Adult BD***, Child Healthy Subject* |
|
| Temporal Cortex | |||||||||
| L Middle Temporal Gyrus | 37 | 229 | −62 | −56 | 2 | 16.68 | 0.000 | Child BD> Adult BD***,Child Healthy Subject*; Adult Healthy Subject> Adult BD*** |
|
| R Middle Temporal Gyrus | 39 | 133 | 48 | −70 | 20 | 14.96 | 0.000 | Child BD> Adult BD**; Adult Healthy Subject> Adult BD** |
|
| R Middle Temporal Gyrus | 22 | 45 | 68 | −40 | 6 | 13.14 | 0.001 | Child BD> Adult BD**; Adult Healthy Subject> Adult BD** |
|
BA = Brodmann's Area
= cluster size at least 20 contiguous voxels, uncorrected
MNI = Montreal Neurological Institute
= P<0.10
= P<0.05
= P<0.01
When examining successful change vs. fixation using the significant clusters found during the successful change vs. go contrast, we found a general pattern of increased activation in CBD compared to ABD and child healthy subjects, and in adult healthy subjects compared to ABD. Yet, many of these differences failed to reach significance (Table 5). While activation in the left superior temporal gyrus was greater in adult healthy subjects vs. ABD (P<0.02) during successful change vs. fixation, this difference was not significant in the left middle temporal gyrus. Similarly, activation in the left paracentral lobule in CBD vs. child healthy subjects was higher (P<0.05) during successful change vs. fixation, but this difference was not seen in the left superior temporal gyrus, left middle temporal gyrus, or right postcentral gyrus.
Table 5. Post-hoc analysis using individual trial types versus fixation using regions of significant activation from primary contrasts.(A) Successful change versus fixation and go versus fixation in regions from successful change versus go contrast; (B) Successful change versus fixation and unsuccessful change versus fixation in regions from successful change versus unsuccessful change contrast.
| (A) Successful change versus fixation and go versus fixation in regions from successful change versus go contrast. | ||||||
|---|---|---|---|---|---|---|
| Post-hoc Contrast | Region | BA | # Voxelsa | F | P | Between group differences |
|
Successful Change versus
Fixation |
Subregion | |||||
| Frontal Cortex | ||||||
| L Insula | 13 | 140 | 2.88 | <0.041 | Child BD>Adult BD* | |
| R PrecentralGyrus | 9 | 102 | 2.40 | <0.074 | Child BD >Adult BD * | |
| Parietal Cortex | ||||||
|
L Precuneus
(Superior Parietal Lobule) |
7 | 210 | 4.45 | <0.006 | Adult Healthy Subject > Adult BD*; Adult Healthy Subject > Child Healthy Subject *** |
|
| L Paracentral Lobule | 5 | 35 | 2.67 | <0.053 | Child BD > Child Healthy Subject ** |
|
| R PostcentralGyrus | 4 | 32 | 3.04 | <0.034 | Adult Healthy Subject > Adult BD * |
|
| Temporal Cortex | ||||||
| L Middle Temporal Gyrus | 22 | 159 | 2.30 | <0.084 | ||
| L Superior Temporal Gyrus | 21 | 45 | 3.44 | <0.021 | Adult Healthy Subject > Adult BD** |
|
| R Superior Temporal Gyrus | 22 | 27 | 2.58 | <0.059 | Adult Healthy Subject > Adult BD* |
|
| Go versus Fixation | Frontal Cortex | |||||
| L Insula | 13 | 140 | 0.51 | = 0.675 | ||
| R PrecentralGyrus | 9 | 102 | 1.97 | = 0.126 | Adult Healthy Subject> Child BD * |
|
| Parietal Cortex | ||||||
|
L Precuneus
(Superior Parietal Lobule) |
7 | 210 | 5.10 | <0.003 | Adult BD >Child BD *; Adult Healthy Subject > Child BD *** |
|
| L Paracentral Lobule | 5 | 35 | 0.15 | = 0.931 | ||
| R PostcentralGyrus | 4 | 32 | 0.64 | = 0.589 | ||
| Temporal Cortex | ||||||
| L Middle Temporal Gyrus | 22 | 159 | 1.31 | = 0.276 | ||
| L Superior Temporal Gyrus | 21 | 45 | 1.58 | = 0.200 | ||
| R Superior Temporal Gyrus | 22 | 27 | 1.78 | = 0.157 | ||
| (B) Successful change versus fixation and unsuccessful change versus fixation in regions from successful change versus unsuccessful change contrast. | ||||||
|---|---|---|---|---|---|---|
| Post-hoc Contrast | Region | BA | # Voxelsa | F | P | Between group differences |
| Successful Change versus Fixation | Subregion | |||||
| Frontal Cortex | ||||||
| R PrecentralGyrus | 9 | 352 | 2.34 | 0.080 | ||
| L PrecentralGyrus | 6 | 21 | 2.27 | 0.087 | Child BD> Adult BD * | |
| L Middle Frontal Gyrus | 6 | 86 | 1.44 | 0.238 | ||
| L Inferior Frontal Gyrus | 45 | 114 | 1.67 | 0.181 | ||
| L Middle Frontal Gyrus | 6 | 76 | 1.70 | 0.173 | ||
| R Medial Frontal Gyrus | 8 | 55 | 0.98 | 0.407 | ||
| Parietal Cortex | ||||||
|
R Precuneus
(Superior Parietal Lobule) |
7 | 333 | 4.11 | 0.009 | Adult Healthy Subject > Child Healthy Subject ***, Adult BD * |
|
|
R Inferior Parietal Lobule
(SupramarginalGyrus) |
40 | 70 | 3.17 | 0.029 | Child BD > Adult BD ** | |
| Temporal Cortex | ||||||
| L Middle Temporal Gyrus | 37 | 229 | 2.17 | 0.097 | ||
| R Middle Temporal Gyrus | 39 | 133 | 2.89 | 0.041 | Adult Healthy Subject > Adult BD * |
|
| R Middle Temporal Gyrus | 22 | 45 | 2.12 | 0.104 | ||
| Unsuccessful change versus Fixation | Frontal Cortex | |||||
| R PrecentralGyrus | 9 | 352 | 0.72 | 0.545 | ||
| L PrecentralGyrus | 6 | 21 | 0.32 | 0.813 | ||
| L Middle Frontal Gyrus | 6 | 86 | 0.38 | 0.769 | ||
| L Inferior Frontal Gyrus | 45 | 114 | 0.91 | 0.443 | ||
| L Middle Frontal Gyrus | 6 | 76 | 1.60 | 0.196 | ||
| R Medial Frontal Gyrus | 8 | 55 | 0.71 | 0.548 | ||
| Parietal Cortex | ||||||
|
R Precuneus
(Superior Parietal Lobule) |
7 | 333 | 3.46 | 0.020 | Adult Healthy Subject > Child BD **; Adult BD > Child BD * |
|
|
R Inferior Parietal Lobule
(SupramarginalGyrus) |
40 | 70 | 1.68 | 0.178 | ||
| Temporal Cortex | ||||||
| L Middle Temporal Gyrus | 37 | 229 | 1.31 | 0.277 | ||
| R Middle Temporal Gyrus | 39 | 133 | 2.03 | 0.117 | ||
| R Middle Temporal Gyrus | 22 | 45 | 0.53 | 0.660 | ||
BA = Brodmann's Area
= cluster size at least 20 contiguous voxels, uncorrected
MNI = Montreal Neurological Institute
= P<0.10
= P<0.05
= P<0.01
For our second post-hoc analysis to decompose the primary contrast, we examined go vs. fixation. Using the same significant clusters found during successful change vs. go, our examination of the go vs. fixation contrast revealed a pattern of hyperactivation across all clusters in adult healthy subject relative to CBD, child healthy subjects, and ABD. However, most of these differences failed to reach significance (Table 5). In the left superior parietal lobule, adult healthy subjects showed greater activation than CBD (P<0.001), while ABD showed a trend toward hyperactivation compared to CBD (P=0.09).
Finally, when GoRT was included as a covariate, the age × diagnosis interactions remained significant in the left insula (P<0.000), right precentral gyrus (P<0.001), left middle temporal gyrus (P<0.000), left superior temporal gyrus (P<0.000), right superior temporal gyrus (P<0.003), left superior parietal lobule (P<0.001), left paracentral lobule (P<0.001), and right postcentral gyrus (P<0.003).
3.3.2 Successful change vs. unsuccessful change
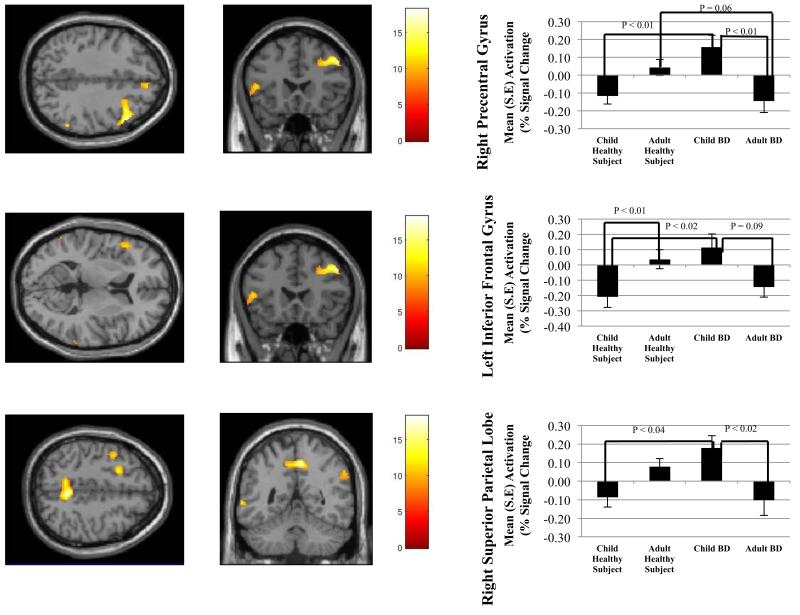
The findings on this contrast were similar to those on the successful change vs. go contrast. That is, there were significant between-group differences in a number of frontal, temporal, and parietal regions, with increased activation in CBD vs. child healthy subjects and ABD in some clusters, and increased activation in adult healthy subjects vs. ABD in others (Figure 2A-C). Specifically, age × diagnosis interactions were detected in right precentral gyrus (BA 9) (Figure 2A), left inferior frontal gyrus (BA 45) (Figure 2B), left precentral gyrus (BA 6), right medial frontal gyrus (BA 8), left middle frontal gyrus (BA 6), left and right middle temporal gyrus (BA 37, 39, and 22), right superior parietal lobule (BA 7) (Figure 2C), and right supramarginal gyrus (BA 40) (Table 4). There were more regions showing abnormal activation in children with BD than in adults with BD (Table 4).
Figure 2.
Mean activation in (A) right precentral gyrus, (B) left inferior frontal gyrus, and (C) right superior parietal lobule on successful change versus unsuccessful change contrast. Between group differences in percent signal change are depicted in the accompanying bar graphs.
Consistent with the results reported above, post-hoc analyses of the successful change vs. fixation contrast using significant clusters from the successful change vs. unsuccessful change contrast showed hyperactivation in CBD compared to ABD and child healthy subjects, and hyperactivation in adult healthy subjects compared to ABD. However, only two of these post-hoc comparisons reached significance (Table 5). In the right superior parietal lobule (Figure 2), adult healthy subjects showed hyperactivation vs. child healthy subjects (P<0.01). In the right supramarginal gyrus, CBD showed hyperactivation compared to ABD (P<0.03). When comparing unsuccessful change vs. fixation between groups using significant clusters from the successful change vs. unsuccessful change, we found hyperactivation in adult healthy subjects compared to CBD in the right superior parietal lobule (P<0.03).
3.4 Association of Clinical Variables with Brain Activation
3.4.1 Effect of Mood State
The age group × diagnosis interactions on the two primary contrasts remained significant in all clusters when only euthymic patients were included.
3.4.2 Comorbid ODD
The age group × diagnosis interactions on the two primary contrasts remained significant in all clusters when patients with comorbid ODD were excluded.
4. Discussion
Using fMRI and a response flexibility task in child and ABD vs. healthy subjects, we confirmed our hypothesis that, across age groups, BD is associated with impaired frontal and parietal activation during executive functioning. Moreover, consistent with the clinical literature suggesting that CBD is more severely impairing than ABD (Perlis, 2004; Birmaher, 2007), we also found that dysfunction is more marked in CBD than in ABD, since child patients had more regions showing abnormal activation than adult patients. Specifically, relative to the three other groups in the study (i.e., healthy youth, ABD, and healthy adults), CBD showed hyperactivation in MFG, IFG, insula, precuneus, and middle and superior temporal gyri. Relative to healthy adults, ABD showed hypoactivation in a more limited number of frontal, temporal, and parietal regions. Thus, our findings indicate that, while all BD patients show brain dysfunction during successful signal detection and response switching, CBD showed increased engagement in regions mediating inhibition, cognitive control, sustained attention, and signal detection, while ABD showed decreased engagement in a subset of these regions. Finally, a previous study of response flexibility in healthy subjects revealed increased frontal, temporal, and parietal cortex activation in children compared to adults (Thomas et al., 2011). This suggests that the observed hyperactivation in CBD and hypoactivation in ABD may represent the extremes of the normal hyper- vs. hypoactivation found in healthy children and adults, respectively. Of note, none of our primary analyses survived whole-brain family-wise error corrected threshold. However, we then adopted a threshold that has been suggested as appropriately balancing Type I and II error (Lieberman and Cunningham, 2009).
We found that successful change trials were associated with precuneus and inferior parietal cortex hyperactivation in CBD, and hypoactivation in ABD. The parietal cortex mediates sustained attention and attention switching, which are central psychological processes in response flexibility tasks (Downar et al., 2001; Tamm et al., 2004; Rubia, et al., 2007a). Previous behavioral studies demonstrate that both adult and pediatric BD patients have deficits in sustained attention and attention flexibility (Dickstein et al., 2004; Iverson et al., 2009) compared with healthy subjects. However, no studies compare adults and children with BD on this or other neuropsychological functions. Such data would be helpful in elucidating the neural underpinnings of the more severe clinical course observed in children vs. adults with BD. The current study is the first to examine parietal cortex activation in ABD during attention switching and response flexibility. Yet, the present parietal cortex findings in CBD support existing evidence that young patients have parietal hyperactivation in tasks requiring attention switching (Dickstein et al., 2010).
A similar pattern was seen in the frontal cortex during successful change trials, with CBD showing hyperactivation relative to healthy youth (and ABD) and adult patients showing hypoactivation relative to healthy adults (and CBD). When successful change trials were contrasted with go trials, this pattern was evident in the right precentral gyrus and insula, whereas when successful change trials were contrasted with unsuccessful change trials, this pattern was present in MFG, bilateral precentral gyri, and IFG. The MFG is activated when detecting a target that guides a motor response (Downar et al., 2001), while the IFG plays a central role in inhibiting motor responses (Aron et al., 2004) and the insula is activated during cognitive conflict (Bunge et al., 2002). Behavioral studies indicate that BD patients are impaired in signal detection (Clark et al., 2002; Doyle et al., 2005) and motor inhibition (Swann et al., 2003). In a partially overlapping sample of CBD and young healthy subjects, we found MFG and insula hyperactivation in BD patients during successful change vs. go trials (Nelson et al., 2007), similar to the MFG and insula hyperactivation we report here in CBD compared to child healthy subjects. In addition, several studies show IFG hypoactivation during motor inhibition in both BD youth and adults (Mazzola-Pomietto et al., 2009; Passarotti et al., 2010); while we found IFG hypoactivation in ABD vs. adult healthy subjects, we found the opposite pattern in CBD vs. child healthy subjects. The current study therefore extends the existing literature, by showing that MFG, insula, and IFG dysfunction exists during response flexibility in both adults and youth with BD, although the direction of the dysfunction varies developmentally.
As in the frontal and parietal lobes, CBD show hyperactivation of middle and superior temporal gyri during successful change trials, while ABD showed hypoactivation. In response flexibility paradigms, the middle and superior temporal gyri mediate the detection of behaviorally salient visual cues (Braver et al., 2001; Tamm et al., 2004). A previous behavioral study showed that ABD are impaired in detecting behaviorally salient visual cues compared to healthy subjects (Clark et al., 2002), while pediatric BD patients showed temporal gyri hypoactivation during a task requiring signal detection to inhibit motor responses, differences that were reversed with pharmacotherapy (Pavuluri et al., 2010). Taken together, temporal gyri dysfunction is important in BD during response flexibility, and may underlie the difficulty in detecting behaviorally salient stimuli important in switching motor responses.
While brain activity differed between BD patients and healthy subjects during response flexibility, BD patients did not differ from healthy subjects in the speed at which they responded to change cues (CSRT) or go cues (GoRT). While our groups did not differ in CSRT, neural measures may be more sensitive than behavioral measures in distinguishing patients from healthy subjects (Wilkinson and Halligan, 2004). Adults showed longer GoRT than children (Table 3). Importantly, our age × diagnosis interaction effects remained significant when including GoRT as a covariate in the analysis, indicating our findings were not due to differences in GoRT between children and adults. The finding of increased change accuracy in adult healthy subjects compared to ABD likely reflects the inability of the task algorithm to completely correct for between-group differences in the speed-accuracy tradeoff.
This study has important limitations. First, we eliminated 44% of scanned subjects; while this potentially limits the generalizability of our findings, this rate of exclusion is not unusual in fMRI studies including young participants (Pliszka et al., 2006). Most of the patients in this study were medicated at the time of testing. Previous work, however, suggests that medication may diminish behavioral and neural differences between BD patients and healthy subjects (Hafeman et al., 2012), and therefore may be more likely to cause Type II errors compared to Type I errors in neuroimaging studies (Phillips et al., 2008). In addition, our patient groups were heterogeneous; we included both BD-I and BD-II patients in a variety of mood states. While pediatric and adult patients did not differ on these clinical variables, future studies should include larger samples to more systematically address the impact of diagnostic subtype and mood state on brain activation across development. In addition, we did not have complete data on socioeconomic status or race from our study groups. Finally, this study is limited in its ability to distinguish whether the age × diagnosis interactions we found are due to changes in the brain activation of early onset patients as the brain develops from childhood into adulthood, or because the neural correlates of the disease are different in early vs. late onset illness. Indeed, since the number of acute illness episodes may be associated with structural neuroanatomical abnormalities in BD (Brambilla et al., 2001; Strakowski et al., 2004; Takahashi et al., 2010), length and severity of illness are potential factors that may contribute to the child BD vs. adult BD differences in the present study. Studies using longitudinal designs comparing imaging data from similarly-aged patients with different ages of onset, relative to an age-matched healthy sample, will be important in disentangling these two possibilities.
This is the first study to compare the neural mechanisms mediating response flexibility in CBD vs. ABD. Our results indicate that the frontal, temporal, and parietal network important in executive functioning, attention, arousal, and emotion regulation is abnormally activated in BD during successful response flexibility, specifically by hyperactivation in CBD and hypoactivation in ABD. This extends existing evidence that the nature of the frontal dysfunction in BD varies developmentally (Weathers et al., 2012) and indicates developmental differences in the direction of temporal and parietal dysfunction in patients with BD. Further, existing literature indicates earlier age at onset of BD illness is associated with higher rates of comorbid illness and number of recurrences, and shorter periods of euthymia (Perlis, 2004; Birmaher, 2007); furthermore, these phenotypic differences may be associated with smaller amygdala volume (Blumberg et al., 2003; Chang et al., 2005). This, together with our findings of increased cortical dysfunction in early onset compared to late onset BD, suggests that the severe course of early relative to late onset illness may be due to both increased cortical dysfunction during executive functioning, and smaller amygdala volume during emotional processing (Blumberg et al., 2003). Future longitudinal studies can determine whether this developmental difference in activation reflects different illness subtypes or, instead, different points on the developmental trajectory of BD.
Disclosures and acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services. Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. We thank the participants and their families who made this research possible and the staff of the Emotion and Development Branch at NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Birmaher MDB. Longitudinal Course of Pediatric Bipolar Disorder. American Journal of Psychiatry. 2007;164:537–539. doi: 10.1176/ajp.2007.164.4.537. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of general psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of basal ganglia in bipolar disorder patients. Psychiatry Research: Neuroimaging. 2001;106:65–80. doi: 10.1016/s0925-4927(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior Cingulate Cortex and Response Conflict: Effects of Frequency, Inhibition and Errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Jean-Baptiste P. Region of interest analysis using an SPM toolbox [abstract]; Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature Frontal Lobe Contributions to Cognitive Control in Children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Fitzgerald KD, Taylor SF, Weissman DH. Removing the effect of response time on brain activity reveals developmental differences in conflict processing in the posterior medial prefrontal cortex. NeuroImage. 2012;59:853–860. doi: 10.1016/j.neuroimage.2011.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced Amygdalar Gray Matter Volume in Familial Pediatric Bipolar Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. The British Journal of Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disorders. 2010;12:707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biological Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The Effect of Task Relevance on the Cortical Response to Changes in Visual and Auditory Stimuli: An Event-Related fMRI Study. NeuroImage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Wilens TE, Kwon A, Seidman LJ, Faraone SV, Fried R, Swezey A, Snyder L, Biederman J. Neuropsychological Functioning in Youth with Bipolar Disorder. Biological Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- First MB, S RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2002. [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Young AH. Rapid Computerized Assessment of Neurocognitive Deficits in Bipolar Disorder. Applied Neuropsychology. 2009;16:207–213. doi: 10.1080/09084280903098778. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory Motor Control in Response Stopping and Response Switching. The Journal of Neuroscience. 2010;30:8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining Clinical Phenotypes of Juvenile Mania. American Journal of Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin J, Anton J, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania✩. Journal of Psychiatric Research. 2009;43:432–441. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. The American journal of psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, Rich BA, Brotman MA, Pine DS, Leibenluft E. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders. 2007;9:810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, et al. Diagnostic Interview for Genetic Studies: Rationale, unique features, and training. Archives of general psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. 2010;181:36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced Prefrontal Function With Pharmacotherapy on a Response Inhibition Task in Adolescent Bipolar Disorder. The Journal of Clinical Psychiatry. 2010;71:1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis R. Long-Term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biological Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Phillips MDM, Travis MDM, Fagiolini MDA, Kupfer MDD. Medication Effects in Neuroimaging Studies of Bipolar Disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka MDS, Glahn PDD, Semrud-Clikeman PDM, Franklin BSC, Perez BSR, Iii, Xiong PDJ, Liotti MDPDM. Neuroimaging of Inhibitory Control Areas in Children With Attention Deficit Hyperactivity Disorder Who Were Treatment Naive or in Long-Term Treatment. American Journal of Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary Studies of the Reliability and Validity of the Children’s Depression Rating Scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal Lobe Dysfunction in Medication-Naïve Boys With Attention-Deficit/Hyperactivity Disorder During Attention Allocation and Its Relation to Response Variability. Biological Psychiatry. 2007a;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007b;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, Howe M, Reiss A. Neural correlates of response inhibition in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Cerullo MA, Eliassen JC, Lamy M, Fleck DE, Lee J-H, DelBello MP. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Intervention in Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2004;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. Journal of Affective Disorders. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Malhi GS, Wood SJ, Yücel M, Walterfang M, Nakamura K, Suzuki M, Pantelis C. Midline brain abnormalities in established bipolar affective disorder. Journal of Affective Disorders. 2010;122:301–305. doi: 10.1016/j.jad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-Related fMRI Evidence of Frontotemporal Involvement in Aberrant Response Inhibition and Task Switching in Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Thomas LA, Hall JM, Skup M, Jenkins SE, Pine DS, Leibenluft E. A developmental neuroimaging investigation of the change paradigm. Developmental Science. 2011;14:148–161. doi: 10.1111/j.1467-7687.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, Brotman MA, Zarate JCA, Connolly ME, Fromm SJ, LeBourdais SB, Pine DS, Leibenluft E. A Developmental Study of the Neural Circuitry Mediating Motor Inhibition in Bipolar Disorder. American Journal of Psychiatry. 2012;169:633–641. doi: 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD) New York Psychiatric Institute; New York: 1988. [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]