Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is associated with Th2-dominant inflammation. Thymic stromal lymphopoietin (TSLP) is a cytokine that triggers dendritic cell-mediated Th2 inflammatory responses and that enhances IL-1-dependent Th2 cytokine production in mast cells. Although elevated levels of TSLP mRNA have been found in nasal polyps (NPs), expression of TSLP protein and its function in CRS have not been fully explored.
Objectives
The objective of this study was to investigate the role of TSLP in CRS.
Methods
We investigated the presence and stability of TSLP protein in NPs by ELISA and western blot, and the function of TSLP in nasal tissue extracts with a bioassay based upon activation of human mast cells.
Results
Although TSLP mRNA was significantly increased in NP tissue from patients with CRSwNP compared to uncinate tissue from patients with CRS or control subjects, TSLP protein was significantly decreased in NP tissue as detected by the commercial ELISA kit. We found that recombinant TSLP was time-dependently degraded by NP extracts and this degradation was completely inhibited by a protease inhibitor cocktail, suggesting that TSLP is sensitive to tissue proteases. Interestingly, NP extract-treated TSLP had higher activity in mast cells, although the amount of full length TSLP was reduced up to 85%. NP extracts significantly enhanced IL-1β-dependent IL-5 production in mast cells compared with uncinate tissue homogenates, and responses were significantly inhibited by anti-TSLP, suggesting that NP contain biologically relevant levels of TSLP activity.
Conclusion
TSLP and its metabolic products may play an important role in the inflammation in CRSwNP.
Keywords: Chronic rhinosinusitis, Nasal polyps, TSLP, Epithelial cells, Mast cells, Th2 cells, IL-5, Proteases
INTRODUCTION
Chronic rhinosinusitis (CRS) is a heterogeneous disease characterized by local inflammation of the upper airways and which persists for at least 12 weeks. CRS is one of the most common chronic diseases in adults in the United States, affecting over 10 million Americans, and has a severe impact on patients' quality of life.1–4 CRS is frequently divided into two groups based on histology and physical examination: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). In general, CRSwNP is associated more closely with clinical complaints of nasal obstruction and olfactory loss and is characterized by eosinophilia and Th2-related inflammation, especially in western countries.5, 6 However the mechanisms underlying the amplification of Th2-related inflammation in CRSwNP have not been identified.
Thymic stromal lymphopoietin (TSLP), an epithelial cell derived cytokine, is now widely recognized as a master regulator of Th2 inflammation. TSLP is an IL-7-like cytokine that stimulates dendritic cells (DCs) to induce naive CD4+ T cell differentiation into Th2 cells.7–13 Although TSLP alone is not sufficient to stimulate mast cells, TSLP synergizes with the inflammatory cytokine IL-1 to potently activate mast cells to produce Th2 cytokines including IL-5 and IL-13.14, 15 TSLP, produced mainly by epithelial cells, is also known to be produced by skin keratinocytes, stromal cells, smooth muscle cells, fibroblasts and mast cells.7, 9, 13, 16 The TSLP receptor is a heterodimeric receptor consisting of the IL-7 receptor alpha chain (IL-7RA) and a common γ-like TSLP specific receptor (TSLPR).11–13 Considerable evidence now implicates TSLP in the pathogenesis of several Th2-related inflammatory diseases including atopic dermatitis, bronchial asthma and eosinophilic esophagitis.9, 17–20
Although CRSwNP is a Th2-related disease, there are limited studies investigating the role of TSLP in CRS and in NPs in particular.21, 22 In this study, we investigated the presence and activity of TSLP in CRS and found that TSLP activity was elevated in NPs. Additionally, we found that TSLP was processed by endogenous proteases in tissue which might convert it to a more active form.
METHODS
Patients and biopsies
CRS patients were recruited from the Allergy-Immunology clinic and the Otolaryngology clinic of the Northwestern Medical Faculty Foundation (NMFF) and the Northwestern Sinus Center at NMFF. Sinonasal and NP tissues were obtained from routine functional endoscopic sinus surgery in patients with CRS. All subjects met the criteria for CRS as defined by the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force.2 Patients with an established immunodeficiency, pregnancy, coagulation disorder, Churg-Strauss syndrome, diagnosis of classic allergic fungal sinusitis or cystic fibrosis were excluded from the study. Details of subjects' characteristics are included in Table I and in the Methods section in the Online Repository. All subjects signed informed consent forms and the protocol governing procedures for this study was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Table 1.
Subject characteristics
| Control | CRSsNP | CRSwNP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total no. of subjects | n=47 (24M) | n=60 (22M) | n=86 (62M) | ||||||
| Age (y), median (range) | 47* (16–78)# | 35 (18–67) | 44 (26–73) | ||||||
| Y | N | U | Y | N | U | Y | N | U | |
| Atopy | 2 | 41 | 4 | 29 | 26 | 5 | 45 | 25 | 16 |
| Asthma | 0 | 46 | 1 | 11 | 47 | 2 | 39 | 47 | 0 |
| Aspirin intolerance | 0 | 47 | 0 | 0 | 60 | 0 | 1 | 85 | 0 |
| Nasal steroid | 0 | 47 | 0 | 6 | 53 | 1 | 10 | 73 | 3 |
| Inhaled steroid | 0 | 47 | 0 | 5 | 54 | 1 | 9 | 74 | 3 |
| Oral steroid | 2 | 45 | 0 | 2 | 57 | 1 | 17 | 66 | 3 |
| Methodologies used | UT | UT | UT | NP | |||||
| Epithelial scrapings | n=21 (13M) | n=32 (12M) | n=22 (17M) | n=36 (28M) | |||||
| Tissue RNA | n=16 (7M) | n=16 (5M) | n=17 (HM) | n=29 (24M) | |||||
| Tissue Extracts | n=17 (8M) | n=25 (9M) | n=24 (15M) | n=35 (24M) | |||||
| TSLP bioassay | n=10 (5M) | n=11 (6M) | n=12 (7M) | n=26 (20M) | |||||
median
(range).
M; male, Y; yes, N; no, U; unknown.
Cell culture
Human primary nasal epithelial cells (PNECs) were collected from the uncinate tissue (UT) or NP tissue by curettage with a Rhinoprobe™ (Arlington Scientific, Springville, UT) as described previously.23 Human peripheral blood-derived mast cells were obtained as described previously.24 Recombinant TSLP was preincubated with BSA (control) or NP extracts for 24 hours and then mast cells were stimulated with those mixtures in the presence of 20 ng/ml IL-1β for 48 hours. Further details can be found in the Methods section in the Online Repository.
Real-time PCR
Real-time RT-PCR was performed using the TaqMan method, as described previously.25 Primer and probe sets were purchased or were synthesized from Applied Biosystems (Foster City, CA). The mRNA expression levels were normalized to the median expression of the housekeeping genes, β-glucuronidase (in vivo) or β-actin (in vitro experiments). Details can be found in the Methods section in the Online Repository.
ELISA, cytometric bead array (CBA) and Western blot
The concentration of TSLP in cell free supernatants was determined by a commercial ELISA kit (R&D systems, Minneapolis, MN). The minimal detection limit for this kit is 15.6 pg/ml. The concentration of TSLP in tissue homogenates was normalized to the concentration of total protein as detected by BCA protein assay kit (ThermoScientific, Rockford, lL). The concentration of IL-5 in cell-free supernatants was measured using a CBA flex set from BD Biosciences (San Jose, CA). The limit of detection is 2.5 pg/ml. Western blot analysis was performed using 50 ng/ml biotinylated goat anti-human TSLP antibody (R&D systems). Further details can be found in the Methods section in the Online Repository.
Statistics
All data are reported as the median (25–75% interquartiles) or as the mean ± SEM. Differences between groups were analyzed using 1-way ANOVA or the paired Student's t test. Correlations were assessed using the Spearman's rank correlation. A p value of less than 0.05 was considered significant.
RESULTS
TSLP expression in CRS
To examine whether nasal epithelial cells can produce TSLP, we collected epithelial scrapings and cultured primary nasal epithelial cells (PNEC). We found that double stranded RNA (dsRNA) strongly induced the production of TSLP (73.8 ± 12.8 pg/ml, n=5, Fig E1, B) and IL-4 synergistically enhanced dsRNA-dependent TSLP production (149.7 ± 10.8 pg/ml, n=3, Fig E1, B) in PNEC (details can be found in the Results section in the Online Repository). This suggests that nasal epithelial cells produce TSLP.
Since we found evidence that the resident epithelial cells in nasal mucosa can produce TSLP, we examined the relevance of TSLP in CRS. To measure the expression of TSLP in CRS, uncinate tissues (UT), nasal polyp (NP) tissues, and epithelial scrapings were collected from patients with CRSsNP and CRSwNP, and also from control subjects. We found that TSLP mRNA was significantly increased in epithelial scrapings from NP compared to UT from controls and CRSsNP (Fig 1, A). We also examined the expression of TSLP and TSLPR in whole sinus tissue and found that mRNAs for TSLP and TSLPR were significantly increased in NP tissue from patients with CRSwNP in comparison to UT from either patients with CRS or control subjects (Fig 1, B and C). Interestingly, TSLP expression positively correlated with TSLPR in sinus tissue (Fig 1, D). However, we found no significant difference in TSLP levels between atopic and non atopic patients or asthmatic and non asthmatic patients (Fig E2). To examine this observation at the protein level, we prepared detergent extracts of UT and NP tissues and measured the concentration of TSLP via ELISA. Surprisingly, TSLP protein was significantly decreased in NP tissue from CRSwNP (n=35; 27.8 ± 6.4 pg/mg) compared to UT from CRSsNP (p<0.001, n=25; 51.2 ± 6.2 pg/mg), CRSwNP (p<0.001, n=24; 41.9 ± 5.0 pg/mg) and controls (p<0.001, n=17; 153.2 ± 31.0 pg/mg) (Fig 1, E). Levels of TSLP protein in control UT were higher than in any other tissue set (p<0.05).
Figure 1.
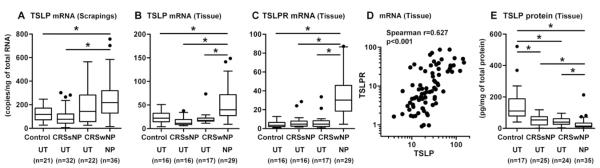
Increased expression of TSLP in nasal polyps. Total RNA was extracted from epithelial scrapings (A) and whole tissue (B–D) from nasal polyps (NP) and uncinate tissue (UT) from control subjects and patients with CRS without NP (CRSsNP) and CRS with NP (CRSwNP). Expression of mRNAs for TSLP and TSLPR was analyzed using real-time PCR (A–D). The correlations were assessed by using the Spearman rank correlation (D). Expression of TSLP protein in tissue homogenates was measured using ELISA (E). TSLP protein concentration was normalized to the concentration of total protein (E). The results are shown as the median (25–75% interquartiles). * p < 0.05, 1-way ANOVA.
Stability of TSLP in nasal polyps
It is known that TSLP is susceptible to mast cell proteases and that mast cells are elevated in NPs.26, 27 In addition, inflammatory cells including eosinophils, neutrophils and macrophages that contain various proteases are known to be elevated in NP,5, 28 and NP contain various tissue proteases.29 To test whether the decreased TSLP protein detected in NP tissue reflects breakdown of TSLP, we incubated recombinant TSLP with 1 mg/ml NP extracts for 24 and 48 hours and determined the concentration of TSLP by ELISA. To protect against the absorption of recombinant TSLP onto the incubation tube, TSLP was also incubated with the equivalent concentration of BSA as a control. We normally generate tissue extracts in the presence of a protease inhibitor cocktail (PIC) to prevent the degradation of molecules during homogenization. In this experiment, we needed to assess the effect of protease activity in the tissue extracts. Therefore we prepared tissue extracts without protease inhibitors for use in the TSLP stability assay and TSLP cleavage assay. We found that immunoreactive TSLP detected in the ELISA was significantly reduced by exposure to NP extracts (p<0.001, 75.2 ± 3.4 % reduction at 24 hours and 82.8 ± 2.6 % reduction at 48 hours, n=7) but not by BSA controls (Fig 2, A). We next assessed whether the observed reduction in immunoreactivity to TSLP was due to complete degradation of TSLP, truncation of TSLP, or both. Recombinant TSLP was incubated with 1 mg/ml NP tissue extracts or BSA for 24 hours and the stability of TSLP was assessed by western blot. We found that TSLP was cleaved by NP extracts, although the extent of truncation and degradation varied among patient samples (Fig 2, B and Fig E3). Importantly, we detected truncated TSLP in all samples and the molecular weight was approximately10–11 KDa (Fig 2, B). We evaluated the cleaved products using SDS-PAGE and Coomassie Blue staining and found that the major truncated product was a 10 KDa protein and minor products were approximately 5 KDa and 3.5 KDa proteins (n=3, data not shown). Interestingly, a cleaved 10 KDa protein was time-dependently generated by NP extracts, while full-length TSLP protein was time dependently reduced (n=3, Fig 2, C and Fig E3, B). When we incubated TSLP and NP extracts in the presence of a PIC or DMSO (vehicle control), we found that PIC completely blocked NP-dependent degradation of TSLP, suggesting that TSLP was truncated by endogenous tissue proteases (n=3, Fig 2, D).
Figure 2.
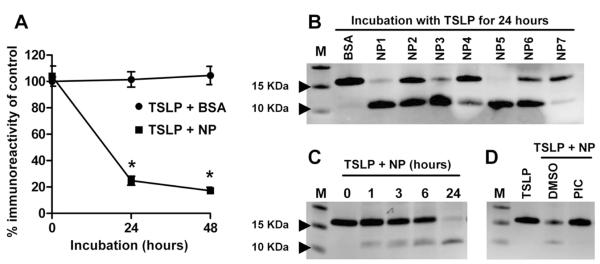
TSLP protein was cleaved by NP extracts. Recombinant TSLP was incubated with 1 mg/ml NP tissue extracts or 1 mg/ml BSA (control) for 24–48 hours, and the stability of TSLP was determined by ELISA (A) (n=5). TSLP was incubated with 1 mg/ml BSA or 1 mg/ml NP tissue extracts from donor 1–7 (NP1–7) for 24 hours, and cleavage of TSLP was determined by western blot (B). TSLP was incubated with 1 mg/ml NP extracts for 0–24 hours (C). TSLP was incubated with NP extracts in the presence of 1% protease inhibitor cocktail (PIC) or 1% DMSO (vehicle control) for 24 hours. The results are representative of three separate experiments with separate donors (B–D). * p < 0.05, paired Student t test.
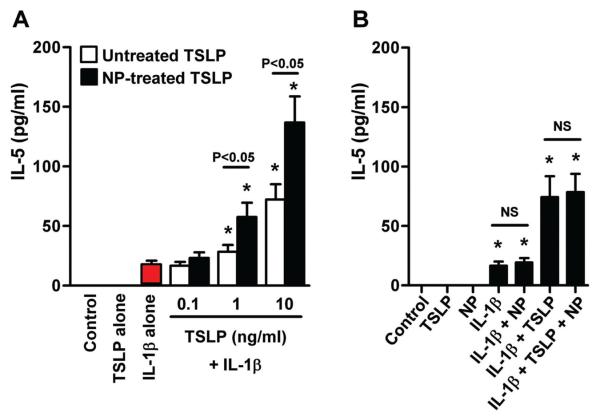
Endogenous proteases are involved in the inactivation of proteins by degradation and participate in processing of inactive pro-proteins into their biologically active forms. Therefore we examined whether NP-treated TSLP possessed biological activity. Allakhverdi et al. have reported that TSLP is a key enhancer of IL-1-dependent Th2 cytokine production in mast cells, and we have recently confirmed their findings.14, 15 Since this response can be inhibited by anti-TSLP antibody,15 we employed human mast cells in a TSLP activity bioassay. To test for TSLP activity, mast cells were stimulated with NP-treated- and untreated-TSLP in the presence of 20 ng/ml IL-1β. TSLP dose dependently enhanced IL-1β-dependent IL-5 production in mast cells (Fig 3, A). Importantly, this enhancement of potency was time dependently increased by preincubation with NP extracts (Fig E4) and potency was significantly higher in 24 hour NP-treated TSLP (136.8 ± 22.1 pg/ml at 10 ng/ml TSLP, n=8, p<0.05) than untreated TSLP (72.2 ± 12.9 pg/ml at 10 ng/ml TSLP, n=8) (Fig 3, A), even though immunoreactivity in the TSLP ELISA kit was reduced up to 85% (Fig 2, A). To exclude the possibility that NP products directly enhanced TSLP or IL-1β activity, mast cells were stimulated with IL-1β and TSLP in the presence of the same concentration of NP extracts (4 μg/ml) but without the preincubation. We found that NP extract did not directly enhance IL-1β- and IL-1β/TSLP-dependent IL-5 production in mast cells (n=6, Fig 3, B) These results suggest that enhanced activity of TSLP requires preincubation with NP extracts and cleaved TSLP may have higher activity than the full-length molecule.
Figure 3.
NP extract-treated TSLP had higher activity in mast cells. TSLP was preincubated with NP extract for 24 hours. Mast cells were then stimulated with NP-treated TSLP and untreated TSLP in the presence of 20 ng/ml IL-1β for 48 hours (n=8) (A). Mast cells were stimulated with 4 μg/ml NP extracts, 20 ng/ml IL-1β 10 ng/ml TSLP or their combination, but without preincubation, for 48 hours (n=5) (B). Concentration of IL-5 was measured by CBA. *; p<0.05 compared to IL-1β alone (A, red bar) or medium control (B). NS, not significant.
TSLP activity in nasal polyps
Our results suggest that TSLP synthesis might be upregulated in NPs and the TSLP produced was truncated by proteases to create more active forms. We therefore examined whether endogenous TSLP activity was elevated in NPs even though TSLP concentration via ELISA was reduced. We generated tissue extracts from UTs and NPs and then stimulated mast cells with 300 μg/ml tissue extracts in the presence of IL-1β. We found that NP extracts significantly enhanced IL-1β-dependent IL-5 production in mast cells (191.5 ± 28.8 pg/ml, n=26, p<0.05) compared with UT from control (76.5 ± 34.3 pg/ml, n=10), CRSsNP (76.0 ± 19.8 pg/ml, n=11) and CRSwNP (106.0 ± 26.8 pg/ml, n=12) and compared with IL-1β alone (24.9 ± 5.2 pg/ml, n=4) (Fig 4, B) or tissue extracts alone (Fig E5). In contrast, tissue extracts alone did not stimulate mast cells (Fig 4, A). Furthermore, we found that NP extract-dependent IL-5 production was significantly inhibited by neutralization with an anti-TSLP antibody (n=10, p<0.05, Fig 4, C). These results suggest that TSLP activity is upregulated in NPs, possibly reflecting a cleavage process leading to a product that has gained bioactivity.
Figure 4.
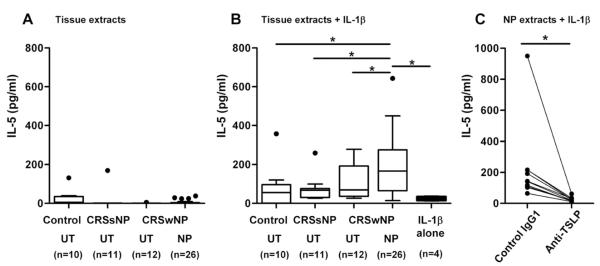
TSLP activity was elevated in NP. Mast cells were stimulated with 300 μg/ml UT tissue extracts or 300 μg/ml NP extracts in the presence (B) or absence (A) of 20 ng/ml IL-1β for 48 hours. Mast cells were stimulated with 300 μg/ml NP extracts and 20 ng/ml IL-1β in the presence of control mouse IgG1 or anti-TSLP (n=10) (C). Concentration of IL-5 was measured by CBA. Concentration of IL-5 in tissue extracts was subtracted. The results are shown as the median (25–75% interquartiles). * p < 0.05, 1-way ANOVA (A, B) and paired Student t test (C).
Since TSLP activity was increased in NPs, we examined whether expression of TSLP correlated with Th2 inflammation in sinus mucosa. TSLP expression positively correlated with markers of Th2 inflammation including IL-5 (r=0.637, p<0.001), IL-13 (r=0.532, p<0.001) and CCL17 (TARC; r=0.619, p<0.001) and with markers of eosinophilia including ECP (r=0.326, p=0.005), and Charcot-Leyden crystal protein (CLC; r=0.614, p<0.001) but not with markers of Th1 (IFN-γ; r=0.063, p=0.581) or Th17 (IL-17A; r=0.032, p=0.783) inflammation in sinus mucosa (n=78, Fig 5 and E6).
Figure 5.
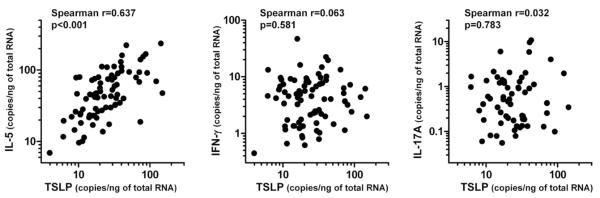
Correlation of TSLP and markers of Th1/Th2/Th17 inflammation in sinus tissue. Messenger RNA for TSLP, IL-5, IFN-γ and IL-17A in sinus tissue that was used in Figure 1B was assessed by real-time PCR (n=78). The correlations were assessed by using the Spearman rank correlation.
DISCUSSION
CRSwNP is characterized by Th2-mediated inflammation and eosinophilia.5, 6 However the role of TSLP in CRSwNP is not well understood. In the current study we demonstrated that TSLP mRNA was significantly increased in NPs and significantly correlated with markers of Th2 inflammation in nasal mucosa (Fig 1 and 5). Although TSLP protein was reduced in NPs as detected by commercial ELISA, TSLP activity was significantly elevated in NPs (Fig 1 and 4). We also found that TSLP protein was truncated by putative endogenous proteases in NPs and that the cleavage product might have more potent activity than its full-length form (Fig 2 and 3).
Although we found that TSLP mRNA was elevated in NPs, the TSLP producing cells in NP are not clearly defined. We therefore initiated immunohistochemistry studies using anti-human TSLP antibody. We found that anti-TSLP antibody did not stain epithelial cells but did stain some inflammatory cells (n=4, Fig E7, A), even though elevated expression of TSLP mRNA was observed in epithelial cells from NPs (Fig 1, A). Therefore we examined whether epithelial cells stored TSLP or released it immediately after stimulation. Primary normal human bronchial epithelial cells were stimulated with dsRNA or dsRNA+IL-4 for 24 hours and subsequently supernatants and cell lysates were collected. We found that TSLP protein was detected in supernatants after stimulation with dsRNA (49.1 ± 4.8 pg/ml) or dsRNA+IL-4 (186.4 ± 30.2 pg/ml), but not in cell lysates (n=3, Fig E7, B). These results suggest that more than 95% of the TSLP protein synthesized was released from epithelial cells within 24 hours, possibly explaining the absence of TSLP protein detected by immunohistochemistry.
Glucocorticoids are widely used in the therapeutic management of inflammatory airway diseases and are known as one of the mainstay drugs in the treatment of CRS. In addition, we previously found that a corticosteroid (fluticasone propionate) inhibited dsRNA-dependent TSLP production in bronchial epithelial cells.7 When we evaluated whether overexpression of TSLP in patients with CRSwNP was associated with glucocorticoid treatment, we found no significant difference in TSLP levels based upon the status of steroid treatment although it was slightly reduced in treated subjects (Fig E2, C). Since patients on steroids tend to have more severe disease and since we did not have large enough subgroups of patients to make firm conclusions about the effect of steroid treatment (n=5), future studies will be required to test this question prospectively in a larger cohort.
We found that TSLP mRNA was increased but TSLP protein was decreased in NP tissues, indicating that TSLP protein might not be stable in tissues. Moreover, we found that TSLP protein was time-dependently degraded by NP extracts in the absence of inhibitors of endogenous proteases. To address the discrepancy between TSLP mRNA and protein in NPs, we initially hypothesized that TSLP was truncated only by NP extracts but not by UT extracts and/or the antibodies used in the ELISA did not recognize truncated TSLP. However we found that UT tissue extracts also degraded recombinant TSLP (Fig E8, A). In addition, both capture and detection antibodies in the ELISA kit detected truncated 10 KDa TSLP by western blot, although antibodies in the ELISA kit had weaker binding to truncated TSLP than full-length TSLP when compared using SDS-PAGE (Fig E8, B and not shown). Although we cannot make a firm conclusion, this apparent reduced affinity of the ELISA antibodies for cleaved TSLP may in part explain why the ELISA shows lower TSLP protein concentration in NPs. We also hypothesized that the TSLP ELISA kit might have more nonspecific reactivity to UT proteins than to NP. We found that nonspecific reactivity appears to be higher in control UT than NP although we did not find a relationship between the concentration detected by ELISA and staining intensity by western blot (Fig E8, B and not shown). These results indicate that the discrepancy of TSLP mRNA and protein may be in part due to reduced sensitivity for truncated TSLP and nonspecific reactivity detected by the commercial ELISA. However we cannot make firm conclusions because western blot is not a quantitative assay system. Future studies using definitive assays such as GC-MS or LC-MS that are highly specific and sensitive for TSLP protein and TSLP degradation products will be required. The important point in this regard is that both TSLP mRNA and TSLP activity are highly elevated in NP tissue.
Several groups have reported that human TSLP is upregulated in Th2-related diseases, however, most groups have shown the elevation of TSLP mRNA by PCR and in situ hybridization or the over expression of TSLP protein using immunohistochemistry.9, 17, 19, 20 Immunohistochemistry is not widely considered a sensitive or reliable quantitative assay, making it challenging to determine the levels of TSLP protein in tissues. However, to date, no investigators have reported successful detection or quantitation of TSLP protein in tissues of patients with asthma or atopic dermatitis. These results suggest that TSLP protein may not be stable in tissues of other Th2-related diseases and/or current TSLP quantification systems may not have sufficient sensitivity and specificity to detect TSLP in tissue, thus underscoring the challenge of reporting the elevation in TSLP protein in tissues by quantitative methods including ELISA.
We provide here the first evidence that TSLP may be posttranslationally modified by proteases in tissue to generate more active forms. Interestingly, we found that a cleaved 10 KDa protein was also time-dependently generated by NP extracts (Fig 2). Comeau et al. identified a putative furin cleavage site in the C-terminus of human TSLP protein that was not present in any rodent sequence identified to date.13, 31–33 Future studies will be required to identify the sequence of cleaved TSLP and to test whether furin is a key enzyme for this truncation.
We also examined whether we can detect endogenous TSLP in tissue extracts by western blot. We detected 25 KDa and 20 KDa proteins by western blot using anti-TSLP and those bands were stronger in NP extracts than control UT extracts (Fig E8, B). Reche et al reported that human TSLP had putative N-glycosylation sites and hTSLP adenovirus vector-infected human 293T cells produced a 23 kDa TSLP protein.34 Future studies will be required to examine whether those protein bands are TSLP.
We recently investigated the presence of mast cells in CRS and found that mast cells were elevated only within the NP epithelium in patients with CRSwNP.27 Furthermore, we reported that airway epithelial cells directly promoted Th2 cytokine production in mast cells during viral infection via the production of IL-1 and TSLP, and this occurred only in a Th2 microenvironment.15 It is conceivable that active forms of TSLP protein in NP tissue may act in concert with inflammatory cytokines to stimulate Th2 cytokine production in mast cells. To this effect, we found NP extracts had significantly enhanced IL-1-dependent IL-5 production in mast cells compared to UT extracts from control subjects and patients with CRS, and this was significantly blocked by anti-TSLP, suggesting that TSLP activity was upregulated in NP (Fig 4). In contrast, Figure 3B showed that NP extracts did not directly enhance IL-1β-dependent IL-5 production in mast cells. This discrepancy can be explained by the fact that we used 75-fold lower concentrations of NP extracts (4 μg/ml) in Figure 3 compared to Figure 4 (300 μg/ml). Indeed we found that NP extracts dose dependently enhanced IL-1-dependent IL-5 production and 4 μg/ml of NP extract was not adequate to significantly enhance this reaction (Fig E9, A). In addition, 4 μg/ml NP extracts could not induce truncation of TSLP (Fig E9, B). It will be important to test whether TSLP activity is elevated and whether cleavage products can be detected in affected tissue in other Th2-related diseases.
We also found upregulation of TSLPR mRNA in NPs (Fig 1). TSLPR is known to be highly expressed on myeloid DCs (mDCs), mast cells and type 2 innate lymphoid cells (ILC2).11,13, 35 Although accumulation of mast cells in NPs has been clearly reported,27 the presence of mDC in NPs is not well understood. Recently a small study in a Chinese cohort by Liu et al. reported that populations of OX40 ligand expressing CD11c+ DCs were higher in NPs than control tissue (n=3 in each groups).22 It will be important to examine whether mDCs are elevated in NPs and whether they are activated by TSLP, since TSLP stimulated mDCs are known to promote Th2 differentiation.11, 13 Finally, Mjösberg et al. recently reported that ILC2, another potential source for Th2 cytokines, were also elevated in NP.36 Importantly, they recently discovered that TSLP can directly activate human ILC2 to produce Th2 cytokines.35 Thus future study will be required to identify the major Th2 cytokine producing cells in NP.
In summary, we report here that TSLP protein is posttranslationally modified by endogenous proteases in NP tissue and that patients with CRSwNP have increased levels of TSLP activity in polypoid tissue. We also found that levels of TSLP correlated with markers of Th2 inflammation in sinus tissue. Our findings indicate that the overproduction and cleavage of TSLP in NPs might contribute to the pathogenesis of CRSwNP, and suggest that a similar process may occur in similar Th2 mediated diseases.
Clinical implications.
Overexpression of TSLP in NPs may have a pathogenic role in CRSwNP.
Acknowledgments
Funding: This research was supported in part by NIH grants, R01 HL078860, R01 AI072570 and R37 HL068546 and by a grant from the Ernest S. Bazley Trust.
Abbreviations
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- DCs
dendritic cells
- dsRNA
Double stranded RNA
- NP
Nasal polyp
- PIC
Protease inhibitor cocktail
- PNEC
Primary nasal epithelial cells
- TSLP
Thymic stromal lymphopoietin
- TSLPR
TSLP receptor
- UT
Uncinate tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–94. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachert C, Van Bruaene N, Toskala E, Zhang N, Olze H, Scadding G, et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis - a GALEN study. Allergy. 2009;64:520–33. doi: 10.1111/j.1398-9995.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 7.Kato A, Favoreto S, Jr., Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–20. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 13.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3:138–47. doi: 10.1038/mi.2009.134. [DOI] [PubMed] [Google Scholar]
- 14.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate T(H)2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, et al. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–7. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 17.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 18.Ying S, O'Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–8. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 19.Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–91. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–11. e1–9. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–93. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Li TL, Zhao F, Xie C, Liu AM, Chen X, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. Am J Med Sci. 2011;341:40–7. doi: 10.1097/MAJ.0b013e3181f20489. [DOI] [PubMed] [Google Scholar]
- 23.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92, e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A, Chustz RT, Ogasawara T, Kulka M, Saito H, Schleimer RP, et al. Dexamethasone and FK506 inhibit expression of distinct subsets of chemokines in human mast cells. J Immunol. 2009;182:7233–43. doi: 10.4049/jimmunol.0801375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–72. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34:425–35. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 27.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–20. e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson S, Poposki JA, Nagarkar DR, Chustz RT, Peters AT, Suh LA, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129:119–27. e9. doi: 10.1016/j.jaci.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Bruaene N, Bachert C. Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2011;11:8–11. doi: 10.1097/ACI.0b013e32834233ef. [DOI] [PubMed] [Google Scholar]
- 30.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–92. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 32.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–66. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–83. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 34.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 35.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]