Abstract
Nanosecond-duration electric stimuli are distinguished by the ability to permeabilize intracellular membranes and recruit Ca2+ from intracellular stores. We quantified this effect in non-excitable cells (CHO) using ratiometric Ca2+ imaging with Fura-2. In a Ca2+-free medium, 10-, 60-, and 300-ns stimuli evoked Ca2+ transients by mobilization of Ca2+ from the endoplasmic reticulum. With 2 mM external Ca2+, the transients included both extra- and intracellular components. The recruitment of intracellular Ca2+ increased as the stimulus duration decreased. At the threshold of 200–300 nM, the transients were amplified by calcium-induced calcium release. We conclude that nanosecond stimuli mimic Ca2+ signaling while bypassing the usual receptor- and channels-mediated cascades. The recruitment of the intracellular Ca2+ can be controlled by the duration of the stimulus.
Keywords: Nanosecond pulses, Electroporation, Electropermeabilization, Calcium signaling, Electric field
1. Introduction
High-intensity electric pulses of nanosecond duration (nsEP), also often called nanosecond pulsed electric fields, were originally introduced to biology as a means to electroporate intracellular membranous structures [1–3]. During the last decade, a number of unique nsEP bioeffects and applications have been reported, although mechanisms of nsEP effects on cells have not been fully understood.
In the case of traditional electroporation with micro- and millisecond duration pulses, charging of the cell plasma membrane (PM) compensates the external electric field and protects the intracellular structures. However, calculations showed that nsEP can charge smaller intracellular structures up to the electroporation threshold faster than it would take for PM to charge and protect them [2–4]. Indeed, independent experimental studies demonstrated the electroporation of mitochondria, endoplasmic reticulum (ER), and vacuoles with nsEP [2–5]. Although these studies claimed the lack of concurrent PM disruption by nsEP, the methods employed to compare the poration of the intracellular structures and of the PM were different. For instance, White et al. [5] used Ca2+ efflux from the ER to assess the poration of ER and the uptake of propidium iodide (PI) to assess the poration of PM. In a study by Tekle et al. [4], permeabilization of vacuoles by nsEP was detected by the efflux of Calcein dye, whereas the PM integrity was judged by the uptake of the ethidium homodimer. In view of later findings of high selectivity of nsEP-formed membrane pores [6, 7], the above data can no longer be taken as a proof of selective intracellular poration.
Further studies focused specifically on the PM have found that it is certainly not exempt from the poration by nsEP. Exposures to nsEP increased the PM electrical conductance [6, 8, 9] and the uptake of small dyes [7, 10], ions [10–13], and water [14]. The formation of small membrane pores (“nanopores”), concurrently in both PM and intracellular membranes was supported by advanced numerical models [15, 16]. The facts that more intense nsEP treatments can actually cause PI uptake [6, 7, 17] and that long pulses can cause intracellular effects once the PM is compromised [18] have further blurred the difference between the traditional electroporation and nsEP.
As of today, it has not been experimentally demonstrated that reducing the width of electric pulses increases their intracellular effect. In addition to the arbitrary and “asymmetrical” choice of the electroporation markers for PM and intracellular poration, the critical question was the choice of comparable parameters for short and long electric stimuli. For example, a 20-Hz train of 100 pulses of 50ns duration at 6.7 kV/cm was arbitrarily compared to a single 400-μs pulse at 1.4 kV/cm [4]. Such comparisons are inconclusive and misleading without proper scaling of parameters from nsEP to longer pulses.
The goal of the present study was to test, under stringent conditions, the theoretical predictions that the intracellular effects of nsEP increase when the pulse duration is decreased. The increase in cytosolic Ca2+ caused by nsEP was chosen as a unique and universal endpoint for this study. In the previous study [11], we have shown that in Chinese hamster ovary (CHO) cells, this increase can occur by either Ca2+ entry from the outside (PM poration) or Ca2+ discharge from the ER (intracellular poration). This relatively simple cell model was chosen for the lack of voltage-gated PM channels and ryanodine receptors, which helps the interpretation of nsEP effects.
In addition, the use of Ca2+ as a criterion of both PM and ER poration has made it possible to utilize ratiometric imaging [19] and measure Ca2+ changes in actual concentration units rather than as fold changes or arbitrary units.
Finally, for a comparison between 10-, 60-, and 300-ns stimuli, we employed a concept of isoeffective treatments. Ca2+ transients were always evoked by a single stimulus. First, in the presence of 2 mM external Ca2+, the intensities of the stimuli were empirically adjusted in order to produce Ca2+ responses of similar amplitude by the stimuli of different duration. Next, these isoeffective intensities of 10-, 60-, and 300-ns pulses were tested in a Ca2+-free medium, in order to compare their potency to recruit the intracellular Ca2+. In agreement with theoretical predictions, we established that the intracellular effect was the highest for 10-ns pulses and minimal for 300-ns pulses.
2. Materials and Methods
2.1. Cell culture
Experiments were performed in CHO-K1 cells obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were propagated at 37 °C with 5% CO2 in air in Ham’s F12K medium supplemented with 10% fetal bovine serum, 100 I.U./ml penicillin, and 0.1 μg/ml streptomycin. The medium and its components were purchased from Mediatech Cellgro (Herdon, VA) except for the serum (Atlanta Biologicals, Norcross, GA).
2.2. Calcium imaging
The detailed procedures employed for loading cells with Fura-2, dye calibration, and time lapse fluorescence imaging were reported elsewhere [11]. Cells were transferred onto glass coverslips 12–24 h prior to experiments. After loading with the dye, cells were placed in a glass-bottomed perfusion chamber mounted on an IX71 microscope (Olympus America, Center Valley, PA).
Cells were continually perfused with a physiological solution containing (in mM): 140 NaCl, 5.4 KCl, 1.5 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.3, ~300 mOsm/kg). For Ca2+-free conditions, CaCl2 was replaced with 2 mM Na-EGTA.
A fast wavelength switcher Lambda DG4 (Sutter Instruments, Novato, CA) was employed for dye excitation alternatively at 340 and 380 nm. Emission was measured at 510 nm with a C9100-02 EM CCD camera (Hamamatsu Photonics, Japan). The cytosolic free Ca2+ concentration ([Ca2+]i) was calculated from Fura-2 emission ratio with a help of Metafluor v.7.5 software (Molecular Devices, Sunnyvale, CA). Ca2+ measurements typically began one minute prior to nsEP exposure.
Fura-2 pentapotassium salt, Fura-2/AM, calcium calibration buffer kit, and Pluronic F-127 (20% solution in DMSO) were purchased from Life Technologies (Grand Island, NY). Thapsigargin (TG), a specific and irreversible blocker of Ca2+-ATPase of the ER, was purchased from Tocris Bioscience (Minneapolis, MN). Other chemicals were from Sigma-Aldrich (St. Louis, MO).
2.3. NsEP exposure and simulation of the local electric field
Nearly rectangular pulses of 10-, 60-, and 300-ns duration were produced by dedicated pulse generators. 300-ns pulses (up to 450 V) were generated in a transmission line-type circuit, by closing a MOSFET switch upon delivery of a TTL trigger pulse [7, 8]. To produce 60-ns pulses (up to 800 V output), we employed a Blumlein line-type circuit [11]. 10-ns pulses of up to 20 kV amplitude were produced by a model FPG 20-1NM pulse generator (FID GmbH, Burbach, Germany).
Pulses were delivered to selected cells on coverslip with a pair of tungsten rod electrodes [7, 11, 20]. The exact pulse shapes and amplitudes were captured and measured with a TDS 3052 oscilloscope (Tektronix, Beaverton, OR). The 10% to 90% rise time was 0.6 ns for 10-ns pulses and 4.5 ns for both 60- and 300-ns pulses. Representative pulse waveforms are shown in Fig. 1.
Fig. 1.
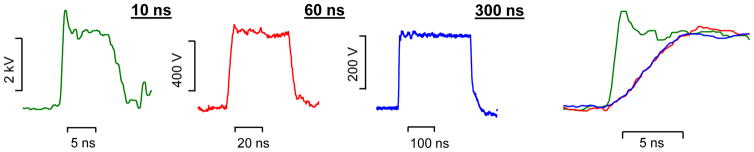
Sample waveforms and comparison of rise times for 10-, 60-, and 300-ns stimuli
NsEP were triggered externally and synchronized with image acquisition by a TTL pulse protocol using Digidata 1440A board and Clampex v. 10.2 software (Molecular Devices). In most experiments, 4–8 cells were stimulated together with nsEP. We did not observe any systematic differences in responses to nsEP that could be attributed to cell size, shape, or the number and configuration of cells in the group. Any group of cells was exposed only once. For statistical analysis, the experiments were repeated 3–10 times in different cell groups and on different coverslips. Different treatment conditions were alternated in a random manner.
The E-field at the cell location between the electrodes was determined as reported previously [7, 11, 20], by 3D simulations with a finite element Maxwell equations solver Amaze 3D (Field Precision, Albuquerque, NM).
2.4. Data analysis
Origin 8.0 (OriginLab Corporation, Northampton, MA) was utilized for smoothing and differentiation of traces, and for statistical analysis. Ca2+ transients shown in Figs. 2–5 have been averaged from 15–50 cells. The data for statistical analysis were measured from the original traces before averaging. The results were expressed as the mean ± s.e. and deemed significant at p < 0.05 (Student’s t-test for unpaired data).
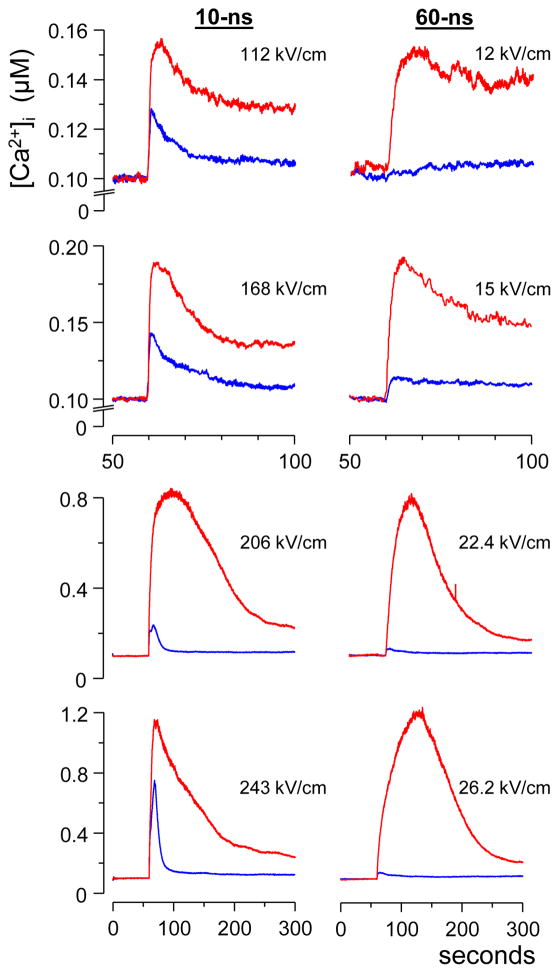
Fig. 2.
Comparison of Ca2+ transients evoked by 10- and 60-ns stimuli. Each trace is the average from at least 15 cells; nsEP was applied at 60 s. The nsEP intensity (kV/cm) was adjusted to evoke similar responses by 10- and 60-ns stimuli in the presence of 2 mM external Ca2+ (red traces). The fraction of Ca2+ coming from the endoplasmic reticulum (ER) was assessed by applying the same nsEP in the absence of external Ca2+ (blue traces). Note that 10-ns pulses consistently evoked higher ER response than 60-ns ones. See text for further details.
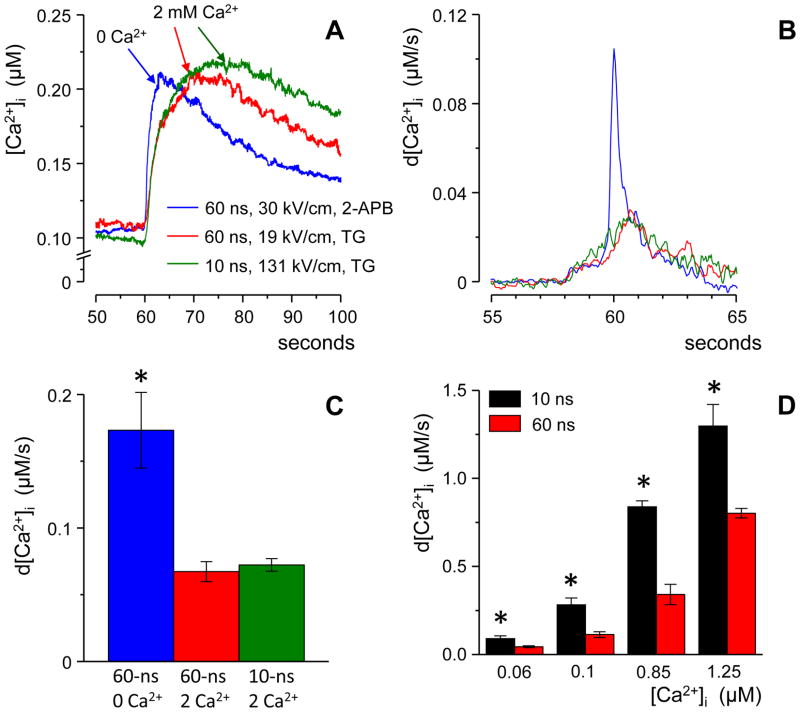
Fig. 5.
Ca2+ discharge from the endoplasmic reticulum (ER) is manifested by a faster rise of the nsEP-induced Ca2+ transient. (A) Traces of Ca2+ transients produced solely by the uptake of the extracellular Ca2+ (red and green) or solely by its discharge from the ER (blue). Each trace is an average for at least 15 cells.. Stimuli were applied at 60 s; the intensities were adjusted to evoke similar responses. (B) Traces from the panel (A) were differentiated in order to emphasize the rate of Ca2+ increase. For clarity, the traces were filtered, and the premature onset of the response is a filtering artifact; see text for details. (C) Peak rates of Ca2+ increase as measured from individual traces without filtering; same data as in (A) and (B). (D) Peak rates of Ca2+ increase for Ca2+ transients evoked by 10- and 60-ns stimuli in the presence of 2 mM external Ca2+ (the data from Fig. 2). Measurements were done without averaging or filtering.. Mean +/− s.e, n≥ 15; * p≤ 0.01.
3. Results and discussion
3.1. PM and ER permeabilization by 10-, 60-, and 300-ns pulses
As we showed earlier [11], nsEP-induced Ca2+ transients in CHO cells may involve Ca2+ entry through PM, Ca2+ discharge from the intracellular lumens, and active amplification of the response by calcium-induced calcium release (CICR). We also demonstrated that these mechanisms can be separated by manipulation of the bath Ca2+ and using pharmacological blockers. The ER was the only significant source of the intracellular Ca2+ in CHO cells.
In the presence of 2 mM external Ca2+, we tuned the intensity of 10- and 60-ns stimuli to evoke Ca2+ transients of similar amplitude. Not surprisingly, shorter stimuli had to be applied at higher intensities to evoke the same Ca2+ response (the difference for 10- and 60-ns stimuli was almost 10-fold, Fig. 2, red traces). These Ca2+ transients were produced by a combination of Ca2+ uptake via PM and Ca2+ discharge from the ER, either without activation of CICR (top panels) or with it (bottom panels). The fraction of the response due to the recruitment of Ca2+ from ER was estimated by the removal of the bath Ca2+ and applying the same 10- and 60-ns stimuli (blue traces). As seen in Fig. 2, for the entire range of tested nsEP intensities, the contribution of Ca2+ from the intracellular stores was profoundly greater for 10-ns stimuli than for 60-ns stimuli. Of note, different shapes of transients induced by the most intense10- and 60-ns EP in the presence of Ca2+ (red traces in the bottom panels) could also be a result of greater ER engagement by 10-ns pulses.
Fig. 3 compares Ca2+ responses to 60- and 300-ns stimuli. Fig. 3A shows Ca2+ traces evoked by 60-ns stimuli of the highest intensity allowed by our pulse generator. The response peaked at 2 μM in the presence of 2 mM external Ca2+ (red trace) and dropped to 0.7 μM in the absence of it (blue trace). The red trace in Fig. 3B shows Ca2+ transient of the same amplitude as in (A), but evoked by a 300-ns stimulus. In contrast to the data in panel A, the removal of external Ca2+ completely abolished the response to 300-ns stimuli. Likewise, 300-ns stimuli did not recruit any intracellular Ca2+ at lower stimulus intensities corresponding to the Ca2+ transients in Fig. 2 (data not shown). However, further increase of the stimulus intensity beyond the level that could be matched by shorter pulses has made it possible to evoke Ca2+ discharge from the ER even by 300-pulses (Fig. 3C).
Fig. 3.
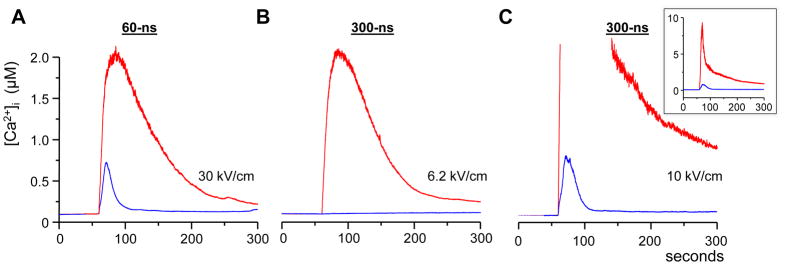
Comparison of Ca2+ transients evoked by 60- and 300-ns stimuli. (A) The transients evoked by a maximum intensity 60-ns pulse in the presence and absence of 2 mM external Ca2+ (hereinafter, shown by red and blue traces, respectively). (B) The intensity of a 300-ns pulse was adjusted, in the presence of 2 mM Ca2+, to evoke a similar Ca2+ response as in panel (A). The removal of external Ca2+ completely eliminated the response to the 300-ns pulse. (C) In principle, 300-ns stimuli were capable of recruiting the intracellular Ca2+, but only at a high intensity that could not be matched with shorter pulses. The inset shows the full amplitude of the response.
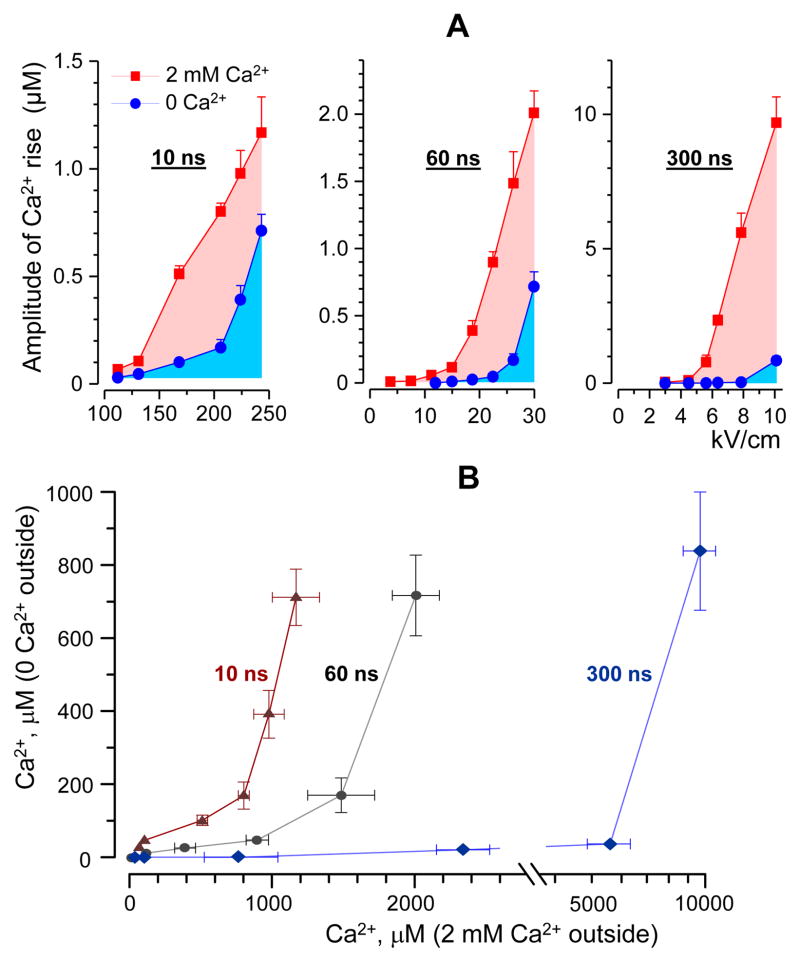
The graphs in Fig. 4A compare the efficiency of 10-, 60- and 300-ns stimuli to produce Ca2+ transients in the presence of external Ca2+ (red symbols) and in the absence of it (blue symbols). Increasing the stimulus intensity increased the amplitude of the response in a characteristically non-linear fashion, reflecting the amplification of the response by CICR once the threshold of 200–300 nM was exceeded [11]. Within the entire tested range of stimulus intensities and irrespective of the emergence of CICR, Ca2+ recruitment from the ER contributed most to the effect of 10-ns stimuli and the least to the effect of 300-ns stimuli.
Fig. 4.
Shorter nsEP are more efficient in recruiting Ca2+ from the intracellular stores. (A) The peak amplitude of Ca2+ transients evoked by 10-, 60- and 300-ns stimuli was plotted against the stimulus intensity (kV/cm), mean +/− s.e.; n ≥ 15. Cells were stimulated in the presence of 2 mM external Ca2+ (red) or its absence (blue). In the latter case, the endoplasmic reticulum was the only significant source of Ca2+. Note the increasing contribution of the intracellular Ca2+ as the stimulus duration is decreased. (B) The amplitude of Ca2+ response due to the discharge from the intracellular stores was plotted against the amplitude of the compound response (due to both the uptake from the outside and the discharge from the stores). The data shown are from the same experiments as in panel (A). For any amplitude of the compound response, shorter stimuli evoked more intracellular Ca2+ discharge.
In the summary graph (Fig. 4B), the stimulus intensity was taken out of the picture. The amplitude of the Ca2+ response for different nsEP stimuli under Ca2+-free conditions was plotted against the response in the presence of 2 mM Ca2+. The characteristic upward bend of the curves corresponds to the threshold of CICR under Ca2+-free conditions. Once again, these graphs show that under all tested conditions 10-ns pulses were the most efficient in recruiting Ca2+ from the intracellular stores.
3.2. The rate of Ca2+ rise as an index of ER involvement
In the above experiments, individual cells within a microscope field of view were selected as “regions of interest” for Ca2+ measurements. [Ca2+]i as measured by this approach was averaged over the entire cell volume. As noted by studies of Ca2+ dynamics in myocytes [21, 22], Ca2+ entry through the PM occurs in a fraction of the cell volume, followed by Ca2+ diffusion; these factors increase the apparent duration of Ca2+ rise when its concentration is averaged over the cell volume. In contrast, membranes of the ER are widely distributed inside the cell, so Ca2+ discharge from the ER spreads throughout the cell volume faster than Ca2+ that enters through the PM. If this rule holds true for CHO cells, the increased rate of nsEP-induced Ca2+ rise can serve as another manifestation of the ER involvement in the response.
To produce a Ca2+ transient solely by Ca2+ entrance through the PM, the ER Ca2+ store was fully depleted by a 30-min preincubation with 100 nM thapsigargin [11]. For comparison, transients caused solely by Ca2+ discharge from the ER were evoked in a Ca2+-free medium. To inhibit possible amplification of the response by CIRC, we blocked inositol-1,4,5-trisphosphate receptors of the ER with 50 μM of 2-APB (2-aminoethoxydiphenyl borate) [11].
Fig. 5, A–C shows that under the above conditions the rate of Ca2+ rise was indeed dependent on the source of Ca2+: The discharge from the ER resulted in a shorter rise time than Ca2+ uptake through the PM (Fig. 5A). In the latter case, the rise time did not depend on whether the nsEP duration was 10 or 60 ns.
The role of the Ca2+ source can be appreciated better by differentiation of the original traces to measure the rate of Ca2+ rise (Fig. 5B). Of note, the percentile filter of Origin 8 that was employed to improve the signal-to-noise ratio has also decreased the peak amplitude and produced an artifact of the premature onset of the response. Hence the traces in Fig 5B serve for illustration purpose only, whereas the quantitative data measured from undistorted traces are provided in Fig. 5C. Indeed, the cell volume-average rate of Ca2+ increase was more than twofold higher when Ca2+ came from the ER as compared to its entry through the PM. Thus, the rate of Ca2+ rise depended on the source of Ca2+ but not on the nsEP duration, and therefore could be utilized to distinguish between PM and ER poration.
These data have laid the ground for rate comparison of Ca2+ transients evoked by 10- and 60-ns stimuli in the presence of 2 mM Ca2+ (Fig. 5D). As noted above, such transients involve both Ca2+ entry from the outside and its discharge from the ER. For different response amplitudes (corresponding to the actual traces in Fig 2), the rate of Ca2+ rise was always higher for 10-ns pulses, thus indicating greater contribution of the ER to the overall response.
Thus, two different approaches have identified 10-ns stimuli as the most efficient to recruit the intracellular Ca2+. These experimental data are in agreement with theoretical predictions that nsEP have the ability to electroporate intracellular structures, and that this ability is higher for shorter pulses. It should be noted that the efficiency was evaluated relative to the ability of the same pulse to electroporate the PM, irrespective of the fact that the required intensity was much higher for shorter nsEP.
The deliberate adjustment of the nsEP duration opens an avenue for accurate control of Ca2+ signaling, by varying the extent of the intracellular Ca2+ recruitment. Ca2+ transients evoked by nsEP seem to be “interpreted” by cells as natural Ca2+ signals and further amplified by CICR, thus mimicking Ca2+ signals that originate from the activation of PM receptors or ion channels. Stimulation of cells by nsEP has the potential to develop into a unique tool for precise but non-chemical activation of Ca2+ signaling mechanisms.
Acknowledgments
The study was supported by R01CA125482 from the National Cancer Institute and R01GM088303 from the National Institute of General Medical Sciences.
Footnotes
Conflict of interest
All co-authors of the following manuscript named “Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: the impact of pulse duration” declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deng J, Stark RH, Schoenbach KH. A nanosecond pulse generator for intracellular electromanipulation. Twenty-Fourth Intern. Power Modulator Symp; Norfolk, VA. 2000. pp. 47–50. [Google Scholar]
- 2.Schoenbach KH, Beebe SJ, Buescher ES. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics. 2001;22:440–448. doi: 10.1002/bem.71. [DOI] [PubMed] [Google Scholar]
- 3.Schoenbach KS, Hargrave B, Joshi RP, Kolb J, Osgood C, Nuccitelli R, Pakhomov AG, Swanson J, Stacey M, White JA, Xiao S, Zhang J, Beebe SJ, Blackmore PF, Buescher ES. Bioelectric Effects of Nanosecond Pulses. IEEE Transactions on Dielectrics and Electrical Insulation. 2007;14:1088–1109. [Google Scholar]
- 4.Tekle E, Oubrahim H, Dzekunov SM, Kolb JF, Schoenbach KH, Chock PB. Selective Field Effects on Intracellular Vacuoles and Vesicle Membranes with Nanosecond Electric Pulses. Biophysical Journal. 2005;89:274–284. doi: 10.1529/biophysj.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White JA, Blackmore PF, Schoenbach KH, Beebe SJ. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J Biol Chem. 2004;279:22964–22972. doi: 10.1074/jbc.M311135200. [DOI] [PubMed] [Google Scholar]
- 6.Pakhomov AG, Pakhomova ON. Nanopores: A distinct transmembrane passageway in electroporated cells. In: Pakhomov AG, Miklavcic D, Markov MS, editors. Advanced Electroporation Techniques in Biology in Medicine. CRC Press; Boca Raton: 2010. pp. 178–194. [Google Scholar]
- 7.Bowman AM, Nesin OM, Pakhomova ON, Pakhomov AG. Analysis of plasma membrane integrity by fluorescent detection of Tl(+) uptake. J Membr Biol. 2010;236:15–26. doi: 10.1007/s00232-010-9269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pakhomov AG, Bowman AM, Ibey BL, Andre FM, Pakhomova ON, Schoenbach KH. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem Biophys Res Commun. 2009;385:181–186. doi: 10.1016/j.bbrc.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakhomov AG, Kolb JF, White JA, Joshi RP, Xiao S, Schoenbach KH. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF) Bioelectromagnetics. 2007;28:655–663. doi: 10.1002/bem.20354. [DOI] [PubMed] [Google Scholar]
- 10.Vernier PT, Sun Y, Gundersen MA. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006;7:37. doi: 10.1186/1471-2121-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenov I, Xiao S, Pakhomov AG. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim Biophys Acta. 2013;1828:981–989. doi: 10.1016/j.bbamem.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Chen J, Chen MT, Vernier PT, Gundersen MA, Valderrabano M. Cardiac myocyte excitation by ultrashort high-field pulses. Biophys J. 2009;96:1640–1648. doi: 10.1016/j.bpj.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beier HT, Roth CC, Tolstykh GP, Ibey BL. Resolving the spatial kinetics of electric pulse-induced ion release. Biochem Biophys Res Commun. 2012;423:863–866. doi: 10.1016/j.bbrc.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 14.Nesin OM, Pakhomova ON, Xiao S, Pakhomov AG. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim Biophys Acta. 2011;1808:792–801. doi: 10.1016/j.bbamem.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gowrishankar TR, Weaver JC. Electrical behavior and pore accumulation in a multicellular model for conventional and supra-electroporation. Biochem Biophys Res Commun. 2006;349:643–653. doi: 10.1016/j.bbrc.2006.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotnik T, Miklavcic D. Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. Biophys J. 2006;90:480–491. doi: 10.1529/biophysj.105.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napotnik TB, Rebersek M, Kotnik T, Lebrasseur E, Cabodevila G, Miklavcic D. Electropermeabilization of endocytotic vesicles in B16 F1 mouse melanoma cells. Med Biol Eng Comput. 2010;48:407–413. doi: 10.1007/s11517-010-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esser AT, Smith KC, Gowrishankar TR, Vasilkoski Z, Weaver JC. Mechanisms for the Intracellular Manipulation of Organelles by Conventional Electroporation. Biophysical Journal. 2010;98:2506–2514. doi: 10.1016/j.bpj.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 20.Pakhomova ON, Gregory BW, Khorokhorina VA, Bowman AM, Xiao S, Pakhomov AG. Electroporation-induced electrosensitization. PloS one. 2011;6:e17100. doi: 10.1371/journal.pone.0017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michailova A, DelPrincipe F, Egger M, Niggli E. Spatiotemporal features of Ca2+ buffering and diffusion in atrial cardiac myocytes with inhibited sarcoplasmic reticulum. Biophys J. 2002;83:3134–3151. doi: 10.1016/S0006-3495(02)75317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thul R, Coombes S, Roderick HL, Bootman MD. Subcellular calcium dynamics in a whole-cell model of an atrial myocyte. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2150–2155. doi: 10.1073/pnas.1115855109. [DOI] [PMC free article] [PubMed] [Google Scholar]