Abstract
Background
The relationship between anti-inflammatory lipoxins and pro-inflammatory leukotrienes may be important in the pathobiology of asthma and its severity.
Objective
To investigate whether exhaled breath condensate (EBC) lipoxin and leukotriene measurements can non-invasively characterize the asthmatic diathesis and its severity.
Methods
We measured lipoxin A4 (LXA4) and leukotriene B4 (LTB4) levels in EBC collected from asthmatics of different severities and from healthy controls.
Results
EBC LXA4 and LTB4 levels are elevated in asthmatics as compared to healthy controls (LXA4 31.40 vs. 2.41 pg/ml EBC respectively, p < 0.001; LTB4 45.62 vs. 3.82 pg/ml EBC, p < 0.001). While both eicosanoids are elevated in asthmatics, the ratio LXA4 to LTB4 decreases with increasing asthma severity. It is 41% lower in severe versus moderate asthmatics (0.52 vs. 0.88, p = 0.034). EBC LXA4 levels correlate with the degree of airflow obstruction measured by FEV1 (r = 0.28, p = 0.018). A cut-off value of 7 pg LXA4/ml EBC provides 90% sensitivity and 92% specificity for the diagnosis of asthma (AUC 0.96, p < 0.001). A cut-off value of 11 pg LTB4/ml EBC provides 100% sensitivity and 100% specificity for the diagnosis of asthma (AUC 1, p < 0.001).
Conclusions
Pro-resolving and pro-inflammatory eicosanoids are generated in airways of all asthmatics. The proportion of pro-resolving compounds declines with asthma severity. These findings support the role for EBC eicosanoid measurements in the non-invasive diagnosis of asthma and suggest that pro-resolving eicosanoid pathways are dys-regulated in severe asthma.
Keywords: Asthma, Biomarkers, Breath Tests, Eicosanoids, Leukotriene B4, Lipoxin A4
INTRODUCTION
Airway inflammation in asthma is characterized by up-regulation of multiple inflammatory mediators and cytokines. Among the most potent pro-phlogistic mediators are arachidonic acid-derived leukotrienes.1 In addition to being potent chemo-attractants and activators of inflammatory cells such as neutrophils and eosinophils, they are also among the most potent broncho-constrictors identified.2
We now understand that accompanying the activation of inflammatory cascades is the generation of pro-resolving mediators which restore tissue homeostasis by antagonism of inflammation and promotion of immune defense mechanisms.3 Among these moieties are lipoxins -- lipoxygenase interaction products of arachidonic acid metabolism. Of particular relevance to asthma, they inhibit eosinophils, neutrophil responses and cytokine signaling.4 They also antagonize the effects of leukotrienes --they inhibit chemotactic responses of human neutrophils and eosinophils stimulated by LTB4, act as endogenous antagonists at the CysLT1 receptor and modulate LTC4-induced airway obstruction.5–9
Lipoxins are generated in the inflamed asthmatic lung; however, their inadequate production and/or function is associated with the more severe phenotype of asthma.10–17 Patients with severe asthma have reduced lipoxin levels in whole blood, bronchoalveolar lavage (BAL) fluid and sputum.10,11,16 We have also found that lipoxin deficiency is directly proportional to loss of lung function in asthma.10 Limitations in procurement of lung-localized specimens have been significant barriers in advancing investigations in the role of dys-regulated eicosanoid metabolism in asthma.
Exhaled breath condensate (EBC) collection offers a non-invasive method to sample the respiratory epithelial lining fluid.18 Biomarkers measured in EBC can provide information about specific biological processes associated with pulmonary diseases, their severity, and response to therapy.19 Lipid-based inflammatory mediators including lipoxins and leukotrienes have been successfully isolated from EBC.19–21 We therefore hypothesized that we could use non-invasively collected EBC to assess the relationship between lipoxin and leukotriene pathways in the asthmatic diathesis and its severity.
Herein we present data that suggests that both inflammatory and pro-resolving eicosanoid pathways are up-regulated in the asthmatic airway, and that relative underproduction of lipoxins occurs with increasing asthma severity. In addition to providing insight into the pathobiology of asthma, these data also suggest that non-invasive EBC measurements may be used as sensitive and specific biomarkers to distinguish asthma from health.
METHODS
Participants
All participants provided written informed consent that was approved by the Institutional Review Board. We recruited adult patients with mild intermittent, moderate and severe asthma to participate in a phenotypic characterization study as part of the NHLBI’s Severe Asthma Research Program (SARP). Severe asthma was defined per the criteria developed by the American Thoracic Society workshop on refractory asthma.22 Moderate asthmatics were required to be on low to medium dose inhaled corticosteroids (ICS) as defined by the National Asthma Education and Prevention Program guidelines (< 880 mcg fluticasone or equivalent).23 Mild asthmatics were patients with intermittent symptoms controlled without the use of anti-inflammatory medications. Patients with history of other respiratory diseases including allergic broncho-pulmonary aspergillosis were excluded from the study. Adults without a prior history of asthma, allergies or chronic respiratory symptoms were recruited as healthy controls. All participants were required to have ≤ 5 pack-years smoking history. Baseline spirometry was performed in asthmatics after bronchodilator withhold if their asthma symptoms permitted (4 hours for short-acting beta-agonists and 12 hours for long-acting beta-agonists). Subjects were not asked to withhold montelukast or ICS, unless the latter was prescribed in combination with their long-acting beta-agonist. Subjects with asthma had to demonstrate either bronchodilator reversibility of at least 12% in the forced expiratory volume exhaled in the first second (FEV1) or a methacholine PC20 (provocative concentration causing 20% fall in FEV1) of ≤ 8 mg/ml or hyperresponsiveness to 3% saline challenge with PD20 (provocative dose causing 20% fall in FEV1) of ≤ 16 ml. Additional phenotypic characterization for participants with asthma included assessment for aspirin sensitivity, serum immunoglobulin E (IgE), peripheral blood eosinophil and fractional exhaled nitric oxide (FeNO) levels.
EBC collection
EBC was collected once from each enrolled study participant by recommended methods using an RTube™ (Respiratory Research, Inc, Austin, TX, USA).18 Participants were required to withhold food and carbonated beverages for one hour prior to EBC collection. The metal sleeve surrounding the collection chamber was chilled to −20° C for at least one hour prior to EBC collection. Samples were collected using tidal breathing through the mouth for 10 minutes without a nose clip. Eicosanoid loss was minimized by rapidly aliquoting and freezing the samples at −80° C under a layer of inert gas immediately subsequent to the 10 minute collection. EBC samples from participants with asthma were stored for approximately 2 years prior to analyses. EBCs from healthy controls were stored for approximately 6 months prior to analyses.
Extraction and measurement of lipid mediators
Eicosanoids were extracted from EBC using C18 Sep-Pak cartridges (Waters, Milford, MA) and concentrated as previously described.10 LXA4, LTB4 and CysLT concentrations were determined using enzyme based immunoassays (Neogen, Lexington, KY and Cayman Chemical, Ann Arbor, MI). Manufacturer-provided lower limits of detection for the assays were 20 pg/ml for LXA4, 13 pg/ml for LTB4 and 34 pg/ml for the CysLTs; however, we were able to detect levels as low as 2 pg LXA4, 2 pg LTB4 and 5 pg CysLT per ml of EBC because the extracted mediators were concentrated up to ten fold prior to measurement. Mediator values were re-calculated to reflect the amount per ml of EBC prior to concentration and normalized to the denominator “per ml of EBC”, independent of the volume of fluid collected from a participant. If mediator levels fell above or below the adjusted limits of detection of an assay for a concentrated sample, we assigned them with values. Those below the lower limit of detection were assigned a value half of the adjusted lower limit for the concentrated sample. Those above the upper limit of detection were assigned a value twice as much as the adjusted upper limit for the concentrated sample.
Statistical analyses
All samples were de-identified prior to measurements. We transformed all mediator values to their logarithm at base 10 to normalize the distributions prior to analysis. Normality was determined by examining the histogram of the distribution of the outcomes and the p value obtained by the Shapiro Wilk test. We performed analyses of covariance for each transformed outcome. Each was adjusted separately for age, gender and race. Categorical data were analyzed by the Chi Square and Fisher’s Exact tests. Student’s t-tests were used for normally distributed continuous data, while non-parametric data were analyzed by the Wilcoxon Rank Sum, Kruskal Wallis and Spearman’s correlation tests. Cut-offs for optimal sensitivity and specificity were determined by examination of receiver-operating curves. We used GraphPad Prism 6.00 (La Jolla, CA, USA) and SAS 9.2 (SAS Institute Inc., Cary, NC, USA) to examine and analyze the data. The significance level used in these tests is set at 95%. For continuous data analyzed by parametric or non-parametric methods, we report the arithmetic means ± standard deviation, with minimum and maximum values for the observations in brackets. For skewed data that were log transformed prior to analyses, we report geometric means with the 95% confidence intervals in brackets. For count data, we report the number in each category, with % of total in brackets.
RESULTS
Participant Characteristics
Tables I and II describe the demographic and phenotypic characteristics of the participants. Severe asthmatics were the oldest among the four groups. Groups were similar with respect to the distribution of gender, race, and ethnicity. As expected, severe asthmatics were on much higher doses of ICS and had lower lung function. There were no other significant differences between the moderate and severe asthmatics, similar to what has been seen in the entire SARP cohort’s phenotypic characterization.24 None of the asthmatics were on oral corticosteroids, anti-IgE therapy or the 5-lipoxygenase inhibitor zileuton.
Table I.
Participants’ demographics
| Healthy controls | Mild asthma | Moderate asthma | Severe asthma | p value for difference between groups |
|
|---|---|---|---|---|---|
| N | 12 | 10 | 23 | 36 | |
| Age*, Years |
27 ± 4 (23 – 36) |
33 ± 12 (18 – 57) |
33 ± 10 (18 – 53) |
42 ± 11 (21 –62) |
< 0.001 |
| Gender, Female:Male(%) |
7(58):5(42) | 6(60):4(40) | 16(70):7(30) | 22(61):14(39) | 0.886 |
| Race, Cauc:AA:other (%) |
7(58):1(9):4(33) | 9(90):0(0):1(10) | 16(69):5(22):2(9) | 19(52):11(31):6(17) | 0.154 |
| Ethnicity, Hispanic: non-hispanic:unknown (%) |
0(0):12(100):0(0) | 1(10):8(80):1(10) | 1(4):21(92):1(4) | 7(19):29(81):0(0) | 0.230 |
| Sensitivity to aspirin, yes: no: unknown (%) |
NA | 0(0):10(100):0(0) | 2(9):15(65):6(26) | 3(8):17(47):16(45) | 0.705 |
| Daily dose of inhaled fluticasone equivalent*, mcg |
NA | NA | 392 ± 142 (176 – 500) |
1004 ± 156 (880 – 1880) |
< 0.001 |
| Montelukast use, yes:no (%) |
NA | NA | 9(39):14(61) | 17(47):19(53) | 0.559 |
Data are presented as arithmetic mean ± standard deviation (minimum - maximum) NA = Not applicable
Data analyzed by analysis of variance, Student’s t test and Fisher’s Exact test
Table II.
Asthma phenotypic characteristics
| Healthy controls | Mild asthma | Moderate asthma | Severe asthma | |
|---|---|---|---|---|
| N | 12 | 10 | 23 | 36 |
| FEV1, L* | ND | 2.93 ± 0.68 | 3.16 ± 0.79 | 2.38 ± 0.79# |
| FEV1, % predicted* | ND | 85 ± 13 | 93 ± 16 | 71 ± 18# |
| FVC, L*& | ND | 3.48 ± 0.91 | 3.91 ± 0.97 | 3.32 ± 0.90# |
| FVC, % predicted*& | ND | 84 ± 18 | 96 ± 14 | 81 ± 14# |
| FEV1/FVC ratio*& | ND | 84 ± 9 | 81 ± 6 | 71 ± 10# |
| 2 puff bronchodilator reversibility, %* |
ND | ND | 8 ± 9 | 13 ± 12 |
| Methacholine PC20
^ mg/ml |
ND | ND | 1.89 (1.00 to 3.6) |
2.40 (1.45 to 4.00) |
| Exhaled nitric oxide, ppb*$ | ND | ND | 22.74 ± 14.27 | 28.53 ± 23.79 |
| Serum IgE level, IU/ml*! | ND | ND | 226 ± 344 | 344 ± 434 |
| Peripheral blood eosinophils, %* |
ND | ND | 4 ± 3 (0 –10) |
4 ± 2 (0 –11) |
Data are presented as arithmetic mean ± standard deviation
PC20: Percent concentration required to cause a 20% decrease in FEV1. Data presented as geometric mean (95% confidence interval). N = 21 in non-severes and 23 in severes for methacholine PC20 results. Data were analyzed after logarithmic conversion.
p < 0.001 compared to moderate asthma for the difference in means calculated by unpaired Student’s t-test
N = 9 for mild asthmatics
N = 22 for moderate and 31 for severe asthmatics
N = 35 for severe asthmatics
ND = Not done
EBC LXA4 levels
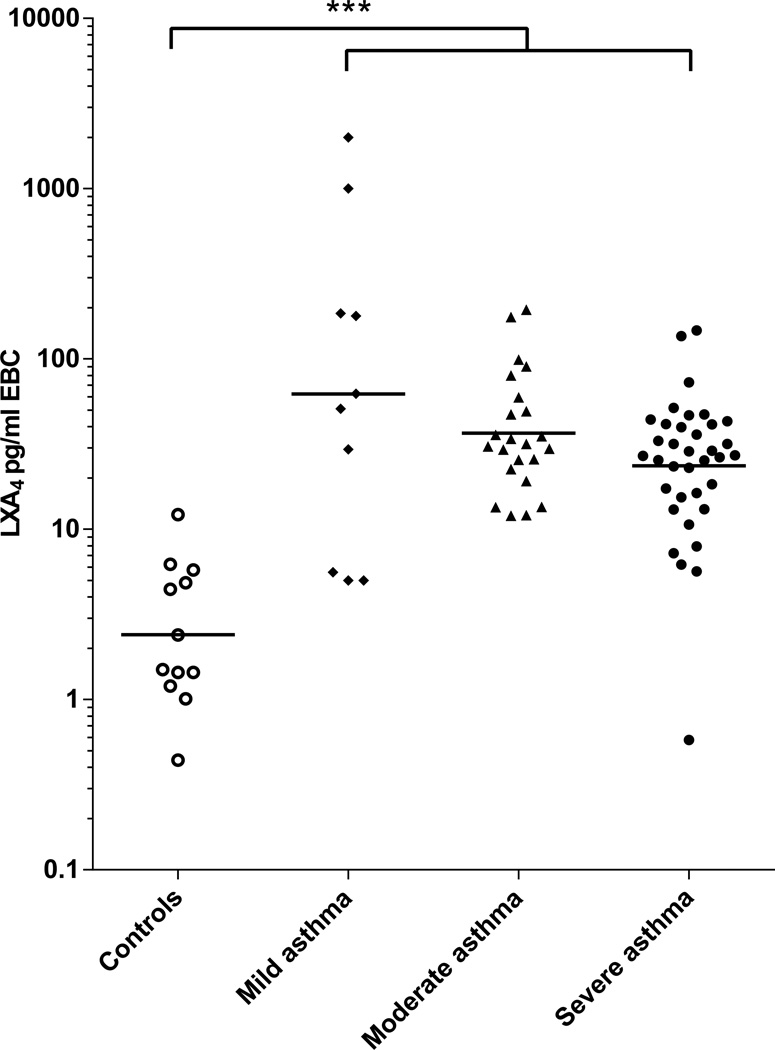
EBC LXA4 levels were more than ten fold higher in the asthmatics as compared to healthy controls (Table III and Figure 1). Geometric mean levels were 31.4 (95% CI 23.58 to 41.81) and 2.41 (1.31 to 4.41) pg/ml EBC respectively (p < 0.001). The difference remained significant after adjustment for age, gender, race, aspirin sensitivity and use of montelukast. A cut-off value of 7 pg/ml EBC provided 90% sensitivity and 92% specificity for the diagnosis of asthma. The area under the receiver-operating curve was 0.96 with p < 0.001. Low lipoxin levels correlated with decreased lung function (r = 0.28, p = 0.018) (Figure 2). Amongst the three groups of asthmatics, the mean EBC LXA4 level was numerically lowest in the group of severe asthmatics (23.54 pg/ml EBC, 95% CI 16.9 to 32.78).
Table III.
Comparison of LXA4 and LTB4 values and their ratio between EBC obtained from healthy controls and participants with mild, moderate and severe asthma
| Healthy controls | All asthmatics | Mild asthma | Moderate asthma | Severe asthma | |
|---|---|---|---|---|---|
| N | 12 | 69 | 10 | 23 | 36 |
| LXA4 pg/ml EBC |
2.41 (1.31 – 4.41) |
31.4 *** (23.58 – 41.81) |
62.22 (13.48 – 287.3) |
36.61 (26.06 – 51.43) |
23.54 (16.9 – 32.78) |
| LTB4 pg/ml EBC |
3.82 ^ (2.30 – 6.35) |
45.62 *** (38.18 – 54.50) |
55.16 (34.10 – 89.23) |
41.81 (31.74 – 55.08) |
45.75 (34.62 – 60.45) |
| EBC LXA4/LTB4 ratio |
0.85 ^ (0.34 – 2.13) |
0.69 (0.52 – 0.92) |
1.13 (0.23 – 5.49) |
0.88 (0.60 – 1.27) |
0.52 ** (0.37 – 0.71) |
Data presented as geometric mean (95% confidence interval of geometric mean) Log transformed data were analyzed by analysis of variance and covariance (adjusted for age, sex and race). None of the covariates were found to change the parameter estimate or the p value of the outcome obtained prior to adjustment. The p value for difference between four groups for both LXA4 and LTB4 was <0.001.
p < 0.001 for the difference in geometric means of EBC LXA4 levels between healthy controls and asthmatics when log transformed values are compared by Student’s t test.
p = 0.034 for the difference in geometric means between participants with moderate asthma and severe asthma when log transformed values are compared by Student’s t test.
N = 8 because EBC was not available for measurement of LTB4 from 4 healthy controls.
Figure 1. LXA4 levels measured in EBC collected from healthy controls and participants with mild, moderate and severe asthma.
Sample size = 12, 10, 23 and 36 in each group respectively. EBC LXA4 values are plotted on a logarithmic scale on the Y axis. Horizontal bars represent geometric means. *** p <0.001 for the difference in geometric means of EBC LXA4 levels between healthy controls (n = 12) and all asthmatics (n = 69) when log transformed values are compared by analysis of variance.
Figure 2. Correlation between EBC LXA4 levels and airflow obstruction measured as FEV1 % predicted.
♦ = mild asthmatics (n = 10) ▲= moderate asthmatics (n = 23) ● = severe asthmatics (n = 36) p = 0.018. r = 0.28 and p = 0.018 by Spearman correlation.
EBC LTB4 levels
The geometric mean of EBC LTB4 levels in the asthmatics was more than ten fold higher than that in the healthy controls -- 45.62 (38.18 to 54.50) versus 3.82 (2.30 to 6.35) pg/ml EBC respectively, p < 0.001 (Table III, Figure 3). The significance was unaffected after adjustment for age, gender, race, aspirin sensitivity and montelukast use. There was no overlap in EBC LTB4 levels between the asthmatics and healthy controls. A cut-off value of 11 pg LTB4/ml EBC provided 100% sensitivity and 100% specificity for the diagnosis of asthma (area under the curve = 1, p < 0.001). Differences in means between the three groups of asthmatics were not significant.
Figure 3. LTB4 levels measured in EBC collected from healthy controls and participants with mild, moderate and severe asthma.
Sample size = 8, 10, 23 and 36 in each group respectively. Measured EBC LTB4 values are plotted on a logarithmic scale on the Y axis. Horizontal bars represent geometric means. *** p <0.001 for the difference in geometric means of EBC LTB4 levels between healthy controls (n = 8) and all asthmatics (n = 69) when log transformed values are compared by analysis of variance.
EBC CysLT levels
Although all EBC samples were concentrated, CysLT levels were below the limit of detection in 62% of the samples and thus could not be reliably interpreted.
Ratio between EBC LXA4 and leukotrienes
Since more than half of the measured CysLT values were below the adjusted lower limit of detection, we examined the ratio of EBC LXA4/LTB4 (Table III, Figure 4). The geometric means for the ratio in healthy controls, mild, moderate and severe asthmatics were 0.85, 1.13, 0.88 and 0.52 respectively. The ratio was significantly lower in the severe asthmatics than that in the moderates − 0.52 (0.37 to 0.71) versus 0.88 (0.60 to 1.27), p = 0.034.
Figure 4. Ratio of EBC LXA4 levels to EBC LTB4 levels in healthy controls and participants with mild, moderate and severe asthma.
Sample size = 8, 10, 23 and 36 in each group respectively. Column bars represent the geometric mean of EBC LXA4/LTB4 values plotted on a logarithmic scale on the Y axis. Vertical lines represent the 95% confidence interval of the geometric mean. ** p = 0.034 for the difference in geometric means of EBC LXA4/LTB4 values between moderate asthmatics (n = 23) and severe asthmatics (n = 36) when log transformed values are compared by Student’s t test.
FeNO levels
FeNO values were available from 22 moderate and 31 severe asthmatics. The mean values were 22.74 ± 14.27 and 28.53 ± 23.79 parts per billion respectively (Table II). 68% of the moderate and 39% of the severe participants in whom such measurements were made had an FeNO value above 25 ppb (the suggested upper value for normal).25 In the same population, 100% of moderates and 90% of severes had an LXA4 level higher than 7 pg/ml EBC. All of these 53 asthmatics had LTB4 levels > 11 pg/ml EBC.
DISCUSSION
Using direct, in vivo measurements from the airways, we demonstrate that the lipoxin and leukotriene pathways are up-regulated in asthma of all severities. Our data also provide in vivo confirmation that relative underproduction of lipoxins occurs with increasing asthma severity. Lastly, our data suggest that non-invasively obtained exhaled breath condensate measurements of representative eicosanoids from these pathways distinguish asthmatics from non-asthmatics with high sensitivity and specificity. These results suggest a pathobiologic role for counter-regulatory eicosanoids in addition to pro-inflammatory eicosanoids in asthma. They further suggest that non-invasively measured eicosanoids may serve as biomarkers for the asthmatic airway diathesis and its severity.
Lipoxins and leukotrienes are biologically active products derived from arachidonic acid with distinct structure and function. Lipoxins are produced by airway structural cells, macrophages and leukocytes during inflammatory responses.26 They exert potent anti-inflammatory and pro-resolving effects and reduce hyper-responsiveness in the airways of patients with asthma, thus functioning as counter-regulatory molecules that maintain tissue homeostasis.3,4 Leukotrienes on the other hand are potent chemo-attractants and activators of inflammatory cells.2 They are elevated in sputum, plasma, bronchoalveolar lavage fluid and EBC from patients with asthma and are postulated to play a role in the pathobiology of the disease.19,27–29 We sought to examine the potential utility of non-invasively obtained measurements of these two countervailing families of eicosanoids as biomarkers for asthma and its severity. We were unable to obtain measurements of CysLTs from more than half of the samples examined; however, we found that we could easily measure LXA4 and LTB4 levels in EBC. Our data show that compared to healthy controls, both pro-resolving and pro-inflammatory pathways, as represented by EBC LXA4 and LTB4 levels respectively, are up-regulated nearly ten fold in asthmatics.
We found EBC LXA4 levels to be elevated in all severities of asthma, including those with mild disease. Considering the broad pro-resolving function played by these eicosanoids as it relates to the pro-inflammatory processes that occur in asthma, the up-regulation of this pathway is not surprising. However, Fritscher and colleagues recently found that EBC LXA4 levels in mild asthmatics are no different than those in healthy controls.20 One explanation for these conflicting results could be that the group of mild asthmatics examined by them were phenotypically different from those examined by us and other groups. Our data with regards to elevated EBC LXA4 levels in mild asthmatics are consistent with findings reported in blood obtained from a pediatric cohort by Wu and colleagues14 and in sputum from adults in two prior reports.12,16
We identified an association between reduced EBC LXA4 levels and declining lung function. Our findings related to the relative production of LXA4 compared to LTB4 are of particular interest. We found that while both LXA4 and LTB4 were elevated in the airways of asthmatics (Table III and Figures 1 and 3), the ratio between the two decreased with increasing asthma severity (Table III and Figure 4). The mean ratio of LXA4 to LTB4 was 41% lower in the severe asthmatics, when compared to the moderates (p = 0.034).
These findings regarding LXA4 in EBC support data suggesting that lipoxin biosynthetic capacity may be defective in patients with severe asthma and thus contribute to the perpetuation of airway inflammation in these patients. They are also consistent with those of Vachier et al. in induced sputum who noted decreased sputum lipoxins with increasing asthma severity,16 and with those of Fritscher et al. in EBC.20 Further, recent data also suggest that these counter-regulatory pathways may be impaired in asthma exacerbations.21,30 Altogether, these data suggest that the pro-resolving pathways play a role not only in the pathophysiology of asthma, but that defects in this pathway may be associated with increasing asthma severity.
Severe asthmatics are treated with significantly higher doses of ICS as compared to those with less severe disease. We have observed that lipopolysaccharide-induced formation of LXA4 by alveolar macrophages is decreased by dexamethasone in both non-severe and severe asthmatics ex vivo.31 It is thus possible that our observations in severe asthma relate to the presence and amounts of ICS rather than the intrinsic disease process. However, we did not find a relationship between inhaled or oral corticosteroid doses and LXA4 levels in BAL fluid obtained from subjects with severe asthma.11 Further, Hasan and colleagues did not see a change in EBC LXA4 levels after 24 hours of systemic glucocorticoid therapy in children who presented with status asthmaticus.21 Finally, while mean EBC LXA4 levels in our cohort were lower in the severe asthmatics on high doses of ICS than the mild intermittent asthmatics not using ICS, they were in fact lowest in the healthy controls. This argues against the effects of steroids on arachidonic acid metabolism leading to biased study results.
We found that exhaled LTB4 levels were more than 10 times higher in asthmatics as compared with non-asthmatics without any overlap in levels between the two groups. These findings are consistent with up-regulation of the leukotriene eicosanoid pathway in asthma and consistent with the findings of others. High LTB4 levels have been reported in blood samples from a pediatric asthmatic cohort when compared to healthy controls.14 Vachier et al. also noted an increase in LTB4 levels in induced sputum in asthmatics versus controls.16 However, their results did not reach statistical significance, possibly related to their small sample size. All studies that have examined EBC LTB4 report elevated levels in moderate to severe asthmatics when compared to controls.20,32,33 However, they differ in their findings in mild asthma. In two studies, LTB4 levels in asthmatics not receiving controller therapy were elevated29,32 while in another study, they were not. In a fourth study, EBC LTB4 levels were elevated in mild persistent asthmatics but not in mild intermittent asthmatics.33 While our data are more consistent with the former studies, the discrepancies suggest that further phenotyping may be necessary to ascertain the potential causes for these observed differences.
EBC collection is completely non-invasive, the equipment is technologically simple and low-cost, and requires minimal operator training.18,19 Hence, it is informative to compare our findings to FeNO – the only other exhaled biomarker that has been accepted as a useful tool in the non-invasive assessment of asthma. Values less than 25 ppb can reliably rule out eosinophilic asthmatic airway inflammation.25 However, it has now become increasingly clear that a subgroup of severe asthmatics have pauci-eosinophilic airway inflammation, which not only makes them refractory to corticosteroid therapy, but also makes them undistinguishable from healthy controls and other asthmatics when using the FeNO cut-off.34 Moreover, the utility of FeNO in patients on inhaled corticosteroids is quite limited due to the profound suppression of FeNO by glucocorticoids even at low doses.35 If the threshold of 25 ppb for FeNO were to be applied to our cohort, 32% of moderate and 61% of severe asthmatics would fall below that threshold. In contrast, almost none of them would have been misclassified if thresholds of 7 pg LXA4/ml EBC and 11 pg LTB4/ml EBC were applied.
While our findings suggest that EBC LXA4 and LTB4 may be useful biomarkers for asthma and its severity, several caveats do need to be considered. While we observed elevations in LXA4 and LTB4 in mild asthma, as discussed above, findings in intermittent asthma by others have been inconsistent.20,29,32,33 Whether these differences relate to phenotypic differences in the populations is unclear and studies in large well-phenotyped cohorts will be required to clarify these observations. Further, it should be noted that elevated eicosanoid levels are not restricted to asthma. Studies have noted elevated levels of LXA4 and LTB4 in blood and respiratory samples of patients with other inflammatory conditions such as chronic obstructive lung disease, cystic fibrosis and scleroderma lung disease.16,20,36–39 These data suggest that pro-resolving eicosanoid responses are not specific to any one particular inflammatory airway disease, but represent the host’s defense mechanisms to counteract eicosanoid-mediated airway inflammation. We propose that EBC LXA4 and LTB4 levels serve as non-invasive biomarkers for the presence of airway inflammation in asthma, identify dys-regulated eicosanoid patterns in those with severe disease, and thus supplement the information obtained by clinical assessment of patients by their physicians.
In summary, our data show that both inflammatory and pro-resolving eicosanoid pathways are up-regulated in asthmatic airways. Further, our in vivo measurements indicate that relative underproduction of lipoxins occurs in the airway with increasing asthma severity. These results provide insight into the pathobiological role of lipid mediator-driven airway inflammation in asthma and suggest that interventions to increase pro-resolving pathways may be of importance in treating severe asthma. Although, they require confirmation in larger cohorts, these data also suggest that non-invasive EBC measurements may be used as sensitive and specific biomarkers to distinguish asthma from non-asthma especially in more severe patients where other tests may have less utility.
Clinical Implications.
Exhaled lipoxin A4 and leukotriene B4 measurements can be used as non-invasive biomarkers for diagnosis of asthma and assessment of its severity. Interventions to increase pro-resolving lipoxins may be of importance in treating severe asthma.
Acknowledgements
Jodi Ann Bonfiglio, Sameer Taneja and Guangli Zhu for technical assistance with sample analyses.
Funded By: HL069349, HL107166, HL109172, AI068084, RR022292, RR025757, RR025758
Abbreviations used
- AUC
Area under the curve
- CysLT
Cysteinyl leukotriene
- EBC
Exhaled breath condensate
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroids
- IgE
Immunoglobulin E
- LT
Leukotriene
- LTB4
Leukotriene B4
- LX
Lipoxin
- LXA4
Lipoxin A4
- PC20
Provocative concentration of methacholine causing a 20% fall in FEV1
- PD20
Provocative dose of 3% saline causing a 20% fall in FEV1
- ROC
Receiver-operating curve
- SARP
Severe Asthma Research Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 2.Borish L. The role of leukotrienes in upper and lower airway inflammation and the implications for treatment. Ann Allergy Asthma Immunol. 2002;88:16–22. doi: 10.1016/s1081-1206(10)62024-8. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Levy B. Novel pathways and endogenous mediators in anti-inflammation and resolution. Chem Immunol Allergy. 2003;83:115–145. doi: 10.1159/000071558. [DOI] [PubMed] [Google Scholar]
- 4.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 5.Bandeira-Melo C, Diaz BL, Cordeiro RS, Jose PJ, Serhan CN, Martins MA, et al. Inhibition of allergen-induced eosinophil migration by lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4. Adv Exp Med Biol. 2002;507:211–216. doi: 10.1007/978-1-4615-0193-0_32. [DOI] [PubMed] [Google Scholar]
- 6.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badr KF, DeBoer DK, Schwartzberg M, Serhan CN. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc Natl Acad Sci U S A. 1989;86:3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 9.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 10.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 13.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 14.Wu SH, Yin PL, Zhang YM, Tao HX. Reversed changes of lipoxin A4 and leukotrienes in children with asthma in different severity degree. Pediatr Pulmonol. 2010;45:333–340. doi: 10.1002/ppul.21186. [DOI] [PubMed] [Google Scholar]
- 15.Celik GE, Erkekol FO, Misirligil Z, Melli M. Lipoxin A4 levels in asthma: relation with disease severity and aspirin sensitivity. Clin Exp Allergy. 2007;37:1494–1501. doi: 10.1111/j.1365-2222.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- 16.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 17.Gras D, Bourdin A, Vachier I, de Senneville L, Bonnans C, Chanez P. An ex vivo model of severe asthma using reconstituted human bronchial epithelium. J Allergy Clin Immunol. 2012;129:1259–1266. doi: 10.1016/j.jaci.2012.01.073. e1. [DOI] [PubMed] [Google Scholar]
- 18.Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 19.Kazani S, Israel E. Exhaled breath condensates in asthma: diagnostic and therapeutic implications. J Breath Res. 2010;4:047001. doi: 10.1088/1752-7155/4/4/047001. [DOI] [PubMed] [Google Scholar]
- 20.Fritscher LG, Post M, Rodrigues MT, Silverman F, Balter M, Chapman KR, Zamel N. Profile of eicosanoids in breath condensate in asthma and COPD. J Breath Res. 2012;6:026001. doi: 10.1088/1752-7155/6/2/026001. [DOI] [PubMed] [Google Scholar]
- 21.Hasan RA, O'brien E, Mancuso P. Lipoxin A4 and 8-isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatr Crit Care Med. 2012;3:141–145. doi: 10.1097/PCC.0b013e3182231644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health. NHLBI/WHO workshop report. Bethesda, Maryland: National Institutes of Health National Heart Lung and Blood Institute; 2007. [Accessed: May 2010]. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. Publication Number 08–4051. [Google Scholar]
- 24.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. 2012;129:S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68–69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–798. doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Claesson HE, Odlander B, Jakobsson PJ. Leukotriene B4 in the immune system. Int J Immunopharmacol. 1992;14:441–449. doi: 10.1016/0192-0561(92)90174-j. [DOI] [PubMed] [Google Scholar]
- 29.Kostikas K, Gaga M, Papatheodorou G, Karamanis T, Orphanidou D, Loukides S. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest. 2005;127:1553–1559. doi: 10.1378/chest.127.5.1553. [DOI] [PubMed] [Google Scholar]
- 30.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E, et al. Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respir Res. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondino C, Ciabattoni G, Koch P, Pistelli R, Trové A, Barnes PJ, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol. 2004;114:761–767. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 33.Csoma Z, Kharitonov SA, Balint B, Bush A, Wilson NM, Barnes PJ. Increased leukotrienes in exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med. 2002;166:1345–1349. doi: 10.1164/rccm.200203-233OC. [DOI] [PubMed] [Google Scholar]
- 34.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A Large Subgroup of Mild-to-Moderate Asthma is Persistently Non-Eosinophilic. Am J Respir Crit Care Med. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest. 2001;119:1322–1328. doi: 10.1378/chest.119.5.1322. [DOI] [PubMed] [Google Scholar]
- 36.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, et al. Serum amyloid A opposes lipoxin A□ to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowal-Bielecka O, Kowal K, Distler O, Rojewska J, Bodzenta-Lukaszyk A, Michel BA, et al. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: an imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis Rheum. 2005;52:3783–3791. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53:160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiron R, Grumbach YY, Quynh NV, Verriere V, Urbach V. Lipoxin A(4) and interleukin-8 levels in cystic fibrosis sputum after antibiotherapy. J Cyst Fibros. 2008;7:463–468. doi: 10.1016/j.jcf.2008.04.002. [DOI] [PubMed] [Google Scholar]