Abstract
Rhabdoid colorectal carcinomas are very rare and only 10 cases have been previously reported. We report two cases of rhabdoid colorectal carcinoma, one arising in the sigmoid colon of a 62-year-old man and another in the rectum of an 83-year-old woman. In both cases, the patients had advanced tumors with lymph node metastases. The tumors mostly showed a diffuse arrangement with rhabdoid features and small glandular regions were combined. Transitional areas from the adenocarcinomas to the rhabdoid tumors were also noted. Adenocarcinoma cells were positive for mixed cytokeratin (CK), CK20 and epithelial membranous antigen (EMA), but focal positive for vimentin. The rhabdoid tumor cells were positive for mixed CK, but focal positive or negative for CK20 and EMA. In addition, they were diffusely positive for vimentin, but negative for desmin. The histological and immunohistologial findings of these two cases suggest that the rhabodid tumor cells originated from dedifferentiated adenocarcinomas.
Keywords: Adenocarcinoma, Colon, Rectum, Rhabdoid tumor
Malignant rhabdoid tumor was first described in 1978 as a rare variant of Wilms tumor.1 This neoplasm was morphologically similar to rhabdomyoblast and had an extremely poor prognosis. Now it is known as malignant renal rhabdoid tumor, which is a distinct clinicopathologic entity from Wilms tumor.2 Tumors with similar clinicopathologic features have been reported in various extrarenal organs. Although these tumors, known malignant extrarenal rhabdoid tumors (MERTs), were reported to be as aggressive as their renal counterparts, the existence of MERTs as an independent disease entity remains controversial.3-5 Colorectal MERTs are very rare, with only 10 cases reported to date.6-15 Among these reports, six cases were pure rhabdoid tumors solely consisting of rhabdoid cells,6-11 and four cases were composite rhabdoid tumors coexisting with adenocarcinomas.12-15 Here, we describe two new cases of colorectal rhabdoid carcinomas arising in the sigmoid colon and rectum, and review the previously reported cases.
CASE REPORT
Case 1
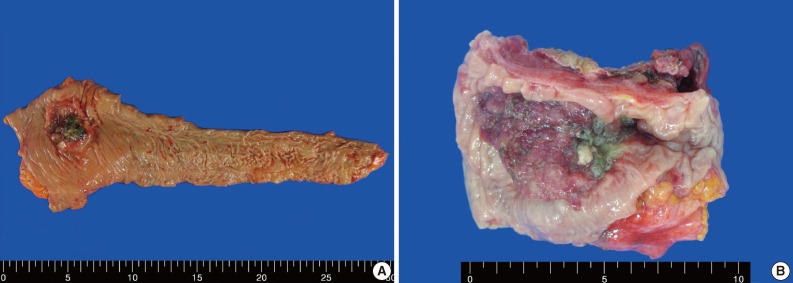
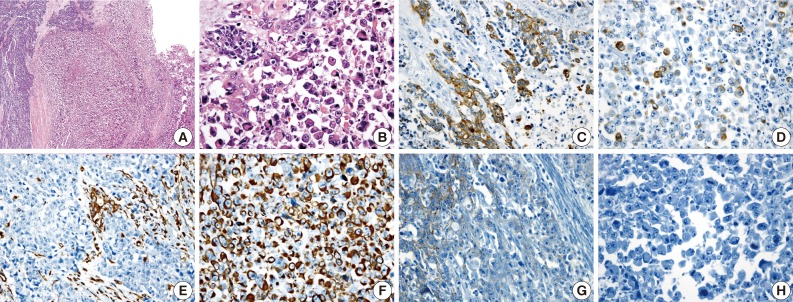
A 62-year-old man was admitted to Konkuk University Medical Center due to occult blood in his stool. He had a six-year history of hypertension and cerebrovascular attacks. Colonoscopic examination revealed a huge mass in the sigmoid colon. Abdominal computed tomography (CT) revealed an ulcerofungating mass in the sigmoid colon and multiple enlarged pericolic lymph nodes. There was no evidence of distant metastasis on chest CT and positron emission tomography-computed tomography (PET-CT). The serum level of cancer antigen 19-9 (CA19-9) was within the normal range (15.75 U/mL). Low anterior resection was performed on a 4.5×4.0×1.2 cm sized ulcerofungating mass in the sigmoid colon (Fig. 1). Microscopically, most of the tumor was composed of sheets of large round and polygonal nuclei with vesicular chromatin along with abundant acidophilic cytoplasm which often contained hyaline-like inclusions. A poorly differentiated adenocarcinoma component was found in the peripheral portion of the tumor, located in the mucosa and submucosa, taking up 5% of the tumor. A transitional area from the poorly differentiated adenocarcinoma to the rhabdoid tumor was noted (Fig. 2). The tumor invaded into the pericolic adipose tissue and 10 out of 24 regional lymph nodes showed metastases. Adenocarcinoma cells were positive for mixed cytokeratin (CK), CK20, and epithelial membranous antigen (EMA), but focally positive for vimentin (Fig. 2). The rhabdoid tumor cells were positive for mixed CK, but focally positive for CK20 and EMA. In addition, the rhabdoid tumor cells were diffusely positive for vimentin, but negative for desmin (Fig. 2). E-Cadherin immunoreactivity was attenuated in the adenocarcinoma cells and negative in the rhabdoid tumor cells (Fig. 2). Immunohistochemical stains for MLH1, MSH2, MSH6, and PMS2 were positive in both types of tumor cells, while the stains for p53 were negative in both types of tumor cells. Immunohistochemical stains for smooth muscle actin (SMA), CD45, CD99, CD117, synaptophysin, neuron-specific enolase (NSE), and human melanoma black 45 (HMB45) were also negative in both types of tumor cells. The patient was treated with 12 cycles of adjuvant chemotherapy (FOLFOX: folinic acid, fluorouracil, oxaliplatin) and was alive without evidence of recurrence or distant metastasis for 36 months after surgery.
Fig. 1.
Gross features of case 1 (A) and 2 (B). (A) An ulcerofungating mass arising in the sigmoid colon. (B) An ulcerofungating mass with luminal narrowing in the rectum.
Fig. 2.
(A) A poorly differentiated adenocarcinoma component (left) and a rhabdoid tumor component (right) are noted. (B) A transitional area from the poorly differentiated adenocarcinoma to the rhabdoid cells is noted. Adenocarcinoma cells are positive for cytokeratin 20 (C), but the rhabdoid tumor cells are focally positive (D). A few adenocarcinoma cells are positive for vimentin (E), but, the rhabdoid tumor cells are diffusely positive (F). E-cadherin immunoreactivity is attenuated in the adenocarcinoma cells (G) and negative in the rhabdoid tumor cells (H).
Case 2
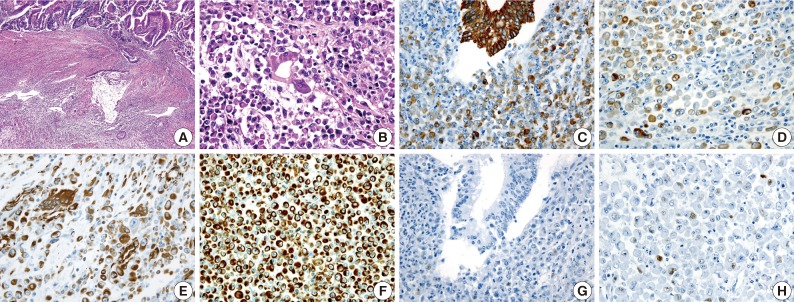
An 83-year-old woman with a history of hypertension was admitted to Konkuk University Medical Center for the further evaluation of rectal cancer. Colonoscopic examination revealed a huge mass in the rectum. Abdominal, chest CT and PET-CT revealed an ulcerofungating mass in the rectum with multiple metastatic lesions in the liver, lung, perirectal, para-aortic and left common iliac lymph nodes. The serum level of CA19-9 was elevated (60.50 U/mL). Lower anterior resection was performed. The tumor was an ulcerofungating mass, measuring 6.5×4.3×4.0 cm, in the rectum (Fig. 1). Microscopically, the tumor composed of sheets of rhabdoid cells, which comprised 70% of the tumor. The remaining portion of the tumor consisted of gland forming adenocarcinoma cells with villous adenoma. The tumor invaded into the mesosalpinx. Although there was no metastasis in 7 regional lymph nodes, 4 out of 4 omental lymph nodes showed metastases. Adenocarcinoma cells were positive for mixed CK, CK20, and EMA, but focal positive for vimentin (Fig. 3). The rhabdoid tumor cells were positive for mixed CK, but negative for CK20 and EMA. In addition, the rhabdoid tumor cells were diffusely positive for vimentin, but negative for desmin (Fig. 3). E-Cadherin immunoreactivity was attenuated in the adenocarcinoma cells and negative in the rhabdoid tumor cells. Immunohistochemical stains for MLH1, MSH2, MSH6, and PMS2 were positive in both types of tumor cells, while the stains for p53 was negative in the adenocarcinoma cells and focal positive in the rhabdoid tumor cells. Immunohistochemical stains for SMA, CD45, CD99, CD117, synaptophysin, and HMB45 were negative in both types of tumor cells, while the stains for NSE was weakly positive in the rhabdoid tumor cells. Unfortunately, the patient died a month after surgery.
Fig. 3.
(A) A gland-forming adenocarcinoma component (upper) and a rhaboid tumor component (lower) are noted. (B) A transition from the malignant gland to the rhabdoid cells is noted. Immunohistochemical stains for cytokeratin (C, D) and vimentin (E, F) are positive in both the adenocarcinoma component and the rhabdoid tumor component. The immunohistochemical stain for p53 is negative in the adenocarcinoma cells (G), but a small subset of the rhabdoid cells are positive (H).
DISCUSSION
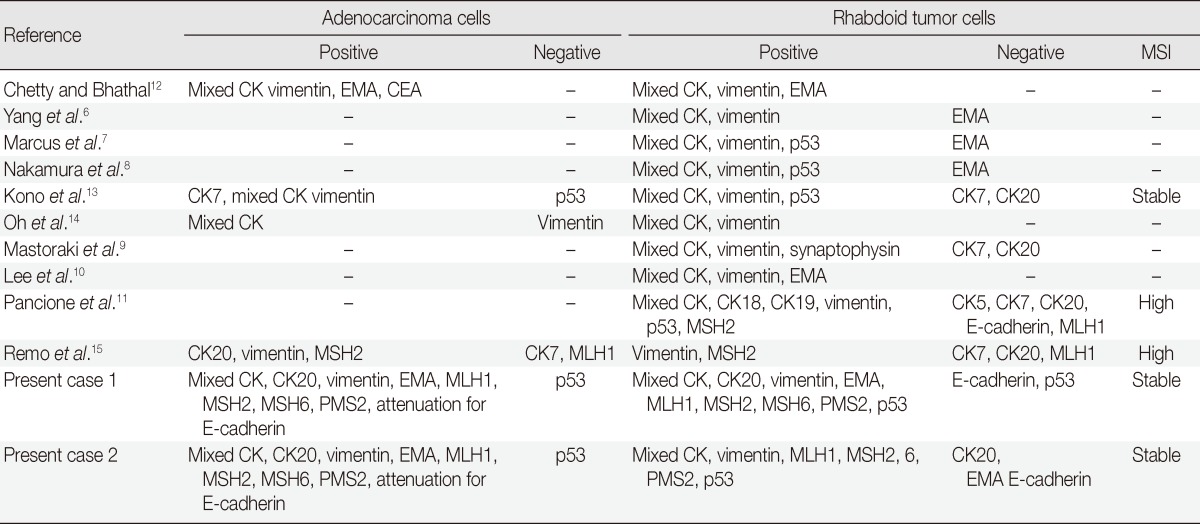
MERT is characterized by the peculiar morphologic features of the proliferating rhabdoid cells, which show an eccentrically located large nucleus with prominent nucleoli and a typical eosinophilic inclusion of aggregated intermediate filaments.16 Although Beckwith and Palmer,1 who described the first case, considered the neoplasm an unusual form of rhabdomyoid sarcoma, there was no ultrastructural or immunohistochemical evidence of myogenic differentiation, so the term "rhabdoid tumor" was adopted.1 Although the histogenesis of rhabdoid tumors is unknown, all reported cases share similar histological, immunohistochemical, and ultrastructural findings.3 The ultrastructural findings of the reported cases showed that the rhabdoid cells had eccentric nuclei containing prominent nucleoli and paranuclear inclusions composed of intermediate filaments of the mesenchymal cytoskeleton.8,9,12,13 Although the percentage of tumor cells with a rhabdoid morphology that are needed in a carcinoma to constitute a composite rhabdoid tumor is still up for debate, the percentage of rhabdoid tumor cells in previously reported cases have been greater than 60%.10,17 The percentage of rhabdoid tumor cells in cases 1 and 2 were 95% and 70%, respectively. Six cases of colorectal MERTs coexisting with adenocarcinoma, including the two cases presented here, suggest that the rhabdoid cells might have been derived from sarcomatous dedifferentiation of malignant epithelial cells.12 The rhabdoid cells in the pure rhabdoid tumors and those in the adenocarcinomas with rhabdoid phenotypes both showed similar morphologic features and immunohistochemical results (Table 1).6-13 In the present cases, the transition from the adenocarcinoma to the rhabdoid cells was noted. Adenocarcinoma cells were positive for mixed CK, CK20, and EMA, but focal positive for vimentin. The rhabdoid tumor cells were positive for mixed CK, but focal positive (case 1) or negative (case 2) for CK20 and EMA. In addition, the rhabdoid tumor cells were diffusely positive for vimentin, but negative for desmin. These results suggest that the rhabdoid tumor cells might have originated from dedifferentiated adenocarcinomas. E-Cadherin is a transmembrane protein confined to epithelial cells and is mainly responsible for the adherence junctions between them. In colon cancer, E-cadherin reduction appears to be associated with dedifferentiation, invasion, metastasis, and poor prognoses.18 In the two cases presented here, E-cadherin immunoreactivity was attenuated in the adenocarcinoma cells and negative in the rhabdoid tumor cells. These results also suggest that the rhabdoid tumor cells might have originated from dedifferentiated adenocarcinomas.
Table 1.
Immunohistochemical findings and microsatellite instability (MSI) results of colorectal malignant extrarenal rhabdoid tumors in the literatures
CK, cytokeratin; EMA, epithelial membranous antigen; CEA, carcinoembryonin antigen.
Colorectal MERTs are very rare and only 10 cases have been reported to date. These cases, as well as the cases presented here, are summarized in Table 2. The majority of MERTs usually occur in childhood, while colorectal MERTs occur in elderly patients. Many of these tumors have been reported to be huge and located proximal to the transverse colon, with extremely poor prognosis.6-15 Colorectal MERTs, including the cases presented here, affect older patients (mean age, 71.3 years; age range, 62-84 years) with no sex preference (male:female ratio=1:1). Of the 10 reported cases, 8 cases were located proximal to the transverse colon. The tumor sizes ranged between 3-15 cm in the largest diameter (mean size, 8.5 cm). Like other MERTs, MERTs of the gastrointestinal tract are rather aggressive and over 75% of the patients die within 6 months following the initial diagnosis.19 In the reported colorectal MERTs, 8 out of 10 patients died within 8 months following the initial diagnosis and Pancione et al.11 and Remo et al.15 reported that adjuvant chemotherapy had no significant benefits. One of the cases presented here also showed an extremely poor prognosis, although the patient in the other case responded to adjuvant chemotherapy and was alive without evidence of recurrence or distant metastasis for 36 months after surgery.
Table 2.
Clinical findings of colorectal malignant extrarenal rhabdoid tumors in the literatures
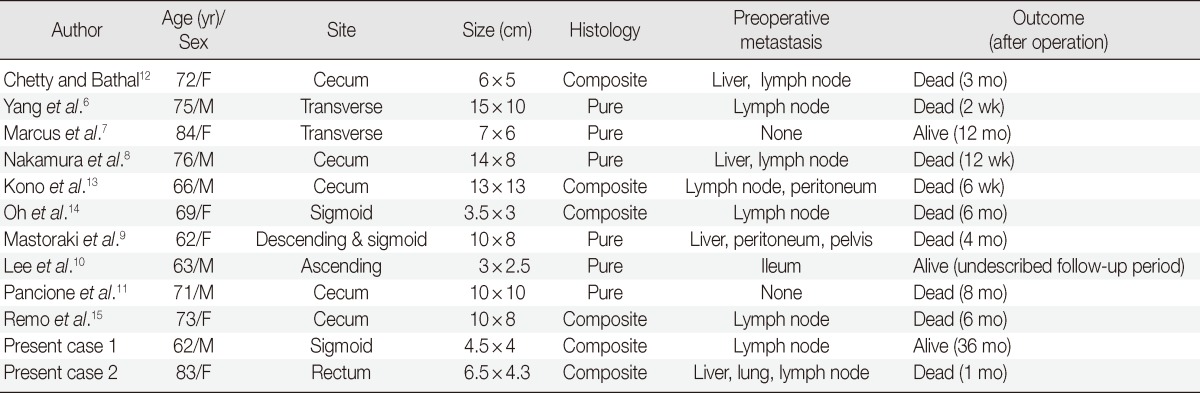
F, female; M, male.
Microsatellite instability (MSI) and p53 gene mutation are two major genetic abnormalities in colorectal cancers. Approximately 15% of all colorectal tumors have MSI and 75-80% of the tumors in this group have the acquired methylation of MLH1.20 MSI-high colorectal tumor are frequently observed in adenocarcinomas of the right sided colon, especially the cecum, of elderly patients.20 Of 10 reported cases, two cases were MSI-high tumors located at the cecum.11,15 One case was a MSI stable tumor13 and the MSI analysis results were not described in the remaining 7 cases. Immunohistochemical analysis of MLH1, MSH2, MSH6, and PMS2 proteins was equivalent in the levels of sensitivity and specificity compared with the MSI analysis.16 The two cases presented here all showed positive immunoreactivity for MLH1, MSH2, MSH6, and PMS2, and therefore might be MSI-stable tumors. There is a possibility that these two MSI-high tumors might not be associated with rhabdoid features but instead are associated with their right side colonic locations. The overexpression of the p53 protein, suggesting mutation of the p53 gene, was noted in four out of the 10 reported cases.7,8,11,13 In the remaining 6 cases, p53 protein results were not described. Kono et al.13 suggested a very restricted role of p53 mutations in the rhabdoid dedifferentiation of adenocarcinoma cells, because p53 protein was not observed in any of the adenocarcinoma cells and was noted in only a small subset of the rhabdoid tumor cells. We found similar p53 protein expression in case 2.
We described two new cases of rhabdoid colorectal carcinoma arising in the sigmoid colon and rectum and reviewed the previously reported cases. Histological and immunohistologial findings of these two cases suggested that the rhabodid tumor cells originated from dedifferentiated adenocarcinoma. These tumors showed no relationship with MSI. However, p53 protein overexpression in a small subset of the rhabdoid cells suggested a role of p53 mutation in the rhabdoid dedifferentiation of adenocarcinoma cells.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms' Tumor Study. Cancer. 1978;41:1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Palmer NF, Sutow W. Clinical aspects of the rhabdoid tumor of the kidney: a report of the National Wilms' Tumor Study Group. Med Pediatr Oncol. 1983;11:242–245. doi: 10.1002/mpo.2950110407. [DOI] [PubMed] [Google Scholar]
- 3.Wick MR, Ritter JH, Dehner LP. Malignant rhabdoid tumors: a clinicopathologic review and conceptual discussion. Semin Diagn Pathol. 1995;12:233–248. [PubMed] [Google Scholar]
- 4.Weeks DA, Beckwith JB, Mierau GW. Rhabdoid tumor. an entity or a phenotype? Arch Pathol Lab Med. 1989;113:113–114. [PubMed] [Google Scholar]
- 5.Ogino S, Ro TY, Redline RW. Malignant rhabdoid tumor: a phenotype? An entity? A controversy revisited. Adv Anat Pathol. 2000;7:181–190. doi: 10.1097/00125480-200007030-00007. [DOI] [PubMed] [Google Scholar]
- 6.Yang AH, Chen WY, Chiang H. Malignant rhabdoid tumour of colon. Histopathology. 1994;24:89–91. doi: 10.1111/j.1365-2559.1994.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 7.Marcus VA, Viloria J, Owen D, Tsao MS. Malignant rhabdoid tumor of the colon: report of a case with molecular analysis. Dis Colon Rectum. 1996;39:1322–1326. doi: 10.1007/BF02055131. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura I, Nakano K, Nakayama K, et al. Malignant rhabdoid tumor of the colon: report of a case. Surg Today. 1999;29:1083–1087. doi: 10.1007/s005950050649. [DOI] [PubMed] [Google Scholar]
- 9.Mastoraki A, Kotsilianou O, Papanikolaou IS, Foukas PG, Sakorafas G, Safioleas M. Malignant rhabdoid tumor of the large intestine. Int J Colorectal Dis. 2009;24:1357–1358. doi: 10.1007/s00384-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Kim TH, Ko DH, et al. Undifferentiated adenocarcinoma of the colon with rhabdoid features. Korean J Gastrointest Endosc. 2010;40:49–53. [Google Scholar]
- 11.Pancione M, Di Blasi A, Sabatino L, et al. A novel case of rhabdoid colon carcinoma associated with a positive CpG island methylator phenotype and BRAF mutation. Hum Pathol. 2011;42:1047–1052. doi: 10.1016/j.humpath.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Chetty R, Bhathal PS. Caecal adenocarcinoma with rhabdoid phenotype: an immunohistochemical and ultrastructural analysis. Virchows Arch A Pathol Anat Histopathol. 1993;422:179–182. doi: 10.1007/BF01607171. [DOI] [PubMed] [Google Scholar]
- 13.Kono T, Imai Y, Imura J, et al. Cecal adenocarcinoma with prominent rhabdoid feature: report of a case with immunohistochemical, ultrastructural, and molecular analyses. Int J Surg Pathol. 2007;15:414–420. doi: 10.1177/1066896907302418. [DOI] [PubMed] [Google Scholar]
- 14.Oh HK, Cho CH, Kum YS. Adenocarcinoma of the sigmoid colon with prominent rhabdoid features: a case report. Korean J Pathol. 2008;42:63–65. [Google Scholar]
- 15.Remo A, Zanella C, Molinari E, et al. Rhabdoid carcinoma of the colon: a distinct entity with a very aggressive behavior: a case report associated with a polyposis coli and review of the literature. Int J Surg Pathol. 2012;20:185–190. doi: 10.1177/1066896911415405. [DOI] [PubMed] [Google Scholar]
- 16.Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney: a distinctive renal tumor of children. Hum Pathol. 1981;12:646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- 17.Al-Nafussi A, O'Donnell M. Poorly differentiated adenocarcinoma with extensive rhabdoid differentiation: clinicopathological features of two cases arising in the gastrointestinal tract. Pathol Int. 1999;49:160–163. doi: 10.1046/j.1440-1827.1999.00839.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K. The E-cadherin adhesion molecule and colorectal cancer: a global literature approach. Anticancer Res. 2008;28:3815–3826. [PubMed] [Google Scholar]
- 19.Tóth L, Nemes Z, Gomba S, et al. Primary rhabdoid cancer of the ileum: a case report and review of the literature. Pathol Res Pract. 2010;206:110–115. doi: 10.1016/j.prp.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Pino MS, Chung DC. Microsatellite instability in the management of colorectal cancer. Expert Rev Gastroenterol Hepatol. 2011;5:385–399. doi: 10.1586/egh.11.25. [DOI] [PubMed] [Google Scholar]