Abstract
Pathologists play an increasingly important role in personalized medicine for patients with lung cancer as a result of the newly recognized relationship between histologic classification and molecular change. In 2011, the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) proposed a new architectural classification for invasive lung adenocarcinomas to provide uniform terminology and diagnostic criteria. This review highlighted the evolution of the classification of lung adenocarcinomas in resected specimens with special respect to both histologic subtyping and invasion. Histologic subtyping of lung adenocarcinoma has been updated based on five major predominant patterns. New concepts of adenocarcinoma in situ and minimally invasive adenocarcinomas have been introduced to define the condition of patients who are expected to have excellent survival. Although the new IASLC/ATS/ERS classification has promising clinical relevance, significant clarification remains necessary for the definitions of subtyping and invasion. More precise definitions and subsequent better education on the interpretation of terminology will be helpful for future studies.
Keywords: Lung, Adenocarcinoma, Classification, Subtyping, Invasion
Lung adenocarcinoma is the most prevalent histologic type of primary lung cancer worldwide, accounting for almost half of all lung cancers.1,2 However, it is a very heterogeneous tumor in the pathologic and molecular perspectives. Due to histology-based therapeutic algorithms for lung cancer, there is a need for a standard for pathologic diagnosis in the rapidly evolving field of lung adenocarcinoma. In 2011, the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) proposed a new architectural classification of invasive lung adenocarcinomas to provide uniform terminology and diagnostic criteria.3 There were several important modifications and changes to the 2004 World Health Organization (WHO) classification that applied to lung adenocarcinoma.4
One of the major changes is that invasive adenocarcinoma subtypes are now classified by their predominant pattern in resected specimens. The new IASLC/ATS/ERS classification allows for five comprehensive subtypes of lepidic, acinar, papillary, solid and micropapillary patterns by a percentage of 5% increments to determine a predominant pattern.3 Since the publication of this new classification, a series of reports have been published demonstrating that this newly proposed classification of lung adenocarcinoma has prognostic value due to the classification of heterogeneous adenocarcinomas according to the predominant pattern of the tumor.5-12
The other major change is the introduction of new concepts of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA). AIS and MIA are defined as localized small (≤3 cm) solitary adenocarcinomas with either a pure lepidic pattern or predominant lepidic pattern with invasion of ≤0.5 cm, respectively.3 These concepts were proposed to define patients who, if they underwent complete resection, would have 100% disease-free survival.5,6,8
Although the new IASLC/ATS/ERS classification is promising, recent studies have stressed that more precise definitions and better education on the interpretation of this classification are required for application to routine daily practice.13-20 Here, we review the current understanding focusing on the clinicopathologic relevance of these two major changes of IASLC/ATS/ERS classification, which addresses resected specimens, and discuss emerging issues on potential problems, which require further clarification (Table 1).
Table 1.
Emerging issues in IASLC/ATS/ERS classification of lung adenocarcinoma in resected specimens with respect to both histologic subtyping and invasion
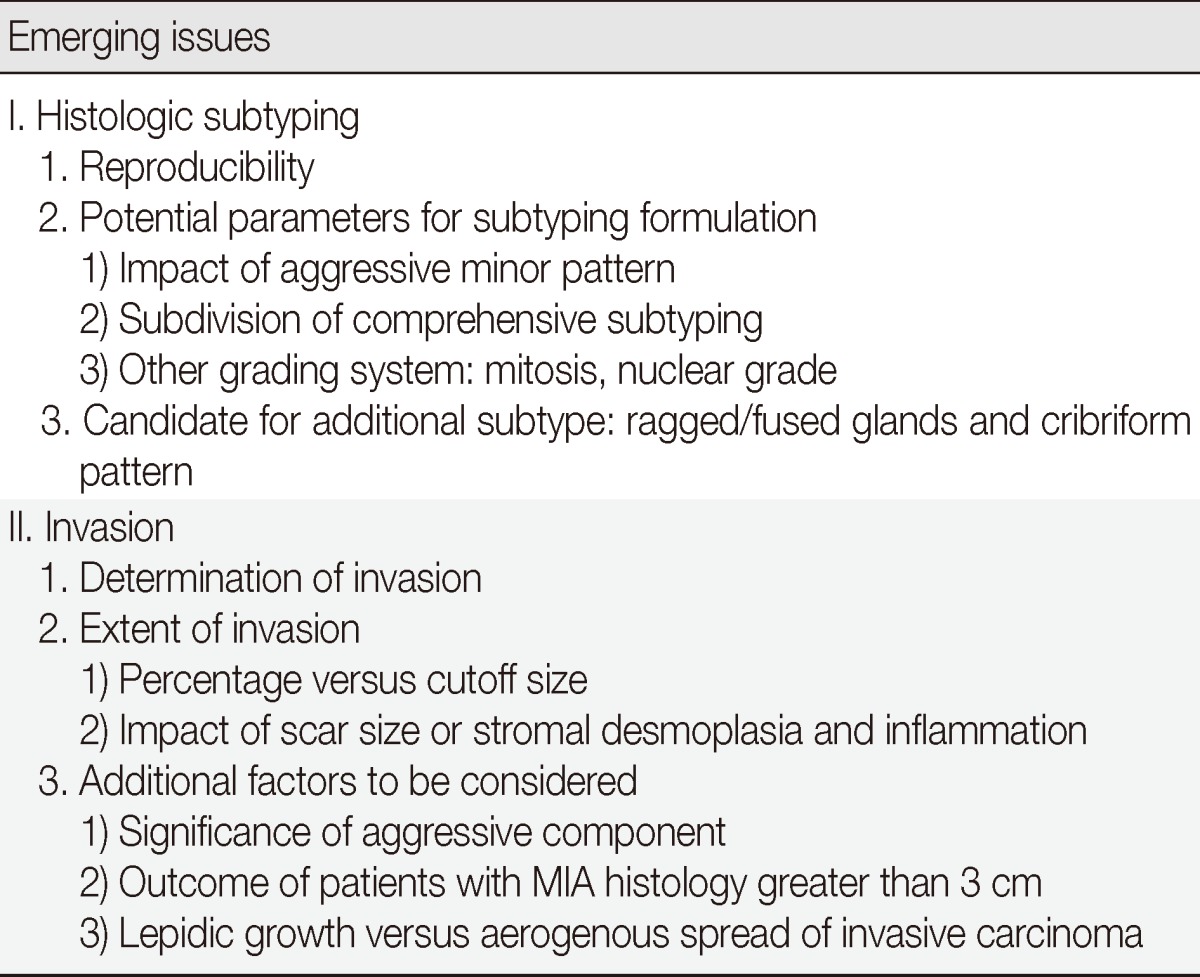
IASLC/ATS/ERS, International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society; MIA, minimally invasive adenocarcinoma.
HISTOLOGIC SUBTYPING
Relevance
For resected invasive adenocarcinomas, comprehensive histologic subtyping with lepidic, acinar, papillary, solid and micropapillary patterns is performed by making a semiquantitative estimation of each of the patterns in 5% increments and choosing a single predominant pattern.3 The terms 'bronchioloalveolar carcinoma (BAC)' and 'mixed subtype adenocarcinoma' have been discontinued, unlike the previous WHO classifications.4,21 The term lepidic replaces BAC for tumors with a predominant component, formerly called nonmucinous BAC. Micropapillary pattern has been added as a new histologic subtype with poor prognosis. The diagnostic criteria of five predominant patterns for invasive adenocarcinoma are briefly summarized in Table 2.
Table 2.
Summarized diagnostic criteria of five predominant patterns for invasive adenocarcinoma in resected specimens according to the new IASLC/ATS/ERS classification3,14
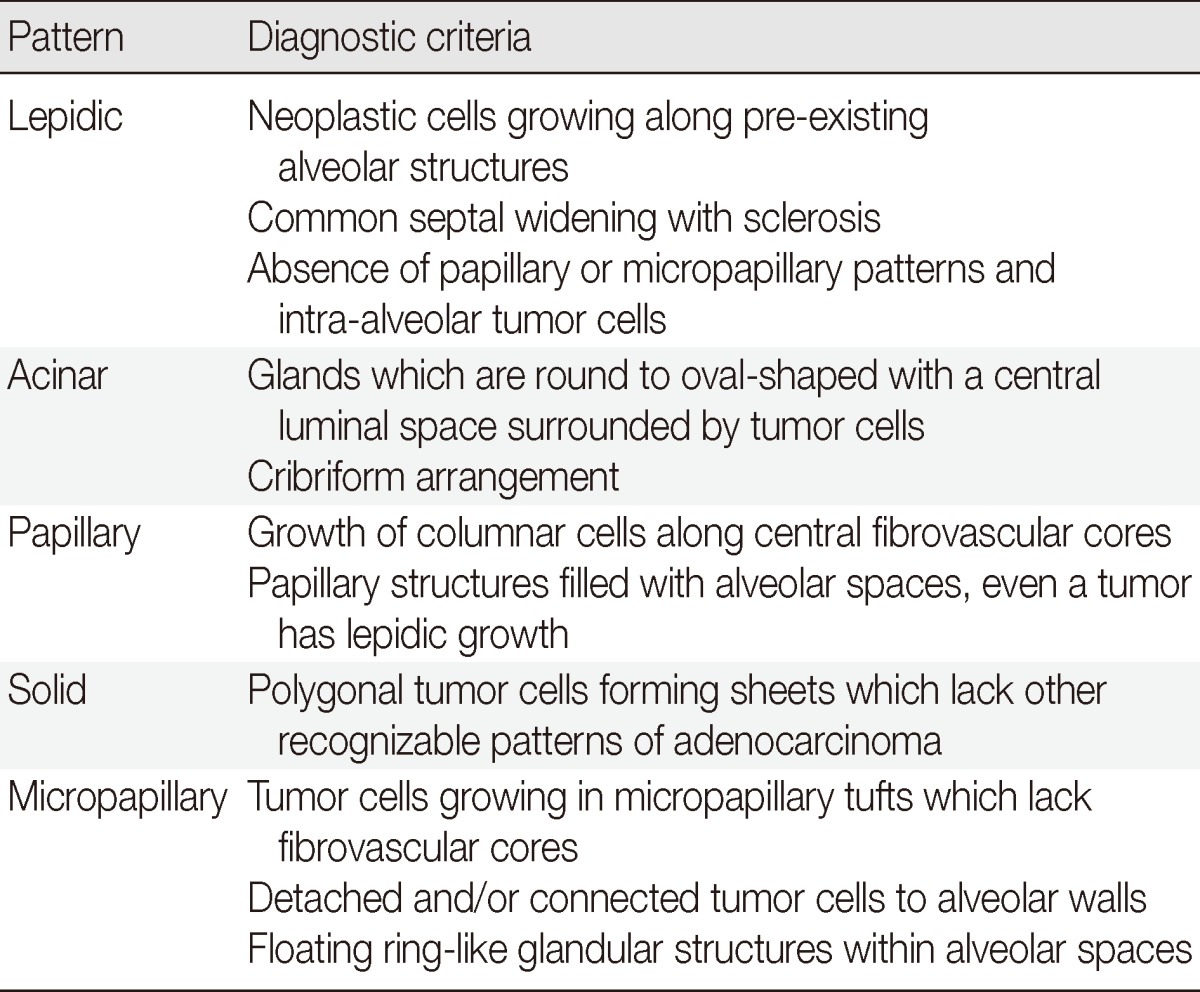
IASLC/ATS/ERS, International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society.
Recently, a growing number of studies have demonstrated the utility of the new IASLC/ATS/ERS classification in identifying prognostic significance and molecular correlations according to the predominant patterns of invasive lung adenocarcinomas across all tumor stages. It has generally shown most favorable prognosis for lepidic predominant adenocarcinomas, intermediate survival for acinar and papillary predominant adenocarcinomas, and poor prognosis for solid and micropapillary predominant adenocarcinomas. Yoshizawa et al.5 examined 514 Western patients with stage I adenocarcinomas, with histological grading of the new IASLC/ATS/ERS classification system defined as low (AIS and MIA), intermediate (lepidic, acinar, and papillary), and high (solid and micropapillary), and demonstrated the prognostic value of this histological grading; low grade AIS and MIA had 100% 5-year disease-free survival; intermediate grade lepidic, acinar, and papillary predominant had 90%, 84%, and 83% 5-year disease-free survival, respectively; and high grade solid and micropapillary predominant had 70% and 67% 5-year disease-free survival, respectively. An analysis of 440 Japanese patients with various stages of lung adenocarcinomas revealed a similar prognostic subset of IASLC/ATS/ERS histologic classification and reaffirmed that the IASLC/ATS/ERS classification is one of the independent parameters for predicting high risk of recurrence and incidence of death by primary lung adenocarcinoma.6 In another Japanese study, stage I lung adenocarcinoma patients with AIS and MIA showed 100% 5-year disease-free survival. The 5-year disease-free survivals of patients with lepidic, acinar, papillary, and solid were 93.5%, 83.7%, 75.0%, and 44.4%, respectively.8
Because most adenocarcinoma subtypes can harbor epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, as well as anaplastic lymphoma kinase (ALK) rearrangement,20 molecular histologic correlation is important in predicting patient prognosis and selecting those who require adjuvant chemotherapy according to molecular changes. It has been reported that the IASLC/ATS/ERS histologic classification has significant correlations with molecular changes.6,22-26 A correlation study between IASLC/ATS/ERS lung adenocarcinoma classification and molecular changes revealed that EGFR mutations were associated with a high frequency of AIS, MIA, lepidic, and papillary predominant subtypes (85.7% AIS, 83.3% MIA, 71.4% lepidic, and 68.5% papillary), followed by acinar (38.4%) and micropapillary (40.1%) predominant subtypes; whereas they were uncommon in the solid predominant subtype (14.3%).6 A Korean study showed a significant phenotype-genotype correlation in that EGFR mutations were associated with lepidic and micropapillary subtypes.22 KRAS mutations were detected more often in acinar (23.1%) and solid (25.0%) predominant subtypes, followed by MIA (8.3%) and papillary (4.5%) predominant subtypes. No KRAS mutations were observed in AIS or lepidic predominant subtypes; whereas all invasive mucinous adenocarcinomas had KRAS mutations.6 ALK rearrangement has been mostly associated with an acinar pattern, including a cribriform morphology, and with signet ring cell features, particularly those with thyroid transcription factor 1 and p63 coexpression.23-25 A correlation study between IASLC/ATS/ERS lung adenocarcinoma classification and ALK rearrangement revealed similar findings in a Korean study where ALK-rearranged adenocarcinoma showed more frequent signet ring cell morphology than ALK-wild type adenocarcinoma.26
Emerging issues
Reproducibility
The term mixed subtype has been discontinued, and invasive adenocarcinomas are now classified according to their predominant subtype. Because invasive lung adenocarcinomas encompass a spectrum of histologic patterns that represent a morphologic continuum, rather than distinct entities, the complex mixture of histologic patterns has presented one of the greatest challenges to the classification of invasive lung adenocarcinomas. A reproducibility study of typical and difficult representative images of the major histologic subtypes by 26 expert lung pathologists revealed that the mean kappa (κ) value for the typical and difficult cases were 0.77±0.07 and 0.38±0.14, respectively.13 A reproducibility study using actual slides of resected tumors performed by 3 pathologists with lung subspeciality training and at least 5 years' experience revealed that the κ value for interobserver agreement was 0.32 for the predominant pattern, 0.26 for the secondary pattern, and 0.26 for the combination of primary/secondary patterns.17 The most common area of discordance was in assigning the acinar vs lepidic predominant pattern. Reproducibility was best for a solid (κ=0.65), followed by micropapillary (κ=0.35), lepidic (κ=0.28), papillary (κ=0.2), and acinar (κ=0.08) patterns.17 In another study, the mean percentages of consensual votes per pattern ranged between 59.6% and 75.0%, with lepidic and solid being the pattern with the most discordant and concordant votes, respectively. Other patterns ranged in between (papillary 65.8%, acinar 67.8%, micropapillary 74.2%).14 Overall, the most frequent problems occurred in the separation of the lepidic from the acinar pattern (pre-existing alveolar structures with thickened septa versus neoplastic acini with desmoplastic stroma) and in the separation of papillary from lepidic (true papillae versus cross sections of branching alveolar walls) and micropapillary (often intermixed with papillary growth).14 Warth et al.7 found that patients with papillary predominant adenocarcinoma had survival rates similar to patients with micropapillary and solid predominant adenocarcinoma, in contrast to the other studies.5,6,8 They explained that these differences might be due to the fact that papillary adenocarcinomas are a rather diverse group with respect to morphology, and it is possible that the different subtypes also differ with respect to prognosis.7 Reproducibility evidently improves, following educational sessions, even among experienced lung pathologists.14 However, further investigation is needed to improve the separation of difficult problems, such as lepidic versus acinar or papillary and micropapillary versus papillary patterns.
Potential parameters for subtyping formulation
First, comprehensive subtyping, followed by selection of a predominant pattern, leads to some questions regarding the impact of minor patterns, even though the prognostic relevance of scores weighting secondary patterns was not evident and had no impact on survival in subgroups built by stratification for predominant patterns by Warth et al.7 Borczuk raised several seeming contradictory questions regarding secondary patterns at the 2013 United States & Canadian Academy of Pathology (USCAP) Companion meeting for the Pulmonary Pathology Society in Baltimore, MD, USA on March 2, 2013.27 For example, if solid and micropapillary patterns are poor prognostic patterns, for what percentage of a secondary pattern is this significant? If a micropapillary component is the critical one regardless of the percentage, should the primary classification be micropapillary adenocarcinoma, even if it is not predominant? All studies on the topic of the micropapillary component of lung adenocarcinoma in patients in early stages have reported that micropapillary is a poor prognostic subtype.28-31 Further research is needed to answer the question of whether, as in the Gleason score in prostate cancer, a minor pattern contributes a significant amount of prognostic information and whether scores weighting all patterns may be helpful.30 The clinical significance of the aggressive micropapillary or solid component, when present as minor component, is likely resolvable through a subgroup analysis and more precise percentage cut-offs, which is essential to classification and nomenclature.
A second concern is the relevance of the predominant label as currently formulated. In the new IASLC/ATS/ERS classification, using 5% increments allows for greater flexibility in choosing a predominant subtype when tumors have two patterns of relatively similar percentages.20 The predominant pattern is defined as the pattern with the largest percentage, not necessarily 50% or greater. Even though it is possible to have equal percentages of two prominent components, a single predominant component should be chosen. At the 2013 USCAP meeting, Borczuk also impugned the differences of the following three examples: acinar predominant (acinar 40%, papillary 30%, solid 30%), papillary predominant (papillary 40%, acinar 30%, solid 30%) and solid predominant (solid 40%, acinar 30%, papillary 30%).27 Does the last tumor have a poorer prognosis than the first two tumors?27 Another issue is that cases in which invasion is greater than 0.5 cm, but still less than 1 cm, in an otherwise lepidic tumor (75% or greater lepidic) are easier to accept as 'predominantly in situ' than those in which the lepidic pattern is predominant (40% lepidic with 20% acinar, 20% papillary, and 20% solid), which is actually 60% invasive and is 'invasive predominant.'27 Borczuk suggested that a middle ground between the lumping of the mixed subtype category by 2004 WHO classification4 and the splitting of comprehensive subtyping is warranted.27
Third, unlike carcinomas of the other organs, such as the breast, prostate, and kidney, there is no established grading system for lung adenocarcinoma in resected specimens. A proposed grading system based on a large Japanese study using 510 lung adenocarcinomas ≤2 cm in diameter has suggested the criteria of lymphovascular invasion, non-BAC or invasive component >1 cm, and solid/cribriform/papillary pattern >30% in the invasive component as predictors of patients outcome and tumor recurrence.32 Recently, it has been reported that not only are histologic subtype and mitotic rate important prognostic factors in lung adenocarcinomas, but also increased risk of recurrence was best predicted by a combined high architectural/mitotic grade after adjusting for clinical factors.9 In another study, nuclear grading based on nuclear area and nuclear dimension was a reliable prognostic indicator for small adenocarcinmas.33 Further investigation is needed to determine whether the optimal grading system should include architectural or nuclear assessment or both to add prognostic value to the predominant pattern.
Candidate for additional subtype
The occurrence of the other patterns that do not fit into these defined morphologies poses a challenge for applying this classification. Joubert et al.18 reported that ragged/fused glands and cribriform patterns were associated with a solid growth pattern, and disease-free survival for tumors containing these complex glandular patterns was similar to that of the high grade tumors. They emphasized that the presence of these variant patterns of adenocarcinoma should be recognized as a distinct subtype and may be considered as a pattern of high grade adenocarcinoma and not be interpreted as acinar adenocarcinoma, with a prognostically intermediate grade. According to the new IASLC/ATS/ERS classification definition, ragged-anastomosing glands and cribriform arrangements are also regarded as a pattern of acinar adenocarcinoma.3 The acinar pattern covers a broad category, such as tubules/glands lined by single-layered or polylayered neoplastic cells and ragged-anastomosing glands. Subclassification of the acinar predominant subtype based on its putative biological significance would further improve the prognostic value.
INVASION
Relevance
Since evidence for a category of MIA with 100% disease-free survival was found in 1995 by Noguchi et al.,34 subsequent studies have been performed such as Sakurai et al.'s study35 of 380 peripheral adenoacrinomas of ≤2 cm diameter and Suzuki et al.'s study36 of 100 lung adenocarcinomas of ≤3 cm and defined subsets of small lung adenocarcinomas associated with 100% disease-free survival using invasion size ≤0.5 cm in the area of BAC growth, respectively. According to the 199921 and 20044 WHO classification, which were strongly affected by the report by Noguchi et al.,34 BAC was strictly defined as an adenocarcinoma with a pure lepidic growth pattern without stromal, vascular, or pleural invasion. Nevertheless, the term BAC has been broadly used and BAC fall into five different entities in the new IASLC/ATS/ERS classification, including AIS, MIA, and lepidic predominant adenocarcinoma, and even other invasive adenocarcinomas with a lepidic component and mucinous adenocarcinoma. The widely varying clinical behavior of the different categories observed in several studies37-39 supports the rationale for the new classification discontinuing the use of the term BAC. Therefore, the newly proposed IASLC/ATS/ERS classification suggests that, in place of BAC, tumors should be subclassified as AIS, MIA, and adenocarcinoma with a lepidic prominent pattern.3
New concepts of AIS and MIA have been introduced to define the condition of patients who will have 100% or near 100% disease-specific survival, respectively, if they undergo complete resection. In the new IASLC/ATS/ERS classification, AIS is defined by a pure lepidic growth pattern with continuous growth of the neoplastic cells along the alveolar septa without disruption of the alveolar structures, except for mild fibrosis or enlargement of the alveolar wall, and lacking stromal, vascular, or pleural invasion, for the resected specimens.3 There should be no papillary or micropapillary patterns and intra-alveolar tumor cells. AIS is typically nonmucinous, consisting of type II pneumocytes and/or Clara cells, but cases of mucinous AIS rarely occur.3 The diagnosis of AIS cannot be established with certainty on cytology or small biopsy specimens. In addition, small tumors (≤3 cm) and tumors with a predominant lepidic pattern should be entirely submitted. It is emphasized that AIS should not be equated with tumors previously classified as BAC. The new term of MIA has been adopted for small (≤3 cm) solitary adenocarcinomas, showing a predominant lepidic pattern with ≤0.5 cm area of parenchymal invasion.3 If multiple microinvasive areas are found in one tumor, the greatest dimension in the largest invasive area should be measured, and it should be ≤0.5 cm in size. The size of invasion is not the summation of all invasive foci.3 The criteria for AIS, as well as MIA, can be applied in the setting of multiple tumors only if the other tumors are regarded as synchronous primary tumors rather than intrapulmonary metastases.3
Recent studies have demonstrated a near 100% 5-year disease-free survival or very favorable overall survival in patients with adenocarcinoma meeting the criteria of AIS and MIA in Western,5 Japanese,6,8 and Korean groups.40 In the future, such patients may represent candidates for limited surgical resection. Certainly, it is very important that AIS and MIA should not be mixed with forms of invasive lepidic predominant adenocarcinoma, because invasive adenocarcinomas with a lepidic predominant pattern still have a compromised prognosis when compared with AIS and MIA.
Emerging issues
Determination of invasion
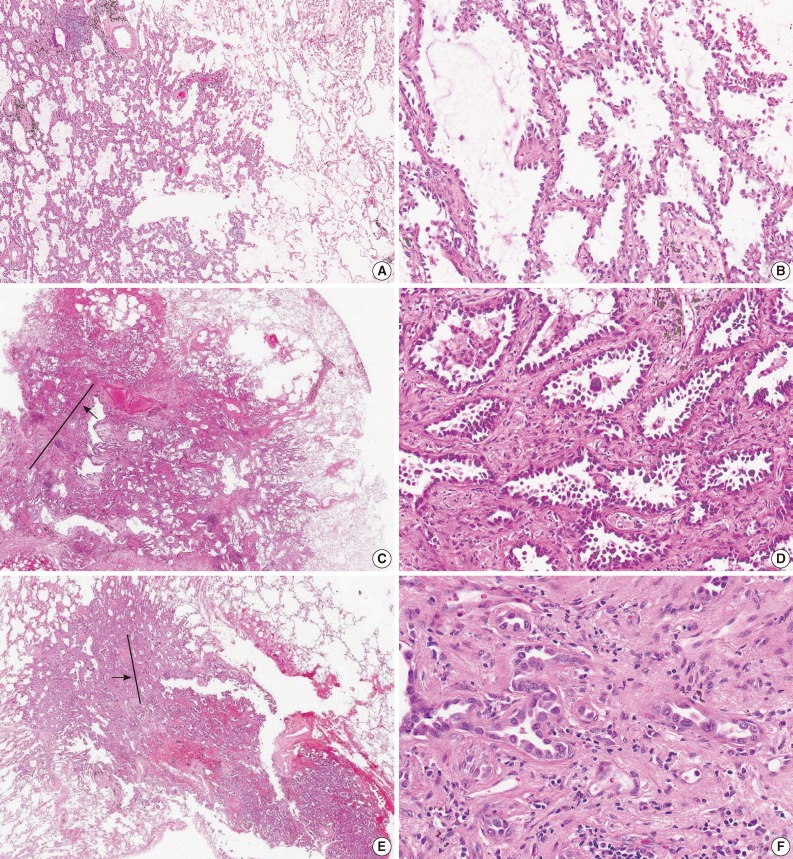
For the diagnosis for MIA, invasion is defined as 1) any histologic subtype other than a lepidic pattern (i.e., acinar, papillary, micropapillary, and/or solid) and/or 2) myofibroblastic stroma associated with invasive tumor cells.20 However, determination of parenchymal invasion is by far the most difficult issue in practical pathologic diagnosis (Fig. 1). Most of the difficulty comes from determining real stromal invasion from its mimickers, like alveolar collapse. AIS with sclerotic septal widening and alveolar collapse (acini of neoplastic cells can be entrapped within the fibrotic scar without eliciting a true desmoplastic stroma) may be difficult and arbitrary to distinguish from the acinar pattern, particularly, the nonmucinous variant.41 Noguchi et al.34 separated alveolar collapse with elastosis from invasion with fibroblast proliferation, emphasizing a good prognosis associated with the former. Furthermore, as adenocarcinoma grows into aerated, alveolar tissue, cross-cutting of growth along the alveolar walls will mimic the papillary structures, and desmoplastic reaction will produce acinar structures, which in reality are collapsed areas lacking invasion.13 This can be further complicated by pre-existing lung architectural changes, such as emphysema or interstitial fibrosis, and inconsistent use of the formalin inflation technique to fix the tumor specimens.13
Fig. 1.
(A) Nonmucinous adenocarcinoma in situ. (B) Tumor shows continuous growth of neoplastic cells along the slightly thickened alveolar septa without disruption of the alveolar structures. (C) Equivocal invasion showing alveolar collapse. Tumor has a central scar (arrow) with a peripheral lepidic growth pattern. (D) The central scar demonstrates thick fibrous septa with intact tumor glands, but less identifiable alveolar architecture and plump, reactive fibroblasts. Some pathologists interpreted a desmoplastic stroma associated with tumor invasion, whereas others considered the same features as benign scarring/fibroelastosis in Thunnissen's reproducibility study.13 (E) Nonmucinous minimally invasive adenocarcinoma. Tumor consists predominantly of lepidic growth with a small invasion focus (arrow). (F) Tumor acini and single cells are invading in the desmoplastic stroma with chronic inflammatory cells.
In a reproducibility study of invasion, the κ value for typical and difficult cases was 0.55±0.06 and 0.08±0.02, respectively, with consistent subdivision by the same pathologists into invasive and non-invasive categories, due to a differing interpretation of the terminology defining invasion.13 It was argued that several morphologic features could be attributed to discrepant interpretation in the judgment of invasion. First, some pathologists interpreted a stromal component as tumor-related stroma with fibroblasts (called desmoplastic stroma), whereas others considered the same features to be benign scarring/fibroelastosis. Second, the presence of elastin was variably weighted as representing the native alveolar wall by some pathologists, but not by others. Third, inflammation in alveolar walls implied invasive disease to some.13
To support the evaluation of parenchymal invasion, the use of elastic stains has been proposed to differentiate invasion (destruction of the elastic structure) from alveolar collapse (maintenance of the elastic structure). However, the routine use of elastic stains would probably add more confusion than clarity in distinguishing true invasion from architectural alterations in the elastic patterns of normal tissue.41 Furthermore, it has also been suggested that early invasion will not disturb the elastic framework of lung parenchyma. Special stains for basement membrane components, such as type IV collagen or laminin, have been reported to be of value, but are difficult to interpret and have not been shown to be useful.39
Given the areas of uncertainty for the diagnostic criteria of AIS and MIA, Andreia suggested that the diagnosis should be established based on the extent of possible invasion at the 2013 USCAP Companion meeting for the Pulmonary Pathology Society in Baltimore, MD, USA on March 2, 2013.27 If confronted with an area of uncertainty, either because of different histological pattern (papillary or acinar) that tends to mimic a lepidic pattern or an area of scar where invasion is not clear, this area of uncertainty is measured. If the area of possible invasion is larger than 0.5 cm, a diagnosis of invasive adenocarcinoma, predominant lepidic pattern is made. He insisted that these tumors are by definition only encountered as stage IA; therefore, patients will not be overtreated, but this approach gives the treating physicians some room for discussion on follow-up and further management.
Recently, Xu et al.41 suggested that the invasive components as defined by the loss of alveolar structures of the lepidic predominant tumor were any of the following three general patterns: 1) complex acinar papillary as defined by enlarged acini with no recognizable alveolar architecture, and septa between complex patterns about the same thickness as alveolar spaces with lepidic growth (around 40 to 60 µm); 2) invasion with desmoplasia and elastosis, with an acinar pattern with open lumina; and 3) invasion with desmoplasis and compressed, angulated glands or solid nests or single-cell invasion. However, more precise definitions and better education on the usage of existing terminology are required to improve the recognition of AIS or MIA, and this is becoming increasingly important.
Extent of invasion
The most current revision of tumor classification focuses on separating invasion tumors with prominent lepidic growth into two groups; those with minimal invasion, and those with invasion of more than 0.5 cm. Since the appearance of stromal invasion versus alveolar collapse is an area of great controversy among pathologists, the introduction of MIA as a new concept is of great assistance in this scenario. The new IASLC/ATS/ERS classification proposes that the greatest diameter of the invasive component determines MIAs.3 However, several emerging practical issues about the optimal method for evaluating the extent of the invasive component should be considered.
First, lepidic predominant adenocarcinoma was found to consist of invasive adenocarcinomas with at least 1 focus measuring >0.5 cm in the greatest dimension without defining the exact amount of lepidic growth required. Therefore, the difficulties inherent in invasion measurement affect only the separation of MIA from lepidic predominant adenocarcinoma. Anami et al.42 found that tumors with a >50% lepidic component were associated with better survival than were tumors with <50% lepidic growth. Other studies showed that invasive size adjustment of gross size may be a better predictor of survival than gross size alone in lepidic predominant adenocarcinomas.7,10,37 Nevertheless, the current guidelines adhere to gross tumor size to determine the size T factor. Although the 0.5 cm cutoff criteria has proven highly predictive of prognostic potential, further investigation is needed to determine whether the diagnosis of MIA is best made using a percentage of the invasive component adjustment of the gross size versus the single largest focus with a 0.5 cm cutoff size of the invasive component or any other size limit.20,37
Second, the impact of scars or prominent stromal desmoplasia and stromal inflammation should be evaluated in determining the size of the invasive component. Adding to the difficulty of demarcating invasion from non-invasion, elastosis may occur in the areas of lepidic spread and may be prominent in central scars which are clearly without invasive glands. Some authors have included the measurement of a scar associated with an invasive component as part of the measurement that defines MIA.35,39 However, this criterion is not mentioned in the consensus document.
Additional factors to be considered
There are a few points that need to be considered for the accurate differentiation of AIS and MIA from invasive adenocarcinoma.
First, the prognostic significance of aggressive micropapillary or solid components as invasive areas in tumors with MIA histology remains to be determined. Xu et al.41 reported that minimally invasive micropapillary adenocarcinoma showed an extensive lymphovascular invasion and lymph node involvement, and the patient died within 16 months with widespread metastases. They suggested that the presence of high grade histology, such as solid or micropapillary, could perhaps affect the prognosis, even in those with MIA.
Second, the new IASLC/ATS/ERS classification recommended that for a tumor larger than 3 cm, particularly if it has not been completely sampled, the term lepidic predominant adenocarcinoma is best applied with a comment that the clinical behavior is uncertain and/or that an invasive component cannot be excluded.3 However, there is insufficient evidence to support the notion that 100% disease-free survival can occur in such tumors with MIA histology >3 cm.
Third, lepidic growth may also be composed of neoplastic cells with nuclear atypia resembling that of the adjacent invasive patterns. Some observers would further argue that such lepidic patterns correspond to an aerogenous spread of tumor cells, but are no longer an in-situ phenomenon.43 Because it has not been established that this is a non-invasive lepidic growth or aerogenous spread of invasive carcinoma, the diagnosis of AIS or MIA should not be made unless the lesion has a discrete circumscribed border; cases with military spread of small foci of tumors into adjacent lung parenchyma and/or with lobar consolidation should be excluded.3 This observation is concerning, and data fine tuning the lepidic predominant percent cutoff associated with survival is an important future direction.
CONCLUSION
This review highlighted the recent evolution of the classification of lung adenocarcinomas in resected specimens with special respect to both histologic subtyping and invasion. Histological subtyping of lung adenocarcinoma has been updated based on five major predominant patterns. The new concepts of AIS and MIA have been introduced to define the condition of patients who will have excellent survival. Although the new IASLC/ATS/ERS classification has promising clinical relevance, significant clarification remains necessary for the designation of subtyping or invasion. Improving the clarity of this area, with more precise definitions and subsequent better education on the interpretation of existing terminologies are needed in future studies.
Acknowledgments
This work was supported by the Dong-A University research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Boyle P, Levin B. World cancer report 2008. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. [Google Scholar]
- 5.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 6.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 7.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 8.Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int. 2012;62:785–791. doi: 10.1111/pin.12016. [DOI] [PubMed] [Google Scholar]
- 9.von der Thüsen JH, Tham YS, Pattenden H, et al. Prognostic significance of predominant histologic pattern and nuclear grade in resected adenocarcinoma of the lung: potential parameters for a grading system. J Thorac Oncol. 2013;8:37–44. doi: 10.1097/JTO.0b013e318276274e. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa A, Sumiyoshi S, Moreira AL, Travis WD. Validation of the IASLC/ATS/ERS lung adenocarcinoma (ADC) classification and use of comprehensive histologic subtyping (CHS) for architectural grading in 432 Japanese patients. Mod Pathol. 2011;24(Suppl 1):429A. [Google Scholar]
- 11.Hwang I, Park KU, Kang YN, et al. Histologic patterns and prognostic significance of invasive adenocarcinoma of lung: analyzed by 2010 IASLC/ATS/ERS classification; The 63rd Annual Fall Meeting of the Korean Society of Pathologists; 2011 Oct 20-21; Seoul, Korea. The Korean Society of Pathologists; [Google Scholar]
- 12.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 13.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma: an international interobserver study. Mod Pathol. 2012;25:1574–1583. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Arch. 2012;461:185–193. doi: 10.1007/s00428-012-1263-6. [DOI] [PubMed] [Google Scholar]
- 15.Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J. 2012;40:1221–1227. doi: 10.1183/09031936.00219211. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Durra HY, Huang Y, Manucha V. Complex acinar pattern: a distinct morphologic subtype and a poor prognostic indicator in primary lung adenocarcinoma. Mod Pathol. 2013;26(Suppl 2):469A. [Google Scholar]
- 17.Wells JM, Mukhopadhyay S, Mani H. Application of the new proposed adenocarcinoma classification: a reproducibility study. Mod Pathol. 2013;26(Suppl 2):469A. [Google Scholar]
- 18.Joubert P, Rekhtman N, Moreira AL. Complex glands (cribriform and fused glands) are patterns of high grade adenocarcinoma in the lung. Mod Pathol. 2013;26(Suppl 2):456A. doi: 10.1016/j.humpath.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137:685–705. doi: 10.5858/arpa.2012-0264-RA. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological typing of lung and pleural tumours. 3rd ed. Berlin: Springer; 1999. [Google Scholar]
- 22.Shim HS, Lee DH, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med. 2011;135:1329–1334. doi: 10.5858/arpa.2010-0493-OA. [DOI] [PubMed] [Google Scholar]
- 23.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida A, Tsuta K, Watanabe S, et al. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer. 2011;72:309–315. doi: 10.1016/j.lungcan.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 25.McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol. 2012;7:348–354. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 26.Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer. 2012;76:403–409. doi: 10.1016/j.lungcan.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 27.USCAP. 102nd USCAP annual meeting [Internet] Augusta: United States & Canadian Academy of Pathology; [cited 2013 Jun 1]. Available from: http://www.uscap.org/index.htm?102nd/index.htm. [Google Scholar]
- 28.Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 31.De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol. 2009;131:694–700. doi: 10.1309/AJCPBS85VJEOBPDO. [DOI] [PubMed] [Google Scholar]
- 32.Maeshima AM, Tochigi N, Yoshida A, Asamura H, Tsuta K, Tsuda H. Histological scoring for small lung adenocarcinomas 2 cm or less in diameter: a reliable prognostic indicator. J Thorac Oncol. 2010;5:333–339. doi: 10.1097/JTO.0b013e3181c8cb95. [DOI] [PubMed] [Google Scholar]
- 33.Nakazato Y, Maeshima AM, Ishikawa Y, et al. Interobserver agreement in the nuclear grading of primary pulmonary adenocarcinoma. J Thorac Oncol. 2013;8:736–743. doi: 10.1097/JTO.0b013e318288dbd8. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung: histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–897. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 37.Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol. 2009;33:462–469. doi: 10.1097/PAS.0b013e318190157c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yim J, Zhu LC, Chiriboga L, Watson HN, Goldberg JD, Moreira AL. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol. 2007;20:233–241. doi: 10.1038/modpathol.3800734. [DOI] [PubMed] [Google Scholar]
- 39.Terasaki H, Niki T, Matsuno Y, et al. Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol. 2003;27:937–951. doi: 10.1097/00000478-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Ahn S, Hwangbo W, Kim H, Kim CH. Naked cuticle Drosophila 1 expression in histologic subtypes of small adenocarcinoma of the lung. Korean J Pathol. 2013;47:211–218. doi: 10.4132/KoreanJPathol.2013.47.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Tavora F, Battafarano R, Burke A. Adenocarcinomas with prominent lepidic spread: retrospective review applying new classification of the American Thoracic Society. Am J Surg Pathol. 2012;36:273–282. doi: 10.1097/PAS.0b013e31823b3eeb. [DOI] [PubMed] [Google Scholar]
- 42.Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol. 2009;4:951–958. doi: 10.1097/JTO.0b013e3181ad8631. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, Tavora F, Burke A. 'Bronchioloalveolar carcinoma': is the term really dead? A critical review of a new classification system for pulmonary adenocarcinomas. Pathology. 2012;44:497–505. doi: 10.1097/PAT.0b013e3283579fda. [DOI] [PubMed] [Google Scholar]