Abstract
We have previously demonstrated that a stable synthetic analog of 20-hydroxyeicosatetraenoic acid (20-HETE), N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE), prevents vascular hyporeactivity, hypotension, tachycardia, and inflammation in rats treated with lipopolysaccharide (LPS) and mortality in endotoxemic mice. These changes were attributed to decreased production of inducible nitric oxide (NO) synthase (iNOS)-derived NO, cyclooxygenase (COX)-2-derived vasodilator prostanoids, and proinflammatory mediators associated with increased cyctochrome P450 (CYP) 4A1-derived 20-HETE and CYP2C23-dependent antiinflammatory mediator formation. The aim of this study was to determine whether decreased expression and activity of iNOS, soluble guanylyl cyclase (sGC), protein kinase G (PKG), COX-2, gp91phox (NOX2; a superoxide generating NOX enzyme), and peroxynitrite production associated with increased expression of COX-1 and CYP4A1 and 20-HETE formation in renal and cardiovascular tissues of rats contributes to the effect of 5,14-HEDGE to prevent vasodilation, hypotension, tachycardia, and inflammation in response to systemic administration of LPS. Mean arterial pressure fell by 28 mmHg and heart rate rose by 47 beats/min in LPS (10 mg/kg, i.p.)-treated rats. Administration of LPS also increased mRNA and protein expression of iNOS and COX-2 associated with a decrease in COX-1 and CYP4A1 mRNA and protein expression. Increased NOS activity, iNOS-heat shock protein 90 complex formation (an index for iNOS activity), protein expression of phosphorylated vasodilator stimulated phosphoprotein (an index for PKG activity), gp91phox, p47phox (NOXO2; organizer subunit of gp91phox), and nitrotyrosine (an index for peroxynitrite production) as well as cGMP (an index for sGC activity), 6-keto-PGF1α (a stable metabolite PGI2) and PGE2 levels (indexes for COX activity), and nitrotyrosine levels by LPS were also associated with decreased CYP hydroxylase activity as measured by 20-HETE formation from arachidonic acid in renal microsomes of LPS-treated rats. These effects of LPS, except iNOS mRNA and COX-1 protein expression, were prevented by 5,14-HEDGE (30 mg/kg, s.c.; 1 h after LPS). A competitive antagonist of vasoconstrictor effects of 20-HETE, 20-hydroxyeicosa-6(Z), 15(Z)-dienoic acid (30 mg/kg, s.c.; 1 h after LPS) reversed the effects of 5,14-HEDGE, except iNOS and COX-1 mRNA and protein expression as well as expression of CYP4A1 mRNA. These results suggest that increased CYP4A1 expression and 20-HETE formation associated with suppression of iNOS/sGC/PKG pathway, COX-2, and gp91phox participate in the protective effect of 5,14-HEDGE against vasodilation, hypotension, tachycardia, and inflammation in the rat model of septic shock.
Keywords: Endotoxin, Hypotension, iNOS/sGC/PKG pathway, COX-2, CYP4A1, gp91phox/NOX2
Introduction
The expression of inducible nitric oxide (NO) synthase (iNOS) is enhanced in many tissues in response to mediators released by the lipid A part of lipopolysaccharide (LPS), also known as endotoxin, which is the most potent microbial mediator in the pathogenesis of septic shock [1]. This leads to increased generation of NO, which contributes to a fall in blood pressure, vascular hyporeactivity, multiple organ failure, and high mortality rate that are associated with septic shock [1]. In many models, endotoxin-induced vascular hyporeactivity to vasoconstrictors is associated with an enhanced formation of NO within the blood vessels, involving activation of not only iNOS, but also endothelial NOS (eNOS) [2]. NO is a potent activator of soluble guanylyl cyclase (sGC) and exerts many of its effects by activating sGC, which produces cyclic guanosine monophosphate (cGMP) [3]. NO plays an important role in cGMP-mediated smooth muscle relaxation by activating protein kinase G (PKG) leading to phosphorylation of vasodilator stimulated phosphoprotein (VASP) [4,5]. NO also reacts with superoxide generated by mainly gp91phox (also known as NOX2) in the presence of p47phox (also known as NOXO2; organizer subunit of gp91phox) to form peroxynitrite, a powerful oxidant and nitrating molecule, and subsequent reaction of peroxynitrite with proteins results in nitrotyrosine formation [6,7]. In vivo, peroxynitrite generation represents a NO-dependent pathogenic mechanism in conditions such as circulatory shock and chronic inflammatory diseases. In addition to NO, increased production of prostanoids by cyclooxygenase (COX)-2 has also been shown to contribute to systemic hypotension and related organ damage and decreased survival in animals and humans with sepsis [1]. Systemic blockade of iNOS or COX-2 opposes the fall in blood pressure in sepsis and septic shock [1]. This is not only due to withdrawal of the vasodilator effects of NO and prostanoids, but also associated with enhanced production of vasoconstrictor mediators including catecholamines, endothelin-1, and 20-hydroxyeicosatetraenoic acid (20-HETE) as well as activation of the renin-angiotensin system and increased sensitivity of baroreceptor reflex mechanisms [1].
20-HETE is an ω-hydroxylation product of arachidonic acid (AA) that is produced by cytochrome P450 (CYP) enzymes, mainly by the CYP4A and CYP4F isoforms in the kidney, heart, liver, brain, lung, and vasculature [1,8–10]. In the vasculature, 20-HETE causes vasoconstriction in several vascular beds, including renal, cerebral, aortic, mesenteric, and coronary arteries [11–15]. Activation of protein kinases, such as mitogen-activated protein kinase (MAPK), MAPK kinase (MEK), and extracellular signal-regulated kinase (ERK) which contribute to the regulation of vascular tone, have been shown to mediate the vasoconstrictor effect of 20-HETE [16–18]. As opposed to its vasoconstrictor effect, 20-HETE has also been reported to produce vasodilation in the vasculature including renal and coronary arteries [19–21]. These vasodilatory responses of 20-HETE have been attributed to NO release [22], conversion of 20-HETE to 20-OH-PGE2 and 20-OH-PGF2α by COX [13,19,23], and increased formation of PGE2 [23] and PGI2 [19–21,23]. In addition, 20-HETE has been shown to activate nuclear factor-κB (NF-κB) signaling and induce expression of cellular adhesion molecules and cytokines, thereby promoting inflammation [24,25]. CYP4A- and CYP4F-derived 20-HETE is also involved in LPS-induced acute systemic inflammation as a proinflammatory mediator [26,27]. In addition, it has been reported that NO inhibits renal CYP ω-hydroxylase activity and the production of 20-HETE [28,29]. Moreover, a NO-induced fall in the endogenous production of 20-HETE has also been found to contribute to the cGMP-independent vasodilator effects of NO in renal and cerebral microcirculations [28,30].
Because of the divergent effects of the NO and eicosanoids in the regulation of vascular tone and inflammation, changes in the functional balance between the production of these mediators might contribute to the pathogenesis and progression of inflammatory diseases, such as sepsis and septic shock. Our previous studies with the use of a stable synthetic analog of 20-HETE, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, 5,14-HEDGE, which mimics the effects of endogenously produced 20-HETE, and a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid, 20-HEDE, in cardiovascular and/or renal tissues of rats suggested that increased expression and/or activity of soluble epoxide hydrolase (sEH) and MEK1/ERK1/2/IκB kinase (IKK) β/inhibitor of κB (IκB)-α/NF-κB pathway as well as formation of NO and vasodilator prostanoids (i.e., PGI2 and PGE2) by iNOS and COX-2, respectively, peroxynitrite by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and proinflammatory cytokines (i.e., tumor necrosis factor [TNF]-α and interleukin [IL-8]) associated with decreased CYP2C23 expression and production of anti-inflammatory mediators (i.e., epoxyeicosatrienoic acids; EETs) contributes to hypotension, tachycardia, inflammation, and mortality in a rodent model of septic shock [31–35]. Therefore, the present study was conducted to determine whether decreased expression and activity of iNOS, sGC, PKG, COX-2, gp91phox, and p47phox, and peroxynitrite production associated with increased expression of COX-1 and CYP4A1 and formation of 20-HETE in renal and cardiovascular tissues of rats contributes to the effect of 5,14-HEDGE to prevent vasodilation, hypotension, tachycardia, and inflammation in LPS-treated rats. The results of this study have been presented in abstract form [33,34].
Materials and methods
Endotoxic shock model
Experiments were performed on Wistar rats (male; 250–330 g; n = 72) (Research Center of Experimental Animals, Mersin University, Mersin, Turkey) fed a standard chow. They were synchronized by maintenance of controlled environmental conditions throughout the experiments. The circadian rhythmicity of the animals was entrained by a standardized 12 h light and 12 h dark cycle. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of Mersin University School of Medicine. Endotoxic shock was induced in rats and mice as previously described by Tunctan et al. [36,37]. Rats were randomly divided into saline (n = 12), LPS (n = 12), 5,14-HEDGE (n = 12), LPS + 5,14-HEDGE (n = 12), 20-HEDE (n = 12), and LPS + 5,14-HEDGE + 20-HEDE (n = 12) groups [32]. In the saline, 5,14-HEDGE, and 20-HEDE groups, animals received saline (4 ml/kg, i.p.) at time 0. Animals in the LPS, LPS + 5, 14-HEDGE, and LPS + 5,14-HEDGE + 20-HEDE groups were treated with LPS (Escherichia coli LPS, O111:B4; Sigma Chemical Co., St. Louis, MO, USA) (10 mg/kg, i.p.; sublethal dose) at time 0. In the 5,14-HEDGE, LPS + 5,14-HEDGE, and LPS + 5,14-HEDGE + 20-HEDE groups, animals were treated with a stable synthetic analog of 20-HETE, 5,14-HEDGE (30 mg/kg, s.c.) [32] and/or a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-HEDE (30 mg/kg, s.c.) [32]1 h after injection of saline or LPS, respectively. 5,14-HEDGE and 20-HEDE were synthesized in the Department of Biochemistry University of Texas Southwestern Medical Center, Dallas, Texas, US. Mean arterial pressure (MAP) and heart rate (HR) of the rats were measured using a tail-cuff device (MAY 9610 Indirect Blood Pressure Recorder System, Commat Ltd., Ankara, Turkey) during a control period at time 0 and 1, 2, 3, and 4 h. All rats survived in the experiments. Rats were euthanized 4 h after the administration of saline or LPS, and blood sample, kidney, heart, thoracic aorta, and superior mesenteric artery were collected from all animals. Detailed method about preparation of serum and tissue samples is reported in the Supplementary material.
Messenger ribonucleic acid (mRNA) isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Complementary deoxyribonucleic acids (cDNAs) for iNOS, COX-1, COX-2, CYP4A1, β-actin, α-skeletal actin, and α-smooth muscle actin were synthesized followed by mRNA isolation from the frozen tissue powders as given in detail in the Supplementary material.
Immunoblotting
Immunoblotting for iNOS, heat shock protein (hsp90), phosphorylated VASP (p-VASP), COX-1, COX-2, CYP4A1, gp91phox, p47phox, nitrotyrosine, β-actin, α-sarcomeric actin, and α-smooth muscle actin proteins were performed according to the method reported in the Supplementary material.
Measurement of NOS, COX, sGC, and CYP hydroxylase activities and nitrotyrosine levels
NOS, COX, and sGC activities and nitrotyrosine levels in the sera and tissue samples were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions in Ultrasensitive Colorimetric Assay for Nitric Oxide Synthase (Oxford Biomedical Research Inc., Oxford, MI, USA), Cyclic GMP Enzyme Immunoassay Kit (Cayman Chemical, Ann Arbor, MI, USA), 6-keto-PGF1α ELISA Kit (Cayman Chemical Co., Ann Arbor, MI, USA), PGE2 ELISA Kit (Cayman Chemical Co., Ann Arbor, MI, USA), and nitrotyrosine ELISA Kit (Northwest Life Science Specialities, LSS, Vancouver, WA, USA), respectively. As an index for CYP hydroxylase activity, the capacity of renal microsomes to produce 20-HETE from (14C)-AA was determined as described in the Supplementary material.
Statistical analysis
Data are expressed as means ± S.E.M. Data were analyzed by one-way ANOVA followed by Student–Newman–Keuls test for multiple comparisons, Kruskal–Wallis test followed by Dunns test for multiple comparisons and Student’s t or Mann–Whitney U tests when appropriate. A P value <0.05 was considered to be statistically significant.
Results
Effect of 5,14-HEDGE on the cardiovascular response to LPS
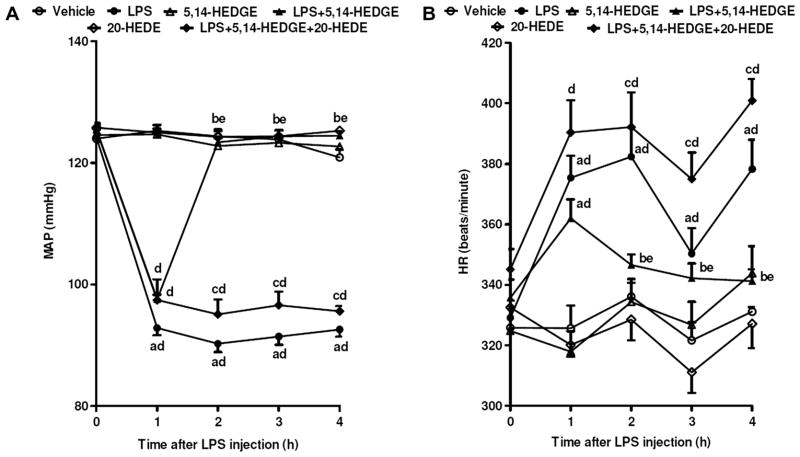
LPS caused a gradual fall in MAP (Fig. 1A) and an increase in HR (Fig. 1B) over the 4 h course of the experiment (p < 0.05). The change in MAP and HR reached a maximum 4 h after the administration of LPS. MAP fell by 28 mmHg (Fig. 1A) and HR rose by 47 bpm (Fig. 1B) in rats treated with LPS. A synthetic analog of 20-HETE, 5,14-HEDGE, which mimics the effects of endogenously produced 20-HETE, prevented the fall in MAP (Fig. 1A) and the increase in HR (Fig. 1B) in rats given LPS (p < 0.05). A competitive antagonist of the vasoconstrictor effects of 20-HETE, 20-HEDE, reversed the ability of 5,14-HEDGE to oppose the effects of endotoxin on MAP (Fig. 1A) and HR (Fig. 1B) (p < 0.05). 5,14-HEDGE or 20-HEDE had no effect on MAP (Fig. 1A) or HR (Fig. 1B) in rats treated with vehicle (p > 0.05).
Fig. 1.
Time course of the effects of 5,14-HEDGE and 20-HEDE on (A) MAP and (B) HR following administration of saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. Data are expressed as means ± S.E.M. of 10 animals. aSignificant difference from the corresponding value seen in rats treated with saline (vehicle) (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05). dSignificant difference from the time 0 h value within a group (p < 0.05). eSignificant difference from the time 1 h value within a group (p < 0.05).
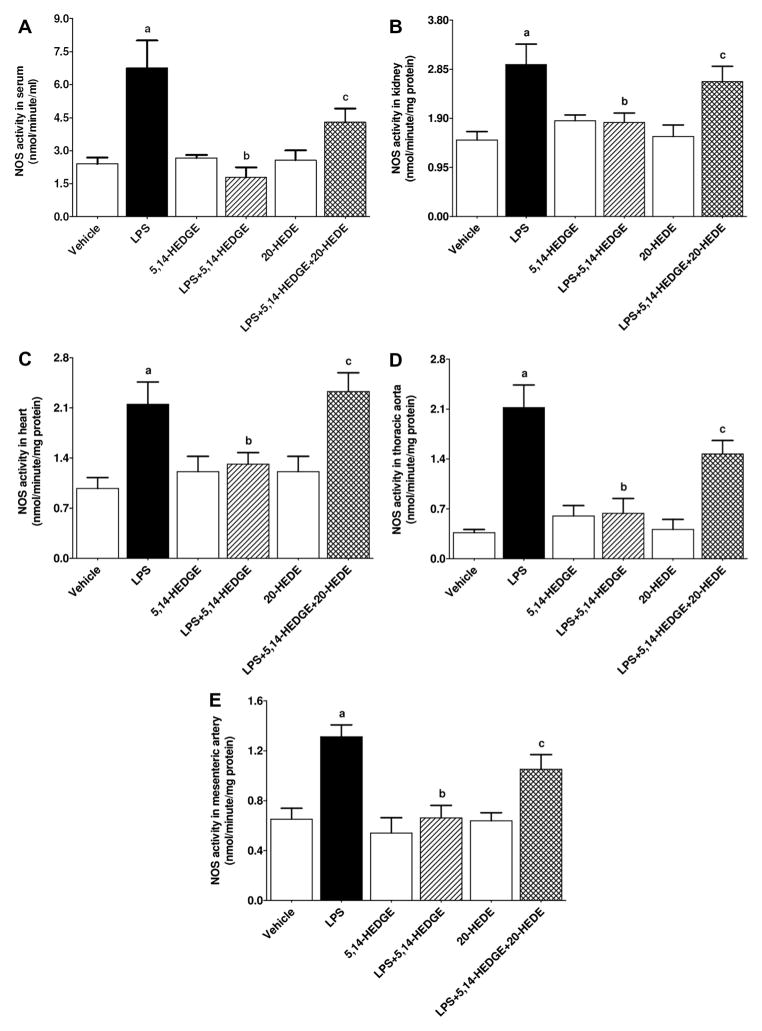
Effect of 5,14-HEDGE on LPS-induced increase in iNOS expression, iNOS-hsp90 complex formation, and NOS activity
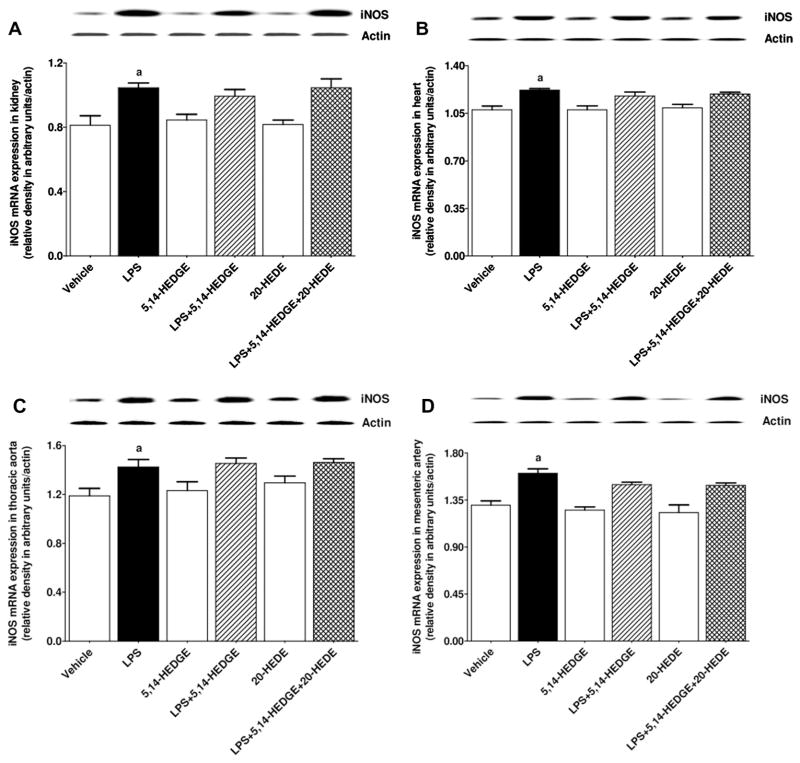
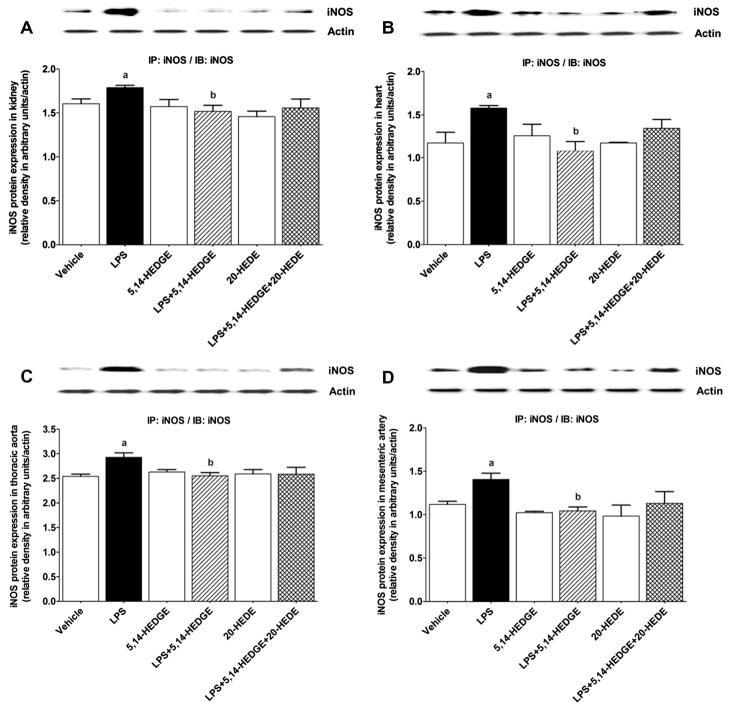
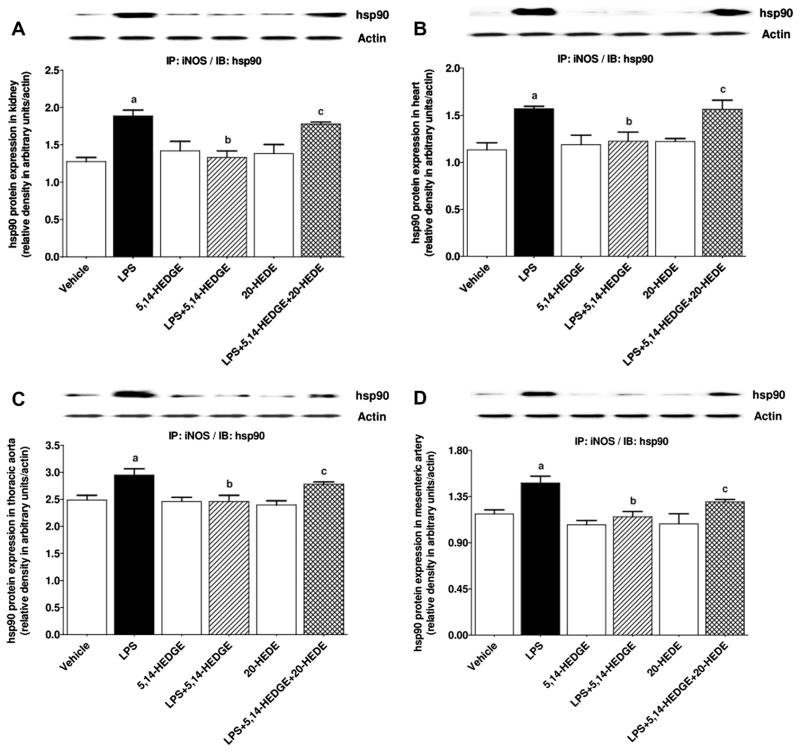
To determine the effect of 5,14-HEDGE on LPS-induced changes in iNOS expression and activity, expression of iNOS mRNA and protein as well as iNOS-hsp90 complex formation and NOS activity (as indexes for iNOS activity) was measured in the serum, kidney, heart, thoracic aorta, and/or superior mesenteric artery of endotoxemic rats. LPS increased expression of iNOS mRNA (Fig. 2) and protein (Fig. 3) as well as iNOS-hsp90 complex formation (Fig. 4) in the kidney, heart, thoracic aorta, and superior mesenteric artery, and NOS activity in the sera and renal and cardiovascular tissues (Fig. 5) (p < 0.05). 5,14-HEDGE prevented the increase in iNOS protein (Fig. 3), but not mRNA (Fig. 2), expression, iNOS-hsp90 complex formation (Fig. 4), and NOS activity (Fig. 5) in the sera and/or tissues of rats caused by LPS (p < 0.05). 20-HEDE reversed the effect of 5,14-HEDGE on iNOS-hsp90 complex formation (Fig. 4) and NOS activity (Fig. 5), but not mRNA (Fig. 2) and protein (Fig. 3) expression, in LPS-treated rats (p < 0.05). 5,14-HEDGE or 20-HEDE had no effect on the basal iNOS expression (Fig. 3), iNOS-hsp90 complex formation (Fig. 4), and NOS activity (Fig. 5) in vehicle-treated rats (p > 0.05).
Fig. 2.
Effects of 5,14-HEDGE and 20-HEDE on changes in iNOS mRNA expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. iNOS mRNA expression in tissue homogenates was measured by RT-PCR. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05).
Fig. 3.
Effects of 5,14-HEDGE and 20-HEDE on changes in iNOS protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. Immunoprecipitation was performed with anti-iNOS antibody and immunoprecipitated fractions were incubated with anti-iNOS antibody in immunoblotting studies. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05).
Fig. 4.
Effects of 5,14-HEDGE and 20-HEDE on changes in iNOS-hsp90 complex formation in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. Immunoprecipitation was performed with anti-iNOS antibody and immunoprecipitated fractions were incubated with anti-hsp90 antibody in immunoblotting studies. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Fig. 5.
Effects of 5,14-HEDGE and 20-HEDE on changes in NOS activity in (A) serum, (B) kidney, (C) heart, (D) thoracic aorta, (E) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. NOS activity in sera and tissue homogenates was measured by the rate of L-arginine to nitrite/nitrate conversion using the Ultrasensitive Colorimetric Assay for Nitric Oxide Synthase ELISA Kit following the manufacturer’s instructions. Data are expressed as means ± S.E.M of 4–6 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
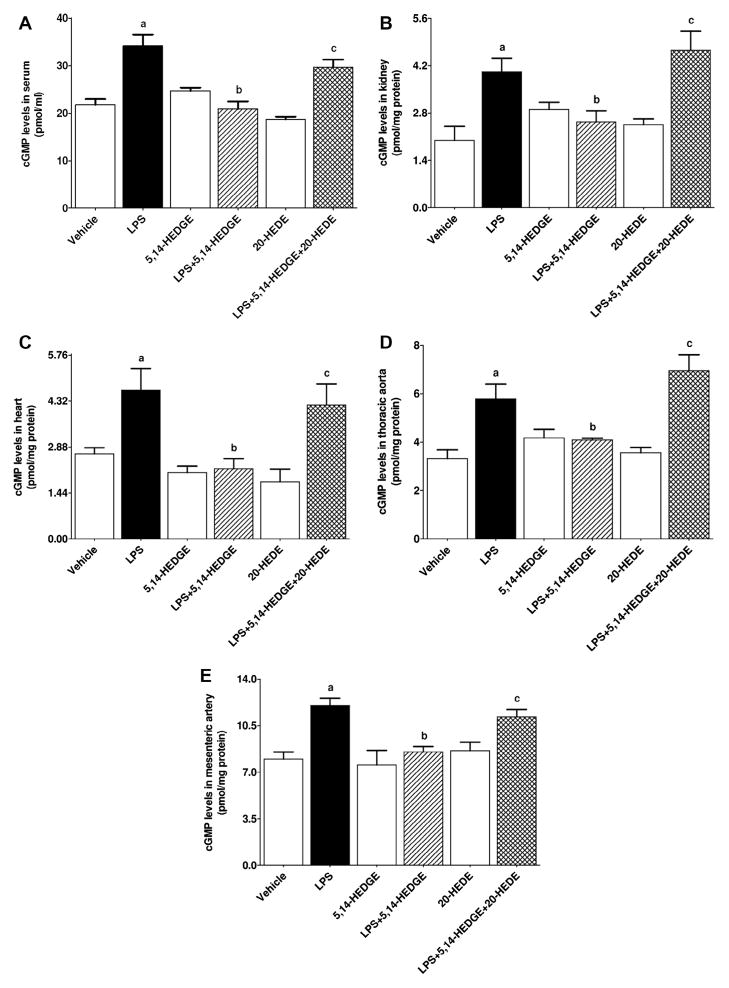
Effect of 5,14-HEDGE on LPS-induced increase in sGC activity
To determine the effect of 5,14-HEDGE on LPS-induced vascular hyporeactivity, cGMP levels (as an index for sGC activity) were measured in the serum, kidney, heart, thoracic aorta, and superior mesenteric artery of endotoxemic rats. LPS increased cGMP levels in the serum (Fig. 6A), kidney (Fig. 6B), heart (Fig. 6C), thoracic aorta (Fig. 6D), and superior mesenteric artery (Fig. 6E) (p < 0.05). The increase in cGMP levels in the sera (Fig. 6A) and tissues (Fig. 6B–E) of rats caused by LPS was prevented by treatment with 5,14-HEDGE (p < 0.05). 20-HEDE reversed the effect of 5,14-HEDGE on cGMP levels in LPS-treated rats (p < 0.05) (Fig. 6). 5,14-HEDGE or 20-HEDE had no effect on the basal cGMP levels in vehicle-treated rats (p > 0.05) (Fig. 6).
Fig. 6.
Effects of 5,14-HEDGE and 20-HEDE on changes in cGMP levels in (A) serum, (B) kidney, (C) heart, (D) thoracic aorta, and (E) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. cGMP levels in sera and tissue homogenates were measured by the Cyclic GMP EIA Kit following the manufacturer’s instructions. Data are expressed as means ± S.E.M of 4–6 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
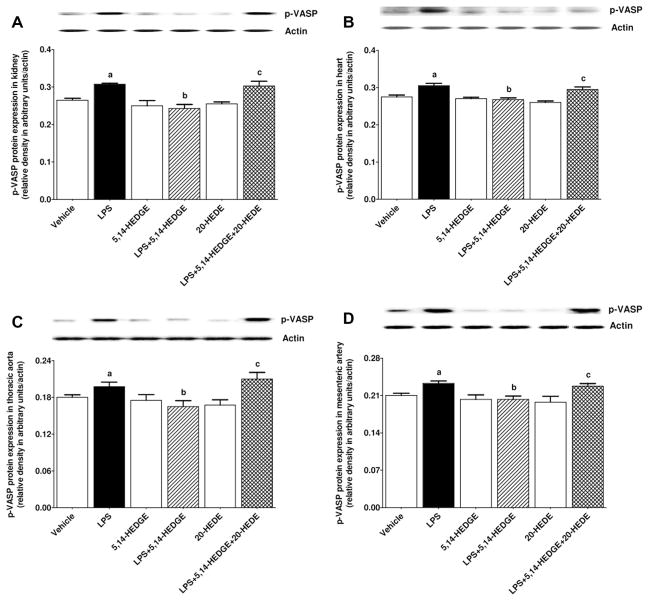
Effect of 5,14-HEDGE on LPS-induced increase in PKG activity
To determine the effect of 5,14-HEDGE on LPS-induced vascular hyporeactivity, p-VASP protein expression (as an index for PKG activity) was measured in the kidney, heart, thoracic aorta, and superior mesenteric artery of endotoxemic rats. LPS increased p-VASP protein expression in the kidney (Fig. 7A), heart (Fig. 7B), thoracic aorta (Fig. 7C), and superior mesenteric artery (Fig. 7D) (p < 0.05); this increase was prevented by 5,14-HEDGE (p < 0.05) (Fig. 7). 20-HEDE reversed the effect of 5,14-HEDGE on p-VASP protein expression in LPS-treated rats (p < 0.05) (Fig. 7). 5,14-HEDGE or 20-HEDE had no effect on the basal p-VASP protein expression in vehicle-treated rats (p > 0.05) (Fig. 7).
Fig. 7.
Effects of 5,14-HEDGE and 20-HEDE on changes in p-VASP (Ser239) protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. p-VASP (Ser239) protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
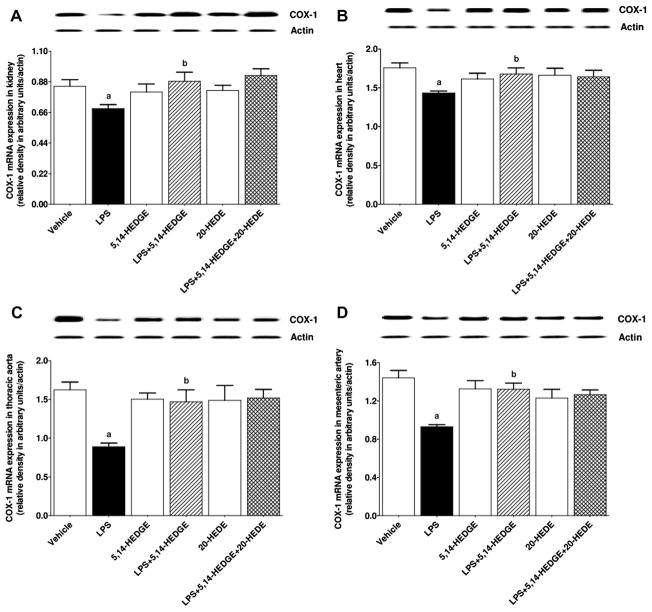
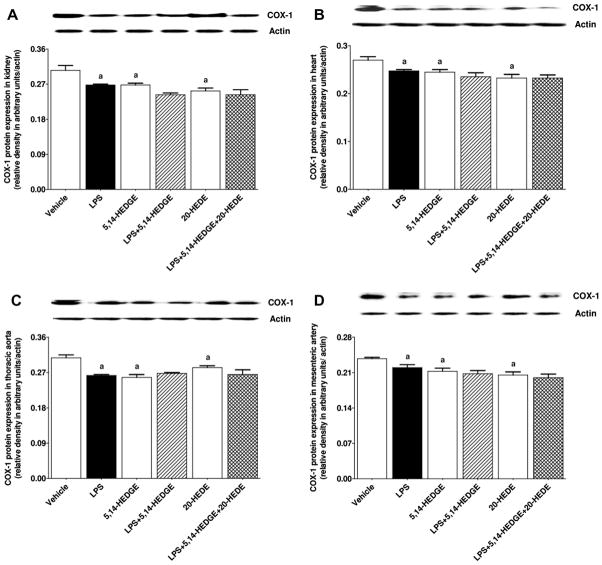
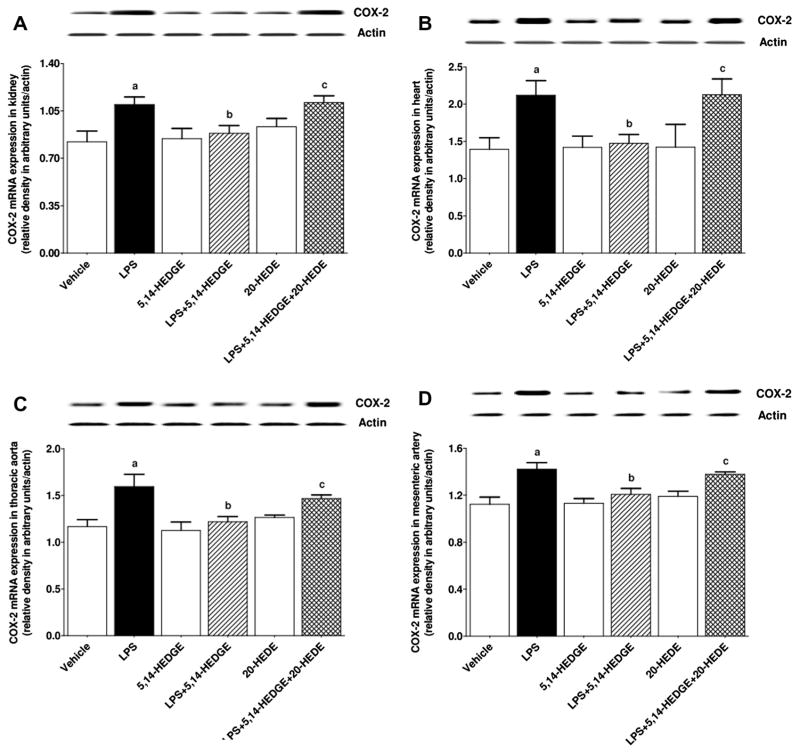
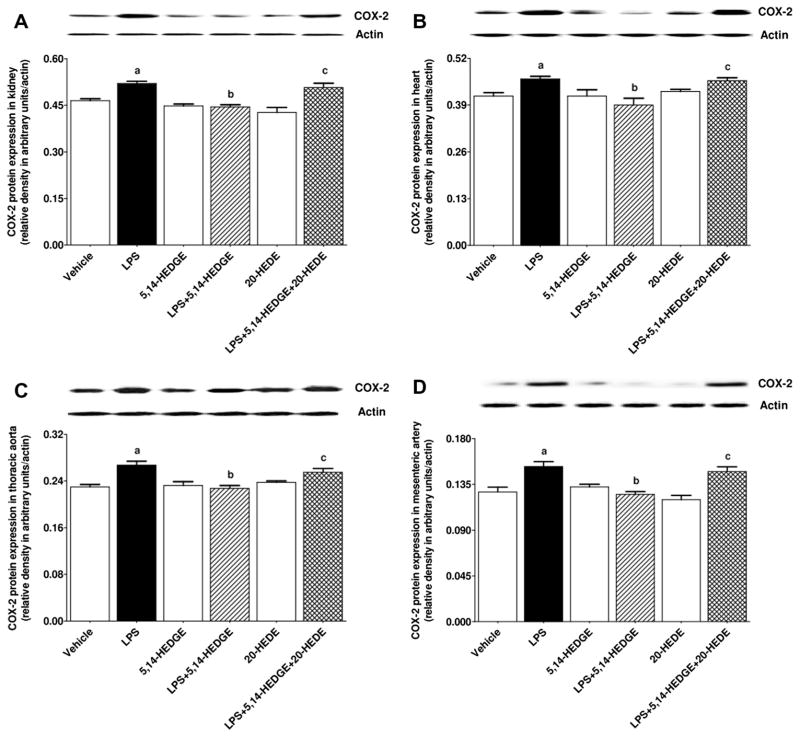
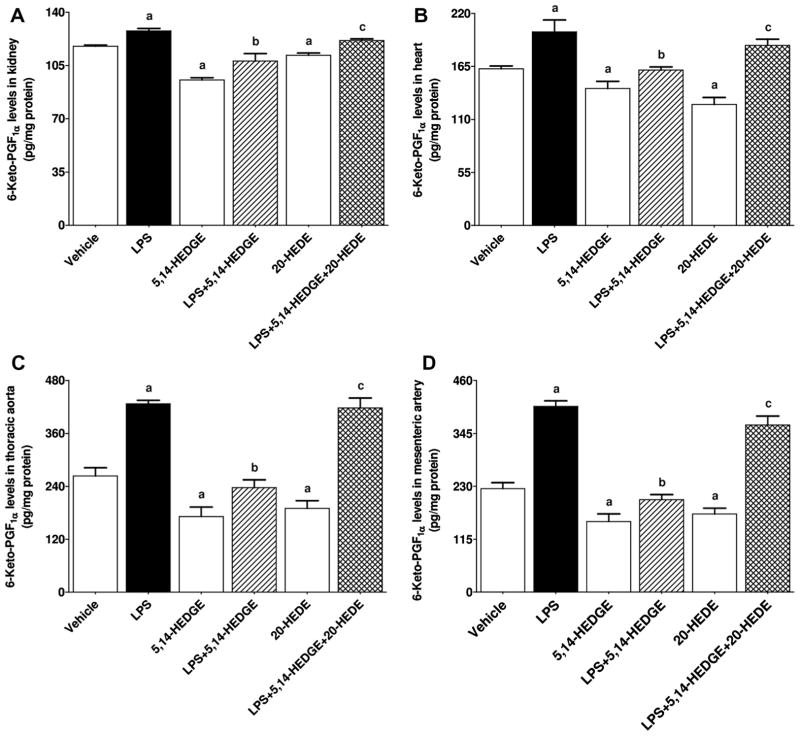
Effect of 5,14-HEDGE on LPS-induced changes in COX-1 and COX-2 expression, and COX activity
To determine the effect of 5,14-HEDGE on LPS-induced changes in COX-1 and COX-2 expression and COX activity, expression of COX-1 and COX-2 mRNA as well as 6-keto-PGF1α and PGE2 levels (as indexes for COX activity) was measured in the serum, kidney, heart, thoracic aorta, and/or superior mesenteric artery of endotoxemic rats. LPS decreased expression of COX-1 mRNA (Fig. 8) and protein (Fig. 9) in the kidney, heart, thoracic aorta, and superior mesenteric artery while expression of COX-2 mRNA (Fig. 10) and protein (Fig. 11) was increased in the renal and cardiovascular tissues (p < 0.05). The LPS-induced increase in COX-2 expression (Figs. 10 and 11) was associated with an increase in 6-keto-PGF1α (Fig. 12) and PGE2 (Fig. 13) levels in the kidney, heart, thoracic aorta, and superior mesenteric artey (p < 0.05). The decrease in COX-1 mRNA (Fig. 8), but not protein (Fig. 9), expression, the increase in COX-2 expression (Fig. 10, Fig. 11) and prostanoid levels (Figs. 12 and 13) in the tissues of rats produced by LPS was prevented by 5,14-HEDGE (p < 0.05). 20-HEDE reversed the effect of 5,14-HEDGE on COX-2 expression (Figs. 10 and 11) and prostanoid levels (Figs. 12 and 13), but not COX-1 expression (Figs. 8 and 9) in LPS-treated rats (p < 0.05). 5,14-HEDGE or 20-HEDE had no effect on the basal COX-1 mRNA (Fig. 8) and COX-2 expression (Figs. 10 and 11) (p > 0.05), however, COX-1 protein expression (Fig. 9) and prostanoid levels (Figs. 12 and 13) were decreased in the tissues of 5,14-HEDGE-or 20-HEDE-treated control rats (p < 0.05).
Fig. 8.
Effects of 5,14-HEDGE and 20-HEDE on changes in COX-1 mRNA expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. COX-1 mRNA expression in tissue homogenates was measured by RT-PCR. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05).
Fig. 9.
Effects of 5,14-HEDGE and 20-HEDE on changes in COX-1 protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. COX-1 protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05).
Fig. 10.
Effects of 5,14-HEDGE and 20-HEDE on changes in COX-2 mRNA expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. COX-2 mRNA expression in tissue homogenates was measured by RT-PCR. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Fig. 11.
Effects of 5,14-HEDGE and 20-HEDE on changes in COX-2 protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. COX-2 protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Fig. 12.
Effects of 5,14-HEDGE and 20-HEDE on changes in 6-keto-PGF1α levels in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. 6-keto-PGF1α levels in tissue homogenates were measured by the 6-Keto-PGF1α ELISA Kit following the manufacturer’s instructions. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
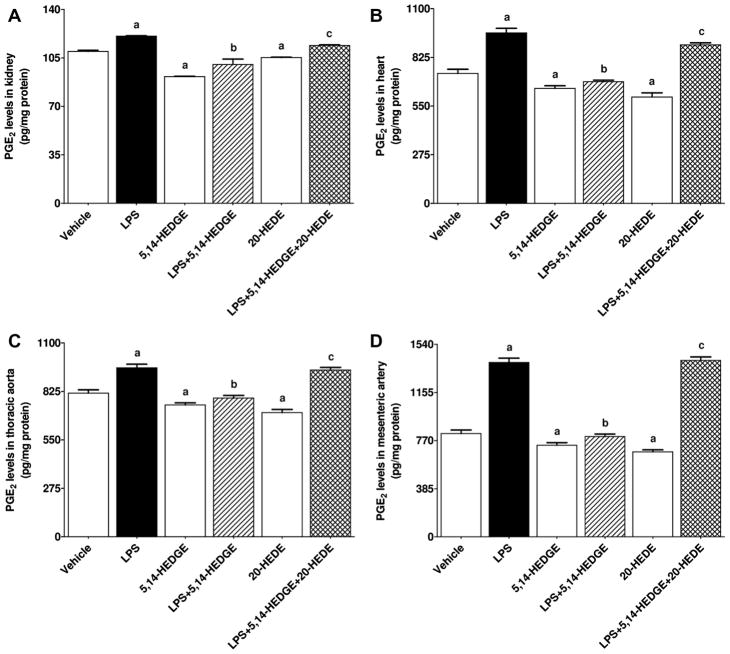
Fig. 13.
Effects of 5,14-HEDGE and 20-HEDE on changes in PGE2 levels in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. PGE2 levels in tissue homogenates were measured by the PGE2 ELISA Kit following the manufacturer’s instructions. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Effect of 5,14-HEDGE on LPS-induced decrease in CYP4A1 expression and CYP hydroxylase activity
To determine the effect of 5,14-HEDGE on LPS-induced decrease in CYP4A1 expression and CYP hydroxylase activity during endotoxemia, expression of CYP4A1 mRNA and protein as well as the capacity of renal microsomes to produce 20-HETE from (14C)AA were measured. LPS decreased expression of CYP4A1 mRNA (Fig. 14) and protein (Fig. 15) as well as 20-HETE formation in renal microsomes (p < 0.05) (Fig. 16); these effects of LPS were prevented by 5,14-HEDGE (p < 0.05) (Figs. 14–16). 20-HEDE reversed the effect of 5,14-HEDGE on CYP4A1 protein (Fig. 15), but not mRNA expression (Fig. 14), and 20-HETE formation in LPS-treated rats (p < 0.05). 5,14-HEDGE or 20-HEDE had no effect on the basal CYP4A1 expression (Figs. 14 and 15) and 20-HETE formation (Fig. 16) in vehicle-treated rats (p > 0.05).
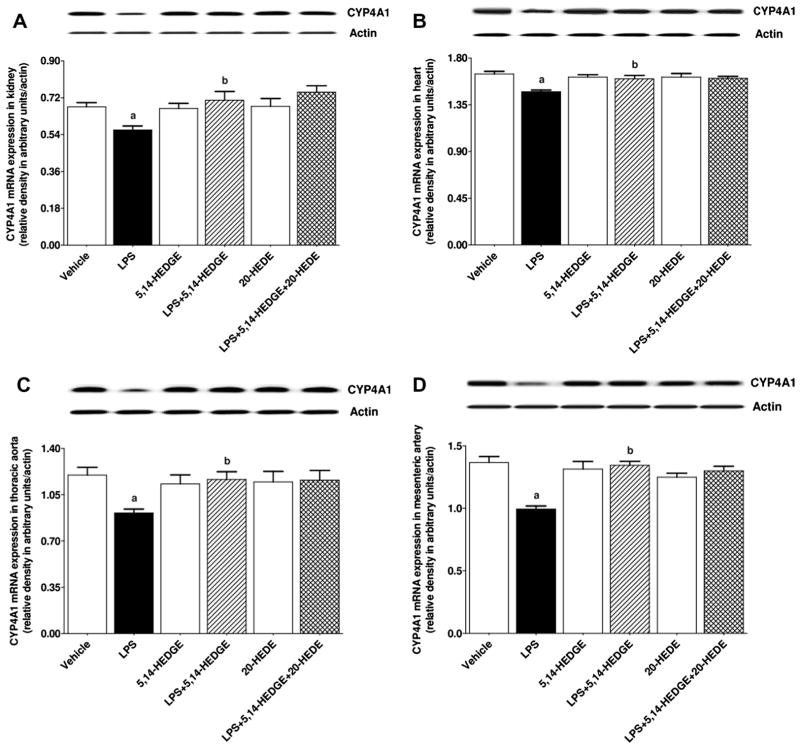
Fig. 14.
Effects of 5,14-HEDGE and 20-HEDE on changes in CYP4A1 mRNA expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. CYP4A1 mRNA expression in tissue homogenates was measured by RT-PCR. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05).
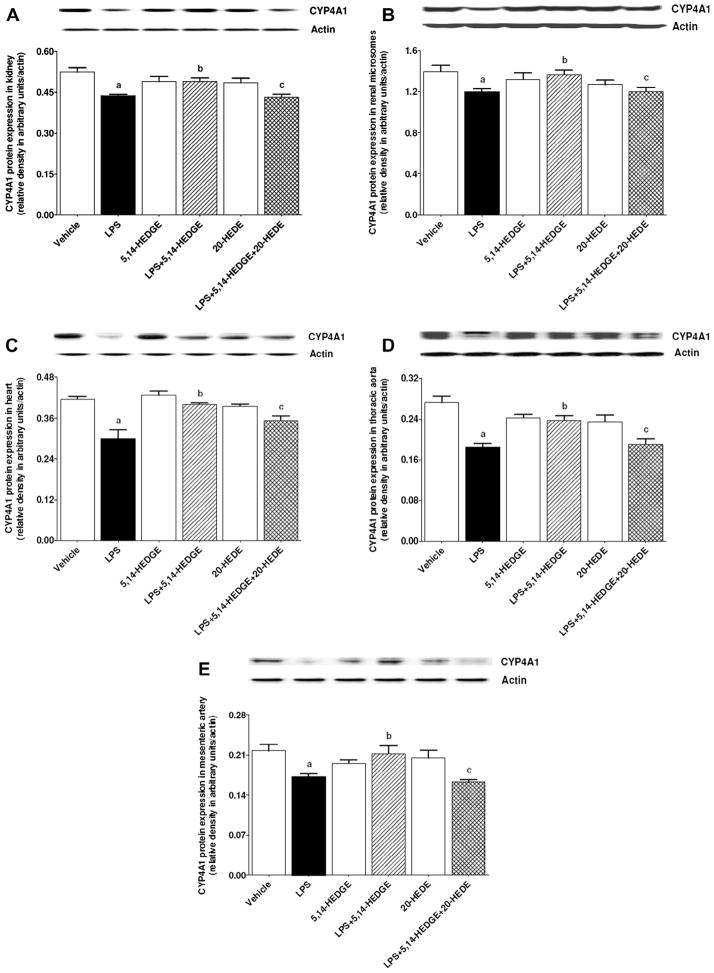
Fig. 15.
Effects of 5,14-HEDGE and 20-HEDE on changes in CYP4A1 protein expression in (A) kidney, (B) renal microsomes, (C) heart, (D) thoracic aorta, and (E) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. CYP4A1 protein levels in tissue homogenates and renal microsomes were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
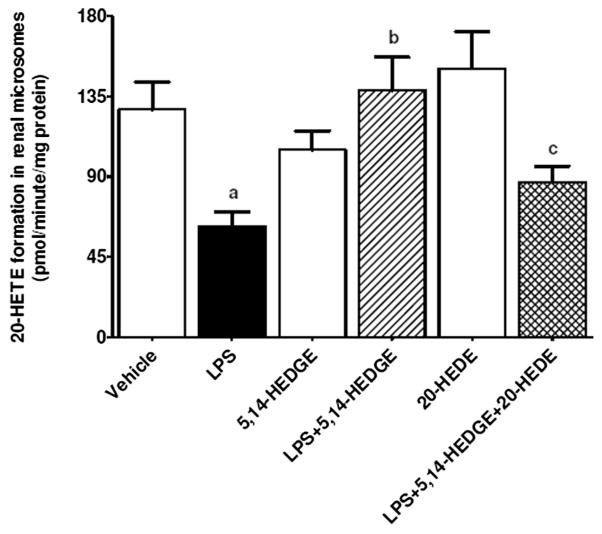
Fig. 16.
Effects of 5,14-HEDGE and 20-HEDE on changes in 20-HETE formation in renal microsomes measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. The capacity of renal microsomes to produce 20-HETE from radiolabeled AA was determined as described in the Supplementary material. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
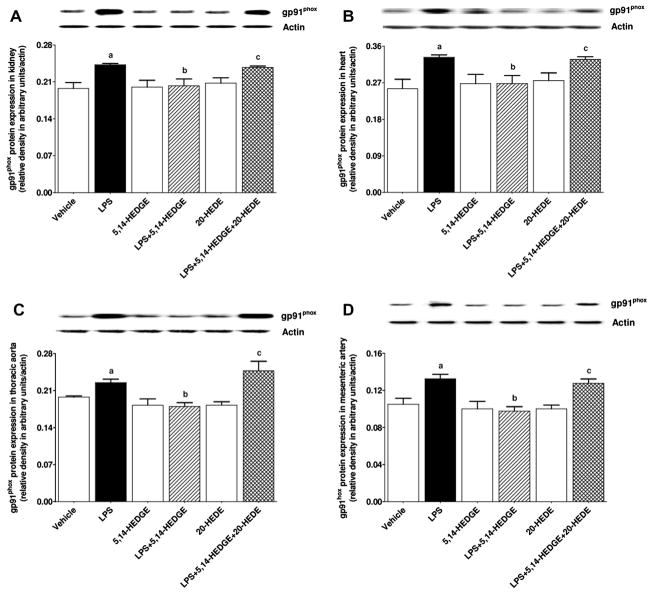
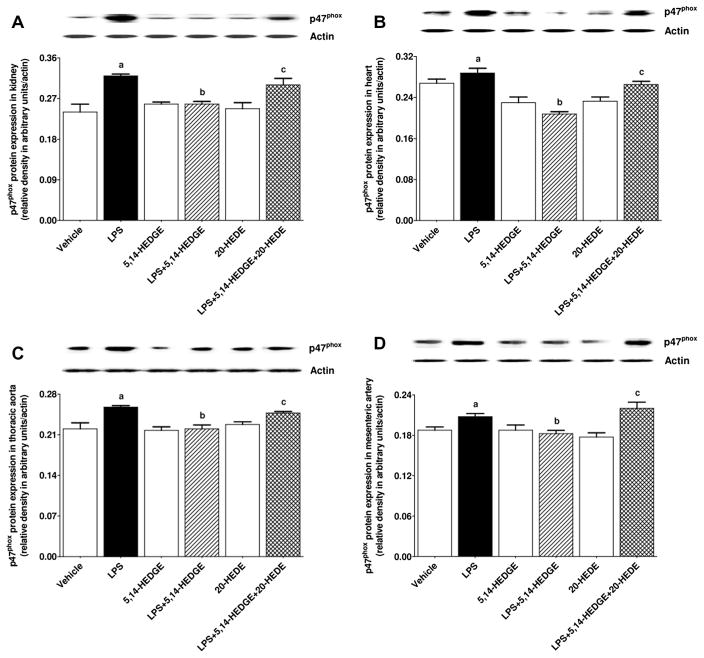
Effect of 5,14-HEDGE on LPS-induced increase in gp91phox protein expression
To determine the effect of 5,14-HEDGE on LPS-induced increase in gp91phox (NOX2; a superoxide producing NOX enzyme), protein expression of gp91phox and p47phox (organizer subunit of gp91phox) was measured in the kidney, heart, thoracic aorta, and superior mesenteric artery of endotoxemic rats. LPS increased expression of gp91phox (Fig. 17) and p47phox (Fig. 18) in the kidney, heart, thoracic aorta, and superior mesenteric artery (p < 0.05); these increases were prevented by treatment with 5,14-HEDGE (p < 0.05) (Figs. 17 and 18). 20-HEDE reversed the effect of 5,14-HEDGE on expression of gp91phox (Fig. 17) and p47phox (Fig. 18) in LPS-treated rats (p < 0.05). 5,14-HEDGE or 20-HEDE had no effect on the basal expression of gp91phox and p47phox in vehicle-treated rats (p > 0.05) (Figs. 17 and 18).
Fig. 17.
Effects of 5,14-HEDGE and 20-HEDE on changes in gp91phox protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. gp91phox protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Fig. 18.
Effects of 5,14-HEDGE and 20-HEDE on changes in p47phox protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. p47phox protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
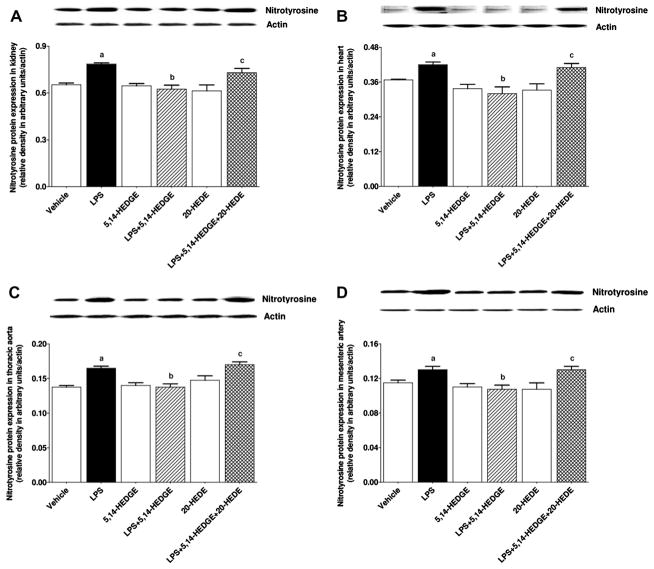
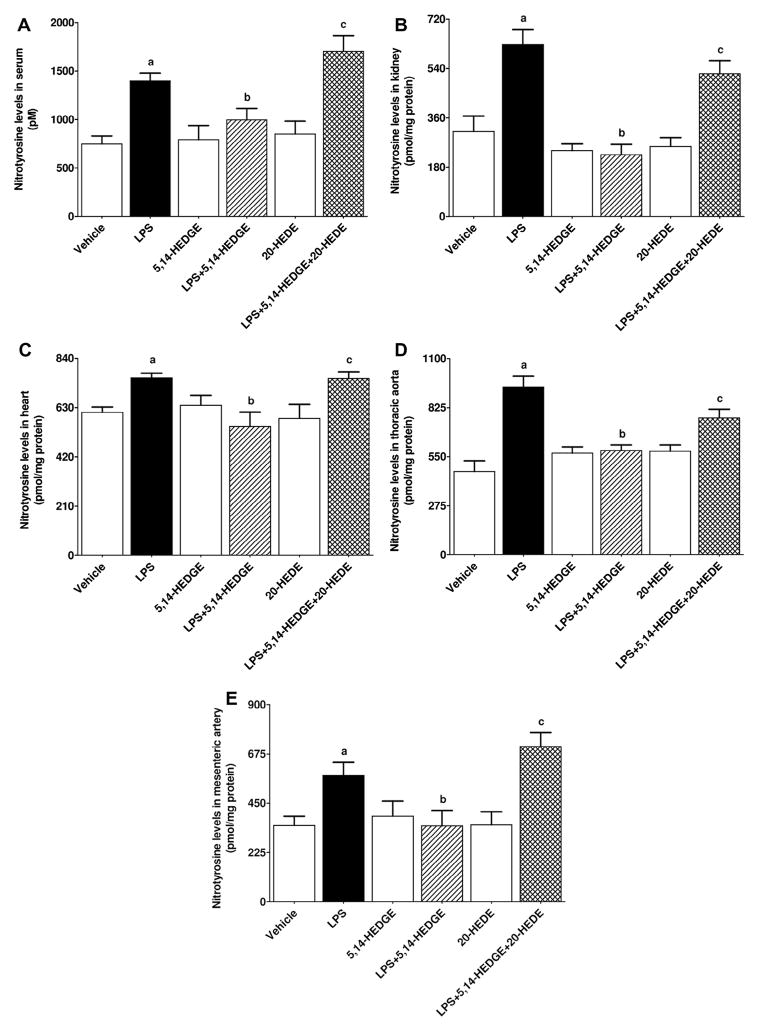
Effect of 5,14-HEDGE on LPS-induced increase in peroxynitrite formation
To determine the effect of 5,14-HEDGE on LPS-induced increase in peroxynitrite formation, nitrotyrosine (a stable end-product of peroxynitrite) levels were measured in the serum, kidney, heart, thoracic aorta, and superior mesenteric artery of endotoxemic rats. LPS increased nitrotyrosine protein expression in the kidney (Fig. 19A), heart (Fig. 19B), thoracic aorta (Fig. 19C), and superior mesenteric artery (Fig. 19D) (p < 0.05). LPS-induced increase in nitrotyrosine expression was also associated with enhanced nitrotyrosine levels in the serum (Fig. 20A), kidney (Fig. 20B), heart (Fig. 20C), thoracic aorta (Fig. 20D), and superior mesenteric artery (Fig. 20E) (p < 0.05). The increase in nitrotyrosine levels in the sera and tissues of rats caused by LPS was prevented by 5,14-HEDGE (p < 0.05) (Figs. 19 and 20). 20-HEDE reversed the effect of 5,14-HEDGE on nitrotyrosine levels in LPS-treated rats (p < 0.05) (Figs. 19 and 20). 5,14-HEDGE or 20-HEDE had no effect on the basal nitrotyrosine levels in vehicle-treated rats (p > 0.05) (Figs. 19 and 20).
Fig. 19.
Effects of 5,14-HEDGE and 20-HEDE on changes in nitrotyrosine protein expression in (A) kidney, (B) heart, (C) thoracic aorta, and (D) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. Nitrotyrosine protein levels in tissue homogenates were measured by immunoblotting. Data are expressed as means ± S.E.M of 4 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Fig. 20.
Effects of 5,14-HEDGE and 20-HEDE on changes in nitrotyrosine levels in (A) serum, (B) kidney, (C) heart, (D) thoracic aorta, and (E) superior mesenteric artery measured 4 h after saline (vehicle) (4 ml/kg, i.p.) or LPS (10 mg/kg, i.p.) injection to conscious rats. 5,14-HEDGE (30 mg/kg, s.c.) and/or 20-HEDE (30 mg/kg, s.c.) were given 1 h after administration of saline or LPS. Nitrotyrosine levels in sera and tissue homogenates were measured by the Nitrotyrosine ELISA Kit following the manufacturer’s instructions. Data are expressed as means ± S.E.M of 4–8 animals. aSignificant difference from the corresponding value seen in rats treated with saline (p < 0.05). bSignificant difference from the corresponding value seen in the rats treated with LPS (p < 0.05). cSignificant difference from the corresponding value seen in the rats treated with LPS and 5,14-HEDGE (p < 0.05).
Discussion
The results of the present study indicate that increased expression and/or activity of iNOS/sGC/PKG pathway, COX-2, gp91phox, and peroxynitrite production associated with decreased CYP4A1 expression and 20-HETE formation participate in the decrease in vascular reactivity, fall in blood pressure, tachycardia, and inflammation in rats treated with LPS. These data also demonstrate that 5,14-HEDGE, a 20-HETE mimetic, prevents vascular hiporeactivity, hypotension, tachycardia, and inflammation which may be due to increased CYP4A1 expression and 20-HETE formation associated with decreased expression and/or activity of the iNOS/sGC/PKG pathway, COX-2, and gp91phox leading to vasodilatory and inflammatory mediator production in the rat model of septic shock.
There are several reports suggesting a direct link between AA metabolites and reactive nitrogen species (e.g., NO and peroxynitrite) under physiological and pathophysiological conditions [1,6,8–10]. The constitutive isoforms of COX and NOS enzymes play an important role in the regulation of several physiological states. On the other hand, under inflammatory conditions such as endotoxic shock, the inducible isoforms of these enzymes as well as the NOX family of reactive oxygen species-generating NADPH oxidases are expressed in a variety of cells and tissues resulting in the production of large amounts of prostanoids and NO. In the present study, we measured changes in the expression and/or activity of iNOS, sGC, PKG, COX-1, COX-2, CYP4A1, and gp91phox (NOX2) and p47phox, organizer subunit of gp91phox, as well as peroxynitrite formation at 4 h after LPS administration. LPS produced a fall in MAP and an increase in HR within 1 h that was sustained for 4 h. Administration of 5,14-HEDGE prevented the LPS-induced fall in blood pressure and increase in HR within 1 h. The effects of LPS on the expression and/or activity of iNOS, sGC, PKG, COX-1, COX-2, CYP4A1, gp91phox, and p47phox as well as peroxynitrite formation, except iNOS mRNA and COX-1 protein expression, were also prevented by 5,14-HEDGE given 1 h after LPS. One might argue that vasodilator mediators (e.g., NO and prostanoids) and decreased levels of 20-HETE generated from an increased expression of iNOS and COX-2 following activation of the MEK1/ERK1/2/IKKβ/IκB-α/NF-κB pathway, and decreased expression of CYP4A1, respectively, contribute to the sustained decrease in blood pressure. It is well established that iNOS and COX-2 expression are induced 1–2 h after LPS administration, and eNOS and COX-1 expression are downregulated [38–40]. It is also known that increased NO production by eNOS is responsible for the fall in blood pressure within 1 h after LPS administration while iNOS-derived NO is responsible for the delayed hypotension and vascular hyporeactivity [40,41]. Compensatory mechanisms, including activation of the renin-angiotensin-aldosteron system, increased sensitivity of baroreceptor reflex mechanisms, and increased production of endothelin-1 and catecholamines, which are known to activate phospholipase A2 and cause release of AA from tissue lipids, and results in prostaglandin synthesis, and also stimulate production of reactive nitrogen (e.g., NO and peroxynitrite) and oxygen (e.g., superoxide) species, have also been reported to be responsible for the changes in the formation of vasoregulatory molecules that could contribute to the fall in blood pressure during the early phase of LPS-induced endotoxemia [38–46]. We have previously demonstrated that selective inhibition of iNOS with phenylene-1,3-bis(ethane-2-isothiourea) dihydrobromide (1,3-PBIT) prevents the fall in MAP, increase in HR, and vascular reactivity to norepinephrine as well as increased sGC and PKG activity associated with systemic and/or aortic NO and nitrotyrosine production 4 h after LPS administration to rats [36,47–49]. Recently, we have reported that an increase in iNOS protein expression is associated with a decrease in eNOS and COX-1 protein expression in the renal, cardiac, and vascular tissues as well as vascular hyporeactivity to norepinephrine in thoracic aorta and superior mesenteric artery 4 h after LPS administration to rats [31,32,50]. These data suggested that overproduction of NO by iNOS associated with an increase in sGC and PKG activity in vascular tissue contributes to the hypotension and vascular hyporeactivity to norepinephrine 4 h after LPS injection to rats. In the present study, administration of LPS to rats caused an increase in mRNA and protein expression of iNOS and COX-2 as well as a decrease in COX-1 and CYP4A1 mRNA and protein expression in the kidney, heart, thoracic aorta, and superior mesenteric artery. Increased NOS activity, iNOS-hsp90 complex formation, protein expression of p-VASP, gp91phox, p47phox, and nitrotyrosine as well as cGMP, PGI2, PGE2, and nitrotyrosine levels associated with decreased 20-HETE formation were also observed in the renal and cardiovascular tissues of LPS-treated rats. Therefore, the results of the present study, together with above-mentioned observations, suggest that the NO, vasodilator prostanoids, and peroxynitrite could cause a decrease in blood pressure which is followed by increased expression of iNOS, COX-2, and gp91phox and decreased expression of CYP4A and 20-HETE formation, resulting in a sustained fall in blood pressure and tachycardia. Furthermore, a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-HEDE, reversed the effects of 5,14-HEDGE, except iNOS and COX-1 mRNA and protein expression as well as expression of CYP4A1 mRNA. These findings also suggest that 20-HEDE is not only an antagonist of vasoconstrictor effects of 20-HETE, but also have additional effects on the 5,14-HEDGE-induced changes in the expression and/or activity of iNOS, COX-2, CYP4A1, and gp91phox during endotoxemia, for example, it reverses the effects of 5,14-HEDGE on iNOS-hsp90 association and NOS activity without a change in iNOS mRNA and protein expression leading to NO production and activation of sGC/PKG pathway resulting in vascular hyporeactivity and hypotension in LPS-treated rats. In the present study, 5,14-HEDGE and 20-HEDE also caused a decrease in COX-1 protein, but not mRNA, expression as well as serum and tissue levels of 6-keto-PGF1α and PGE2 in vehicle-treated rats. A possible mechanism by which 20-HEDE decreases COX-1 protein expression and systemic and tissue PGI2 and PGE2 production in control rats could be through inhibiting the effects of endogenously produced 20-HETE on COX-1 protein expression and prostanoid production. It is also possible that both 5,14-HEDGE and 20-HEDE might directly inhibit COX-1 protein expression and activity at posttranslational level leading to decreased prostanoid levels. However, additional experiments need to be conducted to demonstrate the validity of this hypothesis.
There are conflicting data regarding the effect of LPS on CYP4A expression and activity. Our previous studies suggested that the endotoxemia-induced increase in NO production primarily via iNOS suppresses renal CYP4A expression and activity, and selective inhibition of iNOS with 1,3-PBIT restores renal CYP4A protein and activity and MAP presumably due to increased production of AA metabolites derived from CYP4A [49,51]. The results of the present study together with our previous findings using 5,14-HEDGE and COX inhibitors [31,33,50,52] demonstrate that LPS causes a decrease in CYP4A1 mRNA and protein expression not only in the kidney, but also in the heart, thoracic aorta, and superior mesenteric artery, together with reduced systemic and tissue levels of 20-HETE. In contrast to our findings, Anwar-Mohamed et al. [26] demonstrated that CYP4A1 mRNA expression was increased in the heart of inflamed animals at 6, 12, and 24 h by 400%, 900%, and 6000%, respectively, after injection of LPS (E. coli LPS, O127:B8, 1 mg/kg, i.p.) to Sprague–Dawley rats. Similarly, but with a lower magnitude, renal mRNA expression of CYP4A1 was increased only at 24 h by 100% after injection of LPS (E. coli LPS, O127:B8, 1 mg/kg, i.p.) to rats. The LPS-induced changes in the expression of CYP4A1 as well as enhanced expression of TNF-α and IL-6 genes in cardiac and renal tissues were also associated with increased production of 20-HETE in the heart microsomes of inflamed animals by 40% ex vivo. The authors suggested that acute inflammation causes alteration in cardiac CYP-mediated AA metabolism in favor of 20-HETE formation. However, Theken et al. [27] reported that 20-HETE formation in the kidney, but not in the heart, decreased 6 or 24 h after injection of LPS (E. coli LPS, O111:B4, 1 mg/kg, i.p.) to C57BL/6 mice leading to the conclusion that acute activation of the innate immune response alters CYP expression and eicosanoid metabolism in an isoform-, tissue-, and time-dependent manner. Thus, the contradiction between previously published studies and our results could be attributed to differences in type and strain of animals, dose regimen, strain of LPS, and time points for measurement of enzyme expression and activity which might reflect the differences in the response to LPS treatment.
A chaperone molecule, hsp90, has been identified as a signaling molecule in the activation of all the isoforms of NOS [53]. Hsp90 associates with NOS and facilitates its phosphorylation and, thereby, increases NO production. Vo et al. [54] demonstrated that maximal vascular iNOS expression and its function are achieved via the up-regulation and increased association of eNOS with hsp90, and that in the absence of functional eNOS, the vascular effects of iNOS are delayed during rodent endotoxemia. Yoshida and Xia [55] also reported that hsp90 is an important post-translational modulator of iNOS in iNOS-transfected cells. On the other hand, there are several studies reporting that endotoxin up-regulates iNOS protein, but does not alter basal hsp90 expression in in vitro [56] and in vivo [57] studies. Recently, Cheng et al. [58] have shown that 20-HETE impairs NO production in vitro and its function in vivo by inhibiting association of eNOS with hsp90. It has been shown that increased production of 20-HETE in the vasculature is associated with endothelial dysfunction and increased vascular tone which contributes to the development of hypertension in animal models [59]. At least three different pathways have been suggested to play a role in these responses including increased vascular expression of subunits of reduced nicotinamide-adenine dinucleotide phosphate oxidase by 20-HETE, leading to production of superoxide [60], decreased association of eNOS with hsp90 by 20-HETE, leading to diminished formation of NO and increased formation of superoxide [58,61,62], and increased superoxide production directly by 20-HETE in endothelial cells [62]. We have previously demonstrated that 5,14-HEDGE did not prevent the LPS-induced decrease in endothelium-dependent relaxations induced by acetylcholine in thoracic aorta and superior mesenteric artery, although it reversed the effects of LPS on vascular hyporeactivity to norepinephrine and overproduction of NO in the tissues [32]. More recently, we have demonstrated that the LPS-induced decrease in CYP4A1 protein expression and 20-HETE formation was accompanied with increased iNOS protein expression, decreased expression of eNOS protein, and prostanoid levels without alterations in hsp90 expression in the kidney, heart, thoracic aorta, and/or superior mesenteric artery of endotoxemic rats [31,50]. These effects of LPS, except for eNOS and hsp90 protein expression, were prevented by 5,14-HEDGE. In the present study, 5,14-HEDGE inhibited the iNOS, gp91phox, p47phox, and nitrotyrosine protein expression, iNOS-hsp90 complex formation, and NOS activity as well as nitrotyrosine levels in the renal and cardiovascular tissues of rats. Therefore, the results of the present study suggest that 20-HETE impairs NO synthesis and its function by inhibiting association of iNOS with hsp90 and superoxide formation.
The results of the present study together with our previous observations [31–37,47–51,63–65] suggest that increased CYP4A1 and CYP2C23 expression together with formation of vasoactive eicosanoids (e.g., 20-HETE) and antiinflammatory mediators (e.g., EETs) associated with suppression of not only MEK1/ERK1/2/IKKβ/IκB-α/NF-κB pathway and production of proinflammatory cytokines (e.g., TNF-α and IL-8), but also iNOS, sGC, PKG, COX-2, gp91phox, and superoxide formation participate in the effect of 5,14-HEDGE to prevent LPS-induced vascular hyporeactivity, hypotension, tachycardia, and inflammatory response in endotoxemic rats and mortality in mice. In addition, it seems that transcriptional (e.g., gene expression), translational (e.g., mRNA expression and stability), and posttranslational mechanisms (e.g., protein expression and stability) as well as changes in enzyme activity are involved in the regulation of iNOS, COX-1, COX-2, and CYP4A1 expression by 5,14-HEDGE in the rodent model of septic shock. Although our results demonstrated that 5,14-HEDGE directly increases CYP4A1 expression at both transcriptional and posttranscriptional levels and 20-HETE production in endotoxemic rats, it is also possible that 5,14-HEDGE might increase COX-1 and CYP4A1 expression and activity indirectly by inhibiting expression of iNOS, COX-2, gp91phox, p47phox, and nitrotyrosine, and/or decreasing the effects of NO, superoxide, PGI2, PGE2, 20-HETE, cytokines, and/or transcription factors (e.g., NF-κB) on the expression and activity of COX-1 and CYP4A1. However, additional experiments need to be conducted to demonstrate the validity of the proposed hypothesis. Further characterization of the molecular mechanisms of the effects of 5,14-HEDGE on iNOS, COX-2, CYP4A1, and gp91phox enzymes will provide the framework for extension of this work into understanding the role of AA products in the inflammatory response associated with vascular hyporeactivity, hypotension, tachycardia, inflammation, and mortality during endotoxemia.
In conclusion, the present study provides evidence that endotoxin induces expression and/or activity of iNOS/sGC/PKG pathway, COX-2, and gp91phox associated with decreased CYP4A1 expression and 20-HETE formation during endotoxemia which may result in vascular hyporeactivity, hypotension, tachycardia, inflammation, and mortality in the rodent model of septic shock (Fig. 21). Our findings also suggest that 5,14-HEDGE, a 20-HETE mimetic, prevents vascular hyporeactivity, hypotension, tachycardia, inflammation, and mortality presumably due to increased CYP4A1 expression and 20-HETE formation associated with decreased expression and/or activity of iNOS/sGC/PKG pathway, COX-2, and gp91phox leading to decreased vasodilatory and inflammatory mediator production. The current management of septic shock relies on immediate treatment with antibiotics and strong supportive care to control hypotension, tachycardia, cardiac output, and tissue oxygenation to maintain organ function. However, the failure of conventional therapy is that the pathophysiology of septic shock is the result of a highly complex set of processes in which the host response becomes dysregulated and causes cellular damage, tissue damage, and, ultimately, organ failure [1]. In the light of the important role of 20-HETE in the regulation of renal and cardiovascular hemostasis as well as inflammatory process, further studies with 20-HETE mimetics in experimental models of endotoxemia could provide a novel approach to treat hypotension, tachycardia, inflammation, and mortality which lead to multiple organ failure and death in septic shock.
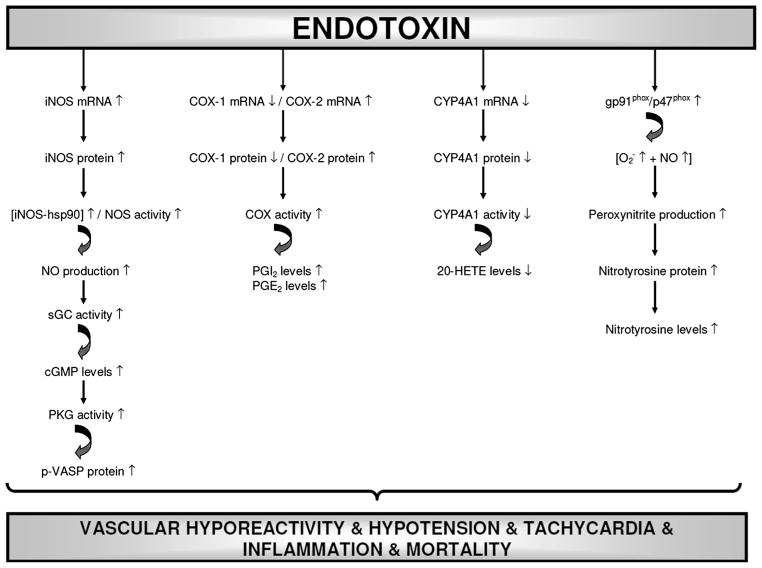
Fig. 21.
Schematic diagram showing the involvement of iNOS, sGC, PKG, COX-1, COX-2, CYP4A1, and gp91phox in endotoxin-induced vascular hyporeactivity, hypotension, tachycardia, and survival based on the results of the present and our previous findings. Endotoxin, the lipid A part of LPS which is the most potent microbial mediator of the pathogenesis of sepsis and septic shock, increases iNOS and COX-2 mRNA expression, iNOS, p-VASP, COX-2, gp91phox (NOX2; a superoxide generating NOX enzyme), p47phox (NOXO2; organizer subunit of gp91phox), and nitrotyrosine protein expression, iNOS-hsp90 complex formation, NOS activity, and levels of cGMP, 6-keto-PGF1α, PGE2, and nitrotyrosine associated with decreased COX-1 and CYP4A1 mRNA expression, COX-1 protein expression, and 20-HETE levels in renal and cardiovascular tissues leading to vascular hyporeactivity hypotension, tachycardia, and mortality in the rodent model of septic shock. 5,14-HEDGE, a 20-HETE mimetic, prevents the effects of endotoxin on the increase in expression of COX-1, COX-2, and CYP4A1 mRNA expression, iNOS, p-VASP, COX-2, CYP4A1, gp91phox, p47phox, and nitrotyrosine protein expression, iNOS-hsp90 complex formation, NOS activity, and levels of cGMP, 6-keto-PGF1α, PGE2, 20-HETE, and nitrotyrosine, and thus, restores blood pressure, prevents tachycardia, and improves survival during rodent endotoxemia. It should be noted that a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-HEDE, prevents the effects of 5,14-HEDGE on blood pressure, HR, p-VASP, COX-2, CYP4A1, gp91phox, p47phox, and nitrotyrosine protein expression, iNOS-hsp90 complex formation, NOS activity, and 6-keto-PGF1α, PGE2, 20-HETE, and nitrotyrosine levels in rats treated with LPS. It can be concluded that decreased expression and activity of iNOS, sGC, PKG, COX-2, and gp91phox associated with increased CYP4A1 expression and activity participate in the protective effect of 5,14-HEDGE against vascular hyporeactivity, hypotension, tachycardia, and mortality in the rodent model of septic shock. (↑), increase; (↑) decrease.
Supplementary Material
Acknowledgments
Financial support was provided by grants from TUBITAK (SBAG-109S121), Mersin University (BAP-ECZ F FB [BT] 2010-5 B), NIH (19134-37 and DK38226), the Robert A. Welch Foundation (GL625910), and The GermanResearch Foundation (SCHU-822/7-1).
Abbreviations
- 20-HEDE
20-hydroxyeicosa-6(Z),15(Z)-dienoic acid
- AA
arachidonic acid
- 20-HETE
20-hydroxyeicosatetraenoic acid
- 5,14-HEDGE
N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine
- 1,3-PBIT
phenylene-1,3-bis(ethane-2-isothiourea) dihydrobromide
- cDNA
complementary deoxyribonucleic acid
- cGMP
cyclic guanosine monophosphate
- COX
cyclooxygenase
- CYP
cyctochrome P450
- EET
epoxyeicosatrienoic acid
- ELISA
enzyme-linked immunosorbent assay
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- HR
heart rate
- hsp
heat shock protein
- IKK
IκB kinase
- IκB
inhibitor of κB
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- i.p
intraperitoneally
- LPS
lipopolysaccharide
- MAP
mean arterial pressure
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase 1
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor-κB
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- PG
prostaglandin
- PKG
protein kinase G
- p-VASP
phosphorylated vasodilator stimulated phosphoprotein
- RT-PCR
reverse transcription-polymerase chain reaction
- s.c
subcutaneously
- sEH
soluble epoxide hydrolase
- sGC
soluble guanylyl cyclase
- TNF
tumor necrosis factor
- VASP
vasodilator stimulated phosphoprotein
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.niox.2013.05.001.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Schunck WH, Falck JR, Malik KU. A novel treatment strategy for sepsis and septic shock based on the interactions between prostanoids, nitric oxide, and 20-hydroxyeicosatetraenoic acid. Anti-Inflammatory Anti-Allergy Agents Med Chem. 2012;11:121–150. doi: 10.2174/187152312803305759. [DOI] [PubMed] [Google Scholar]
- 2.Suba EA, McKenna TM, Williams TJ. In vivo and in vitro effects of endotoxin on vascular responsiveness to norepinephrine and signal transduction in the rat. Circ Shock. 1992;36:127–133. [PubMed] [Google Scholar]
- 3.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–232. doi: 10.1007/s11010-009-0318-8. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann SM, Walter U. Tracking functions of cGMP-dependent protein kinases (cGK) Front Biosci. 2005;10:1313–1328. doi: 10.2741/1621. [DOI] [PubMed] [Google Scholar]
- 5.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Münzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res. 2000;87:999–1005. doi: 10.1161/01.res.87.11.999. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Esposito E, Cuzzocrea S. Superoxide, NO, peroxynitrite and PARP in circulatory shock and inflammation. Front Biosci. 2009;14:263–296. doi: 10.2741/3244. [DOI] [PubMed] [Google Scholar]
- 8.Kroetz DL, Xu F. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu Rev Pharmacol Toxicol. 2005;45:413–438. doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- 9.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;175:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 10.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 11.Escalante B, Omata K, Sessa W, Lee SG, Falck JR, Schwartzman ML. 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol. 1993;235:1–7. doi: 10.1016/0014-2999(93)90812-v. [DOI] [PubMed] [Google Scholar]
- 12.Escalante B, Sessa WC, Falck JR, Yadagiri P, Schwartzman ML. Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–232. [PubMed] [Google Scholar]
- 13.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzman ML, Falck JR, Yadagiri P, Escalante B. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygeanse: formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–11662. [PubMed] [Google Scholar]
- 15.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 16.Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C, Mustafa SJ. Role of ω-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2A-receptor knockout mice. Am J Physiol. 2012;302:R400–R408. doi: 10.1152/ajpregu.00481.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 19.Carroll MA, Capparelli MF, Doumand AB, Cheng MK, Jiang H, McGiff JC. Renal vasoactive eicosanoids: interactions between cytochrome P450 and cyclooxygenase metabolites during salt depletion. Am J Hypertens. 2001;14:159A. [Google Scholar]
- 20.Carroll MA, Garcia MP, Falck JR, McGiff JC. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther. 1992;260:104–109. [PubMed] [Google Scholar]
- 21.Pratt PF, Falck JR, Reddy KM, Kurian JB, Campbell WB. 20-HETE relaxes bovine coronary arteries through the release of prostacyclin. Hypertension. 1998;31:237–241. doi: 10.1161/01.hyp.31.1.237. [DOI] [PubMed] [Google Scholar]
- 22.Yu M, McAndrew RP, Al-Saghir R, Maier KG, Medhora M, Roman RJ, Jacobs ER. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol. 2002;93:1391–1399. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol. 2006;291:H2301–H2307. doi: 10.1152/ajpheart.00349.2006. [DOI] [PubMed] [Google Scholar]
- 24.Cheng J, Wu CC, Gotlinger KH, Zhang F, Falck JR, Narsimhaswamy D, Schwartzman ML. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitricoxide synthase uncoupling. J Pharmacol Exp Ther. 2010;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 26.Anwar-mohamed A, Zordoky BN, Aboutabl ME, El-Kadi AO. Alteration of cardiac cytochrome P450-mediated arachidonic acid metabolism in response to lipopolysaccharide-induced acute systemic inflammation. Pharmacol Res. 2010;61:410–418. doi: 10.1016/j.phrs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab Dispos. 2011;39:22–29. doi: 10.1124/dmd.110.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–F378. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 29.Wang MH, Wang J, Chang HH, Zand BA, Jiang M, Nasjletti A, Laniado-Schwartzman M. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–F302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 31.Cuez T, Korkmaz B, Buharalioglu CK, Sahan-Firat S, Falck J, Malik KU, Tunctan B. A synthetic analogue of 20-HETE, 5, 14-HEDGE, reverses endotoxin-induced hypotension via increased 20-HETE levels associated with decreased iNOS protein expression and vasodilator prostanoid production in rats. Basic Clin Pharmacol. 2010;106:378–388. doi: 10.1111/j.1742-7843.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunctan B, Korkmaz B, Buharalioglu CK, Sahan Firat S, Anjaiah S, Falck J, Roman RJ, Malik KU. A 20-HETE agonist, N-[20-hydroxyeicosa-5(Z), 14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–335. doi: 10.1097/SHK.0b013e31816471c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunctan B, Korkmaz B, Cuez T, Sarı AN, Kacan M, Gilik D, Buharalioglu CK, Sahan-Firat S, Schunck WH, Serin MS, Falck JR, Malik KU. Contribution of iNOS, COX-2 and CYP4A1 to the protective effect of a synthetic analog of 20-HETE, 5, 14-HEDGE, against endotoxin-induced hypotension and mortality in experimental model of septic shock in rats and mice. Inflamm Res. 2011;60:S55. [Google Scholar]
- 34.Tunctan B, Korkmaz B, Sarı AN, Kacan M, Gilik U, Serin MS, Buharalioglu CK, Sahan-Firat S, Cuez T, Schunck WH, Falck JR, Malik KU. A synthetic analog of 20-HETE, 5, 14-HEDGE, reverses endotoxin-induced hypotension and mortality via increased expression and activities of CYP4A1 and CYP2C23 in a rodent model of septic shock: contribution of MEK1/ERK1/2/IKKβ/IκB-α/NF-κB pathway and soluble epoxide hydrolase. Sepsis. 2012;4:158–159. [Google Scholar]
- 35.Tunctan B, Korkmaz B, Sarı AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Cuez T, Schunck WH, Falck JR, Malik KU. 5, 14-HEDGE, A 20-HETE mimetic, reverses hypotension and improves survival in a rodent model of septic shock: contribution of soluble epoxide hydrolase, CYP2C23, MEK1/ERK1/2/IKKβ/IκB-α/NF-κB pathway, and proinflammatory cytokine formation. Prostaglandins Other Lipid Mediat. 2013;102–103:31–41. doi: 10.1016/j.prostaglandins.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1:111–115. [Google Scholar]
- 37.Tunctan B, Altug S, Uludag O, Demirkay B, Abacioglu N. Effects of cyclooxygenase inhibitors on nitric oxide production and survival in a mice model of sepsis. Pharmacol Res. 2003;48:37–48. [PubMed] [Google Scholar]
- 38.Liu SF, Adcock IM, Old RW, Barnes PJ, Evans TW. Differential regulation of the constitutive and inducible nitric oxide synthase mRNA by lipopolysaccharide treatment in vivo in the rat. Crit Care Med. 1996;24:1219–1225. doi: 10.1097/00003246-199607000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Liu SF, Newton R, Evans TW, Barnes PJ. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci. 1996;90:301–306. doi: 10.1042/cs0900301. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi N, Jesmin S, Zaedi S, Shimojo N, Maeda S, Gando S, Koyama A, Miyauchi T. Time-dependent expression of renal vaso-regulatory molecules in LPS-induced endotoxemia in rat. Peptides. 2006;27:2258–2270. doi: 10.1016/j.peptides.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 41.Szabo C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, Sharma AC. Despite minimal hemodynamic alterations endotoxemia modulates NOS and p38-MAPK phosphorylation via metalloendopeptidases. Mol Cell Biochem. 2004;265:47–56. doi: 10.1023/b:mcbi.0000044314.29395.fb. [DOI] [PubMed] [Google Scholar]
- 43.Mazzocchi G, Albertin G, Nussdorfer GG. Adrenomedullin (ADM), acting through ADM(22–52)-sensitive receptors, is involved in the endotoxin-induced hypotension in rats. Life Sci. 2000;66:1445–1450. doi: 10.1016/s0024-3205(00)00455-0. [DOI] [PubMed] [Google Scholar]
- 44.Mitaka C, Hirata Y, Yokoyama K, Nagura T, Tsunoda Y, Amaha K. Pathologic role of endothelin-1 in septic shock. J Cardiovasc Pharmacol. 1998;31:S233–S235. doi: 10.1097/00005344-199800001-00065. [DOI] [PubMed] [Google Scholar]
- 45.Rogausch H, Vo NT, Del Rey A, Besedovsky HO. Increased sensitivity of the baroreceptor reflex after bacterial endotoxin. Ann N Y Acad Sci. 2000;917:165–168. doi: 10.1111/j.1749-6632.2000.tb05380.x. [DOI] [PubMed] [Google Scholar]
- 46.Sharma AC, Sam AD, Alden KJ, Moore SL, Law WR, Ferguson JL. Central versus peripheral mediation of naloxone’s perfusion effects in endotoxic rats. Shock. 2000;14:441–446. doi: 10.1097/00024382-200014040-00004. [DOI] [PubMed] [Google Scholar]
- 47.Korkmaz B, Buharalioglu K, Sahan-Firat S, Cuez T, Demiryurek TA, Tunctan B. Activation of MEK1/ERK1/2/iNOS/sGC/PKG pathway associated with peroxinitrite formation contributes to hypotension and vascular hyporeactivity in endotoxemic rats. Nitric Oxide Biol Chem. 2011;24:160– 172. doi: 10.1016/j.niox.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu CK. Reversal of endotoxin-induced hypotension by inhibition of inducible nitric oxide synthase activity is associated with improved oxidative status in rat heart, aorta and mesenteric artery. Turk J Med Sci. 2006;36:71–80. [Google Scholar]
- 49.Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide and cyclooxygenase of cytochrome P4504A expression and activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide Biol Chem. 2006;14:51– 57. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Buharalioglu CK, Korkmaz B, Cuez T, Sahan-Firat S, Sari AN, Malik KU, Tunctan B. Piroxicam reverses endotoxin-induced hypotension in rats: contribution of vasoactive eicosanoids and nitric oxide. Basic Clin Pharmacol Toxicol. 2011;109:186–194. doi: 10.1111/j.1742-7843.2011.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tunctan B, Yaghini FA, Estes A, Malik KU. Prostaglandins inhibit cytochrome P450 4A activity and contribute to endotoxin-induced hypotension in rats via nitric oxide production. Arch Pharm Res. 2008;31:856–865. doi: 10.1007/s12272-001-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tunctan B, Sari AN, Kacan M, Unsal D, Buharalioglu CK, Sahan-Firat S, Korkmaz B, Falck JR, Malik KU. NS-398 reverses hypotension in endotoxemic rats: contribution of eicosanoids, NO, and peroxynitrite. Prostaglandins Other Lipid Mediat. 2012 doi: 10.1016/j.prostaglandins.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD. Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc. 2007;37:19–35. [PubMed] [Google Scholar]
- 54.Vo PA, Lad B, Tomlinson JA, Francis S, Ahluwalia A. Autoregulatory role of endothelium-derived nitric oxide (NO) on Lipopolysaccharide-induced vascular inducible NO synthase expression and function. J Biol Chem. 2005;280:7236–7243. doi: 10.1074/jbc.M411317200. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem. 2003;278:36953–36958. doi: 10.1074/jbc.M305214200. [DOI] [PubMed] [Google Scholar]
- 56.Chakravortty D, Kato Y, Sugiyama T, Koide N, Mu MM, Yoshida T, Yokochi T. The inhibitory action of sodium arsenite on lipopolysaccharide-induced nitric oxide production in RAW 267.4 macrophage cells: a role of Raf-1 in lipopolysaccharide signaling. J Immunol. 2001;166:2011–2017. doi: 10.4049/jimmunol.166.3.2011. [DOI] [PubMed] [Google Scholar]
- 57.Li FC, Chan JY, Chan SH, Chang AY. In the rostral ventrolateral medulla, the 70-kDa heat shock protein (HSP70), but not HSP90, confers neuroprotection against fatal endotoxemia via augmentation of nitric-oxide synthase I (NOS I)/protein kinase G signaling pathway and inhibition of NOS II/peroxynitrite cascade. Mol Pharmacol. 2005;68:179–192. doi: 10.1124/mol.105.011684. [DOI] [PubMed] [Google Scholar]
- 58.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol. 2008;294:H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 59.Roman RJ, Lombard JH. Does 20-hydroxyeicosatetraenoic acid contribute to sex differences in cardiovascular risk by increasing oxidative stress. Hypertension. 2007;50:37–38. doi: 10.1161/HYPERTENSIONAHA.107.090803. [DOI] [PubMed] [Google Scholar]
- 60.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Medhora M, Falck JR, Pritchard KA, Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol. 2006;291:L378–L385. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 62.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 63.Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 1999;99:1878–1884. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- 64.Tunctan B, Korkmaz B, Dogruer ZN, Tamer L, Atik U, Buharalioglu CK. Inhibition of extracellular signal-regulated kinase (ERK1/2) activity reverses endotoxin-induced hypotension via decreased nitric oxide production in rats. Pharmacol Res. 2007;56:56–64. doi: 10.1016/j.phrs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Tunctan B, Korkmaz B, Cuez T, Buharalioglu CK, Sahan-Firat S, Falck J, Malik KU. Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase-2 inhibitor, NS-398, on endotoxin-induced hypotension in rats. Basic Clin Pharmacol Toxicol. 2010;107:877–882. doi: 10.1111/j.1742-7843.2010.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.