Abstract
Neonatal intensive care unit (NICU) patients are at high-risk for developmental disabilities such as cerebral palsy (CP). Early identification of CP is essential to effective rehabilitation, but diagnosis is often delayed, especially in preterm infants. We hypothesized longitudinal evaluation of motor trajectories in the NICU Follow-up clinic could distinguish in infancy who developed CP by 3 years of age.
Study Design and subjects
Retrospective study of 606 patients in the NICU Follow-up clinic at Vanderbilt University with birthweight <1500 g or a diagnosis of hypoxic ischemic encephalopathy.
Outcomes measures
Assessments included neurologic exams, the Developmental Assessment of Young Children (DAYC), the Bayley Scales of Infant Development (BSID) and the Gross Motor Function Classification Scale.
Results
A decrease in DAYC scores between 6 and 12 months was present in preterm and term infants later diagnosed with CP, but not in children without CP (−23 vs. +1.5, p <0.001). DAYC score decreases in infancy were highly predictive of later CP (p <0.001). BSID scores quantified severe motor delays but did not add to prediction of CP diagnosis.
Conclusion
Standardized assessments of motor milestones quantitatively predict the risk of CP in former NICU patients by 12 months, allowing for timely diagnosis, counseling and therapy in high-risk infants.
Keywords: Neurodevelopment, NICU, Predictive testing, Motor development, Cerebral palsy
1. Introduction
Infants discharged from the neonatal intensive care unit (NICU) are at increased risk for poor neurodevelopmental outcomes[1–4]. Of all infants born before 27 weeks gestational age (GA), 14% develop cerebral palsy (CP), compared to 0.2% of the general population[2]. Almost 2/3 of 11,000 children diagnosed every year with CP in the United States are former preterm infants, or term infants with severe birth-related complications[5–7]. Excellent predictive models exist to help identify which NICU patients will be at highest risk for CP[8–12]. However, for an individual infant, it is essential to establish a diagnosis of CP as early as possible in order to optimize the effectiveness of rehabilitative interventions. Infancy and early childhood are periods of maximal neural plasticity during which therapeutic interventions have the greatest potential for long-term effectiveness[13–17]. Additionally, early identification can help prevent or moderate the complex communication, social and emotional associations that can have functional consequences into adulthood[18–22].
CP is challenging to diagnose in young children due to the complexity of signs, symptoms and developmental progression involved[23]. An initial diagnosis of CP can be especially difficult to make in premature infants whose neurological patterns of maturation and unique pathology complicate their presentation[24,25]. In addition, the developmental surveillance of NICU patients is highly variable, ranging from none to greater than 90% in research studies supporting systematic NICU follow-up[26]. Thus, many NICU patients identified as high-risk for CP will face delayed diagnosis due to a lack of specialized providers and assessments.
Given the implications and challenges of early diagnosis of CP, developing simple tools for early identification and screening in high-risk infants is a priority. Research has focused mainly on highly specialized tools, from imaging to complex neurological assessments, while few studies examine the value of more basic developmental milestone trajectories. Therefore, the goals of this study were to characterize the evolution of motor milestones in the first three years in high-risk infants discharged from the NICU. We hypothesized that the trajectory of motor test scores on an interactive developmental assessment would predict CP in the first year of life, before the administration of gold standard neurodevelopmental tests and neurologic exams at 24 to 36 months.
2. Methods
We conducted a retrospective database review of prospectively acquired data on patients seen in the NICU Developmental Follow-Up Clinic (DFC) at the Monroe Carell Junior Children's Hospital (MCJCH) at Vanderbilt from 2005–2008. Inclusion criteria were infants with birthweights <1500 g, and those with a diagnosis of hypoxic ischemic encephalopathy (HIE) at time of discharge from the MCJCH NICU. Patients were seen at 6, 12, 24 and 36 months chronological age, with interim visits if concerns existed. At the 6 and 12-month visits to the DFC, children were tested using the Developmental Assessment of Young Children (DAYC) [27], a standardized assessment with normative data in the motor development domain. The DAYC is an interactive questionnaire with milestones reported as achieved by parents. These milestones are challenged when a threshold is met by observation and interaction with the patients using common toys (rattle, small blanket, etc.). Most of the observations are made throughout the routine course of the visit as parent and child interact. Some elements of gross motor function such as rolling from side to supine or head up while prone are observed and challenged during the regular physical exam, while others are formally tested. For example, a 6-month milestone would be “does your child transfer a toy from one hand to the other?” If the parent replied yes, and no more advanced skills were reported, the examiner would then state “lets see if she would do this for me too” and hand the child a toy prepared for this purpose. If the child could not perform this milestone, the examiner would then test the preceding milestone. The DAYC was administered by clinic providers who were all trained using standardized observation followed by monitored DAYC administration before independence to maximize inter- and intra-observer reliability. At the 24- and 36-month visits, trained examiners administered the Bayley Scales of Infant Development (BSID) exam[28]. Due to a change in testing formats, the BSID II was used prior to 2007 and the BSID III was used after that year[29]. Therefore, we extracted only composite motor scores from the database instead of scores for fine and gross motor scales.
All patients identified in the DFC database with CP had later concurrence of the diagnosis by pediatric neurologists, movement disorder specialists and rehabilitation providers. CP was defined as a group of permanent disorders of the development of movement and posture, causing activity limitations that are attributed to non-progressive disturbances that occur in the developing fetal or infant brain[23]. CP was classified according to both the algorithm used in the Extremely Low Gestational Age Newborns (ELGAN) study group[25] and according to the Gross Motor Function Classification System (GMFCS)[30] on the basis of the neurological exam at the last visit[31]. Spasticity and dystonia are not part of this classification. The rationale for omitting distinctions of elevated tone is the frequency with which spasticity and dystonia co-occur and the variability of their presentations in infancy[25]. Cranial imaging data were extracted from the medical record and the most severe radiographic findings were reported. Data from these clinic visits were gathered prospectively and maintained in a repository database approved by the Institutional Review Board (IRB) at Vanderbilt University. Vanderbilt IRB approval was subsequently obtained for review and extraction of these data.
3. Statistical Analysis
Baseline descriptive statistics by birth status were compared using the Wilcoxon rank sum test for continuous outcomes and Pearson's Chi-squared test for categorical outcomes. We summarized DAYC and BSID motor scores at each time point using the median and interquartile range (IQR). Using quartiles allowed us to incorporate all motor scores in the analysis, including subjects that were documented too low to be accurately tested. We confirmed from the medical record that “not testable” scores were lower than any measurable score and gave them the lowest possible score on the test. We compared median motor scores at each clinic visit and change in scores from one visit to the next using the Wilcoxon rank sum test. Time from birth to CP diagnosis by birth status was estimated using the log-rank test. Logistic regression analysis was used to determine the probability of CP based on changes in DAYC scores. In the detailed analysis, infants were separated into 2 groups: the first group was composed of preterm infants below 34 weeks GA at birth, the second included late preterm (LPT) (34–36 weeks GA) and term infants (born at 37 weeks GA or above).
4. Results
During the study period, 850 infants with birthweights <1500 g were discharged alive from the MCJCH NICU. Of these, 572 were seen on multiple occasions in the MCJCH DFC for a follow-up rate of 68% at 3 years of age. During this period, 61 infants with a diagnosis of HIE were discharged alive, and 34 were seen in the DFC for a follow-up rate of 56% at 3 years of age. A definitive diagnosis of CP was made in 46 out of the 606 patients seen in the DFC (32 were preterm and 14 late preterm (LPT) or term) for a rate of diagnosis of 7.6%. All children received their documented diagnosis of CP for the first time in the DFC. As expected, preterm and LPT/term infants with CP differed significantly with respect to GA (median 28 weeks, IQR 26–29 vs. median 38 weeks, IQR 37–40) and birthweight (930 g, IQR 729–1404 vs. 3358 g, IQR 2741–3642). The differences between the preterm children with CP and those without were less pronounced, with a median GA of 28 weeks (IQR 26–30) in children without CP vs. a median of 29 weeks (IQR 26–37) in children with CP (p = 0.02). All children diagnosed with CP were referred to rehabilitative specialists and specialized motor disorders clinics at the time of diagnosis. Additionally, the diagnosis was communicated immediately to the state's early intervention program coordinators.
4.1 Characteristics of children with CP (Table 1)
Table 1.
Characteristics of infants diagnosed with cerebral palsy in the DFC.
| PT N = 32 | LPT/T N = 14 | p | |
|---|---|---|---|
| Baseline | |||
| GA at birth in weeks (median, IQR) | 28 (26,29) | 38 (37,40) | <0.001 |
| Birthweight in g (median, IQR) | 930 (729,1404) | 3358 (2741,3642) | <0.001 |
| Cranial imaging | <0.001 | ||
| IVH only, n (%) | 11 (34) | 0 (0) | |
| PVL only, n (%) | 6 (19) | 1 (7) | |
| IVH or/and PVL, n (%) | 22 (69) | 2 (14) | |
| Ischemic findings, n (%) | 1 (3) | 9 (64) | |
| Other, n (%) | 3 (9) | 2 (14) | |
| Normal, n (%) | 6 (19) | 1 (7) | |
|
| |||
| 6-month visit | |||
| DAYC score, median (IQR) | |||
| Corrected age | 96 (86,99) | 78 (68,82) | <0.001 |
| Chronologic age | 75 (68,82) | 77 (68,82) | 0.78 |
|
| |||
| 12-month visit | |||
| DAYC score, median (IQR) | |||
| Corrected age | 74 (49,84) | 48 (40,63) | 0.009 |
| Chronologic age | 54 (40,68) | 40 (40,59) | 0.18 |
|
| |||
| 24-month visit | |||
| Type of CP, n (%) | 0.04 | ||
| Hemiparesis | 4 (12) | 2 (14) | |
| Diparesis | 14 (44) | 1 (7) | |
| Quadriparesis | 14 (44) | 11 (79) | |
| GMFCS score, n (%) | 0.07 | ||
| 1 | 10 (31) | 1 (7) | |
| 2 | 2 (6) | 2 (14) | |
| 3 | 8 (25) | 3 (21) | |
| 4 | 10 (31) | 3 (21) | |
| 5 | 2 (6) | 5 (36) | |
IQR; Interquartile range
All children with CP had cranial imaging performed prior to discharge from the NICU. As expected, findings on cranial imaging were significantly different between the preterm and LPT/term groups (p <0.001). The majority (69%) of the preterm group had intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL) or both, whereas LPT/term infants had findings consistent with ischemic encephalopathy (64%) as ascertained by pediatric radiologists. Twenty percent of preterm infants with CP had no abnormal findings on cranial imaging.
The two groups were significantly different in the overall distribution of CP types (p = 0.04), with 79% of the LPT/term group having quadriparesis and 7% with diparesis. In the preterm group, diparesis and quadriparesis were equally represented (44% for both). Hemiparesis was the least common type of CP in this population, with no differences between preterm and LPT/term groups (12% vs 14%). Infants in the LPT/term group had the least functional types of CP with 93% receiving a GMFCS score of 2 or above vs. 69% in the preterm group. More preterm infants had a GMFCS score of 1 (31% vs. 7% in the LPT/term group) but this difference did not reach statistical significance (p = 0.07).
4.2 Evolution of standardized scores for motor development in the DFC
Median DAYC motor scores for corrected age were significantly lower for children with CP at the 6 and 12-month visits than in children with no CP (Table 2). In children who later developed CP, DAYC scores decreased by a median of 23 points (IQR 36–11) between the 6-month and 12-month visits. However, in children who did not develop CP, the DAYC motor scores were stable (p <0.001). The BSID motor scores were lower in the group with CP at both 24 and 36 month visits; however, there was no change in BSID scores between 24 and 36 months for either group. The group of preterm infants with CP had higher corrected age DAYC scores than the LPT/term group with CP at 6 months (median 96; IQR 86–99 vs. 78; IQR 68–82) and at 12 months (median 74 IQR 49–84 vs 48; IQR 40–63) p <0.01.
Table 2.
Developmental testing motor scores for children referred to the DFC.
| N | CP N = 46 | No CP N = 560 | P | |
|---|---|---|---|---|
| DAYC 6 month | 556 | 78 85 97 | 98 103 107 | 0.01 |
| DAYC 12 month | 480 | 47 61 80 | 95 104 110 | 0.006 |
| DAYC change | 449 | −36 −23 −11 | −6 1 7 | < 0.001 |
| BSID at 24 months | 361 | 46 49 61 | 91 100 106 | < 0.001 |
| BSID at 36 months | 223 | 47 49 61 | 91 100 109 | < 0.001 |
| BSID change | 203 | −6 0 3 | −6 0 8 | 0.41 |
a b c represent the lower quartile, the median, and the upper quartile, respectively.
DAYC and BSID scores are all reported for corrected ages.
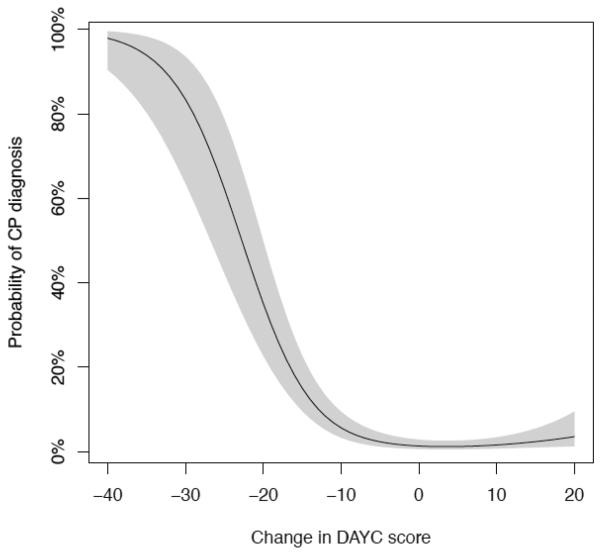
In this population of high-risk infants, we can estimate the probability of developing CP by 3 years of age, based on the decrease in DAYC motor scores between the 6 and 12-month visits (Fig. 1). The probability of developing CP stays low for score decreases that are within 1 standard deviation (SD) of the mean for the test (15 points). However, as the score decrease approaches 20 points, the probability of CP rises to 35.1% (CI [22.7–49.8]). This effect is even more pronounced when the decrease in scores approaches 30 points (i.e. 2 SD below the mean) as the probability of developing CP rises to 83.4% (CI [63.5–93.5]).
Fig. 1. Probability of CP diagnosis by 3 years of age based on decrease in DAYC motor scores between the 6 and 12-month visits.
DAYC score is the motor score for corrected age; shaded area indicates 95% confidence interval. The C-index for this model is C = 0.89, which is highly significant (p < 0.001). The C-index is a statistic that measures the predictive accuracy of a model; it measures the probability that the predicted outcome is the same as the observed outcome. The C-index is equal to the area under the explain ROC (ROC) curve and takes on values between 0 and 1, with a value of 0.5 indicating no predictive accuracy, and a value of 1.0 being a perfect predictor.
4.3 Timing of CP diagnosis based on clinical findings
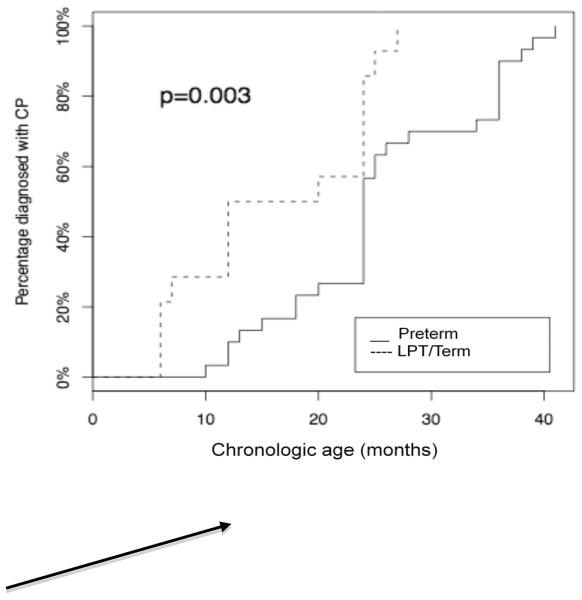
Providers in the clinic assigned a diagnosis of CP based on abnormalities on the neurological and physical exams. As previously noted, all initial CP diagnoses for these children were made in the DFC. At the 24-month visit, all 14 LPT/term infants with CP had been given a diagnosis. This was not the case for preterm infants, where less than half were given this diagnosis by the 24-month visit (Table 2). Fig. 2 illustrates the number of diagnoses as a function of time in both groups. For the preterm population, the median age of CP diagnosis was 24 months (IQR (21.0,35.5)) corresponding to a median corrected age of 20 months. This was significantly later than the median age at diagnosis of 16 months for the LPT/term group (IQR (8.2,24.0); p = 0.004 by Wilcoxon Rank test), and still well after 12 months corrected age in both groups.
Fig. 2. Proportion of infants with diagnosis of CP as a function of chronological age.
P is probability calculated using log-rank test
5. Discussion
This study demonstrates that it is possible to predict a diagnosis of cerebral palsy in infancy in former NICU patients using a simple standardized assessment of motor milestones. Children diagnosed with CP by 3 years of age have a significant and accurately predictive decrease in DAYC motor scores between 6 months and 12 months of chronologic age. This holds true for both preterm infants and for LPT/term infants who are not diagnosed until 16 to 20 months of corrected age using clinical findings alone. The importance of the current approach is very early detection and referral of a condition which can respond to rehabilitation in early childhood.[32,33].
Our results suggest that testing using developmental milestone trajectories can inform the follow-up of high-risk infants without using tools that require a high degree of training and/or resources. The DAYC is a simple questionnaire-based assessment that does not require highly specialized training, personnel or equipment to perform. It is rapidly administered and can be used in many pediatric settings and, as such, is often used by early intervention programs in states where assessment in infancy is funded[27]. While the DFC uses the DAYC, other equally valid and standardized assessments of milestones may potentially also fulfill this purpose, such as the Kent Inventory of Developmental Skills (KIDS-3)[34], Developmental Indicators for the Assessment of Learning (DIAL-3)[35] or the Early Childhood Inventory-4[36].
In contrast, the BSID requires trained providers, is lengthy and expensive to administer and is more accurate after 18 months of age. Additionally, the predictive value of the BSID at school-age has been questioned. The BSID produces valuable data about specific developmental processes and is a benchmark for cognitive, communication and motor development[37,38], as well as an invaluable research tool. However, most children who presented to the DFC with CP were already diagnosed by the time they could be accurately tested with the BSID, and received the lowest scores on the motor assessment at that time.
A complicating factor in the diagnosis of CP lies in the consensus definitions and classifications of many motor disorders often not formalized until at least 2 years of age[23]. In our study, the majority of preterm infants did not meet enough diagnostic criteria for CP until the 24-month visit or later. Until 2 years of age, the variable tone associated with preterm infants' maturing neural pathways and long periods of supine immobilization can mask dystonia and even spasticity. These infants also have motor delays that are directly associated with the length of their hospitalization and to the complications of intensive care[39]. For example, preterm infants without intracranial lesions can exhibit less flexor tone in limbs and increased extensor tone in the neck compared to term infants[40–42]. While sensitive and specific evaluations of movement in infancy such as the Assessment of General Movements can effectively differentiate between the effects of prematurity and other neurologic conditions[43–46], they require access to either highly specialized providers or resources[47–49]. Until further maturation has occurred, it may be difficult for a non-specialized pediatric provider to differentiate increased abnormal tone due to mild CP rather than due to complications of prematurity.
The types of CP that are more common in preterm infants in our study are similar to those observed in other larger studies[50]; diparesis and quadriparesis are equally represented and much more frequent than hemiparesis. The findings on cranial imaging of our preterm population are also consistent with reported associations with IVH and/or PVL in almost 70% of the preterm population with CP[51–54]. In particular, preterm infants often have CP with higher levels of functionality, as 38% have a GMFCS ≤2. This supports studies on outcomes of prematurity[55] and especially periventricular hemorrhagic infarction[11]. Predictive functionality curves have been established for the GMFCS and help providers understand the potential for rehabilitation[56]. By combining these curves with the results from our study, the counseling of parents of premature infants with CP in the DFC is potentially more optimistic than for term infants, who mostly develop quadriparetic CP.
Finally, it is evident that this study builds on the work of numerous studies that have established predictive models at discharge from the NICU, as they are the basis for the selection of patients requiring developmental follow-up. While neuroimaging findings or electrophysiologic measures can be very helpful in identifying high-risk patients[57–59] [60,61], their predictive value often depends on the criteria used to read these tests or even accessibility issues. However, models incorporating clinical variables with imaging findings are extremely valuable to the follow-up of children at high-risk for CP[62]. In particular, Himpens[9] showed that gestational age and gender may not be as important as imaging and clinical variables (for example perinatal asphyxia) when considering the population of NICU patients as whole.
Our study had the limitations inherent in a retrospective analysis, which precludes attributing cause. One of the concerns for bias is the range of pediatric providers who administered the DAYC in our clinic, including pediatric and neonatal nurse practitioners, general and developmental pediatricians and neonatologists. We attempted to alleviate this bias by minimizing inter-observer variability through a standardized training process. An additional source of potential bias is the suboptimal follow-up rate of 68% at 3 years, which can be attributed to the vast geographic regions served by the NICU at Vanderbilt University, a tertiary care referral center for an area encompassing several states with large rural populations. There was a potential cultural sample bias in that significantly more of the patients missing from follow-up were from surrounding states than from Tennessee. Of note, neuroimaging, socioeconomic status and gestational age at birth were not significantly different in the patients lost to follow up and those who were seen to age 3 years. We also chose to extract only the motor trajectories of the patients to obtain a complete timeline of easily reproducible data. However, CP also involves interacting communication, cognition and social-adaptive components that should not be overlooked; moreover, language acquisition, social-emotional adaptation and behavior are crucial elements of NICU follow-up in a population at risk for most developmental delays and disorders. Finally, a newer version of the DAYC, the DAYC-2 has recently been published, wherein the motor domain is divided into fine and gross motor scales for added precision. The DAYC-2 has been tested on a 2010 normative sample of the population and 87% of the items on the new motor domain are taken from the original version of the DAYC. However, as with most neurodevelopmental assessment tools, it has not yet been compared to the original version. It is possible that only the gross motor scale will prove of similar predictive value in infants whose more complex manual milestones emerge after the first 12 months.
In summary, the trajectory of motor milestones on a standardized clinic assessment can predict CP in the first year of life in a high-risk NICU population, before complex neurodevelopmental tests and specialized neurologic exams can be performed. The ease and value of this finding allowed quick implementation of trajectory monitoring in the NICU follow-up clinic population, allowing earlier referrals to rehabilitative interventions and neurologic or developmental specialists, as well as community outreach and training of primary care providers. Future studies in this context will focus on tools to extend this approach to cognitive, communication and social-emotional domains.
Table 3.
Timing of CP diagnosis based on clinical variables.
| PT group % with diagnosis (N = 32) | LPT/T group % with diagnosis (N = 14) | |
|---|---|---|
| Visit date (in months) | ||
| 6 | 3 | 29 |
| 12 | 22 | 50 |
| 24 | 47 | 100 |
| 36 | 100 | 100 |
Acknowledgements
we would like to thank Dr Donna Daily for her groundbreaking work in developing the NICU Follow-Up Clinic at Vanderbilt and first designing the database used in this study.
Abbreviations
- BSID
Bayley Scales of Infant Development
- CP
Cerebral palsy
- DAYC
Developmental Assessment of Young Children
- DFC
Developmental Follow-up Clinic
- NICU
Neonatal Intensive Care Unit
- IVH
Intraventricular hemorrhage
- PCP
Primary care provider
- PVL
Periventricular leukomalacia
- GMFCS
Gross Motor Function Classification System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement: We have no conflicts of interest.
References
- 1.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr. Opin. Neurol. 2008 Apr.21(2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 2.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008 Jan.371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 4.Hintz SR, Poole WK, Wright LL, Fanaroff AA, Kendrick DE, Laptook AR, et al. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed. 2005 Mar.90(2):F128–33. doi: 10.1136/adc.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of Cerebral Palsy in 8-Year-Old Children in Three Areas of the United States in 2002: A Multisite Collaboration. Pediatrics. 2008 Mar.121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 6.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011 Jun.127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 7.Platt MJ, Cans C, Johnson A, Surman G, Topp M, Torrioli MG, et al. Trends in cerebral palsy among infants of very low birthweight. The Lancet. 2007 Jan.369(9555):43–50. doi: 10.1016/S0140-6736(07)60030-0. [DOI] [PubMed] [Google Scholar]
- 8.Spittle AJ, Boyd RN, Inder TE, Doyle LW. Predicting motor development in very preterm infants at 12 months' corrected age: the role of qualitative magnetic resonance imaging and general movements assessments. Pediatrics. 2009 Feb.123(2):512–517. doi: 10.1542/peds.2008-0590. [DOI] [PubMed] [Google Scholar]
- 9.Himpens E, Oostra A, Franki I, Vansteelandt S, Vanhaesebrouck P, Broeck den CV. Predictability of cerebral palsy in a high-risk NICU population. Early Hum. Dev. 2010 Jul.86(7):413–417. doi: 10.1016/j.earlhumdev.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Majnemer A, Rosenblatt B. Prediction of Outcome at School Age in Neonatal Intensive Care Unit Graduates Using Neonatal Neurologic Tools. Journal of Child Neurology. 2000 Oct.15(10):645–651. doi: 10.1177/088307380001501002. [DOI] [PubMed] [Google Scholar]
- 11.Maitre N. More favorable neurodevelopmental outcoems of infants with unilateral compared to bilateral periventricular hemorrhagic infarction. Pediatrics. 2009 doi: 10.1542/peds.2009-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badr LK, Bookheimer S, Purdy I, Deeb M. Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical and environmental factors. Early Hum. Dev. 2009 May;85(5):279–284. doi: 10.1016/j.earlhumdev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009 Jan.31(1):1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15(2):94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 15.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003 May;(41 Suppl):7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 16.Stein DG, Hoffman SW. Concepts of CNS plasticity in the context of brain damage and repair. J Head Trauma Rehabil. 2003 Jul-Aug;18(4):317–341. doi: 10.1097/00001199-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AL, di Maggio A. Rehabilitation for children after acquired brain injury: current and emerging approaches. Pediatr. Neurol. 2012 Jun;46(6):339–344. doi: 10.1016/j.pediatrneurol.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Msall ME. Optimizing early development and understanding trajectories of resiliency after extreme prematurity. Pediatrics. 2009 Jul.124(1):387–390. doi: 10.1542/peds.2009-1149. [DOI] [PubMed] [Google Scholar]
- 19.Msall ME. The panorama of cerebral palsy after very and extremely preterm birth: evidence and challenges. Clin Perinatol. 2006 Jun.33(2):269–284. doi: 10.1016/j.clp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 20.King S, Teplicky R, King G, Rosenbaum P. Family-centered service for children with cerebral palsy and their families: a review of the literature. YSPEN. 2004 Mar.11(1):78–86. doi: 10.1016/j.spen.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Spittle AJ. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. 2007 Dec.31:1–62. doi: 10.1002/14651858.CD005495.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Krakovsky G, Huth MM, Lin L, Levin RS. Functional changes in children, adolescents, and young adults with cerebral palsy. Res Dev Disabil. 2007 Jun.28(4):331–340. doi: 10.1016/j.ridd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007 Feb.109:8–14. [PubMed] [Google Scholar]
- 24.Donohue PK, Graham EM. Earlier markers for cerebral palsy and clinical research in premature infants. J Perinatol. 2007 May;27(5):259–261. doi: 10.1038/sj.jp.7211741. [DOI] [PubMed] [Google Scholar]
- 25.Kuban K, Allred E, O'Shea M, Paneth N, Pagano M, Leviton A. An Algorithm for Identifying and Classifying Cerebral Palsy in Young Children. The Journal of Pediatrics. 2008 Oct.153(4):466–472. e1. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Follow-up Care of High-Risk Infants. Pediatrics. 2004 Jan.114(Supplement 5):1377–1397. [Google Scholar]
- 27.Voress JMT. Developmental Assessment of Young Children. PRO-ED; Austin, TX: 1998. [Google Scholar]
- 28.Bayley N. Bayley Scales of Infant Development. Second The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 29.Albers C GA. Test Review: Bayley Scales of Infant and Toddler Development, Third Edition. Journal of Psychoeducational Assessment. 2007;25(2):180–190. [Google Scholar]
- 30.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental medicine and child neurology. 2008 Oct.50(10):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuban KCK, O'Shea M, Allred E, Leviton A, Gilmore H, DuPlessis A, et al. Video and CD-ROM as a Training Tool for Performing Neurologic Examinations of 1-Year-Old Children in a Multicenter Epidemiologic Study. Journal of Child Neurology. 2005 Oct.20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 32.Arpino C, Vescio MF, De Luca A, Curatolo P. Efficacy of intensive versus nonintensive physiotherapy in children with cerebral palsy: a meta-analysis. Int J Rehabil Res. 2010 Jun.33(2):165–171. doi: 10.1097/MRR.0b013e328332f617. [DOI] [PubMed] [Google Scholar]
- 33.Cauraugh JH, Naik SK, Hsu WH, Coombes SA, Holt KG. Children with cerebral palsy: a systematic review and meta-analysis on gait and electrical stimulation. Clin Rehabil. 2010 Nov.24(11):963–978. doi: 10.1177/0269215510371431. [DOI] [PubMed] [Google Scholar]
- 34.Reuter J, Katoff L. Western Psychological Services. 2000. Kent Inventory of Developmental Skills: KIDS : Administration Booklet. [Google Scholar]
- 35.Elkins K. The Developmental Indicators for the Assessment of Learning - 3rd Edition (Dial-3): A Screening Tool for Sensory Integrative Dysfunction. Louisiana Tech University; 2006. [Google Scholar]
- 36.Sprafkin J, Volpe RJ, Gadow KD, Nolan EE, Kelly K. A DSM-IV-Referenced Screening Instrument for Preschool Children: The Early Childhood Inventory-4. Journal of the American Academy of Child & Adolescent Psychiatry. 2002 May;41(5):604–612. doi: 10.1097/00004583-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Hack M. Poor Predictive Validity of the Bayley Scales of Infant Development for Cognitive Function of Extremely Low Birth Weight Children at School Age. Pediatrics. 2005 Aug.116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 38.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010 Apr.164(4):352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 39.Allen MC, Alexander GR. Using gross motor milestones to identify very preterm infants at risk for cerebral palsy. Developmental Medicine & Child Neurology. 1992 Mar.34(3):226–232. doi: 10.1111/j.1469-8749.1992.tb14995.x. [DOI] [PubMed] [Google Scholar]
- 40.Mercuri E, Guzzetta A, Laroche S, Ricci D, vanhaastert I, Simpson A, et al. Neurologic examination of preterm infants at term age: comparison with term infants. The Journal of pediatrics. 2003 Jun.142(6):647–655. doi: 10.1067/mpd.2003.215. [DOI] [PubMed] [Google Scholar]
- 41.Forslund M, Bjerre I. Neurological assessment of preterm infants at term conceptional age in comparison with normal full-term infants. Early Human Development. 1983 Oct.8(3–4):195–208. doi: 10.1016/0378-3782(83)90002-6. [DOI] [PubMed] [Google Scholar]
- 42.Ricci D, Romeo DMM, Haataja L, van Haastert IC, Cesarini L, Maunu J, et al. Neurological examination of preterm infants at term equivalent age. Early Human Development. 2008 Nov.84(11):751–761. doi: 10.1016/j.earlhumdev.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Einspieler C, Prechtl HFR. Prechtl's assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005;11(1):61–67. doi: 10.1002/mrdd.20051. [DOI] [PubMed] [Google Scholar]
- 44.Bruggink JL, Einspieler C, Butcher PR, Van Braeckel KN, Prechtl HF, Bos AF. The quality of the early motor repertoire in preterm infants predicts minor neurologic dysfunction at school age. The Journal of Pediatrics. 2008 Jul.153(1):32–39. doi: 10.1016/j.jpeds.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 45.Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D. An early marker for neurological deficits after perinatal brain lesions. Lancet. 1997 May 10;349(9062):1361–1363. doi: 10.1016/S0140-6736(96)10182-3. [DOI] [PubMed] [Google Scholar]
- 46.Romeo DMM, Guzzetta A, Scoto M, Cioni M, Patusi P, Mazzone D, et al. Early neurologic assessment in preterm-infants: integration of traditional neurologic examination and observation of general movements. Eur. J. Paediatr. Neurol. 2008 May;12(3):183–189. doi: 10.1016/j.ejpn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Palmer FB. Strategies for the early diagnosis of cerebral palsy. The Journal of pediatrics. 2004 Aug.145(2 Suppl):S8–S11. doi: 10.1016/j.jpeds.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Stahl A, Schellewald C, Stavdahl Ø , Aamo OM, Adde L, Kirkerød H. An optical flow-based method to predict infantile cerebral palsy. IEEE Trans Neural Syst Rehabil Eng. 2012 Jul.20(4):605–614. doi: 10.1109/TNSRE.2012.2195030. [DOI] [PubMed] [Google Scholar]
- 49.Garcia JM, Gherpelli JLD, Leone CR. The role of spontaneous general movement assessment in the neurological outcome of cerebral lesions in preterm infants. J Pediatr (Rio J) 2004 Jun.80(4):296–304. [PubMed] [Google Scholar]
- 50.Kuban KC, Allred EN, O'Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. Journal of Child Neurology. 2009 Jan.24(1):63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laptook AR. Adverse Neurodevelopmental Outcomes Among Extremely Low Birth Weight Infants With a Normal Head Ultrasound: Prevalence and Antecedents. Pediatrics. 2005 Mar.115(3):673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 52.O'Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008 Sep.122(3):e662–9. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larroque B, Marret S, Ancel PY, Arnaud C, Marpeau L, Supernant K, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. The Journal of pediatrics. 2003 Oct.143(4):477–483. doi: 10.1067/S0022-3476(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 54.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet neurology. 2009 Jan.8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemming K, Colver A, Hutton J, Kuringzhuc J, Pharoah P. The Influence of Gestational Age on Severity of Impairment in Spastic Cerebral Palsy. The Journal of Pediatrics. 2008 Aug.153(2):203–208. e4. doi: 10.1016/j.jpeds.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 56.Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Developmental Medicine & Child Neurology. 2009 Apr.51(4):295–302. doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 57.Holmefur M, Krumlinde-Sundholm L, Bergstrom J, Eliasson AC. Longitudinal development of hand function in children with unilateral cerebral palsy. Developmental medicine and child neurology. 2010 Apr.52(4):352–357. doi: 10.1111/j.1469-8749.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 58.Mirmiran M, Barnes PD, Keller K, Constantinou JC, Fleisher BE, Hintz SR, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics. 2004 Oct.114(4):992–998. doi: 10.1542/peds.2003-0772-L. [DOI] [PubMed] [Google Scholar]
- 59.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006 Aug.355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi-Kurahashi N, Kidokoro H, Kubota T, Maruyama K, Kato Y, Kato T, et al. EEG for Predicting Early Neurodevelopment in Preterm Infants: An Observational Cohort Study. Pediatrics. 2012 Jan. doi: 10.1542/peds.2012-1115. [DOI] [PubMed] [Google Scholar]
- 61.Wikström S, Pupp IH, Rosén I, Norman E, Fellman V, Ley D, et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatrica. 2012 Jan.101(7):719–726. doi: 10.1111/j.1651-2227.2012.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broitman E, Ambalavanan N, Higgins RD, Vohr BR, Das A, Bhaskar B, et al. Clinical Data Predict Neurodevelopmental Outcome Better than Head Ultrasound in Extremely Low Birth Weight Infants. The Journal of Pediatrics. 2007 Nov.151(5):500–505. e2. doi: 10.1016/j.jpeds.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]