Abstract
Objective
Lesion-based mapping of speech pathways has been possible only during invasive neurosurgical procedures using direct cortical stimulation (DCS). However, navigated transcranial magnetic stimulation (nTMS) may allow for lesion-based interrogation of language pathways noninvasively. Although not lesion-based, magnetoencephalographic imaging (MEGI) is another noninvasive modality for language mapping. In this study, we compare the accuracy of nTMS and MEGI with DCS.
Methods
Subjects with lesions around cortical language areas underwent preoperative nTMS and MEGI for language mapping. nTMS maps were generated using a repetitive TMS protocol to deliver trains of stimulations during a picture naming task. MEGI activation maps were derived from adaptive spatial filtering of beta-band power decreases prior to overt speech during picture naming and verb generation tasks. The subjects subsequently underwent awake language mapping via intraoperative DCS. The language maps obtained from each of the 3 modalities were recorded and compared.
Results
nTMS and MEGI were performed on 12 subjects. nTMS yielded 21 positive language disruption sites (11 speech arrest, 5 anomia, and 5 other) while DCS yielded 10 positive sites (2 speech arrest, 5 anomia, and 3 other). MEGI isolated 32 sites of peak activation with language tasks. Positive language sites were most commonly found in the pars opercularis for all three modalities. In 9 instances the positive DCS site corresponded to a positive nTMS site, while in 1 instance it did not. In 4 instances, a positive nTMS site corresponded to a negative DCS site, while 169 instances of negative nTMS and DCS were recorded. The sensitivity of nTMS was therefore 90%, specificity was 98%, the positive predictive value was 69% and the negative predictive value was 99% as compared with intraoperative DCS. MEGI language sites for verb generation and object naming correlated with nTMS sites in 5 subjects, and with DCS sites in 2 subjects.
Conclusion
Maps of language function generated with nTMS correlate well with those generated by DCS. Negative nTMS mapping also correlates with negative DCS mapping. In our study, MEGI lacks the same level of correlation with intraoperative mapping; nevertheless it provides useful adjunct information in some cases. nTMS may offer a lesion-based method for noninvasively interrogating language pathways and be valuable in managing patients with peri-eloquent lesions.
Keywords: transcranial magnetic stimulation, magnetoencephalography, language mapping, direct cortical stimulation, speech arrest
1. Introduction
The localization of essential cortical language regions is of great relevance across a wide spectrum of basic, translational, and clinical neuroscience. Retrospective localization studies relied largely on lesion-symptom mapping, in which existing injuries were correlated with clinical symptoms (Adolphs et al., 2000; Bates et al., 2003; Chao and Knight, 1998; Dronkers, 1996; Friedrich et al., 1998; Naeser and Hayward, 1978). The advent of intraoperative direct cortical stimulation (DCS) mapping revolutionized this field, allowing clinicians to interrogate a region with a temporary electrical lesion. While this technique is effective, it is also highly invasive, requiring a craniotomy for cortical exposure, and is thus performed only when necessary for the surgical management of an existing lesion. A technique for noninvasive, lesion-based identification of critical language sites would therefore be of great benefit to clinicians. Such a technique would also be important for neurophysiologists interested in testing cognitive models of language, including language streams (Hickok and Poeppel, 2004) and connectome-based models (Lemaire et al., 2012).
1.1. Navigated Transcranial Magnetic Stimulation for Precise Brain Mapping
Navigated transcranial magnetic stimulation (nTMS) is a lesion-based technique which may address this need. The distinction of this nTMS system as compared with other systems is its demonstration, in realtime, of the precise location and strength of the magnetic pulse. By integrating a frameless stereotactic navigational system (such as those used commonly in neurosurgical and other procedures) with a TMS coil, we can co-register a structural MRI or CT brain scan to a subject’s anatomy using fiducial markers or anatomical landmarks. This advancement allows us to deliver TMS pulses with unprecedented precision under image guidance (Julkunen et al., 2009; Krings et al., 2001a; Krings et al., 2001b; Picht et al., 2009). Furthermore, the strength and directionality of these pulses are calculated on-the-fly according to a dynamic spherical model which takes into account the preset parameters of stimulation as well as the subject’s scalp/skull thickness (Sarvas, 1987; Tarkiainen et al., 2003). As a result, when we position the stimulation coil over the subject’s scalp, we can visualize the targeted cortical region, the strength and orientation of the magnetic dipole, and the cone of activation generated by the magnetic pulse.
nTMS therefore allows us, for the first time, to pinpoint precisely the cortical region that is being targeted. This capacity for accurate localization suggests a number of novel applications for TMS. In particular, it offers the possibility of mapping essential cortical regions associated with language function. Prior studies (Epstein et al., 1996; Epstein et al., 1999; Epstein et al., 2000; Jennum et al., 1994; Michelucci et al., 1994; Pascual-Leone et al., 1991; Wassermann et al., 1999) (see Table 1 for details) have examined the use of repetitive TMS to cause speech arrest and lateralize language. However, these efforts were not stereotactically guided, and showed repetitive TMS to be an unreliable technique for determining language laterality, largely because of a high false-positive rate for speech arrest sites on the supposedly non-dominant hemisphere. It should be noted that most of these studies did report finding dominant-hemisphere positive language disruption sites in almost all their subjects (see Table 1, “# Pts with +SA”). The significance of these speech arrest sites vis-à-vis intraoperative DCS sites remains heretofore undetermined.
Table 1.
Characteristics of previously published repetitive-TMS studies. (CRRMS = Cadwell Rapid-Rate Magnetic Stimulator, CHSMS = Cadwell High-Strength Magnetic Stimulator, IAT = Intracarotid amobarbital test (a.k.a. Wada test); SA = speech arrest)
| Date | # Pts |
# Pts with +SA |
rTMS freq (Hz) |
Train length (s) |
Task | Primary Pathology |
Coil | Design | |
|---|---|---|---|---|---|---|---|---|---|
| Pascual-Leone | 1991 | 6 | 6 | 25 | 10 | counting | epilepsy | CRRMS | 15 positions bilat (10/20), c/w IAT |
| Michelucci | 1994 | 14 | 7 | 16–20 | 6–10 | counting | epilepsy | CRRMS | 9 positions bilat (10/20), c/w IAT |
| Jennum | 1994 | 21 | 14 | 30 | 1 | counting, reading | epilepsy | MagPro biphasic | 4 positions bilat (10/20), c/w IAT |
| Wassermann | 1999 | 14 | 13 | 15, 5–18 | 2–3 | naming, reading | epilepsy | CHSMS | 1cm grid, c/w IAT in 10 pts. |
| Epstein | 1996 | 5 | 4 | 4 | 1–5 | counting | none | CHSMS | Continuous over lat frontal region |
| Epstein | 1999 | 10 | 10 | 4 | 2–5 | counting | none | Custom circular | Continuous Over Lat frontal region |
| Epstein | 2000 | 17 | 16 | 4 | N/A | counting | epilepsy | Custom circular | Continuous Over lat frontal region |
1.2. Magnetoencephalographic Imaging and Language Dominance
While efforts to determine language laterality with TMS have yet to succeed, magnetoencephalographic imaging (MEGI) has shown promise in this regard. Our group has demonstrated excellent correlation between MEGI-derived language laterality and intra-arterial amobarbital procedure (IAP, also known as the Wada test), the current gold standard for preoperative language lateralization (Findlay et al., 2012). MEG studies of language lateralization using dipole analysis have demonstrated 69 – 86% concordance with IAP, (Doss et al., 2009; Papanicolaou et al., 2004) while other MEGI studies based on newer algorithms have also shown promising results (Hirata et al., 2010; Hirata et al., 2004; Kim and Chung, 2008). Functional magnetic resonance imaging (fMRI) has also been investigated as a technique for preoperative language localization. Several studies have demonstrated the use of fMRI for determining language lateralization with strong correlations with IAP results (Binder, 2011; Binder et al., 1996; Desmond et al., 1995; Dym et al., 2011; Jones et al., 2011; Lehericy et al., 2000; Liu et al., 2009; Woermann et al., 2003; Wood et al., 2011), for a summary see (Abou-Khalil, 2007). However, thus far the technique lacks the spatial and temporal resolution necessary to be useful in identifying critical language sites that correlate with intraoperative DCS.
1.3. Study Rationale and Objectives
Thus, it seems that nTMS and MEGI may complement each other well: nTMS for mapping individual language sites, and MEGI for more global analysis of language lateralization. It would be valuable, then, for nTMS language maps to undergo a systematic validation with those of MEGI and DCS, as well as with surgical outcomes data. For nTMS-derived language maps to play a role in language localization studies, they must undergo systematic validation against these historical techniques.
To that end, the aim of this study is to determine the significance of cortical sites that cause speech disruption with nTMS by validating them against MEGI and DCS. We also observe whether subjects find low-frequency repetitive TMS to be tolerable. Because repetitive TMS can cause significant discomfort, we examine whether adjusting stimulator intensity on a per-patient basis to optimize comfort still allows for successful mapping. Finally, we present aggregated maps of positive language sites acquired with nTMS, MEGI, and DCS to give an overall view of the distribution of positive sites in the left hemisphere.
2. Materials and Methods
2.1. Subject population
We prospectively enrolled 12 adult subjects with brain tumors in cortical language areas, which were defined as lesions involving dominant temporal, inferior and middle frontal, supramarginal and angular gyri, as well as those involving related subcortical structures. All subjects were referred for clinical MEG scanning at the University of California, San Francisco (UCSF) Biomagnetic Imaging Laboratory. Subjects had to be scheduled for subsequent craniotomy with awake language mapping. Subjects with seizure frequency greater than 1 per week were excluded from the study, as were subjects with cardiac pacemakers or implanted metal devices in the cranial region. Subjects underwent neurological and neuropsychological examination before and after surgery. Subjects were followed for 3 months postoperatively and their clinical exams recorded.
All subjects gave written informed consent to participate in the research and the study was conducted under Institutional Review Board approval (reference number 10-02932).
2.2. Structural MR Images
High-resolution MRI was performed at 1.5 Tesla to provide necessary anatomic detail for surgical planning and intraoperative neuronavigation. The protocol typically included the following sequences: (1) a T1-weighted, 3-dimensional spoiled gradient-recalled echo in a steady-state sequence with TR = 34 msec, TE = 3–8 msec, and flip angle = 30 degrees; and (2) a T2-weighted, 3-dimensional fast spin-echo sequence with TR = 3,000 msec and TE = 105 msec. Both sequences had slice thickness = 1.5 mm, matrix size = 256 × 256 × (108 to −140), and field of view = 260 mm × 260 mm with skin-to-skin coverage to include the nasion and preauricular points.
2.3. MEGI
2.3.1 Recordings
Magnetic fields were recorded in a magnetically shielded room using a whole-head CTF Omega 2000 system (VSM MedTech, Coquitlam, British Columbia, Canada). The system consisted of 275 axial gradiometers and 29 reference sensors used for computing synthetic third-order gradiometer measurements. The MEG signals were collected continuously and digitized at a sampling rate of 1,200 Hz. Head localization was performed at the beginning and ending of the collection to register head position and to measure head movement during the task.
2.3.2. Tasks
Each individual lay on a comfortable bed with the head fit snugly inside the MEG dewar helmet. First, a verb-generation task was administered. This task consisted of 100 nouns presented at a comfortable volume through earphones every 4 seconds. Subjects were instructed to think of a verb associated with the noun and to speak into a microphone at the foot of the bed. Auditory stimuli and spoken responses were recorded on separate analog-to-digital (ADC) channels for post-processing analysis.
Second, a picture naming task was administered. This task consisted of 120 images presented on a screen every 5 seconds. The images were drawn from a standardized set of 50 black-and-white line drawings (Snodgrass and Vanderwart, 1980). These images were also used for the picture naming task in the nTMS and DCS studies. Subjects were instructed to name each image as it was presented. Spoken responses and image presentation markers were recorded on separate ADC channels for postprocessing analysis.
2.3.3. Post-processing
Onsets of stimuli and response were marked using amplitude threshold detection on the ADCs and verified by eye. Data were formatted into separate trials for each stimulus-response pair, excluding trials without a response. Artifact detection was done by visually examining all trials for many different sensors; the specifics for artifact detection procedures have been published elsewhere (Findlay et al., 2012).
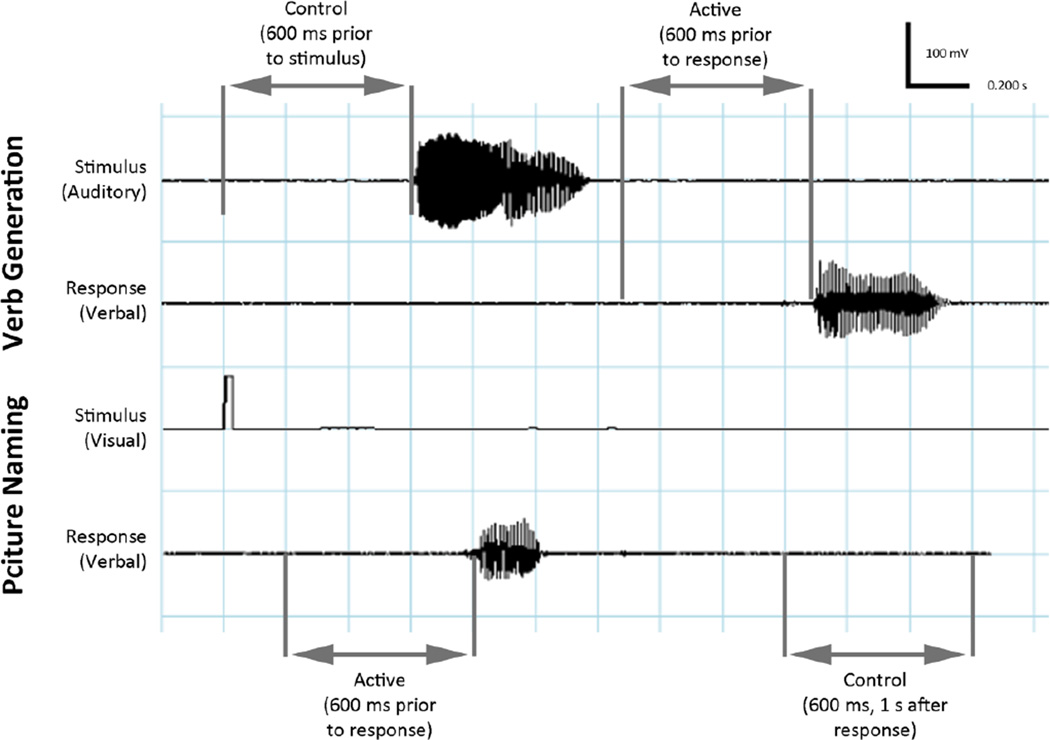
MEG responses prior to overt speech production were examined using adaptive spatial filtering. In the verb-generation task, a window of 600ms preceding the stimulus was used as the control period, and a window of 600ms preceding the response was used as the active period. In the picture naming task, a window from 1,000 – 1,600ms after the response was used as the control period, while the 600ms preceding the response was used as the action period (Figure 1).
Figure 1.
(a) Auditory verb generation task. Subjects heard a noun and overtly generated a verb. The time window chosen for baseline was 600 ms prior to noun onset, and the active window was 600 ms prior to response. (b) Visual object naming task. Subjects were shown an image and asked to name it. The time window chosen for baseline was 600 ms 1 s after response, and the active window was 600 ms prior to response.
The MEG data were bandpass filtered in the β frequency band (15–30 Hz). Sensor data covariance was computed in a window beginning ≈600 ms before the onset of movement, designated as the active period. Sensor data covariance was also computed for a 600-msec baseline control period 1–2 seconds after movement onset. Details of the adaptive spatial filtering algorithm used are described elsewhere (Sekihara et al., 2004; Sekihara et al., 2001; Vrba and Robinson, 2001). In brief, an estimate of the source power at each voxel in the brain based on the MEG data are computed for the active and control time periods using a forward-field, which has been computed assuming a multiple local-sphere spherical volume conductor model making use of the sensor data covariance. Source power estimates are obtained at a 5-mm resolution across the entire brain for the active and control periods, and a pseudo-F ratio is calculated. Negative values of the pseudo-F ratio indicate a decrease in β-band power, also referred to in the literature as an event-related desynchronization (ERD); positive values indicate an increase in β-band power, also referred to as an event-related synchronization (ERS) (Crone et al., 2001; Sekihara et al., 2001).
The process of adaptive spatial filtering was done using a commercially available synthetic aperture magnetometry software package (VSM MedTech Ltd.) and integrated with custom-built in-house software package (NUTMEG)(Dalal et al., 2004; Dalal et al., 2011) which runs in the MATLAB environment (2007a, The MathWorks, Natick, MA). Using NUTMEG time-frequency tools, images of β-band power changes were averaged across time at each voxel and displayed. For each individual, the ERD/ERS images were overlaid on the preoperative high-resolution MR images as described above, which were also normalized to standard Montreal Neurologic Institute (MNI) brain space using SPM2 (Tzourio-Mazoyer et al., 2002). Normalization was verified by eye, comparing ventricles, tumor volumes, and other anatomical and pathological boundaries. The transformation matrix derived from this normalization was then applied to each individual subject’s MEGI results. Prior studies have described NUTMEG time-frequency analysis in detail (Dalal et al., 2008; Dalal et al., 2007; Dalal et al., 2004). Peaks of these activation maps were then extracted to derived “MEGI” sites which indicate sites that are involved in motor speech preparation. Peaks are selected based primarily on intensity, with the highest peaks being selected first. Relative peaks that are in peri-lesional areas are also included. For each subject, 2 to 3 peaks are selected for each language task.
2.4. nTMS Mapping
2.4.1 Subject Preparation
Prior to starting the mapping, all subjects were prepared for the experience with a short verbal description of the expected sensations associated with single-pulse and repetitive nTMS. Subjects were asked to give feedback about the level of discomfort associated with the nTMS mapping process so that the stimulation intensity could be decreased if necessary. They were also encouraged at multiple points to ask for a break if needed, and to opt out of the mapping altogether if they felt any significant discomfort. Finally, subjects were prepared for a possible speech arrest with an explanation of the purpose of mapping, a basic description of the underlying neurophysiological processes, and an assurance that any language disturbance was a result of the stimulation and would spontaneously resolve.
2.4.2. Device Description
We used a navigated transcranial magnetic stimulation (nTMS) system (NBS System 3.2, Nexstim, Helsinki, Finland) for mapping of motor and language areas. This device utilized a figure-of-eight coil with a coil diameter of 40mm, an overall length of 173mm and width of 99mm, and winding diameters of 50mm (inner) and 70mm (outer). The device was used in single pulse stimulation mode with a biphasic pulse shape and a pulse length of 230 µs. The strength and directionality of this field was calculated according to a dynamic spherical model which took into account the preset parameters of stimulation as well as the subject’s scalp/skull thickness (Sarvas, 1987; Tarkiainen et al., 2003).
2.4.3. Procedure in Detail
A high-resolution T1 MRI series was acquired as described above and uploaded to the nTMS software, which recreated a 3-dimensional model of the subject. This anatomical scan was then co-registered to the subject’s head using anatomical landmarks and surface matching (Hannula et al., 2005). The mapping surface was located between 23 and 28mm deep to the scalp; the peeling depth was adjusted on a case-by-case basis to best reveal the cortical anatomy.
Surface electrodes (Neuroline 720; Ambu, Ballerup, Denmark) were attached to the subject’s muscles for EMG recording. All subjects had electrodes affixed to abductor digiti quinti (ADQ) and abductor pollicis brevis (APB). The most likely location of the hand knob was identified anatomically. This area was then stimulated in a grid pattern with 5mm spacing while systematically varying the rotation, tilt and yaw of the magnetic field. The location of maximal motor evoked potential (MEP) was identified. Resting motor threshold (RMT) was then determined using this location. RMT was defined as the minimum stimulation intensity capable of generating an MEP in 50% of cases (Rossini et al., 1994).
Language mapping was then performed at 110% of RMT. Subject responses were recorded and language disruptions were categorized as described in Section 2.6. The rTMS protocol consisted of a train of 10 pulses at 5 Hz lasting a total of 2 s. The inter-stimulus interval was maintained at 4s with the image being visible for 3 seconds. The rTMS pulse train was automatically triggered at stimulus onset by an electronic trigger. If subjects were unable to tolerate mapping at this intensity, it was lowered in 10% increments until the stimulation trains were tolerated.
Using this configuration, the tumoral and peritumoral regions were covered systematically in a grid pattern with stimulations occurring at ≈5mm spacings. The electrical field strength was optimized by the software, which dictated the tilt of the stimulator coil. All mapping was conducted with a minimum incident electrical field strength of 65 V/m. The orientation of the dipole was largely maintained in perpendicularity to the fibers of the temporalis muscle to minimize discomfort. Secondary attention was paid to maintaining perpendicularity to the adjacent sulcus. The mapping was performed over 3 passes, during which all cortical regions were mapped, and between which subjects were given a 2 minute break. All cortical regions were covered at least three times. Mapped regions included the area of anticipated exposure plus at least a 2 cm margin.
2.5. Surgical Procedure and DCS Mapping
All subjects underwent surgery for tumor resection with DCS mapping 24 hours after the MEGI and TMS were performed. Neuronavigation was used in all cases; the craniotomy was tailored according to the exact extension of the tumor with a 3-cm margin of surrounding brain tissue. A positive language site was defined as an area that induced language disturbance when stimulated. Full details of our DCS mapping protocol have been previously described (Berger and Ojemann, 1992; Berger et al., 1990; Sanai et al., 2008). Object naming was performed with the same Snodgrass image set used in the MEGI and the nTMS studies (Snodgrass and Vanderwart, 1980). A bipolar electrode with 5-mm spaced tips delivering a biphasic current (square-wave pulses in 4-second trains at 60 Hz, with single-pulse phase duration = 1 ms) was applied to the brain. Cortical mapping was initiated at 1.5 mA, and increased to a maximum of 6 mA. Stimulation sites were identified using sterile numbered labels distributed per square centimeter of exposed cortex. Language sites were categorized using the same criteria as described above, although mild language disturbances such as paraphasias did not alter the surgical plan. After the cortical mapping was completed, the tumor was removed in a tailored fashion with subcortical mapping where appropriate. When a functional site was detected, a 1-cm margin of tissue was always preserved around this site (Haglund et al., 1994). The exact locations of the functional sites were registered with a navigational MRI system; this was performed just after the cortical mapping and before any tumor removal that could have been a source of spatial mis-localization by brain shift (Hill et al., 1998). Additionally, a photograph was taken of the cortical surface map before and after resection.
2.6. Analysis of Language Disruptions
In both the nTMS and the DCS language mapping studies, the language mapping task consisted of object naming with the same set of images that were used in the MEGI (Snodgrass and Vanderwart, 1980). Subjects were presented with the images randomly and were instructed to name the object as part of a phrase (e.g. “this is a…”) so as to distinguish speech arrest from anomia. During testing, subject responses were recorded on audio-visual equipment for offline analysis by two experts, one neurosurgeon and one speech and language pathologist. Disagreements on categorization were resolved by a third expert, a speech and language pathologist. All analysis of language disruptions was conducted with the experts blinded to the cortical region being stimulated. A positive site was recorded if stimulation resulted in a given error in at least 2 out of 3 trials. Errors were categorized into 3 groups. Speech arrest was defined as a complete inability to produce language. Anomia was defined as the ability to produce the lead-in phrase without the ability to name the object. All other speech errors, including semantic and phonemic paraphasias, hesitation, and stuttering were categorized as “other,” due to the relatively low numbers of each of these types of error.
2.7. Analysis of MEGI, nTMS, and DCS Sites
Each subject’s MRI was spatially normalized to the Montreal Neurological Institute (MNI) atlas template, using the toolbox Statistical Parametric Mapping 8 (SPM8) for MATLAB (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). In cases where tumor growth or peri-tumoral edema distorted the brain anatomy, reference points were placed on identifiable anatomic structures such as the Sylvian fissure, inferior frontal sulcus, superior temporal sulcus, and central sulcus to assist the normalization procedure. After normalization, the volumes were then visually inspected for correctness using mri3dX, an open-source application for 3-dimensional reconstruction and analysis of MRI, which is documented and available for free download (http://www.cubric.cf.ac.uk/Documentation/mri3dX/). As a final check, each 3-dimensional volume was then compared against intraoperative photographs of the cortical surface. The cortical regions of interest (ROIs) of nTMS mapping, surgical mapping, and surgical resection were delineated for each subject. All positive speech preparation MEGI, nTMS and DCS points were also marked.
Using the MNI coordinate space, the ROIs and mapping points were overlaid onto a template Freesurfer brain (“Bert”) for data aggregation and group analysis. Freesurfer is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). To aid in group analysis and optimize data representation, the ROIs and points were transferred to Photoshop CS4 (Adobe Inc, Delaware, USA), with each subject’s ROIs and mapping points occupying a separate layer. A 1cm2 grid was also overlaid on this composite ROI and language map.
Descriptive statistics were used to report the baseline characteristics and outcome profiles for all subjects. Aggregate matrix representations of ROIs and mapping points were constructed using MATLAB. For each cortical site, the percentage of nTMS and DCS stimulations that were positive (i.e., that caused interruption in language function) and negative were recorded. Analysis of co-positive nTMS, DCS, and MEGI points was done on a per-subject basis. Sites were considered to be co-positive if the distance between them was less than 10mm. Using nTMS as the experimental and DCS as the gold-standard modalities, overall sensitivity, specificity, positive, and negative predictive values were calculated.
3. Results
3.1. Demographics and Tumor Characteristics
In a 6-month period from August, 2011 to January, 2012, 12 sequential craniotomies were performed with awake speech mapping. Of these subjects, all met the inclusion criteria and none refused participation. There were 7 male and 5 female subjects, and their ages ranged from 29 to 65 with a median age of 45 years. Ten subjects were right handed, 1 was left handed, and 1 reported himself as ambidextrous (although he wrote predominantly with his left). Wada testing was performed on the 1 purely left handed patient and showed left hemispheric speech. Eleven subjects demonstrated left hemispheric speech by MEGI-based language laterality index(Findlay et al., 2012), while 1 right handed subject demonstrated right hemispheric speech by MEGI-based laterality index (this subject had a prior partial left frontal lobectomy).
Six subjects were newly presenting and 6 had recurrence. Four subjects had frontal lesions, 7 had temporal lesions, 1 had a parietal lesion, and 1 subject’s lesion spanned multiple lobes. No subjects had significant neuropsychological impairment on preoperative testing. At the time of mapping, 7 subjects were on no anti-epileptic drugs, 5 were on levetiracetam, 1 was on valproate, and 1 was on both lacosamide and oxcarbazepine. Final pathology results demonstrated glioblastoma multiforme (WHO IV) in 5 subjects, anaplastic astrocytoma (WHO III) in 1 subject, anaplastic oligoastrocytoma (WHO III) in 2 subjects, oligoastrocytoma (WHO II) in 2 subjects, anaplastic ganglioglioma (WHO III) in 1 subject, and ganglioglioma (WHO I) in 1 subject (Table 2).
Table 2.
Demographics and clinical characteristics. (LI = laterality index; AA = anaplastic astrocytoma; OA = oligoastrocytoma; AOA = anaplastic oligoastrocytoma; GG = ganglioglioma; AGG = anaplastic ganglioglioma; GBM = glioblastoma multiforme)
| Age | Sex | Handed- ness |
MEGI- based LI |
Location | Recurrent? | Pathology | AED | |
|---|---|---|---|---|---|---|---|---|
| 1. | 44 | M | L/R | L | frontal | yes | AA (WHO III) | - |
| 2. | 37 | F | R | L | temporal | yes | OA (WHO II) | valproate |
| 3. | 31 | F | R | L | frontal | no | OA (WHO II) | levetiracetam |
| 4. | 42 | M | R | L | temporal | yes | GBM (WHO IV) | - |
| 5. | 56 | F | R | L | frontal | no | AOA (WHO III) | levetiracetam |
| 6. | 56 | M | R | R | frontal | yes | GBM (WHO IV) | - |
| 7. | 34 | F | R | L | temporal | no | AGG (WHO III) | levetiracetam |
| 8. | 61 | M | R | L | temporal | yes | GBM (WHO IV) | - |
| 9. | 63 | M | R | L | parietal | no | GBM (WHO IV) | - |
| 10. | 23 | M | R | L | multiple | yes | AOA (WHO III) | lacosamide, oxcarbazepine |
| 11. | 29 | M | L | L | temporal | no | GG (WHO I) | levetiracetam |
| 12. | 65 | F | R | L | temporal | no | GBM (WHO IV) | levetiracetam |
3.2. nTMS Mapping Tolerability and Safety
Subjects underwent rTMS language mapping starting at 110% of RMT using a pulse train of 10 pulses at 5 Hz. All subjects were able to conclude the mapping session with a minimum of 3 passes over each cortical site. Seven subjects complained of discomfort during the procedure resulting in a decrease in the stimulation intensity until continued mapping was tolerable. As a result, 5 subjects were mapped at 110% of RMT, 5 at 90% of RMT, and 2 at 80% of RMT. Within this range of intensities, there did not appear to be any correlation between stimulator intensity and number of language disturbance sites identified. No subjects exhibited emotional disturbance such as spontaneous crying after speech arrest as has been previously reported. Two subjects reported a headache following mapping for which they received acetaminophen. No subject exhibited signs of seizure during or after the mapping process.
3.3. Analysis of Positive Language sites
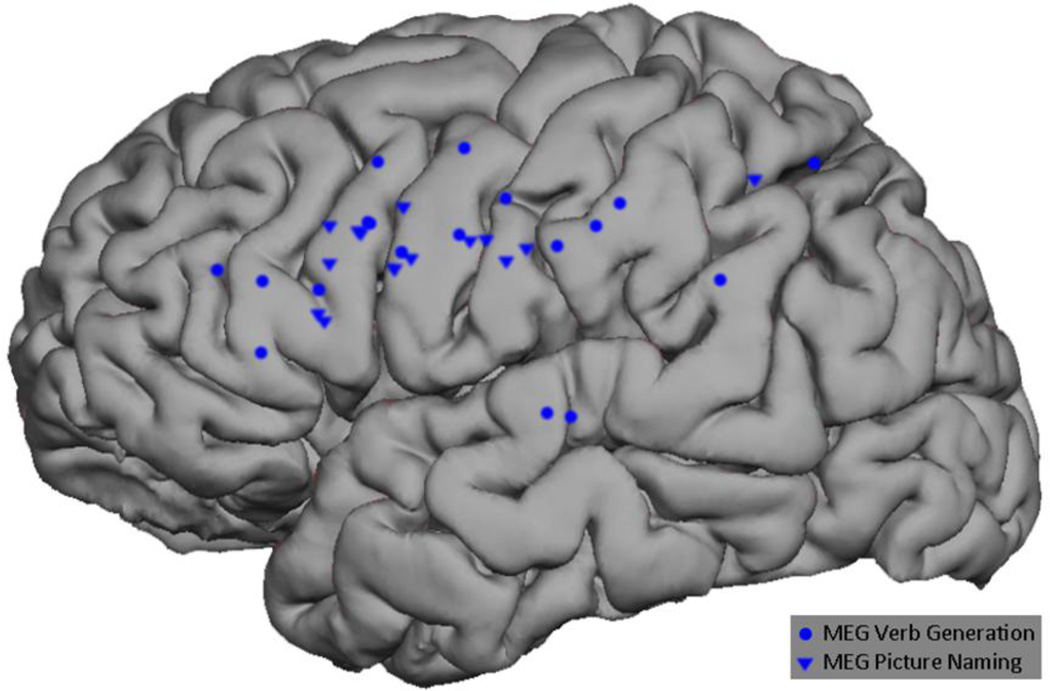
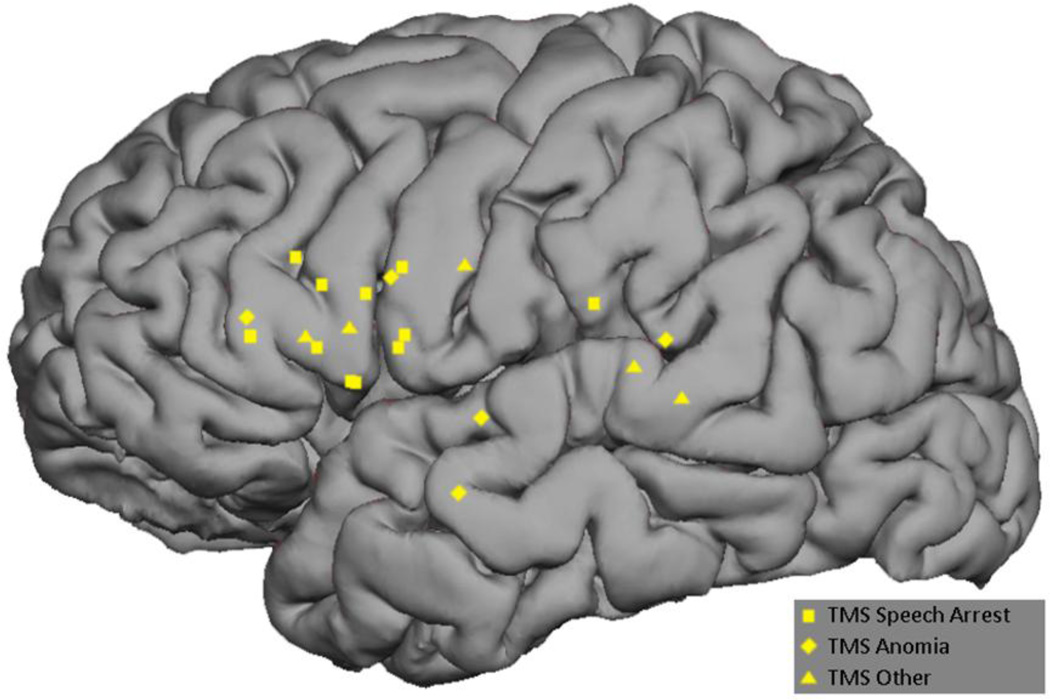
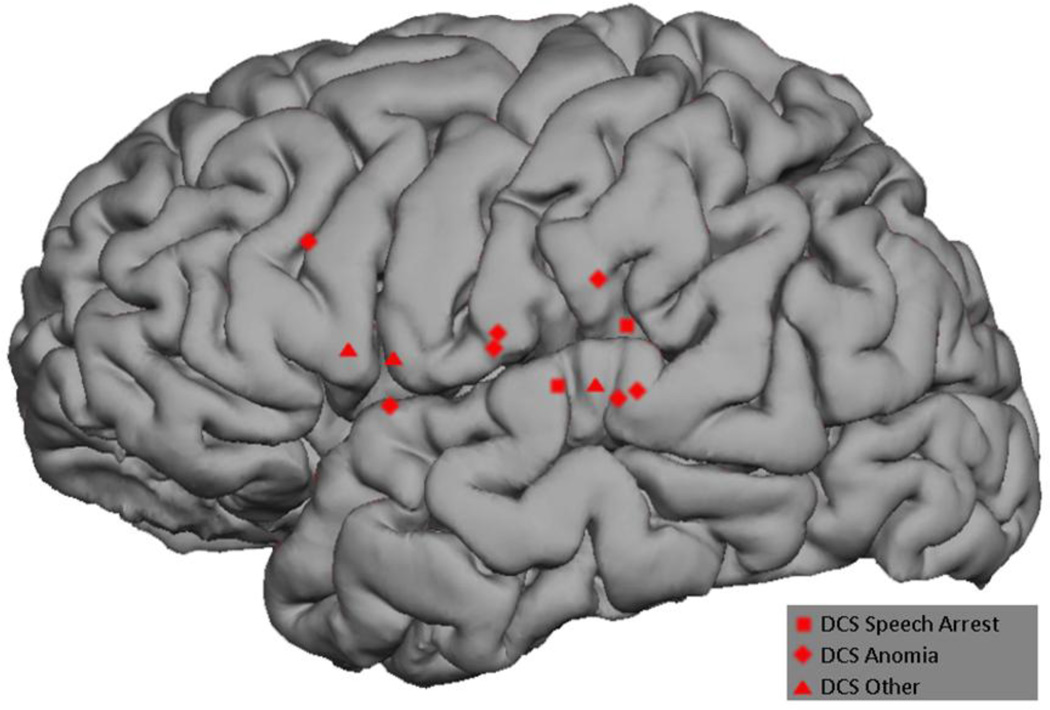
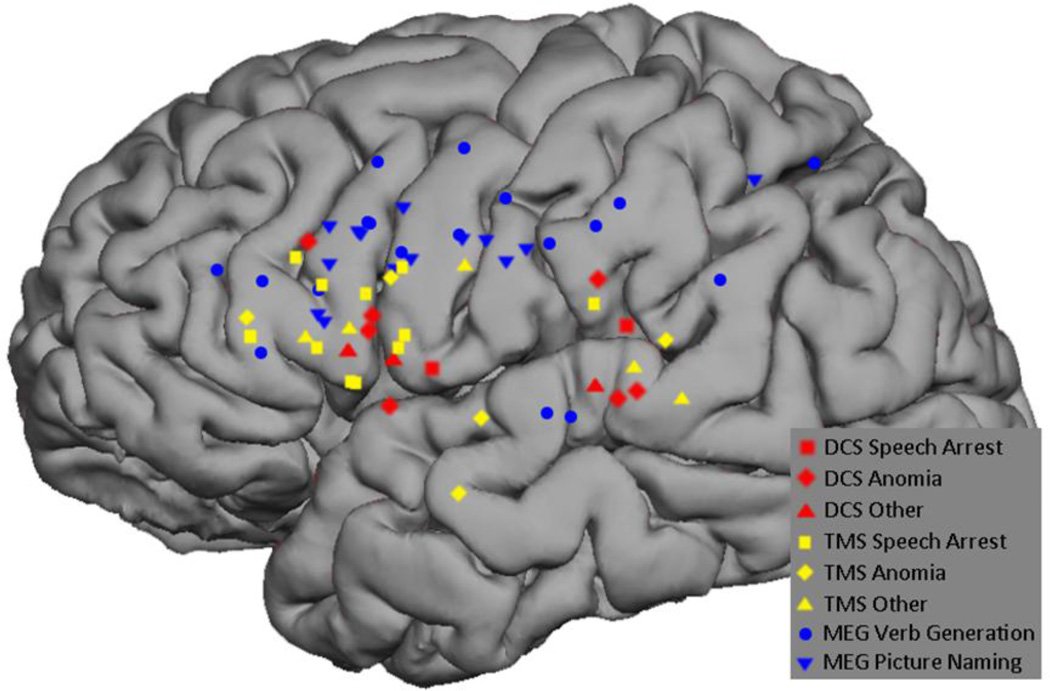
All 12 subjects underwent MEGI and nTMS mapping as described above. The MEGI algorithms successfully localized at least one site each for verb-generation and picture naming in every subject. In total, 18 verb-generation and 14 picture naming points were recorded (Figure 2). nTMS was able to localize at least 1 site of language disruption in 10 subjects; 2 subjects had a completely negative nTMS map. A total of 21 positive sites were found with nTMS. Of these, 11 caused speech arrest, 5 caused anomia, and 5 caused other disruptions including hesitation and paraphasia (Figure 3). Intraoperatively, 9 subjects had at least 1 positive DCS site and 3 had negative maps. A total of 12 DCS points were identified; speech arrest accounted for 2, anomia for 7, and other disruptions including hesitation and paraphasia made up the final 3 (Figure 4). A composite overlay of all points is depicted in (Figure 5). Although formal inter-observer reliability statistics were not calculated, disagreements regarding the categorization of language errors occurred in 3 individual language errors, requiring consultation with a third expert as described in Section 2.6.
Figure 2.
Composite representation of all MEGI-based verb-generation and object naming points across subjects.
Figure 3.
Composite representation of all nTMS-based positive speech disruption points across subjects.
Figure 4.
Composite representation of all DCS-based positive speech disruption points across subjects.
Figure 5.
Composite representation of all MEGI-, nTMS-, and DCS-based positive speech disruption points across subjects.
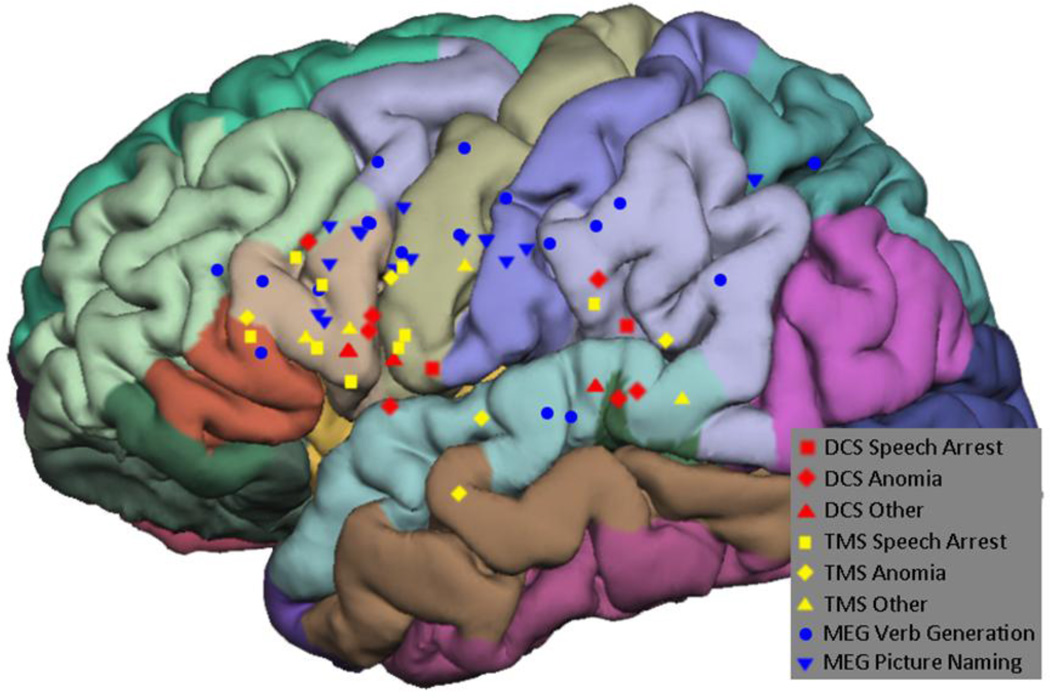
The sites were also categorized by anatomical location (Figure 6). MEGI sites were distributed widely over the left hemisphere, with 28% of sites located in the pars operculum, 25% in the pre-central gyrus, and 13% in the supramarginal gyrus. Of the nTMS positive sites, 43% were located in the pars operculum, 24% were in the pre-central gyrus, and 14% were in the superior temporal gyrus. A sub-analysis by type of speech disruption showed that 55% of speech arrest sites were located in the pars operculum and 27% were in the pre-central gyrus; thus, these two locations accounted for more than 80% of nTMS speech arrest sites. The 5 anomia sites were distributed evenly across multiple regions. DCS sites were located in the superior temporal gyrus in 33% of cases and the pars operculum in 25% of cases, while the pre-central gyrus and supramarginal gyrus accounted for 17% of cases each. The 2 DCS speech arrest sites were split between the pre-central gyrus and the supramarginal gyrus, while anomia sites were in the superior temporal gyrus in 50% of cases and in the pars orbitalis in 33% of cases. The complete breakdown is shown in Table 3.
Figure 6.
All positive mapping points overlaid on anatomic regions
Table 3.
Breakdown of positive language sites by modality and anatomic location – no. (%). (MFG (c) = caudal middle frontal gyrus; MFG (r) = rostral middle frontal gyrus; Post-CG = post-central gyrus; Pre-CG = pre-central gyrus; PO = pars opercularis; PT = pars triangularis; SMG = supramarginal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; SPL = superior parietal lobule; SA = speech arrest)
| MFG (c) |
MFG (r) |
Post- CG |
Pre- CG |
PO | PT | SMG | MTG | STG | SPL | |
|---|---|---|---|---|---|---|---|---|---|---|
| DCS | - | 1 (8) | - | 2 (17) | 3 (25) | - | 2 (17) | - | 4 (33) | - |
| -SA | - | - | - | 1 (50) | - | - | 1 (50) | - | - | - |
| -Anomia | - | 1 (17) | - | - | 2 (33) | - | - | - | 3 (50) | - |
| -Other | - | - | - | 1 (25) | 1 (25) | - | 1 (25) | - | 1 (25) | - |
| nTMS | - | - | - | 5 (24) | 9 (43) | 1 (5) | 2 (10) | 1 (5) | 3 (14) | - |
| -SA | - | - | - | 3 (27) | 6 (55) | 1 (9) | 1 (9) | - | - | - |
| -Anomia | - | - | - | 1 (20) | 1 (20) | - | 1 (20) | 1 (20) | 1 (20) | - |
| -Other | - | - | - | 1 (20) | 2 (40) | - | - | - | 2 (40) | - |
| MSI | 1 (3) | 2 (6) | 3 (9) | 8 (25) | 9 (28) | 1 (3) | 4 (13) | - | 2 (6) | 2 (6) |
| -Verb-gen | 1 (6) | 1 (6) | 1 (6) | 3 (17) | 4 (22) | 1 (6) | 4 (22) | - | 2 (11) | 1 (6) |
| -Naming | - | 1 (7) | 2 (14) | 5 (36) | 5 (36) | - | - | - | - | 1 (7) |
3.4. Comparison of Language Maps
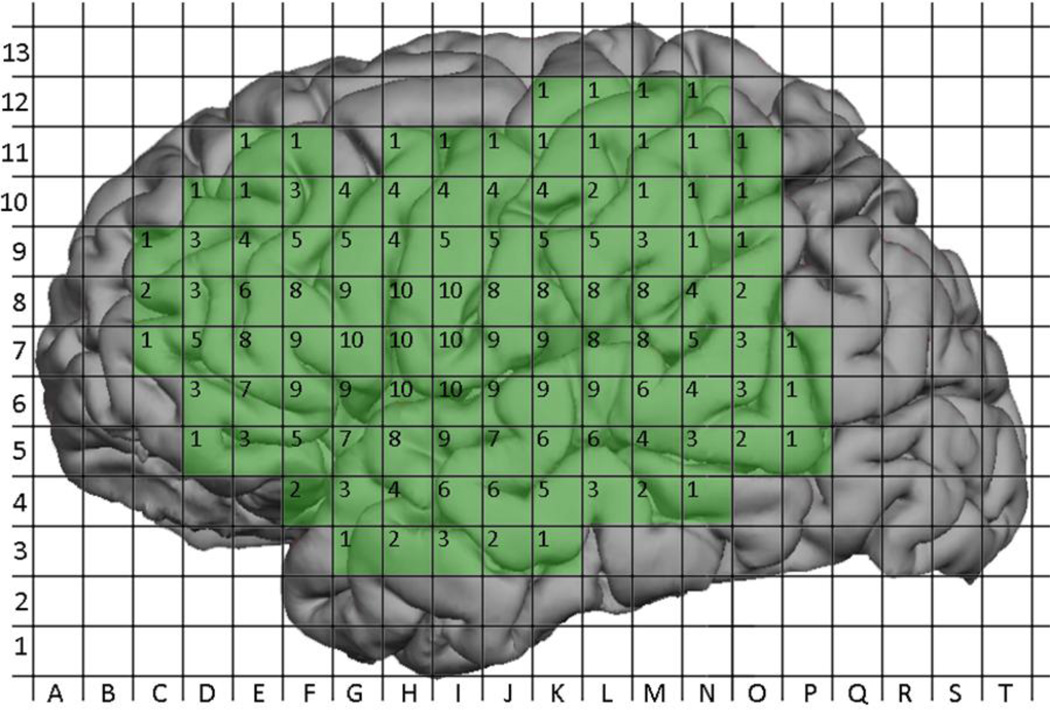
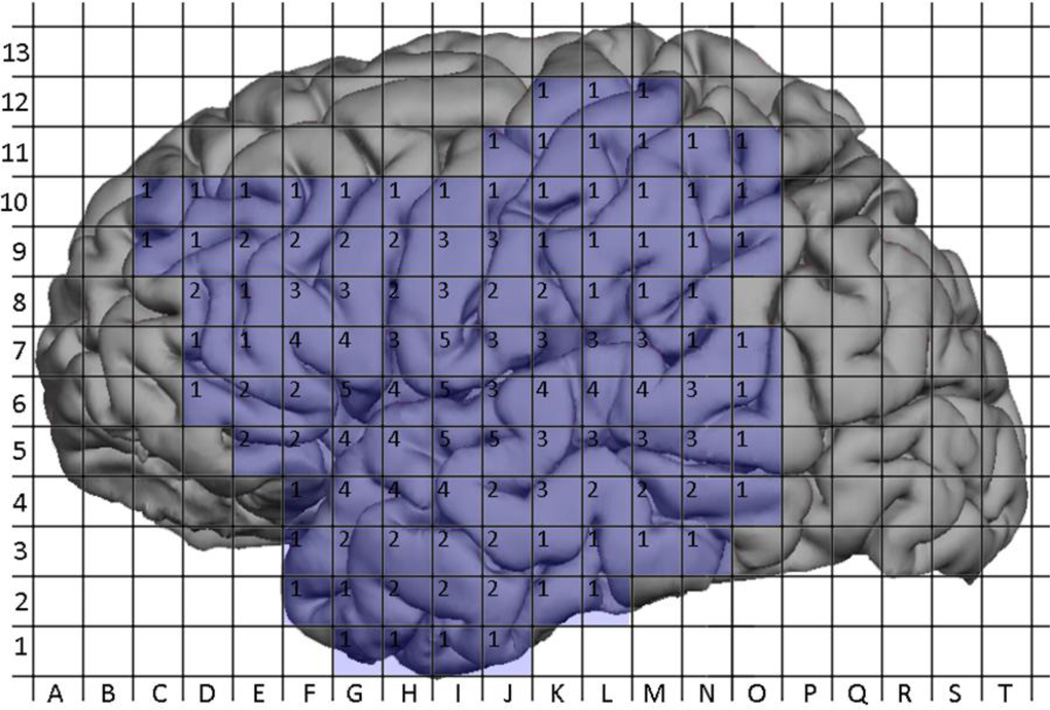
The aggregation of nTMS maps and DCS maps generated a composite map representing the total number of cortical sites mapped using nTMS or DCS (Figure 7 and Figure 8). A total of 465 sites were mapped with nTMS, while 221 sites were mapped with DCS; 183 sites were mapped with both modalities. The probability of positivity at each site was calculated for nTMS and DCS and is depicted in (Figure 9 and Figure 10). The 183 sites that were mapped with both nTMS and DCS were analyzed for co-positivity as described above. Of these sites, 9 were nTMS positive / DCS positive (true positive) and 169 were nTMS negative / DCS negative (true negative). There were 4 nTMS positive / DCS negative sites (false positive) and 1 nTMS negative / DCS positive (false negative) site. Thus the sensitivity was 90%, specificity was 98%, the positive predictive value was 69%, and the negative predictive value was 99% (Table 3).
Figure 7.
Aggregated coverage of nTMS mapping across all subjects. Labels within grid squares represent the number of patients who underwent nTMS mapping at that location.
Figure 8.
Aggregated coverage of DCS mapping across all subjects. Labels within grid squares represent the number of patients who underwent DCS mapping at that location.
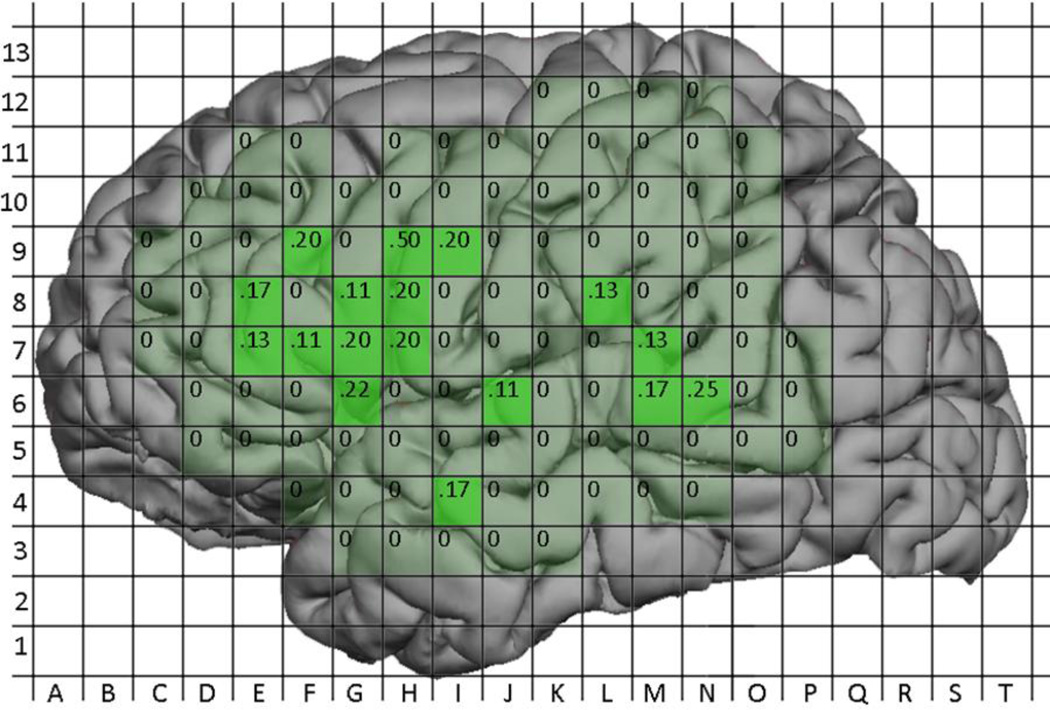
Figure 9.
Probabilites of a positive nTMS site by location. Labels within grid squares represent the probability of a positive nTMS site at that location (No. positive / No. mapped).
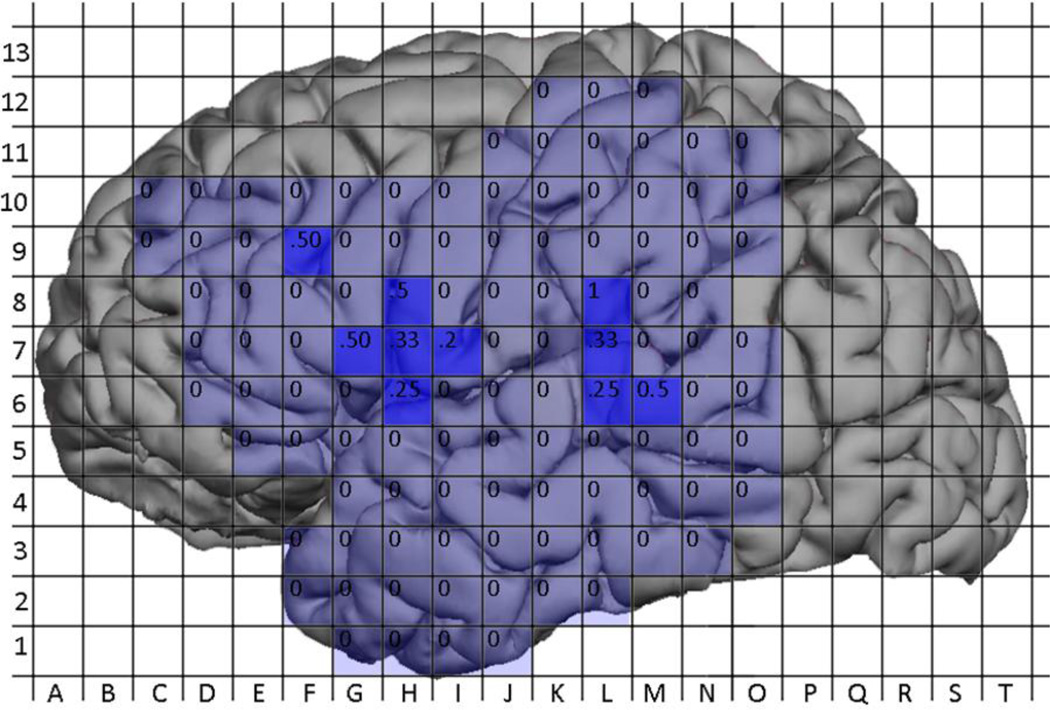
Figure 10.
Probabilites of a positive DCS site by location. Labels within grid squares represent the probability of a positive DCS site at that location (No. positive / No. mapped).
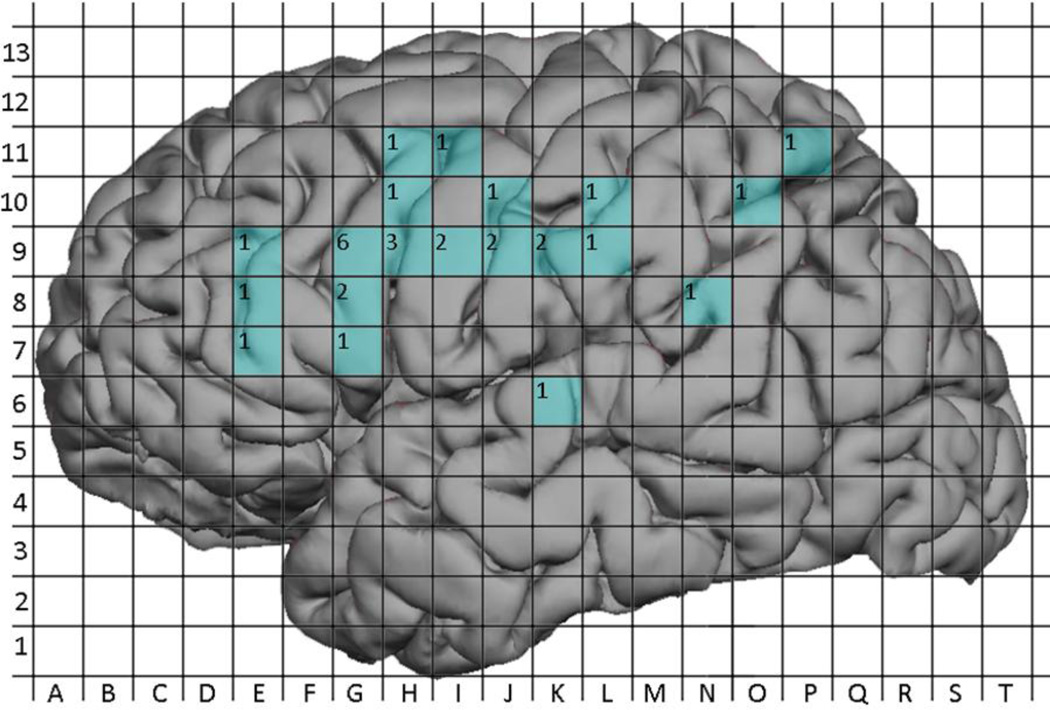
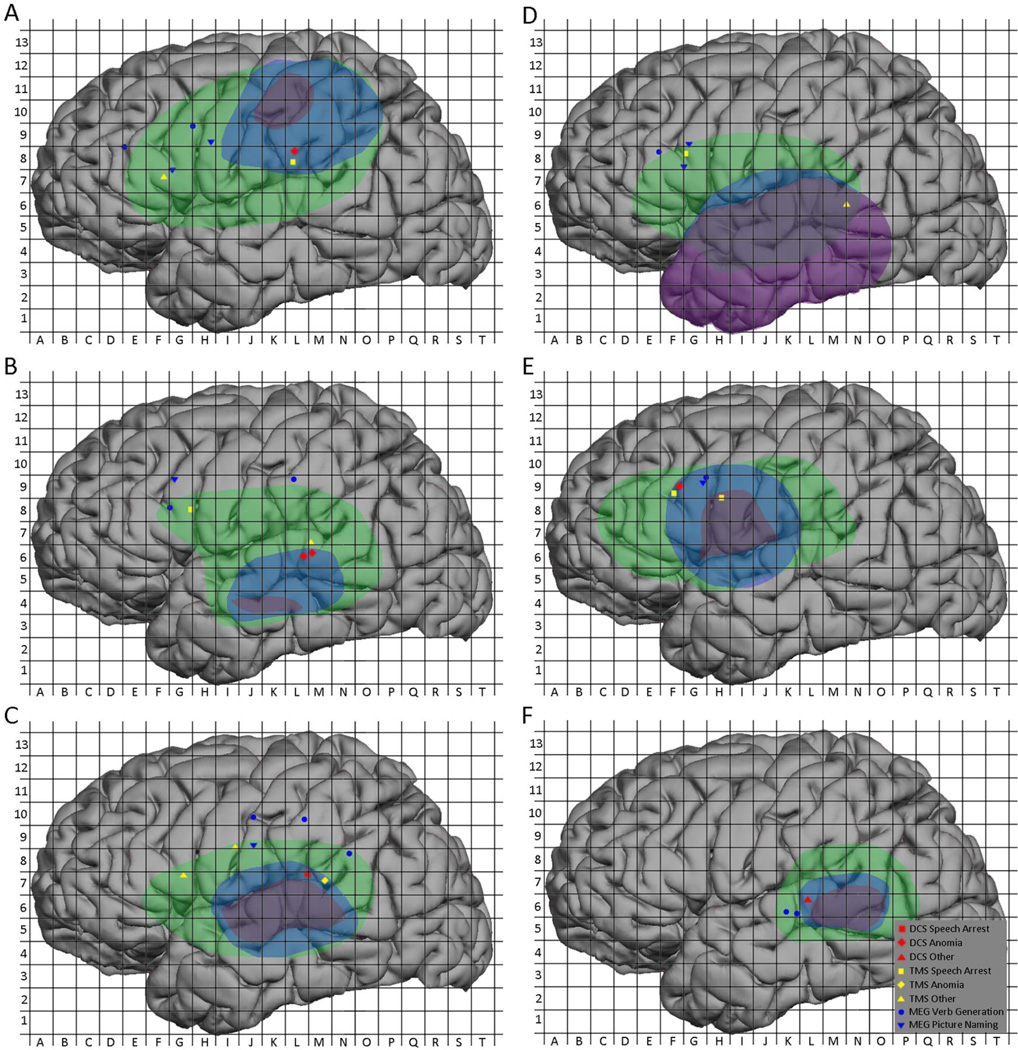
MEGI sites were also aggregated into a composite map (Figure 11) but because MEGI is a whole-brain mapping technique while nTMS and DCS are regional, calculations of sensitivity and specificity were not performed for MEGI. Instead, the 32 MEGI sites were compared with nTMS and DCS maps on a per-subject basis. In 4 subjects (#2, #3, #9, and #12) an MEGI object naming site correlated with an nTMS language site while in 1 subject (#3) an object naming site correlated with a DCS language site. Similarly, in 3 subjects (#2, #3, and #11) an MEGI verb generation site correlated with an nTMS language site, while in 2 subjects (#3 and #8) a verb generation site correlated with a DCS language site. Overall, 7 MEGI sites correlated with nTMS language sites in 5 distinct subjects and with DCS language sites in 2 distinct subjects. These subjects’ individual maps are depicted in Figure 12A–F.
Figure 11.
Aggregated MEGI language sites across all subjects. Labels within grid squares represent the number of patients with positive MEGI mapping sites at that location.
Figure 12.
Case examples. (A) 63-year-old man with a left parietal GBM and good concordance between nTMS, DCS, and MEGI; (B) 29-year-old man with a left temporal ganglioglioma and good concordance between nTMS, DCS, and MEGI; (C) 65-year-old woman with GBM and good concordance between nTMS, DCS, and MEGI; (D) 37-year-old woman with left temporal anaplastic ganglioglioma and false positive nTMS site in the posterior temporal region; (E) 31-year-old woman with left frontal oligoastrocytoma and false positive nTMS site in inferior precentral gyrus; (F) 61-year-old man with left temporal GBM and false negative nTMS site in posterior superior temporal gyrus.
4. Discussion
4.1
Since its introduction, DCS remains the gold-standard for lesion-based cortical language mapping. In the past, these surgeries involved large craniotomies to maximize the chance of finding one or more positive language sites (Duffau et al., 1999; Haglund et al., 1994; Ojemann et al., 1989). Currently, however, it has been shown that a negative language map may be relied upon; thus, smaller craniotomies exposing only the peritumoral area are now preferred. However, despite the significant advances made in the field, the rate of permanent neurologic morbidity following surgery has been reported at 3.2 – 20% (Brell et al., 2000; Chang et al., 2003; Devaux et al., 1993; Sanai et al., 2008; Sawaya et al., 1998; Taylor and Bernstein, 1999; Vorster and Barnett, 1998). Additionally, it cannot be performed in every case: anxiety or psychiatric disturbance, intolerance of conscious sedation, and young age are all relative contraindications. Furthermore, intraoperative seizure induced by DCS can render further mapping impossible.
Although we did identify a site of nTMS speech arrest in several subjects, we could not correlate all of these sites to DCS maps because not all of them fell within the surgical exposure. However, our objective was not to find a speech arrest site in every subject. Rather, it was to explore the utility of nTMS as a modality for noninvasive lesion-based language mapping and to compare it against DCS and MEGI. According to this study, nTMS language mapping may play a valuable role in both basic science and clinical localization studies.
4.2. Sensitivity, Specificity, and Predictive Value
We have shown that nTMS has a high rate of concordance with intraoperative DCS—in this series, nTMS has 90% sensitivity, and 98% specificity when compared with DCS. The single false negative site (discussed below) and the 169 true negative sites yielded a promising 99% negative predictive value for the technique. However, it should be noted that any given subject will have (at most) only a few positive language sites, so most cortical regions will be negative. The large number of true negatives is therefore understandable; in such cases it is expected to have a good negative predictive value.
The positive predictive value, in contrast, was much lower at 69%. The 4 false positive nTMS sites out of 13 total sites reflect what we believe is a tendency of nTMS to “over-call” positive language sites (see Section 4.6 for a discussion of why this may be). This tendency is likely the reason why previous attempts to utilize TMS for language lateralization failed: there were too many false-positive sites (in those cases, the false-positives were on the contralateral, non-dominant hemisphere). Indeed, in our series, 3 of the 4 false-positive dominant hemisphere sites went on to be resected with no clinical sequelae.
These results are in contrast to those of nTMS for motor mapping. Multiple prior studies have shown excellent correlation between TMS- and DCS-generated motor maps (Krieg et al., 2012; Picht et al., 2011; Tarapore et al., 2012; Vitikainen et al., 2009). The language system, however, is far more complex than the motor system, and this difference is unsurprising. As a presurgical language mapping tool, this propensity for over-identification of positive sites is preferable to the opposite. Having identified the location of candidate language sites preoperatively, the surgeon can quickly hone in on these sites intraoperatively and perform confirmatory DCS testing. In its current form, nTMS mapping is best utilized in this manner: to generate a map of likely language sites that can then be confirmed with DCS intraoperatively.
4.3. Specific Cases
While much of this report focuses on aggregated group statistics, the inherent variability of language sites between subjects makes an individual analysis instructive. We thus present 6 cases with varying degrees of correlations between mapping modalities. Three subjects (#9, #11, #12) that had good nTMS, DCS and MEGI concordance are presented in Figure 12A–C, respectively. Subject #9, a 63-year-old man with a left parietal GBM, had excellent co-localization between the nTMS and DCS points in the inferior supramarginal gyrus (grid square L8). Similarly, this subject had concordance between an nTMS and MEGI picture naming point in the pars opercularis (grid squares F7 and G7), although there were 3 other MEGI peaks that did not have corresponding nTMS points. Subject #11, a 29-year-old man with a left temporal ganglioglioma, demonstrated colocalization between posterior superior temporal gyrus nTMS and DCS sites(grid squares M7, L6, and L7), both of which produced anomia. He also showed proximity between an MEGI verb generation peak and a TMS speech arrest site in the pars opercularis (grid square G8). Two other MEGI sites fell outside the region of nTMS mapping. Finally, subject #12, a 65-year-old woman with GBM, showed concordance between nTMS and DCS sites in the parieto-temporal region (grid squares L7 and M7), as well as between nTMS and MEGI picture naming in the inferior Rolandic region (grid squares I9 and J9). This subject also had one positive nTMS site in the pars opercularis that did not coincide with an MEGI site and fell outside the region of intraoperative mapping.
Examination of false positive and negative sites is, perhaps, of even more value. Subject #2 (Figure 12D), a 37-year-old woman with a left temporal anaplastic ganglioglioma, had a negative map of the temporal lobe by DCS but had 1 positive anomia site by nTMS (grid square N6). Interestingly she also had good concordance between a pars operculum nTMS site and 3 MEGI sites. Subject #3 (Figure 12E), a 31-year-old woman with left frontal oligoastrocytoma, showed excellent colocalization between nTMS DCS sites, on the periphery of the exposure (grid square F9). Two MEGI sites for verb generation and picture naming were also found close by (grid square G9). However, 1 nTMS speech arrest site had no DCS correlation and fell within the region of resection; this subject went on to have some word finding difficulties that improved markedly on 3-month follow-up. Finally, subject #8 (Figure 12F), a 61-year-old man with left temporal GBM had a negative map by nTMS, but demonstrated 1 positive DCS anomia site in the superior temporal gyrus that correlated with 2 MEGI verb generation (grid squares K6 and L6).
4.4. Comparison with Prior Studies
Although the use of nTMS for language mapping is in its infancy, in the last 3 decades, several eminent research groups have examined TMS as a technique for studying the lateralization of language function (Epstein et al., 1996; Epstein et al., 1999; Epstein et al., 2000; Jennum et al., 1994; Michelucci et al., 1994; Pascual-Leone et al., 1991; Wassermann et al., 1999). In the first reported study of rTMS and the interruption of language function, Pascual-Leone et al showed that speech arrest may be obtained in all 6 of 6 test subjects. Subsequent studies by Michelucci et al. (1994) and Jennum et al. (1994) had less convincing results, finding speech arrest sites in 50% and 67% of their study subjects, respectively. Wassermann et al. (1999) showed that rTMS of left-sided targets would preferentially interfere with object naming. Finally, Epstein et al. (2000) showed that rTMS was inferior to the Wada test in determining language laterality. One other report of particular significance was that of Epstein et al, which showed that low-frequency repetitive TMS (4–5 Hz) can be equally effective as high frequency repetitive TMS (15–30 Hz) in causing language disruption, because most subjects are unable to tolerate the discomfort of high-frequency repetitive TMS (Epstein et al., 1996).
There are a few distinctions to point out between these previous studies and the current study which make direct comparison of results difficult. One is the difference in stimulation parameters. The initial TMS language studies utilized high frequencies of 15–30Hz with long trains of up to 10s in their stimulation protocols. These parameters, in addition to being intensely uncomfortable to subjects, would not be approved by most modern ethical review committees because of the risk of provoking seizure. Indeed, Wassermann et al chose to change the stimulation protocol in the midst of their study when it became known that low frequency stimulation was equally effective.
Another important difference between this and prior studies is the integration with a frameless stereotactic image guidance system. In our opinion, image guidance is a critical component if any comparison is to be attempted between nTMS sites and those of other modalities. Precise targeting of the stimulus pulse is clearly important, but we have found that the orientation of the dipole is also critical. In fact we have noted on several occasions that small rotations of just 10–15° can alter the amplitude of a motor evoked potential or the positivity of a language site. In general, we maintained the dipole in perpendicularity with fibers of the temporalis muscle (see below) and the adjacent sulcus.
Two final differences are our use of the figure-of-8 coil and the ability to optimize the electrical field strength in real-time. Regarding the coil shape there is not much to point out except that a figure-of-8 coil offers improved ability to focus the magnetic field on a small cortical region. Regarding field optimization, our system allows us to visualize in real-time the theoretical field strength for a proposed stimulation pulse given the angle of the coil and the targeted cortical region. We are thus able to maximize the effective cortical depolarization for a given stimulator output intensity, thus ensuring improved consistency between pulse trains.
In recent months, one other group has compared nTMS language mapping with DCS (Picht et al., 2013). Although the findings of this study are promising, there are several critical methodological differences between our study and the aforementioned one. First, we utilize an nTMS stimulation onset that is simultaneous with presentation of the object, while Picht et al employ a 300ms delay between object presentation and nTMS stimulation. Our nTMS protocol is therefore identical to the intraoperative DCS protocol, which also has no delay between presentation and stimulation. Second, we employ a standardized normalization algorithm using SPM8 and mri3dX, as described in the Methods section, projecting each brain onto the MNI template. This allows for standardized data analysis as well as precise and accurate localization of positive points within anatomic subregions of the cortex. Third, our analysis of co-positive nTMS and DCS points utilizes much higher granularity. Picht et al use a simple anatomic cortical parcellation system, counting as co-positive any pair of nTMS and DCS points that fall within the same parcel. Points in different parcels, regardless of actual proximity, are considered not co-positive. In our analysis, on the other hand, points were considered co-positive based only on the geometric distance between them: if they were within 10mm of one another, they were considered co-positive. Thus, our analysis more closely reflects the actual co-localization of nTMS and DCS sites. This methodological difference also accounts for the improved specificity in our study (98% vs. 24%): by improving our granularity to 10mm, we avoided the large number of false-positives that decreased the specificity in the Picht et al analysis. Finally, we include MEGI data in our study, which provides additional depth regarding the utility of each modality in obtaining a high-quality, reliable language map.
4.5. Subject Safety and Tolerability
While seizures are a theoretical risk in any TMS procedure, we saw none in our series. This zero rate is likely a combination of several factors, including a low-frequency protocol and careful subject selection (no subjects had uncontrolled seizures). It is likely not attributable to pharmacology, because more than half of our subjects were on no anti-epileptic drugs at the time of mapping.
As we have alluded to elsewhere in this report, optimizing subject comfort is of great importance to make nTMS a viable modality for preoperative language mapping. The inherent issue is that the majority of language sites fall in the inferior frontal and temporal regions, underneath the temporalis muscle and facial nerve. In targeting these regions, the magnetic pulse must travel through the muscle fibers; in so doing it triggers a brief but powerful spasm of the temporalis muscle. It can also depolarize the facial nerve, resulting in similar contractions of the orbicularis oculus muscle. Thus, in a repetitive TMS train of 10 pulses at 5 Hz, the subject can experience a jaw-rattling sensation, the description of which ranges from “uncomfortable” to “painful.” We attempt to mitigate this effect by maintaining the dipole of our induced field in perpendicularity to the temporalis muscle fibers. Nevertheless, half of subjects required some reduction in the stimulation intensity.
Not only is the jaw contraction unpleasant for the subject, but it can also confound the examiner’s ability to distinguish true speech errors from subject withdrawal. Indeed, this side effect is the reason why we chose not to use dysarthria as a specific type of speech disturbance; it was often impossible to differentiate between true dysarthria from cortical disruption and “secondary” dysarthria from temporalis interference. Thus, when a questionable site was encountered, we made a note to repeat stimulation; interestingly, all sites eventually resolved themselves as speech arrest or as temporalis interference.
4.6. Limitations and Future Directions
While the results of this series are promising, there are several limitations than bear mentioning. The first of these relate to the precision of the measurements used to compare mapping sites. The tolerance of registration on the nTMS system is estimated at 2–3mm; given that the guidance system is not frame-based it is possible that the actual error is higher. Similarly, intraoperative navigation has a tolerance of at least 2–3mm. Add in the brain shift and sag that are inherent to any intradural operation and it is likely that the error is greater. Thus, between nTMS and intraoperative DCS, the error is at least 5mm. To mitigate this error, the intraoperative positive DCS points have been confirmed by both navigation and photography in each case.
One inherent limitation in the specificity of nTMS is transsynaptic excitation of downstream (and, possibly, upstream) neuronal units. Neurophysiological and neuroimaging studies have shown that repetitive TMS of a given brain region induces distributed activation of neural circuitry via transsynaptic spread, which follows established functional networks (Bestmann, 2008; Paus et al., 1997; Valero-Cabre and Pascual-Leone, 2005). Thus, behavioral effects may be a result of activation not in the target region but in a distant, functionally connected region. The “over-calling” seen with nTMS is likely a result of this limitation. Nevertheless, it should be pointed out that the overall nTMS language map does reflect the distribution of DCS language sites both from this series and from larger ones published previously (Sanai et al., 2008). Additionally, it seems to correlate with prior studies of un-navigated rTMS mapping of language sites.
Another inherent limitation to TMS is the spread of the magnetic field itself. In our TMS system, the figure-of-8 coil generates a conical magnetic field. The field is therefore roughly circular at the cortical surface with a diameter of about 2cm (and greatest intensity at the center with a sharp fall-off at the edges) and tapers toward its apex which occurs approximately 4.5cm from the coil surface. It is much less prone to interference and dispersion than the DCS electrical field, which is roughly spherical and generates a clinically significant effect of about 1cm radius from the stimulating bipolar electrode. As a result, nTMS might be disrupting subcortical white matter tracts which DCS is unable to reach.
Furthermore, a given neuron’s orientation, volume, axonal and dendritic organization, and innate threshold affect the likelihood of a magnetic pulse generating an action potential (Pashut et al., 2011). DCS, on the other hand, relies less on the neuronal morphology, activating an obloid volume between the electrodes. Thus, nTMS and DCS positive sites are not “points”—that is a misnomer; it is more accurate to say that they are regions, and to be aware that closely approximated regions of positivity on the map may all be associated with a single eloquent cortical site.
While the convergence between nTMS and DCS language maps was relatively high for the opercular region, there was lower convergence between the two modalities for the posterior region. We believe that this discrepancy represents an opportunity for refining the nTMS mapping protocol, perhaps by selecting tasks that are particularly suited for testing a particular region. Object naming, for example, while typically associated with temporal activity, does seem to involve inferior frontal sites as well in a subset of patients (Lubrano et al., 2012). Thus, we hypothesize that a different, purely temporal task may work better for posterior temporal sites—a semantic task, for example, such as identification of famous faces, (Gorno-Tempini and Price, 2001) or categorization of objects (Peelen and Caramazza, 2012).
It is also possible that a task with auditory stimuli might be needed, such as the verb-generation task that we use for generating MEGI maps. It may not be coincidence that the false negative nTMS site of Subject #8 and 3 of 4 false positive nTMS sites (Subjects #2, #7, and #10) were all located in the posterior temporal region. Further experimentation with more specialized tasks will likely improve the accuracy of nTMS mapping.
Finally, the basic parameters of nTMS stimulation should be examined more systematically and thoroughly. It is possible that relatively small adjustments in the frequency or number of pulses could improve results. Similarly, the timing of the onset of the pulse train is potentially important. We initiated the pulse train just before the presentation of the stimulus, largely because that is the protocol we use for DCS. It is possible, however, that initiating the pulse train with, or just after the stimulus might reduce the false positive rate. These variations in task and parameter should be chosen carefully; the endless potential variations in protocol combined with the relative scarcity of subjects make it impossible to run through every possible configuration.
With regard to the limitations of MEGI mapping, the relatively poor correlation between MEGI and nTMS/DCS in this study suggests that there are several. Firstly, MEGI was conducted using both object naming and verb generation. Object naming was also used for DCS and nTMS studies, but verb generation was not. Our group and others have found that, while object naming and verb generation activate overlapping cortical regions associated with semantic association and speech production, verb generation also involves a speech perception phase that involves posterior temporal regions (Edwards et al., 2010; Herholz et al., 1997; Ojemann et al., 2002; Petersen et al., 1988; Thiel et al., 1998; Thompson-Schill et al., 1998). Thus, difference in tasks is one likely source of discrepancy between MEGI verb generation sites and nTMS or DCS sites. It should be noted, however, that the MEGI object naming task was identical to that used in the nTMS and DCS studies. Although language mapping with MEGI correlated marginally with nTMS/DCS, MEGI-based laterality index, as described in a previous publication from our group (Findlay et al., 2012), correlated very well with nTMS- and DCS-based findings of hemispheric dominance. MEGI-based laterality index correctly identified left hemispheric speech in 11 of 12 subjects, including 2 with left-handedness and confirmed by IAP. The remaining subject had undergone multiple prior resections amounting to a radical subtotal left frontal lobectomy; in this case, the lack of left-sided parenchyma rendered impossible an accurate comparison of left- and right-sided language activation sites.
There is also a mechanistic reason for mismatch between MEGI and nTMS/DCS. Because MEGI identifies regions of cortical activity (and, by derivation, ERD and ERS), it quantifies language processing differently from DCS and nTMS, which are lesion-based modalities that identify necessary regions. From prior work in our group, we know that MEGI language sites correlate better with frontal, motor regions than with temporal regions, perhaps because we used p-desynchronization in our analysis. Other studies based on current density imaging in the 1 – 50Hz frequency range (Bowyer et al., 2005; Bowyer et al., 2004) and dipole analysis (Levelt et al., 1998) have demonstrated activity in more ventral regions. Also, the adaptive spatial filtering algorithm that was used for our analysis is not optimized for sparse solution sets; it is possible that, by using other algorithms such as Champagne (Owen et al., 2012; Wipf et al., 2010) we will see improved correlation between MEGI and DCS / nTMS. Finally, we chose to examine only the largest activations in the MEGI data which were in the beta-band prior to speech onset. Further examination of other frequency bands such as high-y power increases and across multiple other time-windows may yield greater consistency between MEGI, navigated rTMS and DCS. It should be mentioned, however, that in prior analyses of this data, the high-γ band was found to be inconsistent and the β band was chosen as a result.
4.7. Clinical Relevance of Multimodal Mapping
It should be mentioned that speech arrest can be caused by lesioning in several different brain regions which demonstrate significant inter-subject variability (Amunts et al., 1999). Inferior frontal gyrus, in which resides the canonical Broca’s region, is considered essential for speech production, and functional imaging of this region demonstrates activity during phonation(Penfield and Rasmussen, 1950; Poeppel, 1996). On the other hand, stimulation of the orofacial motor region of the precentral gyrus results in vocal fold adduction and vocalization responses. Lesioning of this region can also result in speech arrest, although in this case it is motoric in origin. Both these varieties of speech arrest can be achieved with DCS or nTMS, and both are clinically relevant in the perioperative setting (Sanai et al., 2008).
While these early results are encouraging, nTMS is not a replacement for DCS. Indeed, it is unlikely that DCS will be replaced in the near future, because the ability to perform intraoperative interrogation of a given cortical region is indispensable. The real contribution of nTMS is in the preoperative preparation that it allows. By mapping a subject before surgery, the surgeon can generate a precise map of potentially positive language sites, which then may be swiftly interrogated with DCS during surgery. Additionally, if the preoperative nTMS map shows clear speech arrest sites distant from the region of surgical exposure, the surgeon may have increased confidence in the reliability of a negative intraoperative DCS map.
From the perspective of subject preparation, nTMS mapping can help with preoperative education and discussions. If, for example, an nTMS speech arrest site falls directly over a tumor, the surgeon might spend more time preparing the subject for the possibility of subtotal resection and postoperative language deficits. Furthermore, the subject is exposed to the tasks and techniques associated with language mapping in a comfortable, low-stress environment, allowing for a “practice run” before surgery. Many subjects reported feeling more at ease with intraoperative speech arrest after having experienced it during nTMS mapping.
5. Conclusion
nTMS and MEGI can both be performed safely and effectively for lesion-based identification of positive language sites. The presence of anti-epileptic drugs has no impact on these maps. The pars opercularis is the most likely location of finding a positive language sites regardless of modality. Maps of language function generated with nTMS correlate well with those generated by DCS. Negative nTMS mapping also correlates with negative DCS mapping. In our study, MEGI lacks the same level of correlation with intraoperative mapping, but provides useful adjunct information with regard to language lateralization and confirmation of DCS maps in some cases. nTMS is a useful modality for generating language maps noninvasively. It is thus of immediate interest in the clinical management of subjects with eloquent brain tumors; it also has wide-ranging implications in basic science and translational studies of cortical language representation and physiology.
Table 4.
Sensitivity and specificity of nTMS language mapping as compared with DCS. (Sens = sensitivity; Spec = specificity; PPV = positive predictive value; NPV = negative predictive value)
| DCS + | DCS − | ||
|---|---|---|---|
| nTMS + | 9 | 4 | PPV 69% |
| nTMS − | 1 | 169 | NPV 99% |
| Sens 90% | Spec 98% |
Highlights.
Navigated TMS is a noninvasive method for lesion-based mapping of language pathways.
nTMS is safe, well-tolerated by patients, and can be performed in a lab environment.
nTMS-based language maps correlate well with maps from direct cortical stimulation.
nTMS maps are less well correlated with maps from magnetoencephalographic imaging.
nTMS is useful for interrogating language pathways for research and clinical purposes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia. 2007;48:442–455. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Berger MS, Ojemann GA. Intraoperative brain mapping techniques in neuro-oncology. Stereotactic and functional neurosurgery. 1992;58:153–161. doi: 10.1159/000098989. [DOI] [PubMed] [Google Scholar]
- Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurgery clinics of North America. 1990;1:65–80. [PubMed] [Google Scholar]
- Bestmann S. The physiological basis of transcranial magnetic stimulation. Trends Cogn Sci. 2008;12:81–83. doi: 10.1016/j.tics.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Binder JR. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2011;20:214–222. doi: 10.1016/j.yebeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Bowyer SM, Fleming T, Greenwald ML, Moran JE, Mason KM, Weiland BJ, Smith BJ, Barkley GL, Tepley N. Magnetoencephalographic localization of the basal temporal language area. Epilepsy Behav. 2005;6:229–234. doi: 10.1016/j.yebeh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bowyer SM, Moran JE, Mason KM, Constantinou JE, Smith BJ, Barkley GL, Tepley N. MEG localization of language-specific cortex utilizing MR-FOCUSS. Neurology. 2004;62:2247–2255. doi: 10.1212/01.wnl.0000130385.21160.7a. [DOI] [PubMed] [Google Scholar]
- Brell M, Ibanez J, Caral L, Ferrer E. Factors influencing surgical complications of intra-axial brain tumours. Acta neurochirurgica. 2000;142:739–750. doi: 10.1007/s007010070088. [DOI] [PubMed] [Google Scholar]
- Chang SM, Parney IF, McDermott M, Barker FG, 2nd, Schmidt MH, Huang W, Laws ER, Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. Journal of neurosurgery. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. Journal of cognitive neuroscience. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, Gordon B. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Berger MS, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. NeuroImage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS. Spatial localization of cortical time-frequency dynamics. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2007;2007:4941–4944. doi: 10.1109/IEMBS.2007.4353449. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Zumer JM, Agrawal V, Hild KE, Sekihara K, Nagarajan SS. NUTMEG: a neuromagnetic source reconstruction toolbox. Neurology & clinical neurophysiology : NCN. 2004;2004:52. [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Zumer JM, Guggisberg AG, Trumpis M, Wong DD, Sekihara K, Nagarajan SS. MEG/EEG source reconstruction, statistical evaluation, and visualization with NUTMEG. Computational intelligence and neuroscience. 2011;2011:758973. doi: 10.1155/2011/758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, Gabrieli JD, Morrell MJ. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118((Pt 6)):1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- Devaux BC, O'Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. Journal of neurosurgery. 1993;78:767–775. doi: 10.3171/jns.1993.78.5.0767. [DOI] [PubMed] [Google Scholar]
- Doss RC, Zhang W, Risse GL, Dickens DL. Lateralizing language with magnetic source imaging: validation based on the Wada test. Epilepsia. 2009;50:2242–2248. doi: 10.1111/j.1528-1167.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez J, Faillot T, Abdennour L, Law Koune JD, Dadoun S, Bitar A, Arthuis F, Van Effenterre R, Fohanno D. Intra-operative direct electrical stimulations of the central nervous system: the Salpetriere experience with 60 patients. Acta neurochirurgica. 1999;141:1157–1167. doi: 10.1007/s007010050413. [DOI] [PubMed] [Google Scholar]
- Dym RJ, Burns J, Freeman K, Lipton ML. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology. 2011;261:446–455. doi: 10.1148/radiol.11101344. [DOI] [PubMed] [Google Scholar]
- Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal imaging of cortical activation during verb generation and picture naming. NeuroImage. 2010;50:291–301. doi: 10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CM, Lah JJ, Meador K, Weissman JD, Gaitan LE, Dihenia B. Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology. 1996;47:1590–1593. doi: 10.1212/wnl.47.6.1590. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Meador KJ, Loring DW, Wright RJ, Weissman JD, Sheppard S, Lah JJ, Puhalovich F, Gaitan L, Davey KR. Localization and characterization of speech arrest during transcranial magnetic stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 1999;110:1073–1079. doi: 10.1016/s1388-2457(99)00047-4. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Woodard JL, Stringer AY, Bakay RA, Henry TR, Pennell PB, Litt B. Repetitive transcranial magnetic stimulation does not replicate the Wada test. Neurology. 2000;55:1025–1027. doi: 10.1212/wnl.55.7.1025. [DOI] [PubMed] [Google Scholar]
- Findlay AM, Ambrose JB, Cahn-Weiner DA, Houde JF, Honma S, Hinkley LB, Berger MS, Nagarajan SS, Kirsch HE. Dynamics of hemispheric dominance for language assessed by magnetoencephalographic imaging. Annals of neurology. 2012;71:668–686. doi: 10.1002/ana.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12:193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. doi: 10.1093/brain/124.10.2087. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. discussion 576. [DOI] [PubMed] [Google Scholar]
- Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, Carlson S. Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Human brain mapping. 2005;26:100–109. doi: 10.1002/hbm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K, Reulen HJ, von Stockhausen HM, Thiel A, Ilmberger J, Kessler J, Eisner W, Yousry TA, Heiss WD. Preoperative activation and intraoperative stimulation of language-related areas in patients with glioma. Neurosurgery. 1997;41:1253–1260. doi: 10.1097/00006123-199712000-00004. discussion 1260-1252. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hill DL, Maurer CR, Jr, Maciunas RJ, Barwise JA, Fitzpatrick JM, Wang MY. Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery. 1998;43:514–526. doi: 10.1097/00006123-199809000-00066. discussion 527-518. [DOI] [PubMed] [Google Scholar]
- Hirata M, Goto T, Barnes G, Umekawa Y, Yanagisawa T, Kato A, Oshino S, Kishima H, Hashimoto N, Saitoh Y, Tani N, Yorifuji S, Yoshimine T. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. Journal of neurosurgery. 2010;112:528–538. doi: 10.3171/2009.7.JNS09239. [DOI] [PubMed] [Google Scholar]
- Hirata M, Kato A, Taniguchi M, Saitoh Y, Ninomiya H, Ihara A, Kishima H, Oshino S, Baba T, Yorifuji S, Yoshimine T. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. NeuroImage. 2004;23:46–53. doi: 10.1016/j.neuroimage.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Jennum P, Friberg L, Fuglsang-Frederiksen A, Dam M. Speech localization using repetitive transcranial magnetic stimulation. Neurology. 1994;44:269–273. doi: 10.1212/wnl.44.2.269. [DOI] [PubMed] [Google Scholar]
- Jones SE, Mahmoud SY, Phillips MD. A practical clinical method to quantify language lateralization in fMRI using whole-brain analysis. NeuroImage. 2011;54:2937–2949. doi: 10.1016/j.neuroimage.2010.10.052. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Saisanen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, Kononen M. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage. 2009;44:790–795. doi: 10.1016/j.neuroimage.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chung CK. Language lateralization using MEG beta frequency desynchronization during auditory oddball stimulation with one-syllable words. NeuroImage. 2008;42:1499–1507. doi: 10.1016/j.neuroimage.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, Ringel F. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. Journal of neurosurgery. 2012;116:994–1001. doi: 10.3171/2011.12.JNS111524. [DOI] [PubMed] [Google Scholar]
- Krings T, Chiappa KH, Foltys H, Reinges MH, Cosgrove GR, Thron A. Introducing navigated transcranial magnetic stimulation as a refined brain mapping methodology. Neurosurgical review. 2001a;24:171–179. doi: 10.1007/s101430100151. [DOI] [PubMed] [Google Scholar]
- Krings T, Foltys H, Reinges MH, Kemeny S, Rohde V, Spetzger U, Gilsbach JM, Thron A. Navigated transcranial magnetic stimulation for presurgical planning--correlation with functional MRI. Minimally invasive neurosurgery : MIN. 2001b;44:234–239. doi: 10.1055/s-2001-19935. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, Hertz-Pannier L, Le Bihan D, Marsault C, Baulac M. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Lemaire JJ, Golby A, Wells WM, 3rd, Pujol S, Tie Y, Rigolo L, Yarmarkovich A, Pieper S, Westin CF, Jolesz F, Kikinis R. Extended Broca's Area in the Functional Connectome of Language in Adults: Combined Cortical and Subcortical Single-Subject Analysis Using fMRI and DTI Tractography. Brain Topogr. 2012 doi: 10.1007/s10548-012-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ, Praamstra P, Meyer AS, Helenius P, Salmelin R. An MEG study of picture naming. Journal of cognitive neuroscience. 1998;10:553–567. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano V, Filleron T, Demonet JF, Roux FE. Anatomical correlates for category-specific naming of objects and actions: A brain stimulation mapping study. Human brain mapping. 2012 doi: 10.1002/hbm.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelucci R, Valzania F, Passarelli D, Santangelo M, Rizzi R, Buzzi AM, Tempestini A, Tassinari CA. Rapid-rate transcranial magnetic stimulation and hemispheric language dominance: usefulness and safety in epilepsy. Neurology. 1994;44:1697–1700. doi: 10.1212/wnl.44.9.1697. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Hayward RW. Lesion localization in aphasia with cranial computed tomography and the Boston Diagnostic Aphasia Exam. Neurology. 1978;28:545–551. doi: 10.1212/wnl.28.6.545. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Ojemann GA, Lettich E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. Journal of neurosurgery. 2002;97:33–38. doi: 10.3171/jns.2002.97.1.0033. [DOI] [PubMed] [Google Scholar]
- Owen JP, Wipf DP, Attias HT, Sekihara K, Nagarajan SS. Performance evaluation of the Champagne source reconstruction algorithm on simulated and real M/EEG data. NeuroImage. 2012;60:305–323. doi: 10.1016/j.neuroimage.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, Billingsley RL, Buchanan S, Wheless J, Maggio V, Maggio WW. Magnetocephalography: a noninvasive alternative to the Wada procedure. Journal of neurosurgery. 2004;100:867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pashut T, Wolfus S, Friedman A, Lavidor M, Bar-Gad I, Yeshurun Y, Korngreen A. Mechanisms of magnetic stimulation of central nervous system neurons. PLoS Comput Biol. 2011;7:e1002022. doi: 10.1371/journal.pcbi.1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Caramazza A. Conceptual object representations in human anterior temporal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15728–15736. doi: 10.1523/JNEUROSCI.1953-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man—A Clinical Study of Localization of function. New York: Macmillan; 1950. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Picht T, Krieg SM, Sollmann N, Rosler J, Niraula B, Neuvonen T, Savolainen P, Lioumis P, Makela J, Deletis V, Meyer B, Vajkoczy P, Ringel F. A Comparison of Language Mapping by Preoperative Navigated Transcranial Magnetic Stimulation and Direct Cortical Stimulation During Awake Surgery. Neurosurgery. 2013 doi: 10.1227/NEU.0b013e3182889e01. [DOI] [PubMed] [Google Scholar]
- Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery. 2009;65:93–98. doi: 10.1227/01.NEU.0000348009.22750.59. discussion 98–99. [DOI] [PubMed] [Google Scholar]
- Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, Karhu J, Vajkoczy P, Suess O. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011;69:581–588. doi: 10.1227/NEU.0b013e3182181b89. discussion 588. [DOI] [PubMed] [Google Scholar]
- Poeppel D. A critical review of PET studies of phonological processing. Brain Lang. 1996;55:317–3051. doi: 10.1006/brln.1996.0108. discussion 352–385. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and clinical neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. The New England journal of medicine. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Physics in medicine and biology. 1987;32:11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. discussion 1055-1046. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Nagarajan SS, Poeppel D, Marantz A. Asymptotic SNR of scalar and vector minimum-variance beamformers for neuromagnetic source reconstruction. IEEE transactions on bio-medical engineering. 2004;51:1726–1734. doi: 10.1109/TBME.2004.827926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE transactions on bio-medical engineering. 2001;48:760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of experimental psychology. Human learning and memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS. Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. Journal of neurosurgery. 2012;117:354–362. doi: 10.3171/2012.5.JNS112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkiainen A, Liljestrom M, Seppa M, Salmelin R. The 3D topography of MEG source localization accuracy: effects of conductor model and noise. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114:1977–1992. doi: 10.1016/s1388-2457(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. Journal of neurosurgery. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, von Stockhausen HM, van Leyen-Pilgram K, Pietrzyk U, Kessler J, Wienhard K, Klug N, Heiss WD. Localization of language-related cortex with 15O-labeled water PET in patients with gliomas. NeuroImage. 1998;7:284–295. doi: 10.1006/nimg.1998.0334. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Pascual-Leone A. Impact of TMS on the primary motor cortex and associated spinal systems. IEEE Eng Med Biol Mag. 2005;24:29–35. doi: 10.1109/memb.2005.1384097. [DOI] [PubMed] [Google Scholar]
- Vitikainen AM, Lioumis P, Paetau R, Salli E, Komssi S, Metsahonkala L, Paetau A, Kicic D, Blomstedt G, Valanne L, Makela JP, Gaily E. Combined use of non-invasive techniques for improved functional localization for a selected group of epilepsy surgery candidates. NeuroImage. 2009;45:342–348. doi: 10.1016/j.neuroimage.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Vorster SJ, Barnett GH. A proposed preoperative grading scheme to assess risk for surgical resection of primary and secondary intraaxial supratentorial brain tumors. Neurosurgical focus. 1998;4:e2. doi: 10.3171/foc.1998.4.6.5. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Blaxton TA, Hoffman EA, Berry CD, Oletsky H, Pascual-Leone A, Theodore WH. Repetitive transcranial magnetic stimulation of the dominant hemisphere can disrupt visual naming in temporal lobe epilepsy patients. Neuropsychologia. 1999;37:537–544. doi: 10.1016/s0028-3932(98)00102-x. [DOI] [PubMed] [Google Scholar]
- Wipf DP, Owen JP, Attias HT, Sekihara K, Nagarajan SS. Robust Bayesian estimation of the location, orientation, and time course of multiple correlated neural sources using MEG. NeuroImage. 2010;49:641–655. doi: 10.1016/j.neuroimage.2009.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Wood JM, Kundu B, Utter A, Gallagher TA, Voss J, Nair VA, Kuo JS, Field AS, Moritz CH, Meyerand ME, Prabhakaran V. Impact of brain tumor location on morbidity and mortality: a retrospective functional MR imaging study. AJNR Am J Neuroradiol. 2011;32:1420–1425. doi: 10.3174/ajnr.A2679. [DOI] [PMC free article] [PubMed] [Google Scholar]