Abstract
Background
Perinatal changes in maternal sleep patterns may modify circadian phase. Our objectives were to (a) measure changes in circadian phase and phase angle between salivary dim light melatonin onset (DLMO) and sleep onset across the perinatal period; and (b) prospectively examine associations between circadian measures and depressed mood in women with a history of major depressive disorder (MDD).
Methods
Twelve women (age±SD = 26.9±5 years) who fulfilled DSM-IV criteria for history of MDD (but not in a mood episode at enrollment) were studied from third trimester of pregnancy through postpartum week 6. Participants completed sleep diaries, wore wrist actigraphs and light sensors, and had mood assessed with the Hamilton Depression Rating Scale (HAMD-17) during 3 separate weeks of the perinatal period; they gave saliva samples at 33 weeks gestation and 6 weeks postpartum to determine DLMO phase.
Results
Nine women had DLMO phase shifts ≥ 30 minutes. On average±SD, new mothers phase delayed 42±80 minutes (range = 163 min phase delay to 144 min phase advance). The time interval between average actigraphic sleep onset and DLMO was shorter at 6 weeks postpartum compared to 3rd trimester in 9 of 12 women, indicating that most new mothers were going to bed closer to the onset of endogenous melatonin secretion. Circadian measures were associated with depressed mood at postpartum weeks 2 and 6.
Limitations
These data are preliminary findings from a small sample and require replication.
Conclusions
We observed individual differences in magnitude and direction of circadian phase shifts and their timing relative to sleep across the perinatal period. These measures were correlated with postpartum depressive symptoms. These preliminary data indicate that changes in perinatal circadian rhythms may contribute to the development of postpartum mood disorders.
Keywords: pregnancy, postpartum, depression, circadian, sleep, DLMO, phase angle
Introduction
Previous research highlights changes in sleep behavior and sleep timing in the postpartum period, including increased daytime napping distributing sleep across the day (Signal et al., 2007; Swain et al., 1997) and later rise times in the weeks following delivery compared to third trimester. One group of 36 women followed with sleep diaries, for example, reported average delay in wake time of 52 minutes from 3rd trimester of pregnancy to 2–4 weeks postpartum (Wolfson et al., 2003).
Few studies have examined perinatal circadian rhythms. One cross-sectional study of postpartum women showed altered patterns of urinary 6-sulfatoxymelatonin excretion compared to non-pregnant, nulliparous controls; differences included blunted circadian rhythm amplitude along with elevated melatonin levels in daytime urine samples (Thomas & Burr, 2006). Another study compared plasma melatonin profiles in two groups – (1) depressed and non-depressed pregnant women near the end of third trimester and (2) depressed and non-depressed new mothers (Parry et al., 2008). Results showed lower average 24-hour melatonin levels in depressed pregnant women compared to healthy controls; in contrast, depressed postpartum women exhibited higher melatonin levels than controls, with no significant difference in time of average melatonin onset or offset observed between depressed and non-depressed women in either group. We emphasize that all of these studies of melatonin secretion patterns in perinatal women were cross-sectional samples, and none examined differences in circadian phase or phase angle across the perinatal period.
We speculate that sleep pattern changes that occur in the early postpartum period alter light-dark exposure and may thus modify circadian phase. The primary aim of this study was to measure circadian phase before and after the birth of a child to examine whether women experience circadian phase shifts across the perinatal period. We hypothesized that women who delay their sleep also experience delays in salivary dim light melatonin onset (DLMO) phase at postpartum week 6 relative to third trimester of pregnancy.
Given the growing body of work in perinatal sleep and postpartum depression, (e.g., (Bei et al., 2010; Coble et al., 1994; Lee et al., 2000; Okun et al., 2009; Okun et al., 2011)), our second aim was to explore whether circadian measures were associated with postpartum depressed mood in our sample of women with a history of MDD.
Methods
Participants
We recruited participants who telephoned the laboratory in response to flyers, brochures, newspaper advertisements, and a direct mailing. We studied women ages 18–40 who fulfilled DSM-IV criteria for history of MDD but who were not in a current mood episode at 33 weeks gestation calculated by last menstrual period. Mood disorder history and absence of a mood episode at enrollment were confirmed by a Structured Clinical Interview for DSM Disorders (SCID I/P, (First et al., 2002)). We excluded those with a primary Axis I diagnosis other than MDD; learning disability, mental retardation, or developmental delay; diagnosis of a primary sleep disorder (including insomnia); high risk pregnancy; current employment as night shift worker; disability that interfered with testing; current alcohol/drug dependence; expectation that infants would not be living in the home or would have a nighttime caregiver; and women taking hypnotics for insomnia. Women with comorbid anxiety disorders were not excluded. We did not select participants on the basis of parity, feeding plans, tobacco use, or use of antidepressants, anxiolytics, antipsychotics, or mood stabilizers. The Rhode Island Hospital and Women and Infants Hospital institutional review boards approved the study. Participants gave signed informed consent and were paid for participating.
Circadian Phase Measures
We measured dim light salivary melatonin onset phase (DLMO) at approximately 33 weeks gestation and 6 weeks postpartum. Participants wore wrist actigraphs and kept sleep diaries for one week before each DLMO phase assessment to record bedtimes and wake times (see below). Spring and fall DLMO phase assessments were completed at least two weeks after daylight savings time.
On the day of DLMO phase assessment, researchers went to participants’ homes with a saliva collection kit that included labeled tubes, saliva sample log, and a scale to weigh samples. Participants also were given dark welder’s glasses (Uvex, Smithfield, RI) to wear continuously during saliva collection to avoid light-induced melatonin suppression. Saliva was collected using Salivettes (Sarstedt, Nümbrecht, Germany) every 30 minutes from ~2.5 hours before the predicted DLMO phase time to ~3 hours after predicted DLMO phase, determined from sleep diary data (Burgess & Eastman, 2005). A researcher telephoned the participant at each sample time to prompt saliva collection and to confirm welder’s glasses were worn. Participants were instructed to log the time and weight of each sample (target weight was 9 gm including the Salivette to ensure adequate sample quantity). Participants refrigerated Salivettes overnight; samples were collected the next day, centrifuged, and frozen at −20°F.
Saliva samples were assayed for melatonin using radioimmunoassay (Alpco, Salem, NH). We defined threshold for melatonin production onset as 4 pg/ml (Lewy et al., 1998; Lewy et al., 1999). Figure 1 illustrates the circadian measures. DLMO phase was computed by linear interpolation between the times of saliva samples before and after the melatonin levels reached threshold. Phase shifts between 3rd trimester and 6 weeks postpartum were determined by subtracting the DLMO phase at 6 weeks postpartum from the 3rd trimester DLMO phase. Thus, a negative value indicates a phase delay shift (i.e., a later DLMO phase postpartum). We considered DLMO time changes larger than the 30-minute saliva sample collection interval circadian phase shifts. The phase angle between DLMO and sleep pattern was calculated by subtracting DLMO time from sleep onset time averaged for the 7 nights before saliva collection. Thus, a positive value indicates the time interval after DLMO that sleep was initiated; larger values indicate falling asleep later relative to melatonin secretion onset.
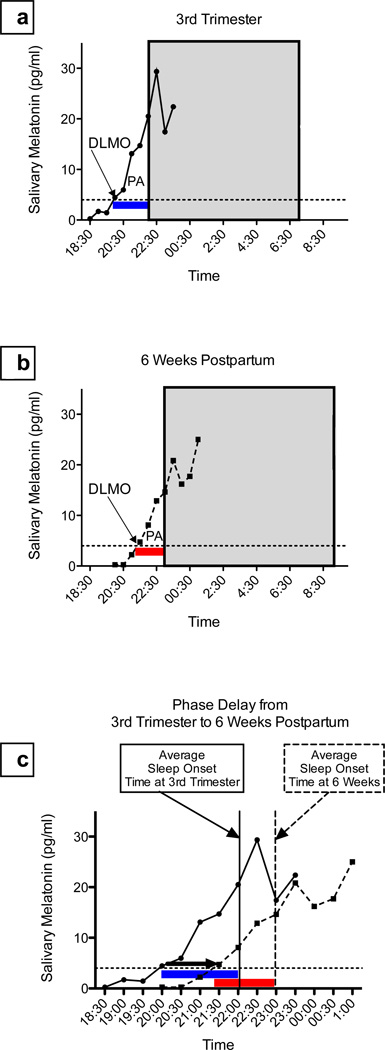
Figure 1. Determination of circadian measures.
In each figure, DLMO threshold is shown with the fine dotted line and the gray box indicates the participant’s average sleep time measured with actigraphy. DLMO = dim light melatonin onset, calculated by linear interpolation across the time points above and below the onset threshold (4 pg/ml); PA= phase angle (duration of time) between melatonin onset and average sleep onset.
Panel 1a shows an example of a DLMO determination for an individual participant at 3rd trimester. Solid line indicates serial melatonin concentrations; DLMO is indicated with the arrow; and the blue PA bar shows phase angle (duration of time) between melatonin onset and average sleep onset.
Panel 1b shows the same participant at 6 weeks postpartum. The dashed line indicates serial melatonin concentrations, and the red PA bar shows a shorter phase angle between DLMO and sleep onset than during 3rd Trimester.
Panel 1c illustrates this participant’s 1.42 hour phase delay in DLMO from 3rd Trimester to 6 weeks postpartum (thick black arrow). Phase angle shortened from 3rd trimester (blue bar) to postpartum week 6 (red bar).
Circadian phase preference was measured at enrollment with the Horne-Östberg Morningness-Eveningness Questionnaire (MEQ; (Horne & Östberg, 1976)); scores range from 16–86 with lowest scores indicating an evening (night owl) preference and highest scores a morning (early bird) preference.
Sleep and Light Measurements
Participants wore a wrist actigraph (Octagonal Basic or Micro Motionlogger Watch, AMI, Ardsley, NY) continuously on the nondominant wrist for one week each at ~33 weeks gestation and at postpartum weeks 2 and 6. (Settings were 1-minute bins in zero crossing mode with a filter setting of 18). Activity data were analyzed using Action-W software (AMI) algorithm, which has been validated with polysomnography (Sadeh et al., 1994). We derived the following sleep measures from actigraphy: sleep onset time (first of three continuous epochs of sleep occurring after the bedtime reported on the sleep diary); sleep offset time (last epoch of 5 continuous epochs of sleep occurring before the wake time reported on the sleep diary); and sleep minutes (number of minutes of estimated sleep occurring between sleep onset and sleep offset).
We measured ambient light exposure with a Micro Light Sensor (AMI, Ardsley, NY) worn continuously pinned/clipped to the shirt or with the built-in light sensor on the Micro Motionlogger Watch (3 participants). Light data (lux) were analyzed with Action-W software; measures included the average and maximum levels over 24 hours and during the actigraph-defined sleep periods.
In addition to wearing actigraphs/light sensors, participants completed a daily sleep/wake diary developed for perinatal women (Wolfson et al., 2003) and called into the laboratory’s time-stamped voicemail each day when they woke up. Diary items include bedtime, rising time, amount of sleep, naps, and times when they removed the actigraph.
Mood Measures
We assessed symptoms of depressed mood with the seventeen-item Hamilton Rating Scale for Depression (HAMD, (Hamilton, 1960)) performed by a trained, board-certified psychiatrist (KMS) at the end of each monitoring week. The rater was not blind to condition but was blind to DLMO phase.
Statistical Analyses
We used SPSS Version 19 (IBM, Chicago, IL) for data analysis. Data are summarized with means and standard deviations. Differences in circadian measures at 3rd trimester and 6 weeks postpartum were tested with paired-samples T-tests. Associations between circadian measures and depressive symptoms were examined with Pearson correlations. We correlated 3rd trimester HAMD scores with 3 concurrent circadian measures (DLMO Phase at 3rd trimester, magnitude of phase angle between DLMO phase and sleep onset at 3rd trimester, and MEQ score). The 3rd trimester circadian measures also were correlated with postpartum week 2 HAMD scores. Finally, we correlated HAMD score at 6 weeks postpartum with the three 3rd trimester circadian measures, the concurrent circadian measures (DLMO phase at postpartum week 6 and magnitude of phase angle between DLMO phase and sleep onset at postpartum week 6), and changes in circadian phase and phase angle from 3rd trimester to 6 weeks postpartum. Because of the small sample size, the exploratory nature of the associations between circadian measures and mood, and the number of correlations examined (total=13), interpretation of correlations was based on Cohen’s effect sizes, where r > 0.3 was considered a medium effect size and r > 0.5 was considered a large effect size (Cohen, 1988).
Results
Participants
Twelve women (mean age 26.9±5.0 years) participated. Average number of lifetime mood episodes was 1.8±0.6. Seven participants were nulliparous, and the median number of children among those who were already mothers was 1 (range 1–3 children). Five participants (46.1%) reported working for pay at least part-time during 3rd trimester of pregnancy, 58.3% reported exposure to traumatic life events, and 83.3% were involved with or living with their baby’s father. One participant had a past history of drug abuse. One participant had twins during the study. Average MEQ score was 51±8.8 including 3 moderate morning-types, 6 neither types, and 3 moderate evening-types (Horne & Östberg, 1976).
With respect to medication use, 8 participants reported no antidepressant, anxiolytic, mood stabilizer, or antipsychotic use during the study. One participant took sertraline and lorazepam as needed at postpartum week 2 but discontinued before postpartum week 6; one took fluoxetine at week 6 postpartum; one took sertraline at all time points; and one took bupropion and clonazepam as needed at all time points.
Circadian Measures at 3rd Trimester of Pregnancy and 6 Weeks Postpartum
Nine women had circadian phase shifts ≥ 30 minutes from 3rd trimester of pregnancy to 6 weeks postpartum (see Figure 2a). We observed a phase delay in DLMO > 30 minutes in 7 women and a phase advance > 30 minutes in 2 women. Average phase shift among all participants was −42±80 minutes (range −163 to 144 minutes). Seven of 12 women had phase shifts greater than one hour (6 delayed; 1 advanced). Among women with a phase delay > 30 minutes, average±SD phase shift was −94±33 minutes (range −59 to −163 minutes), and average DLMO time shifted from 20:55±60 minutes at 3rd trimester to 22:30±31 minutes at 6 weeks postpartum (t = −7.34, df=6, p=.001). Two women showed phase advances > 30 minutes: one advanced 31 minutes and one advanced 144 minutes. Of note, these two participants were the only two in this sample who had their 3rd trimester assessments in the summer—during a relatively longer photoperiod—and their 6-week assessments in the late autumn/early winter during a shorter photoperiod.
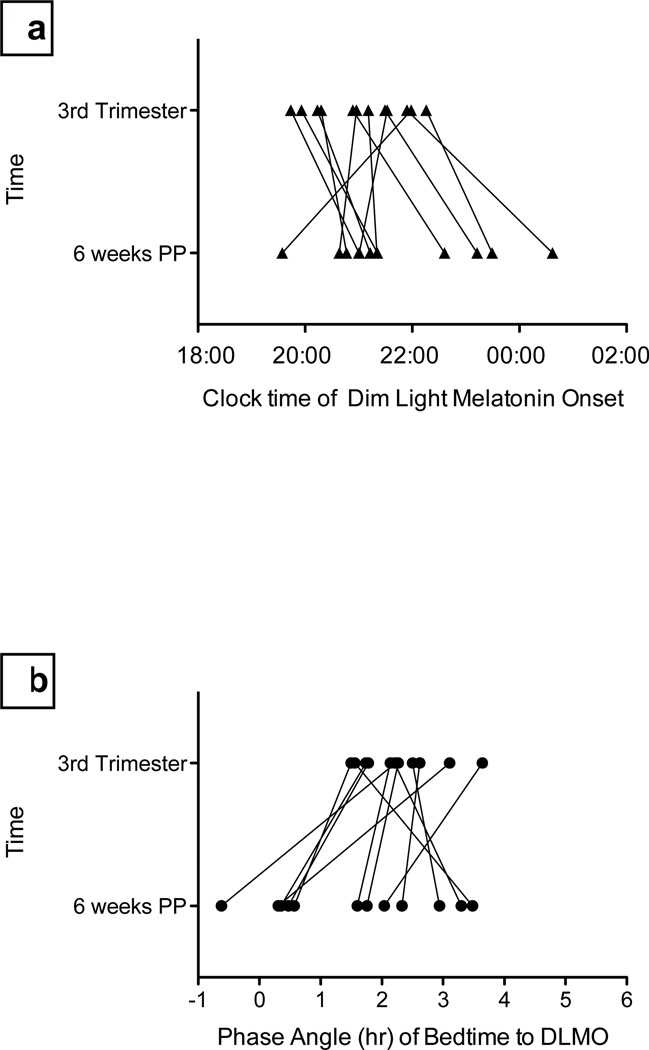
Figure 2. Changes in circadian phase and phase angle from 3rd Trimester to 6 weeks Postpartum.
In Fig. 2a, triangles represent the participants DLMO phase at 3rd Trimester (top) and 6 weeks postpartum (bottom). Most women phase delayed across the perinatal period. In Fig. 2b, circles represent the participants’ phase angles at 3rd trimester (top) and 6 weeks postpartum (bottom). Most women had a decrease in phase angle across the perinatal period, indicating they were going to sleep at an earlier circadian time, i.e., closer to the onset of nocturnal melatonin secretion.
In addition to clock time of melatonin onset, we also were interested in the relation between the time of melatonin onset and the timing of sleep. Phase angle was shorter at postpartum week 6 compared to 3rd trimester in 9 of 12 women, indicating that these new mothers were falling asleep closer to the onset of endogenous melatonin secretion than during pregnancy. Average absolute change ±SD in phase angle was 78±52 minutes (range = 18–170 minutes). Seven of 12 women had phase angle changes > 1 hour. Figure 2b shows the changes in phase angle position from 3rd trimester to 6 weeks postpartum.
Light Exposure
Average light levels during the actigraphic sleep period and across the 24-hour day are shown in Table 1. Women had lower average light levels over 24 hours at 6 weeks postpartum compared to 3rd trimester (t=2.55, df=11, p=.027). Light levels were not correlated with circadian phase shifts or changes in phase angle between DLMO and sleep onset.
Table 1. Circadian, Sleep, and Light Measures during the Perinatal Period (n=12).
Sleep and light averages were calculated from actigraph and light sensor recordings and sleep diaries completed for 7 days at each time point.
| Measure | 3rd Trimester | 6 weeks postpartum |
T (df) | p |
|---|---|---|---|---|
| Diary Bedtime (SD in minutes) | 23:06 (56) | 23:22 (71) | −.90 (11) | .368 |
| Diary Wake Time (SD in minutes) | 7:44 (94) | 8:12 (89) | −.99 (11) | .345 |
| Actigraphy sleep onset time (SD in minutes) | 23:18 (57) | 23.16 (54) | .10 (11) | .922 |
| Actigraphy sleep offset time (SD in minutes) | 7:56 (82) | 8:04 (50) | −.33 (11) | .744 |
| Estimated sleep minutes (SD) | 416 (81) | 397 (59) | .93 (11) | 373 |
| DLMO Clock Time (SD in minutes) | 21:02 (50) | 21:44 (86) | −1.81 (11) | .098 |
| Phase Angle with Bedtime, minutes (SD) | 136 (38) | 92 (79) | 1.78 (11) | .103 |
| Average Lux Level during Sleep (SD) | 0.9 (2.1) | 1.4 (1.7) | −.60 (11) | .562 |
| Highest Lux Level during Sleep (SD) | 33.0 (55.7) | 47.3 (42.7) | −.61 (11) | .553 |
| Average Lux per 24 hours (SD) | 173.1 (96.3) | 100.8 (60.2) | 2.55 (11) | .027 |
| Highest Lux per 24 hours (SD) | 2763.5 (1329.2) | 2099.0 (997.8) | 1.87 (11) | .089 |
Sleep
We observed no significant changes in average diary bedtimes or wake times, actigraphic sleep onset or sleep offset times, or sleep minutes estimated with actigraphy between 3rd trimester and 6 weeks postpartum (see Table 1).
Mood
Average±SD HAMD-17 scores were 6.6±3.7 at 3rd trimester (range =2 to 14), 6.8±6.6 at 2 weeks postpartum (range=3 to 25), and 6.5±3.2 at 6 weeks postpartum (range = 2 to 12). Correlation analyses showed that circadian measures were associated with depressive symptoms. At 3rd trimester, HAMD had a large association with concurrent DLMO phase (r=.518) and a moderate association with phase angle (r=.392) and MEQ (r=−.370): later DLMO phase, longer phase angle between DLMO phase and sleep onset, and evening circadian preference were associated with more depressive symptoms. Similarly, at postpartum week 2, all three 3rd trimester circadian measures showed large associations with HAMD score (DLMO r= 0.625; Phase angle r= 0.705; and MEQ r= −.624). HAMD scores at 6 weeks postpartum showed large associations to DLMO phase at 3rd trimester (r=.539) and MEQ (r=−.676) and a moderate association with phase angle at 3rd Trimester (r=0.445). We observed moderate associations between HAMD at postpartum week 6 and concurrent phase measures (DLMO phase at 6 weeks (r=0.320), and phase angle at 6 weeks (r=−.429)). HAMD at 6 weeks also showed a large association with change in phase angle from 3rd trimester to 6 weeks postpartum (r=−.556). The only circadian parameter that did not show at least a moderate relationship with HAMD at 6 weeks was change in DLMO phase from 3rd trimester to postpartum week 6 (r=−0.045).
Discussion
The major novel finding of this preliminary study of circadian rhythms across the perinatal period is the observation that new mothers experienced meaningful changes in circadian phase position and phase angle between DLMO and bedtime from 3rd trimester of pregnancy to 6 weeks postpartum. We hypothesized that new mothers would experience circadian phase delays, given previous data showing later wake times in the early postpartum period with respect to 3rd trimester (Wolfson et al., 2003). Indeed, most women showed phase delays across this time period. Two women, however, both studied at 3rd trimester in late summer and at 6 weeks postpartum in late fall/winter, had a phase advance > 30 minutes. We speculate that their phase advances may be related to relatively later “starting” phase positions that could be seasonally mediated through photoperiod.
Seven of 12 participants had phase shifts > 1 hour, equivalent to one time-zone change or to the phase shift experienced at transitions to and from daylight saving time. Phase shifts of this magnitude might be expected to bring about changes in alertness and performance and possible mood dysregulation (Yang et al., 2001). One potential concern about our observed phase shifts is that they may represent physiologic drift in the circadian system over time or statistical regression toward the mean. On the other hand, Wyatt and colleagues have demonstrated stability of DLMO phase, albeit over shorter ranges of time (Wyatt et al., 2006). Future work will seek to confirm stability of DLMO in non-perinatal women over a similar time span.
The average phase angle change between DLMO and bedtime from 3rd trimester to postpartum week 6 was > 1 hour on average. If DLMO is considered as the time when the “sleep gate” opens (Shochat et al., 1997), these data indicate that the relation between this biologically “permissive” time for sleep and when women fall asleep changes across the perinatal period. The majority of women in our study went to bed “earlier” with respect to DLMO at postpartum week 6 compared to 3rd trimester. This may be an adaptive way that women maximize sleep, given the inevitable nighttime awakenings they experience as a result of caring for their infants.
Though our data set is small and we want to be circumspect in our interpretation of the findings, our data raise the possibility that circadian timing during pregnancy and in the early postpartum weeks is associated with depressive symptoms. In particular, later circadian phase and a more evening circadian preference were associated most consistently with depressive symptoms. An association between delayed circadian phase and depression severity has been observed in non-perinatal women (Emens et al., 2009). Although more data are needed, these prospective data suggest that perinatal circadian rhythm alterations may contribute to the development of postpartum mood disorders. In addition, these data support circadian interventions that could be used to prevent postpartum depressive symptoms in a subset of women showing circadian vulnerability, e.g., bright light therapy (Epperson et al., 2004; Oren et al., 2002; Wirz-Justice et al., 2011).
We did not observe differences in the timing of sleep across the perinatal period in our participants as has been shown by Wolfson and colleagues (Wolfson et al., 2003). This may be due to differences in measurement times, as their postpartum assessment was completed at 2–4 weeks compared to 6 weeks in our participants. Lack of significant differences in sleep timing may be a function of our small sample size. Other potential factors that could have contributed to few differences in sleep timing include a lower percentage of women working full time and our inclusion of women with a history of MDD.
Study strengths include the use of objective measures of sleep, light levels, and mood, as well as our use of a prospective, repeated-measures (rather than cross-sectional) design.
The major limitation is that this analysis included a relatively small sample of women with a history of MDD. The small sample precluded examination of seasonal influences. A third limitation is the inclusion of women who used medication with the potential to affect sleep and circadian rhythms. Future work should examine circadian rhythms and mood in a larger sample of perinatal women and include women with no previous history of mental illness.
In conclusion, in this prospective longitudinal study of circadian rhythms, sleep, and mood across the perinatal period in women with a history of MDD, we found evidence for large circadian phase shifts and associations between circadian measures and depressed mood.
Acknowledgements
The authors thank Kayla Beaucage, Ellen Ferriter, and Sharon Driscoll for assistance with melatonin assays and Aubree Hoepper, Ijeoma Iko, Julie Quattrucci, and Emily Mepham for assistance with data collection. We are grateful to the participants and their families for the time and effort they put forth to complete the study protocol.
Role of funding sources:
This study was funded by a grant from the Brown University/Women and Infants Hospital National Center of Excellence in Women’s Health to KMS and TP, and a J. Christian Gillin Award from the Sleep Research Society Foundation and K23-MH086689 to KMS. None of the funding sources had a role in study design; in data collection, analysis, or interpretation; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: KMS, TBP, and MAC designed the study and interpreted the results. KMS collected data, performed statistical analyses, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript
Conflict of Interest:
KMS and MAC declare that they have no conflict of interest. TBP has been a consultant for Ironwood Pharmaceuticals and she receives research support (medication and matching placebo) from Pfizer for an unrelated project.
References
- Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coble PA, Reynolds CF, 3rd, Kupfer DJ, Houck PR, Day NL, Giles DE. Childbearing in women with and without a history of affective disorder. I. Psychiatric symptomatology. Compr Psychiatry. 1994;35:205–214. doi: 10.1016/0010-440x(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, Wisner KL. Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry. 2004;65:421–425. doi: 10.4088/jcp.v65n0319. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Lee KA, McEnany G, Zaffke ME. REM sleep and mood state in childbearing women: sleepy or weepy? Sleep. 2000;23:877–885. [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav Sleep Med. 2009;7:106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130:378–384. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, Terman M. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry. 2002;159:666–669. doi: 10.1176/appi.ajp.159.4.666. [DOI] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Kripke DF. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry. 2008;165:1551–1558. doi: 10.1176/appi.ajp.2008.08050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Shochat T, Luboshitzky R, Lavie P. Nocturnal melatonin onset is phase locked to the primary sleep gate. The American journal of physiology. 1997;273:R364–R370. doi: 10.1152/ajpregu.1997.273.1.R364. [DOI] [PubMed] [Google Scholar]
- Signal TL, Gander PH, Sangalli MR, Travier N, Firestone RT, Tuohy JF. Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and post-partum. Aust N Z J Obstet Gynaecol. 2007;47:16–22. doi: 10.1111/j.1479-828X.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- Swain AM, O'Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90:381–386. doi: 10.1016/s0029-7844(97)89252-6. [DOI] [PubMed] [Google Scholar]
- Thomas KA, Burr RL. Melatonin level and pattern in postpartum versus nonpregnant nulliparous women. [Comparative Study Research Support, NIH., Extramural] J Obstet Gynecol Neonatal Nurs. 2006;35:608–615. doi: 10.1111/j.1552-6909.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, Riecher-Rossler A. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. [Randomized Controlled Trial Research Support, Non-U.S. Gov't] J Clin Psychiatry. 2011;72:986–993. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in Sleep Patterns and Depressive Symptoms in First-Time Mothers: Last Trimester to 1-Year Postpartum. Behavioral Sleep Medicine. 2003;1:54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. [Research Support, NIH., Extramural] Sleep. 2006;29:1075–1080. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]
- Yang CM, Spielman AJ, D'Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]