Introduction
HIV associated neurocognitive disorder (HAND) is a condition that encompasses cognitive deficits as well as motor symptoms. HIV is thought to enter the CNS early at the time of primary infection by crossing the blood brain barrier. The virus primarily infects perivascular macrophages and microglia (both of which express CD4); while the neurons, which do not express CD4, sustain injury secondary to the resulting inflammation(Gonzalez-Scarano and Martin-Garcia 2005). HAND MRI findings classically include symmetric, periventricular hyperintense lesions on T2-weighted sequences.
We describe an HIV+ patient with an absolute CD4+ T-cell count in the normal range and well-controlled plasma HIV viral load (vl) who developed progressive cognitive decline and gait difficulty.
Case
A 32-year-old HIV+ Brazilian woman with a history of treated CNS toxoplasmosis, presented to the neurology clinic for evaluation of cognitive complaints. Her CD4+ T-cell count was 769 cells/uL and plasma HIV vl 395 copies/mL. She was diagnosed four years earlier with concurrent HIV and CNS toxoplasmosis and started on lamivudine, zidovudine, and efavirenz which were discontinued due to poor compliance. Her CD4+ T-cell nadir at diagnosis was 6/uL. She was started on tenofovir, didanosine, and ritonavir-boosted atazanavir 20 months later when her HIV plasma vl was 272,000 copies/mL. On exam, she was inattentive and had decreased psychomotor speed. She scored 11/16 on the HIV dementia scale(Power et al. 1995). Brain MRI showed several areas of subcortical and periventricular white matter hyperintensities (figure-A, arrows). In addition, there was an old enhancing discoid lesion along the corpus callosum (figure-B&C, arrowheads), attributed to treated CNS toxoplasmosis.
Figure 1.
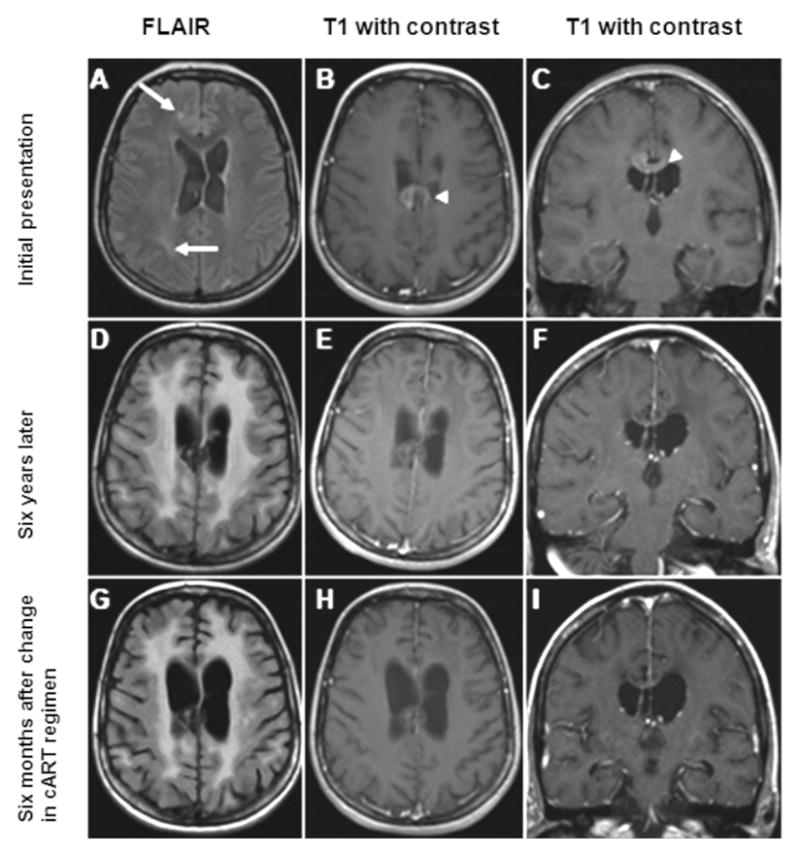
(A–C) Images from initial presentation; (D–F) Images obtained 6 years later; (G–I) Images obtained 6 months after a change in the patient’s cART regimen. The FLAIR image (A) demonstrates subcortical and periventricular hyperintense signal [arrows]. There is no enhancement seen involving the subcortical lesions (B, C); however, there is an enhancing mass that is visible on the axial (B) and coronal (C) cuts inferior to the corpus callosum [arrowhead]. Six years later, the FLAIR sequence (D) demonstrates atrophy as well as confluent areas of bilateral symmetric white matter hyperintense signal which does not enhance (E, F). The mass inferior to the corpus callosum is visible (E, H) and minimally enhances. Six months later, the axial FLAIR sequence (G) demonstrates a modest improvement in white matter disease with a similar degree of atrophy coinciding with a significant clinical improvement. There is no parenchymal enhancement in the contrast enhanced sequences (H, I); while a faint degree of enhancement is seen in the mass abutting the corpus callosum (I).
Diagnostic workup of the white matter lesions and memory complaints included serologies for Lyme, trypanosoma cruzi and cysticercosis, RPR, CSF PCR for JC virus, Epstein-Barr virus, Toxoplasma gondii and cytological examination were negative. However, the CSF WBC count (lymphocytic predominant) and protein concentration were elevated (Table). HIV vl in the CSF was 801 copies/mL. This clinical presentation, MRI, and CSF results were consistent with a diagnosis of HIV associated neurocognitive disorder (HAND). Abacavir and lamivudine were added to her regimen in order to improve CSF penetration and, with these changes, her CPE (CNS Penetration Effectiveness-2010) score increased from 5 to 10.
Table 1. Clinical and virological evolution over time.
The patient’s laboratory values, cART regimen, HIV dementia scale scores, and HIV genotype are presented, starting with her initial diagnosis of HIV-infection, and subsequent care within the infectious disease and HIV/neurology clinics. Medications marked in bold were those added to the cART regimen based on genotype testing at that time. Didanosine was discontinued at month 54 due to nausea. Only mutations found exclusively in the cerebrospinal fluid and not in the plasma are included. D67N refers to a mutation in which the Aspartic acid (D) normally at amino acid position 67 is substituted by Asparagine (N). N348I refers to a mutation in which Asparagine normally at position 348 is substituted by Isoleucine (I). K20R refers to a mutation in which the Lysine (K) normally at position 20 is substituted by Arginine (R). E399D refers to a mutation in which Glutamic acid (Q) normally at position 399 is substituted by Aspartic acid (E).
| Nadir/HIVdx (month 0) | month 20 | month 48 | month 54 | month 61 | months 109–112 | months 115–118 | months 124–126 | |
|---|---|---|---|---|---|---|---|---|
| CD4 count (cells/uL) | 6 | 769 | 696 | 989 | 559 | 725 | ||
| plasma viral load (cps/mL) | 272,000 | 395 | undetectable | 511 | 263 | 75 | ||
| CSF viral load (cps/mL) | 801 | 294 | 6950 | <20 | ||||
| CSF WBC (cells/uL) | 11 | 5 | 13 | 1 | ||||
| CSF protein (mg/dL) | 57 | 56 | 101 | 51 | ||||
| cytology | neg | neg | neg | |||||
| abacavir3 | darunavir/r5 | |||||||
| lamivudine4 | abacavir | raltegravir9 | ||||||
| lamivudine | tenofovir | tenofovir2 | lamivudine | zidovudine7 | ||||
| zidovudine | didanosine | atazanavir/r1 | tenofovir | lamivudine8 | ||||
| didanosine2 | tenofovir6 | |||||||
| cART regimen | efavirenz | atazanavir/r | atazanavir/r | |||||
| CPE score (2010) | 9 | 5 | 10 | 8 | 13 | |||
| HIV dementia scale | 11 | 15 | 13 | 12 | 14.5 | |||
| Plasma genotype | + | + | ||||||
| CSF genotype | − | + | ||||||
| Unique CSF mutations | + | |||||||
| NRTI | D67N | |||||||
| NNRTI | N348I | |||||||
| PI | K20R | |||||||
| other | E399D |
cART= combination antiretroviral therapy; CPE= CNS penetration effectiveness; cps= copies; Dx= diagnosis; neg= negative; NNRTI= non-nucleoside reverse transcriptase inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; r= ritonavir boosted;
Susceptibility testing results of the CSF was similar to that in the plasma.
susceptible
low-level resistance
intermediate resistance
high level resistance
susceptible
low-level resistance
intermediate resistance
high level resistance
no resistance profile available
One year after the changes in cART (Table, month 61) her cognition had improved significantly. CSF exam showed a stable protein count, normalization of WBC, and decreased CSF HIV vl. Furthermore, MRI of the brain demonstrated a decrease in size of white matter hyperintensities.
However, approximately four years later (Table, month 109), she complained of worsening in her cognition as well as gait difficulties. An MRI of the brain demonstrated atrophy and bilateral increase in hyperintense signal (figure-D). CSF analysis showed an HIV vl of 6950 copies/mL. The CSF WBC count and protein concentration were both elevated. CSF and plasma HIV genotypes showed a similar yet slightly different drug resistance pattern. Several HIV mutations found in the CSF were not identified in the plasma (Table). One mutation found in both compartments was M184V mutation, driven by lamivudine. Viruses with this mutation are known to be more susceptible to several nucleoside reverse transcriptase inhibitors such as zidovudine. Based on these results, abacavir was replaced by zidovudine, and ritonavir-boosted atazanavir was replaced by ritonavir-boosted darunavir. Raltegravir was added as a new integrase inhibitor. These changes increased the CPE score of the cART regimen from 8 to 13.
Six months later, her gait and cognition had improved. CSF analysis showed normalization of WBC and protein, while the CSF HIV vl was undetectable. MRI of the brain showed a decrease in the hyperintense white matter disease (figure-G).
Discussion
We report a case of an HIV+ patient who developed HAND in the setting of a suppressed plasma HIV replication while on cART. She had a CD4+ T-cell count nadir of 6/uL. It is not uncommon for patients with a history of a low CD4+ T-cell count to develop neurocognitive disorder as the CD4 nadir is a predictor for HAND(Ellis et al. 2011).
It has recently been shown that new neurologic symptoms occurring in the setting of an undetectable or low plasma HIV vl may indicate the presence of CSF viral escape(Peluso et al. 2012). This finding should alert infectious disease specialists, neurologists, and other HIV providers to remain vigilant for neurologic complaints and symptoms and to prompt an appropriate work up, including imaging, measurement of HIV vl in the CSF, and genotype testing, regardless of the plasma HIV vl. However, in the absence of neurologic complaints, symptoms, or exam findings, the presence of CSF viral escape has not been associated with neurocognitive disease(Valero et al. 2012) and might not require a change in the antiretroviral regimen.
The discordance in the mutations seen in the CSF compared to those seen in the plasma in our patient highlights the fact that the CNS can act as an independent reservoir for HIV(Takahashi et al. 1996; Lambotte et al. 2003), and is consistent with findings indicating that viral replication occurs within the CNS compartment(Ritola et al. 2005; Schnell et al. 2009). Following a change in the antiretroviral regimen, the patient improved clinically, concomitant to suppression of viral replication in the CSF. This observation highlights the need for an antiretroviral regimen with good penetration through the blood-brain-barrier (high CPE score)(Smurzynski et al. 2011) and the usefulness of genotype testing in CSF to adapt cART regimen. Therefore, CNS penetration ability and the CSF resistance profile of HIV should be considered in adjusting the cART regimen in patients with HAND(Peluso et al. 2012).
Finally, the HIV Dementia Scale is a valuable screening tool to detect HAND and was used in our patient. However, the proposed raw cut-off of ≤ 10 provides a sensitivity of only 24% as shown recently by the CHARTER group(Sakamoto et al. 2013), and might not identify those patients with subtle or asymptomatic neurocognitive impairment. When the cut-off score was increased to ≤ 14, the sensitivity improves (66%(Sakamoto et al. 2013) – 83%(Simioni et al. 2010)) at the expense of specificity. Our patient’s neurological function correlated with the fluctuations in her score. Further investigations using various HIV Dementia Scale cut-off points and factoring in CSF HIV vl are needed to establish a more accurate clinical tool in the diagnosis of HAND.
Acknowledgments
The authors would like to thank Dr. Sarah Gheuens for her input on the final manuscript.
References
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews Immunology. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain pathology. 2003;13(1):95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, Gisslén M, Angoff N, Price RW, Cinque P, Spudich S. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1995;8(3):273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R. Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. Journal of virology. 2005;79(16):10830–10834. doi: 10.1128/JVI.79.16.10830-10834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Marcotte TD, Umlauf A, Franklin D, Jr, Heaton RK, Ellis RJ, Letendre S, Alexander T, McCutchan JA, Morgan EE, Woods SP, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson D, Grant I CHARTER Group. Concurrent Classification Accuracy of the HIV Dementia Scale for HIV-Associated Neurocognitive Disorders in the CHARTER Cohort. Journal of acquired immune deficiency syndromes. 2013;62(1):36–42. doi: 10.1097/QAI.0b013e318278ffa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS pathogens. 2009;5(4):e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Annals of neurology. 1996;39(6):705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Valero IP, Letendre S, Ellis R, Deutsch R, Franklin D, Clifford D, Heaton R, McCutchan J, McArthur J, Morgello S, Gelman B, Collier A, Grant I. Prevalence and risk factors for HIV CSF Viral Escape: Results from the CHARTER and HNRP cohorts. Journal of the International AIDS Society. 2012;15(6):18189. [Google Scholar]


