Abstract
AIM
To describe chest radiographic abnormalities and assess their usefulness for predicting causes of fever in a resource-limited setting.
MATERIALS AND METHODS
Febrile patients were enrolled in Moshi, Tanzania, and chest radiographs were evaluated by radiologists in Tanzania and the United States. Radiologists were blinded to the results of extensive laboratory evaluations to determine the cause of disease.
RESULTS
Of 870 febrile patients, 515 (59.2%) had a chest radiograph available; including 268 (66.5%) of the adults and adolescents, the remainder were children and infants. One hundred and nineteen (44.4%) adults and 51 (20.6%) children were human immunodeficiency virus (HIV)-infected. Among adults, radiographic abnormalities were present in 139 (51.9%), including 77 (28.7%) with homogeneous and heterogeneous lung opacities, 26 (9.7%) with lung nodules, 25 (9.3%) with pleural effusion, 23 (8.6%) with cardiomegaly, and 13 (4.9%) with lymphadenopathy. Among children, radiographic abnormalities were present in 87 (35.2%), including 76 (30.8%) with homogeneous and heterogeneous lung opacities and six (2.4%) with lymphadenopathy. Among adults and adolescents, the presence of opacities was predictive of Streptococcus pneumoniae and Coxiella burnetii, whereas the presence of pulmonary nodules was predictive of Histoplasma capsulatum and Cryptococcus neoformans.
CONCLUSIONS
Chest radiograph abnormalities among febrile inpatients are common in northern Tanzania. Chest radiography is a useful adjunct for establishing a causal diagnosis of febrile illness and may provide useful information for patient management, in particular for pneumococcal disease, Q fever, and fungal infections.
Keywords: Tanzania, thoracic radiography, thorax, etiology, HIV
INTRODUCTION
Febrile illness is a common condition among adults and children seeking hospital care in Africa.1-5 Human immunodeficiency virus (HIV)-associated conditions and other infectious diseases are common and have been leading causes for hospital admission.5 Despite the burden of infectious diseases, diagnostic laboratory services are limited6 and often clinicians must rely on their clinical diagnosis. In these circumstances, inaccurate diagnosis may lead to over-treatment of some conditions and inadequate treatment of others.7 This can result in poor outcomes and increased morbidity and mortality.5,7
In addition to HIV infection, opportunistic diseases associated with immune suppression, such as bacterial pneumonias, tuberculosis, invasive salmonelloses,3,5 bacterial zoonoses, and fungal diseases,5 may be encountered in febrile patients. Chest films will frequently demonstrate abnormalities associated with these conditions and may play an important role in diagnosing pulmonary disease, providing clues to the cause and influencing empiric and specific treatment.8,9 Chest radiography may be a useful adjunct along with clinical history and physical examination in providing diagnoses, especially when laboratory confirmation is not possible.
The purpose of the present study was to describe the spectrum of radiographic abnormalities among febrile hospitalized patients in northern Tanzania, an area with a generalized HIV epidemic and a range of other endemic infections. The role of chest radiography in predicting the cause of specific lung diseases was also examined.
MATERIALS AND METHODS
Study population
The study was conducted at Kilimanjaro Christian Medical Centre (KCMC) and Mawenzi Regional Hospital (MRH) in Moshi, Tanzania, from September 2007 through August 2008. Eligible participants (adults ≥13 years and children <13 years) included those admitted to the medical wards with an oral temperature ≥38°C. Patients were screened and enrolled on the day of hospital admission. Informed consent was obtained from either the consenting adult patient or the parent or guardian of the child. A standardized clinical history and physical examination were done. Blood was collected for HIV testing, blood culture, and malaria smear. Acute and convalescent serum was collected along with a urine sample. A posterior–anterior chest radiograph was obtained. Patients underwent HIV pre-test counselling. Further details of the study have been described elsewhere.10–14
Radiology
A posterior–anterior chest radiograph was taken by trained radiographers at KCMC and MRH. All chest radiographs were independently evaluated by radiologists at KCMC (H.C.D.) and Duke University Medical Center (DUMC; P.C.G.). If there was disagreement between evaluations, the radiograph was re-evaluated by the DUMC radiologist and a consensus was reached. Both radiologists were initially blinded to all clinical, laboratory, and diagnosis results. An assessment was made of the quality of the radiograph, and the absence or presence and extent of pulmonary abnormalities, including heterogeneous and homogeneous opacification, nodules, cavitation, and pleural effusion, was recorded, and the number and distribution of lobes affected. In addition, pleural and mediastinal abnormalities were also recorded. Homogeneous opacification is defined by the overall general uniformity in “whiteness” and indistinct margins throughout the abnormality, as often seen in the air bronchograms. Heterogeneous opacification is defined as non-uniformity in “whiteness” and is generally seen as a combination of linear, reticular (cross-hatched), and/or small nodular opacities throughout the abnormality.
Laboratory methods
HIV antibody testing was performed on whole blood using both Capillus (Trinity Biotech, Bray, Wicklow, Ireland) and Determine (Abbott Laboratories, Abbott Park, IL, USA) rapid HIV antibody tests. After March 2008, Capillus was replaced with SD Bioline HIV-1/2 3.0 (Standard Diagnostics, Kyonggi-do, Korea). If the test results were discordant, a blood sample was tested with enzyme-linked immunosorbent assay (ELISA; Vironostika Uni-Form II plus O Ab, bioMérieux, Durham, NC, USA). If the ELISA was negative, no additional testing was done. If the ELISA was positive, a Western blot (Genetic Systems HIV-1 Western Blot kit, Bio-Rad, Hercules, CA, USA) was undertaken to confirm the result.15
Bloodstream infections were detected by blood culture using the bioMérieux BacT/ALERT standard aerobic (SA) bottle and mycobacterial (MB) bottle for adults and adolescents, and the paediatric fan bottle for infants and children. Bottles were evaluated for volume and incubated in the BacT/ALERT 3D Microbial Detection system (bioMérieux). Positive bottles were evaluated further by standard methods. Results of direct examination and identity of isolates were reported.
Urine was collected and tested for Histoplasma capsulatum using the MVista H. capsulatum Quantitative Antigen EIA (Miravista Diagnostics, Indianapolis, IN, USA).16 Urine was also tested for Streptococcus pneumoniae using the Binax NOW S. pneumoniae Antigen Test (Binax, Scarborough, ME, USA). Malarial parasites were sought and identified using thick and thin blood films stained with Giemsa and examined for blood parasites by oil immersion microscopy.
Serum samples collected for serological investigation for Coxiella burnetii, spotted fever group rickettsioses (SFGR), and typhus group rickettsioses (TGR) were evaluated at the Rickettsial Zoonosis Branch of the US Centers for Disease Control and Prevention (CDC). For C. burnetii, sera were screened using C. burnetii IgG ELISA against the phase II antigen (Inverness Medical Innovations). If positive or indeterminate, paired sera were tested by indirect immunofluorescence antibody (IFA) IgG assay to C. burnetii phase I and phase II antigens. A fourfold or greater increase in IFA reciprocal titre to phase II antigen defined acute C. burnetii. For SFGR and TGR, sera were tested by IgG IFA to Rickettsia conorii (Moroccan strain) and to Rickettsia typhi (Wilmington strain), respectively. Among paired sera, a fourfold or greater increase in IFA titre defined infection. 12
Leptospirosis laboratory diagnosis was undertaken using the standard microscopic agglutination test (MAT) and performed at the CDC. Live leptospiral cell suspensions were incubated with serially diluted serum specimens. Resulting agglutination titres were read using dark-field microscopy. The reported titre was the highest dilution of serum that agglutinated at least 50% of the cells. Among paired sera, a fourfold or greater increase in the MAT titre defined infection.13,17
Brucellosis laboratory diagnosis was made using the standard MAT and performed at the CDC. Standardized B. abortus strain 1119-3 killed antigen (National Veterinary Services Laboratory, Ames, IA, USA) was used for the MAT at a 1:25 working dilution. Among paired sera, a fourfold or greater increase in the MAT titre defined infection.18
Diagnosis of H. capsulatum or S. pneumoniae disease was determined either by positive urine antigen test or blood culture.19 Malaria was identified by positive smear. Cryptococcal antigen level was measured using the Latex Cryptococcal Antigen Detection System assay (Immuno-Mycologics, Norman, OK, USA).10,11 Other diagnoses were determined by positive blood culture. Sputum specimens were not collected; therefore, a positive result for M. tuberculosis was determined by blood culture and defined as disseminated disease. History of Kaposi sarcoma (KS) was determined by clinical evaluation.
Statistical analysis
Descriptive analyses were performed separately on both cohorts. However, subsequent analyses were performed only on the adult and adolescent cohort due to limited substantial findings in the infant and child cohort. Univariate analyses were performed using Fisher's exact tests. To determine potential predictors of pulmonary disease, patients with the specific pulmonary abnormality were compared to those without the specific abnormality. The p-values were based on two-tailed test results, and a p-value ≤0.05 was used to define statistical significance. Odds ratios and 95% confidence intervals were calculated to evaluate the univariate risk for having a specific radiographic abnormality due to disease diagnosis. A multivariate logistic regression model was undertaken to identify predictors of pulmonary disease after adjusting for potential confounding (age and HIV infection status). Statistical analyses were conducted using SAS, Version 9.2 (SAS Institute, Cary, NC, USA).
Research ethics
This study was approved by the KCMC Ethics Committee, the Tanzania National Institutes for Medical Research (NIMR) Ethics Committee, and the DUMC Institutional Review Board (IRB).
RESULTS
Participant characteristics
Overall, 870 participants were enrolled including 403 (46.3%) adults and adolescents, the remainder were children and infants. Chest radiographs were available for 515 (59.2%) participants, including 268 (66.5%) of the adults and adolescents. Among adults and adolescents, the median age (range) of the participants with a chest radiograph was 38 years (range 14–86) years, 139 (51.9%) were female, and 119 (44.4%) were HIV infected. Of HIV-infected participants, the median CD4+ T-lymphocyte count (CD4 count) was 98.5 (range 1–1105) cells/mm3. Not including HIV infection, laboratory evaluations established diagnoses among 105 (39.2%) participants; 84 (31.3%) had one infection, 18 (6.7%) had two infections, and three (1.1%) had three infections.
Among 467 children and infants, chest radiographs were available for evaluation for 247 (52.9%). The median age was 1.3 (range 0.2–13) years, 93 (37.7%) were less than 1 year of age, 104 (42.1%) were female, and 51 (20.6%) were HIV-infected. Of HIV-infected participants, the median CD4 count was 380 (range 2–3959) cell/mm3 and percent CD4 was 15% (range 0–56&), respectively. Not including HIV infection, laboratory evaluations established a diagnosis among 34 (13.8%) participants.
Radiographic findings and infectious causes
Among adults and adolescents, 139 (51.9%) were reported to have a radiographic abnormality. Specific abnormalities included 56 (20.9%) with homogeneous opacities, 48 (18%) with heterogeneous opacities, 26 (9.7%) with pulmonary nodules, 25 (9.3%) with pleural effusion, 23 (8.6%) with cardiomegaly, 13 (4.9%) with lymphadenopathy, and 12 (4.5%) with cavitation. Sixty-six (24.7%) participants had unilateral or lobar homogeneous or heterogeneous opacities, whereas 38 (14.2%) had bilateral or perihilar homogeneous or heterogeneous opacities. Lower lobe involvement was the most common, followed by mixed lobe involvement (Table 1).
Table 1.
Chest radiographic abnormalities among febrile inpatients, northern Tanzania, 2007–2008
| Adult and adolescents n (%a) | Children and infants n (%a) | |
|---|---|---|
| Any chest radiographic abnormality | 139 (51.9) | 87 (35.2) |
| Any opacity | 77 (28.7) | 76 (30.8) |
| Homogeneous opacities | ||
| Unilateral or lobar | 42 (15.7) | 39 (15.8) |
| Bilateral or perihilar | 14 (5.2) | 8 (3.2) |
| Heterogeneous opacities | ||
| Unilateral or lobar | 24 (9) | 31 (12.6) |
| Bilateral or perihilar | 24 (9) | 10 (4) |
| Lobe involvement | ||
| Upper lobes only | 9 (3.4) | 21 (8.5) |
| Lower lobes only | 32 (11.9) | 27 (10.9) |
| Middle lobe only | 3 (1.1) | 0 (0) |
| Mixed lobes | 29 (10.8) | 18 (7.3) |
| Other abnormalities | ||
| Nodules | 26 (9.7) | 1 (0.4) |
| Pleural effusion | 25 (9.3) | 4 (1.6) |
| Cardiomegaly | 23 (8.6) | 5 (2) |
| Lymphadenopathy | 13 (4.9) | 6 (2.4) |
| Cavitation | 12 (4.5) | -- |
| Hyperinflation | 11 (4.1) | 7 (2.8) |
| Large pulmonary arteries | 4 (1.5) | -- |
| Bronchiectasis | 2 (0.7) | 1 (0.4) |
| Mass | 2 (0.7) | 1 (0.4) |
Percent (%) based on all participants who had a chest radiograph
Among children and infants, 87 (35.2%) had a radiographic abnormality. Specific abnormalities included 47 (19%) with homogeneous opacities and 41 (16.6%) with heterogeneous opacities. Lower lobe involvement was most common. Other abnormalities included six (2.4%) with lymphadenopathy, five (2%) with cardiomegaly, and four (1.6%) with pleural effusion. Seventy (28.4%) had unilateral or lobar homogeneous or heterogeneous opacities, whereas 18 (7.2%) had bilateral or perihilar homogeneous or heterogeneous opacities (Table 1).
Among adults and adolescents, 129 diagnoses other than HIV infection were identified. Bacterial diagnoses included S. pneumoniae in 20 (7.5%), leptospirosis in 13 (9.1%), C. burnetii in 13 (9.2%), SFGR in 13 (9.2%), and Salmonella serovar Typhi in 12 (4.5%). These diagnoses accounted for 71 (55%) of all diagnoses other than HIV infection. Nine (7.5%) participants reported a prior a history of KS. C. neoformans was identified in 13 (4.9%), disseminated M. tuberculosis in eight (3%), and malaria in eight (3%) participants. As expected, the fungal infections, C. neoformans and H. capsulatum, along with disseminated M. tuberculosis and prior history of KS have a high likelihood of HIV co-infection (Table 2). Among children and infants, 34 diagnoses other than HIV infection were identified and included SFGR in 13 (9.2%) and leptospirosis in 10 (6.9%) (Table 3). Although not confirmed microbiologically, based on radiographic findings, the radiologist suspected pulmonary tuberculosis in 22 (8.2%) adults and adolescents and six (2.4%) infants and children.
Table 2.
Laboratory diagnoses and chest radiographic abnormalities among febrile adult and adolescent inpatients, northern Tanzania, 2007–2008
| Laboratory diagnoses | No. diagnosed/no. tested | No. HIV co-infecteda | Any CXR abnormality n (%b) | Any opacity n (%b) | Nodules n (%b) | Pleural effusion n (%b) | Cavitation n (%b) | Lymphadenopathy n (%b) |
|---|---|---|---|---|---|---|---|---|
| HIV infection | 119 / 268 | -- | 79 (66.4) | 48 (40.3) | 16 (13.4) | 14 (11.8) | 5 (4.2) | 11 (9.2) |
| Streptococcus pneumoniae | 20 / 267 | 14 (70) | 15 (75) | 11 (55) | 1 (5) | 2 (10) | 1 (5) | -- |
| Coxiella burnetii | 13 / 142 | 6 (46.2) | 9 (69.2) | 7 (53.8) | 1 (7.7) | 1 (7.7) | 2 (15.4) | -- |
| Leptospira spp. | 13 / 143 | 2 (15.3) | 6 (46.2) | 3 (23.1) | 1 (7.7) | 1 (7.7) | 1 (7.7) | -- |
| SFGR | 13 / 142 | 2 (15.3) | 8 (61.5) | 6 (46.2) | 1 (7.7) | 1 (7.7) | 2 (15.4) | |
| Cryptococcus neoformans | 13 / 267 | 13 (100) | 9 (69.2) | 3 (23.1) | 4 (30.8) | 2 (15.4) | 1 (7.7) | -- |
| Salmonella Typhi | 12 / 267 | 0 (0) | 1 (8.3) | -- | 1 (8.3) | -- | 1 (8.3) | 2 (16.7) |
| Kaposi sarcoma | 9 / 120 | 9 (100) | 8 (88.9) | 6 (66.7) | 3 (33.3) | -- | -- | 2 (22.2) |
| Mycobacterium tuberculosis | 8 / 267 | 8 (100) | 7 (87.5) | 4 (50) | 1 (12.5) | 3 (37.5) | 1 (12.5) | -- |
| Plasmodium spp. | 8 / 266 | 0 (0) | 1 (12.5) | -- | -- | 1 (12.5) | -- | -- |
| Brucella spp. | 7 / 143 | 1 (14.3) | 3 (42.9) | 2 (28.6) | 1 (14.3) | 1 (14.3) | -- | 1 (14.3) |
| Escherichia coli | 4 / 267 | 3 (75) | 1 (25) | 1 (25) | -- | -- | -- | -- |
| Histoplasma capsulatum | 4 / 212 | 3 (75) | 3 (75) | 1 (25) | 2 (50) | -- | -- | 1 (25) |
| Staphylococcus aureus | 3 / 267 | 1 (33.3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | -- | -- | -- |
| TGR | 2 / 142 | 1 (50) | -- | -- | -- | -- | -- |
Percent (%) based on number HIV-infected diagnosed with condition (co-infection) by total number diagnosed.
Percent (%) based on number with specific abnormality by number of diagnosed with condition.
HIV, human immunodeficiency virus; SFGR, spotted fever group rickettsioses; TGR, typhus group rickettsioses.
Table 3.
Laboratory diagnoses of and chest radiographic abnormalities among febrile children and infants inpatients, northern Tanzania, 2007–2008
| Laboratory diagnoses | No. diagnosed/no. tested | No. HIV co-infecteda | Any CXF abnormality n (%b) | Any opacity n (%b) | Nodules n (%b) | Pleural effusion n (%b) | Cavitation n (%b) | Lymphadenopathy n (%b) |
|---|---|---|---|---|---|---|---|---|
| HIV infection | 55 / 247 | -- | 32 (58.2) | 30 (54.5) | 1 (1.8) | 1 (1.8) | -- | 4 (7.3) |
| SFGR | 13 / 141 | 2 (15.4) | 5 (38.5) | 5 (38.5) | -- | -- | -- | 1 (7.7) |
| Leptospira spp. | 10 / 145 | 3 (33.3) | 3 (30) | 3 (30) | -- | -- | -- | 1 (10) |
| Coxiella burnetii | 3 / 142 | 1 (33.3) | 1 (33.3) | -- | -- | -- | -- | -- |
| Plasmodium spp. | 3 / 245 | 0 (0) | 1 (33.3) | 1 (33.3) | -- | -- | -- | -- |
| Cryptococcus neoformans | 1 / 246 | 1 (100) | -- | -- | -- | -- | -- | -- |
| Brucella spp. | 2 / 147 | 0 (0) | 1 (50) | 1 (50) | -- | -- | -- | -- |
| Histoplasma capsulatum | 1 / 175 | 0 (0) | -- | -- | -- | -- | -- | -- |
| Streptococcus pneumoniae | 1 / 246 | 1 (100) | -- | -- | -- | -- | -- | -- |
Percent (%) based on number HIV-infected diagnosed with condition (co-infection) by total number diagnosed.
Percent (%) based on number with specific abnormality by number of diagnosed with condition.
HIV, human immunodeficiency virus; SFGR, spotted fever group rickettsioses.
Homogeneous and heterogeneous opacities were the most common abnormality and were seen in varied proportions of patients who had specific diagnoses established. Among adults and adolescents, these opacities were identified in six (66.7%) patients with a history of KS, 11 (55%) with S. pneumoniae, seven (53.8%) with C. burnetii, and four (50%) with M. tuberculosis (Table 2). Among children and infants, these opacities were identified in 30 (54.5%) with HIV infection and five (38.5%) with SFGR (Table 3).
Among adults and adolescents, the presence of other lung abnormalities was associated with a laboratory-confirmed diagnosis of specific infections. Pulmonary nodules were associated with a diagnosis of H. capsulatum, C. neoformans, and history of KS. Pleural effusion was associated with a diagnosis of disseminated M. tuberculosis; and lymphadenopathy was associated with HIV infection, M. tuberculosis, and a history of KS (Table 4).
Table 4.
Univariate analysis of participant characteristics and diagnoses associated with radiographic abnormalities in febrile adult and adolescent inpatients, northern Tanzania, 2007–2008
| Any CXR abnormality OR (95% CI) | Any opacity OR (95% CI) | Nodules OR (95% CI) | Pleural effusion OR (95% CI) | Cavitation OR (95% CI) | Lymphadenopathy OR (95% CI) | |
|---|---|---|---|---|---|---|
| Age (≥50 years) | 2.4 (1.3, 4.3)a | 1.4 (0.8, 2.5) | 0.5 (0.2, 1.6) | 1.2 (0.5, 3.1) | 1 (0.3, 4) | 0.3 (0, 2) |
| Sex (male) | 0.9 (0.5, 1.4) | 0.9 (0.6, 1.6) | 2.3 (0.9, 5.4) | 0.8 (0.4, 1.9) | 0.7 (0.2, 2.1) | 0.9 (0.3, 2.9) |
| HIV infection | 2.9 (1.8, 4.8)a | 2.8 (1.6, 4.8)a | 2.2 (0.9, 5) | 1.7 (0.7, 3.8) | 0.9 (0.3, 2.9) | 6.7 (1.4, 31.4)a |
| Streptococcus pneumoniae | 3 (1.1. 8.6) | 3.4 (1.3, 8.5)a | 0.5 (0.1, 3.6) | 1.1 (0.2, 5) | 1.1 (0.1, 9.2) | -- |
| Leptospirosis | 0.8 (0.3, 2.4) | 0.7 (0.2, 2.7) | 0.8 (0.1, 6.1) | 0.8 (0.2, 6.3) | 1.8 (0.2, 15.5) | -- |
| Coxiella burnetii | 2.2 (0.6, 7.2) | 3.1 (1, 9.5)a | 0.8 (0.1, 6.1) | 0.8 (0.1, 6.4) | 4.5 (0.9, 22.8) | -- |
| Salmonella Typhi | 0.1 (0, 0.6)a | -- | 0.8 (0.1, 6.8) | -- | 2 (0.2, 17.1) | -- |
| SFGR | 1.5 (0.5, 4.8) | 2.2 (0.7, 6.8) | 0.8 (0.1, 6.1) | 0.8 (0.1, 6.4) | 1.8 (0.2, 15.5) | 1.8 (0.2, 15.5) |
| Cryptococcus neoformans | 2.2 (0.6, 7.2) | 0.7 (0.2, 2.7) | 4.7 (1.3, 16.5)a | 1.8 (0.4, 8.8) | 1.8 (0.2, 15.5) | 4.5 (0.9, 22.8) |
| Kaposi sarcoma | 7.8 (1, 63.4)a | 5.3 (1.3, 21.7)a | 5.1 (1.2, 21.9)a | -- | -- | 7.1 (1.3, 38.7)a |
| Mycobacterium tuberculosis | 6.8 (0.8, 56) | 2.6 (0.6, 10.5) | 1.3 (0.2, 11.4) | 6.5 (1.5, 29)a | 3.2 (0.4, 28.6) | 8.3 (1.5, 46.6)a |
| Malaria | 0.1 (0, 1) | -- | -- | 1.4 (0.2, 11.9) | -- | -- |
| Brucellosis | 0.7 (0.2, 3.1) | 1 (0.2, 5.2) | 1.6 (0.2, 13.6) | 1.6 (0.2, 14.2) | -- | -- |
| Escherichia coli | 0.3 (0, 3) | 0.8 (0.1, 8.1) | -- | -- | -- | 7.7 (0.7, 79.8) |
| Histoplasma capsulatum | 2.8 (0.3, 27.5) | 0.8 (0.1, 8) | 10 (1.3, 74.2)a | -- | -- | -- |
| Staphylococcus aureus | 1.9 (0.2, 21) | 1.2 (0.1, 13.9) | 4.8 (0.4, 54.8) | -- | -- | 11.5 (1, 137.2) |
| TGR | -- | -- | -- | -- | -- | -- |
p≤0.05
OR, odds ratio; 95% CI, 95% confidence interval; HIV, human immunodeficiency virus; SFGR, spotted fever group rickettsioses; TGR, typhus group rickettsioses.
After adjusting for potential confounders of older age and HIV status, the presence of homogeneous or heterogeneous opacities were predictive of S. pneumoniae (aOR 2.8; 95% CI 1.1–7.2) and C. burnetii (aOR 3.6; 95% CI 1.1–11.8) infection. The presence of pulmonary nodules were predictive of H. capsulatum (aOR 7.8; 95% CI 1–59.5) and C. neoformans (aOR 3.8; 95% CI 1–14.5) infection. Pleural effusion was predictive of having disseminated tuberculosis (aOR 5.6; 95% CI 1.2–26.8). The positive predictive value (PPV), the probability of the diagnosis when the specific radiographic abnormality was present, ranged from 9.5% to 21.9%. (Table 5) The radiographic appearance of pulmonary nodules and lung opacities are described in Figs. 1 and 2.
Table 5.
Multivariable analysis of predictors of pulmonary disease among febrile adult and adolescent inpatients, northern Tanzania, 2007–2008
| Radiographic finding | Laboratory diagnosis | Adjusted ORa (CI 95%) | p-Value | PPV (CI 95%) |
|---|---|---|---|---|
| Any opacity | Streptococcus pneumoniae | 2.8 (1.1, 7.2) | 0.03 | 14.5 (7.5, 24.4) |
| Coxiella burnetii | 3.6 (1.1, 11.8) | 0.03 | 21.9 (9.3, 40) | |
| Kaposi sarcoma | 3.2 (0.8, 13.6) | 0.11 | 14.6 (5.6, 29.2) | |
| Pulmonary nodules | Histoplasma capsulatum | 7.8 (1, 59.5) | 0.05 | 9.5 (1.5, 30.4) |
| Cryptococcus neoformans | 3.8 (1, 14.5) | 0.05 | 15.4 (4.5, 34.9) | |
| Kaposi sarcoma | 3.8 (0.8, 17.4) | 0.08 | 21.4 (4.9, 50.8) | |
| Pleural effusion | Mycobacterium tuberculosis | 5.6 (1.2, 26.8) | 0.03 | 12 (2.7, 31.3) |
| Lymphadenopathy | Mycobacterium tuberculosis | 4.1 (0.7, 24.2) | 0.12 | 16.7 (206, 48.4) |
Adjusted for older age and HIV status.
PPV, positive predictive value; OR, odds ratio; 95% CI, 95% confidence interval; HIV, human immunodeficiency virus.
Figure 1.
Radiographic appearance of pulmonary nodules with (a) H. capsulatum. Coned-down view of the right lower lung demonstrates uniform sized (1–2 mm in diameter) nodules, a miliary pattern, that was seen bilaterally in this patient with histoplasmosis. Again this is a pattern that is not unexpected with disseminated fungal infection (or occasionally tuberculosis). (b) C. neoformans. Anteroposterior chest film reveals several distinct bilateral lung nodules. The margins are discreet and in some cases well defined. The sizes range from approximately 1–1.5 cm in diameter. These larger nodules (as compared to the disseminated examples of histoplasmosis) have been described in patients with wide-spread pulmonary cryptococcosis.
Figure 2.
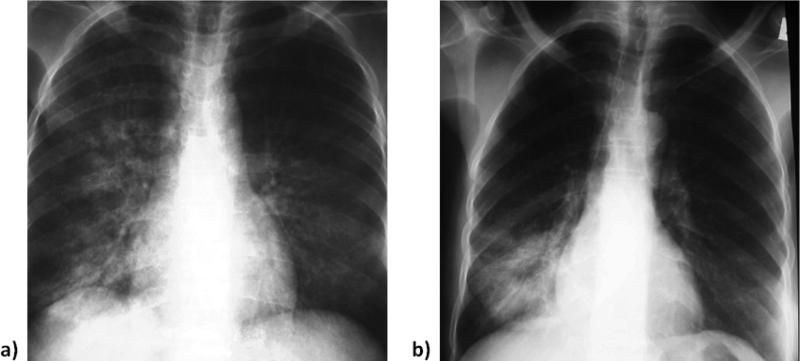
Radiographic appearance of lung opacities with (a) C. burnetii. Mildly cropped anteroposterior film demonstrates coarse right perihilar and lower lobe linear and reticular heterogeneous opacities. Less well visualized are fine-medium left mid-lung reticular and small nodular opacities. (b) S. pneumoniae. Minimally croppedanteroposterior chest film demonstrates both homogeneous and heterogeneous opacities in the right lower lobe. Centrally the opacity is more uniform or homogeneous, whereas peripherally the pneumonia is more a combination of linear and reticular opacities or heterogeneous. This example was also typical of the type of abnormality seen on chest radiographs in patients with bacterial pneumonia, (e.g., S. pneumoniae).
DISCUSSION
In a large cohort of febrile adult and adolescent patients who received chest radiography as well as extensive laboratory evaluation upon hospital admission, HIV infection, S. pneumoniae, C. burnetii, leptospirosis, and SFGR were the most common infections. Among children and infants who received chest radiography, HIV infection, SFGR, and leptospirosis were the most common infections. In both cohorts, homogeneous and heterogeneous opacities were the most common abnormality at chest radiography. In the adult and adolescent cohort, after taking into account HIV status and older age, the presence of these opacities on chest films were predictive of pneumococcal disease and Q fever. The presence of pulmonary nodules was predictive of the fungal infections: histoplasmosis and cryptococcal disease. Evidence of pleural effusion on the chest radiograph was predictive of disseminated tuberculosis. Despite the small number of some diagnoses and the diversity of conditions diagnosed (small predictive values), these radiographic findings may provide additional insight for an evaluating clinician when targeting the cause of the illness.
Pneumonia was a common radiographic diagnosis and S. pneumoniae was the leading causative agent. This was followed closely by C. burnetii despite only slightly more than half of the participants being tested for this infection. Although S. pneumoniae is a common infection and frequently diagnosed throughout sub-Saharan Africa,3,8,20,21 lack of diagnostics and limited clinician awareness14 suggest that C. burnetii may be underappreciated in many areas. As both S. pneumoniae and C. burnetii demonstrated a similar pattern of opacification on the chest radiograph,22 clinicians should also have a high index of suspicion for C. burnetii in patients. The present findings suggest that empiric treatment strategies for community-acquired pneumonia in sub-Saharan Africa should include antimicrobial agents with activity against C. burnetii as well as S. pneumoniae. Although C. burnetii infection is often self-limited, antimicrobial treatment may shorten the severity and duration of illness and prevent chronic disease. Furthermore, tetracyclines, active against C. burnetii, are widely available and inexpensive in sub-Saharan Africa. 12
In the present study, pulmonary nodules were the second most common abnormality found on chest radiographs. C. neoformans and H. capsulatum were both associated with pulmonary nodules and were also common HIV co-infections. The association of C. neoformans with pulmonary nodules has been described in other studies both in HIV-infected and non-HIV infected persons.23–31 However, many studies have found more extensive pulmonary disease among HIV-infected persons compared to non-HIV infected persons with cryptococcal disease, including the presence of pleural effusion, cavitation, and other lung opacities in addition to pulmonary nodules.24,28–30 The lack of association between more extensive pulmonary disease and cryptococcal disease in the present study may have been due to the limited number of patients diagnosed. In another study, pulmonary nodules were also found in HIV-infected patients with histoplasmosis,32 along with pleural effusions and other abnormalities. Again, more extensive pulmonary disease was not common in the present series. However, consistent with other studies, the present findings support the consideration that pulmonary nodules on chest radiographs, especially in those with HIV infection, could represent fungal infection and should warrant further investigation in determining a specific diagnosis.
The present study had several limitations. Testing for specific diagnoses was not performed on all participants. For example, due to lack of availability of a convalescent serum sample or limited original volume, testing for C. burnetii, SFGR, TGR, and Brucella spp. was only performed on approximately half of the participants. Therefore, the actual number of such infections may have been underestimated in this population. Although a large number of conditions were tested for to identify the cause of the febrile illness, all possible conditions were not tested for, and the cause of the febrile illness remained unknown for many participants (35.1% of the adult cohort). For example, respiratory samples were not evaluated, and thus, some pathogens, such as P. jiroveci, which is common in Tanzania, may not have been detected or their role may have been underestimated.33 Furthermore, the diagnosis of S. pneumoniae in adults and adolescents was augmented by use of S. pneumoniae urine antigen testing, and the assay was not used in the infant and child cohort due to concern for false-positive results.34 Finally, the diagnosis of disseminated tuberculosis was made solely on the basis of mycobacterial blood culture, as sputum samples were not collected as part of the study.35,36 Therefore, pulmonary tuberculosis was not microbiologically sought despite the radiologist's suspicion, most likely underestimating the prevalence of M. tuberculosis. It may be possible that many without a confirmed diagnosis would have had a positive sputum culture for pulmonary tuberculosis.
In conclusion, chest radiographic abnormalities are common among hospitalized febrile inpatients in northern Tanzania and provide useful information for patient management. A chest radiograph may help to guide empiric management, for example, by confirming the syndrome of community-acquired pneumonia, and in some instances, may contribute to making an aetiological diagnosis. However, the combination of clinical information, including knowledge of HIV serostatus (co-infection), along with laboratory testing and evaluation of radiographic abnormalities is likely to offer the best approach for diagnosis of pulmonary disease. Finally, results from the present study indicate that C. burnetii is a leading cause of community-acquired pneumonia in northern Tanzania, and it is recommended that antimicrobial management includes coverage of C. burnetii in this setting.
ACKNOWLEDGEMENTS
This research was supported by an International Studies on AIDS Associated Co-infections (ISAAC) award, a United States National Institutes of Health (NIH) funded program (U01 AI062563). Authors received support from NIH awards ISAAC (J.A.C., A.B.M., J.A.B., V.P.M.), AIDS International Training and Research Program D43 PA-03-018 (J.A.C., J.A.B., V.P.M., G.D.K.), the Duke Clinical Trials Unit and Clinical Research Sites U01 AI069484 (S.P.F., J.A.C., J.A.B., V.P.M., G.D.K.), the Duke Center for AIDS Research P30 AI 64518 (J.A.B.), and the Center for HIV/AIDS Vaccine Immunology U01 AI067854 (J.A.C., J.A.B.). The authors thank Ahaz T. Kulanga, MBA, for providing administrative support to this study and Pilli M. Chambo, Beata V. Kyara, Beatus A. Massawe, Anna D. Mtei, Godfrey S. Mushi, Lillian E. Ngowi, Flora M. Nkya, and Winfrida H. Shirima for reviewing and enrolling study participants. The authors are grateful to the leadership, clinicians, and patients of KCMC and MRH for their contributions to this research. The authors thank Inverness Medical for donating Binax NOW® Streptococcus pneumoniae Antigen Test kits and the Binax® NOW Legionella Urinary Antigen Test kits for the study. The authors acknowledge the Hubert-Yeargan Center for Global Health at Duke University for critical infrastructure support for the Kilimanjaro Christian Medical Centre–Duke University Collaboration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Petit PL, van Ginneken JK. Analysis of hospital records in four African countries, 1975–1990, with emphasis on infectious diseases. J Trop Med Hyg. 1995;98:217–27. [PubMed] [Google Scholar]
- 2.Bahwere P, Levy J, Hennart P, et al. Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis. 2001;5:180–8. doi: 10.1016/s1201-9712(01)90067-0. [DOI] [PubMed] [Google Scholar]
- 3.Peters RP, Zijlstra EE, Schijffelen MJ, et al. A prospective study of bloodstream infections as cause of fever in Malawi: clinical predictors and implications for management. Trop Med Int Health. 2004;9:928–34. doi: 10.1111/j.1365-3156.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 4.Parker TM, Murray CK, Richards AL, et al. Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hyg. 2007;77:390–2. [PubMed] [Google Scholar]
- 5.Reddy EA, Shaw AV, Crump JA. Community acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–32. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis. 2001;7:302–5. doi: 10.3201/eid0702.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyburn H, Redempta M, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania; a prospective study. BMJ. 2004;329:1212–5. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boersma WG, Daniels JM, Löwenberg A, Boeve WJ, van de Jagt EJ. Reliability of radiographic finds and the relation to etiologic agents in community-acquired pneumonia. Respir Med. 2006;100:926–32. doi: 10.1016/j.rmed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Marrie TJ. Community-acquired pneumonia. Clin Infect Dis. 1994;18:501–515. doi: 10.1093/clinids/18.4.501. [DOI] [PubMed] [Google Scholar]
- 10.Crump JA, Ramadhani HO, Morrissey AB, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52:341–8. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump JA, Ramadhani HO, Morrissey AB, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011;16:830–7. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhu M, Nicholson WL, Roche AJ, et al. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. 2011;53:e8–315. doi: 10.1093/cid/cir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biggs HM, Bui DM, Galloway RL, et al. Leptospirosis among hospitalized febrile patients in Northern Tanzania. Am J Trop Med Hyg. 2011;85:275–81. doi: 10.4269/ajtmh.2011.11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertz JT, Munishi OM, Eong Ooi E, et al. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–7. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayhood MK, Afwamba IA, Odhiambo CO, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and Determine HIV-1/2 rapid HIV antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–51. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly PA, Durkin MM, LeMonte AM, Hackett EJ, Wheat LJ. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol. 2007;14:1587–91. doi: 10.1128/CVI.00071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikken H, Kmety E. Serological typing methods of leptospires. In: Bergan T, Norris JR, editors. Methods in Microbiology. Academic Press; London: 1978. pp. 259–307. [Google Scholar]
- 18.Bouley AJ, Biggs HM, Stoddard RA, et al. Brucellosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;87:1105–11. doi: 10.4269/ajtmh.2012.12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lofgren SM, Kirsch EJ, Maro VP, et al. Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans Royal Soc Trop Med Hyg. 2012;106:504–7. doi: 10.1016/j.trstmh.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JAG, Hall AJ, Muyodi C, et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet. 2000;355:1225–30. doi: 10.1016/s0140-6736(00)02089-4. [DOI] [PubMed] [Google Scholar]
- 21.French N, Williams G, Williamson V, Bhatt SM, Gilks CF. The radiographic appearance of pneumococcal pneumonia in adults is unaltered by HIV-1 infection in hospitalized Kenyans. AIDS. 2002;10:2095–6. doi: 10.1097/00002030-200210180-00020. [DOI] [PubMed] [Google Scholar]
- 22.Gikas A, Kofteridis D, Bouros D, Voloudaki A, Tselentis Y, Tsaparas N. Q fever pneumonia: appearance on chest radiographs. Radiology. 1999;210:339–43. doi: 10.1148/radiology.210.2.r99fe20339. [DOI] [PubMed] [Google Scholar]
- 23.Chenchani V, Kamholz SL. Pulmonary manifestations of disseminated cryptococcosis in patients with AIDS. Chest. 1990;98:1060–6. doi: 10.1378/chest.98.5.1060. [DOI] [PubMed] [Google Scholar]
- 24.Chang W, Tzao C, Hsu H, et al. Pulmonary cryptococcosis. Chest. 2006;129:333–40. doi: 10.1378/chest.129.2.333. [DOI] [PubMed] [Google Scholar]
- 25.Haramati LB, Jenny-Avital ER. Approach to the diagnosis of pulmonary disease in patients infected with the human immunodeficiency virus. J Thorac Imaging. 1998;13:247–60. doi: 10.1097/00005382-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Castañer E, Gallardo X, Mata JM, Esteba L. Radiologic approach to the diagnosis of infectious pulmonary diseases in patients infected with the human immunodeficiency virus. Eur J Rad. 2004;51:114–29. doi: 10.1016/j.ejrad.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Edinburgh KJ, Jasmer RM, Huang L, et al. Multiple pulmonary nodules in AIDS: usefulness of CT in distinguishing among potential causes. Radiology. 2000;214:427–32. doi: 10.1148/radiology.214.2.r00fe22427. [DOI] [PubMed] [Google Scholar]
- 28.Lindell RM, Hartman TE, Nadrous HF, Ryu JH. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology. 2005;236:326–31. doi: 10.1148/radiol.2361040460. [DOI] [PubMed] [Google Scholar]
- 29.Khoury MB, Godwin JD, Ravin CE, Gallis HA, Halvorsen RA, Putman CE. Thoracic cryptococcosis; immunologic competence and radiologic appearance. AJR Am J Roentgenol. 1984;142:893–6. doi: 10.2214/ajr.142.5.893. [DOI] [PubMed] [Google Scholar]
- 30.Roebuck DJ, Fisher DA, Currie BJ. Cryptococcosis in HIV negative patients: findings on chest radiography. Thorax. 1998;53:554–7. doi: 10.1136/thx.53.7.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron ML, Bartlett JA, Gallis HA, Waskin HA. Manifestations of pulmonary cryptococcosis in patients with acquired immunodeficiency syndrome. Rev Infect Dis. 1991;13:64–7. doi: 10.1093/clinids/13.1.64. [DOI] [PubMed] [Google Scholar]
- 32.Conces DJ, Stockberger SM, Tarver RD, Wheat LJ. Disseminated histoplasmosis in AIDS: findings on chest radiographs. AJR Am J Roentgenol. 1993;160:15–19. doi: 10.2214/ajr.160.1.8416614. [DOI] [PubMed] [Google Scholar]
- 33.Kibiki GS, Beckers P, Mulder B, et al. Aetiology and presentation of HIV/AIDS-associated pulmonary infections in patients presenting for bronchoscopy at a referral hospital in northern Tanzania. E African Med J. 2007;84:420–427. doi: 10.4314/eamj.v84i9.9551. [DOI] [PubMed] [Google Scholar]
- 34.Hamer DH, Egas J, Estrella B, MacLeod WB, Griffiths JK, Sempértegui F. Assessment of the Binax NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage. Clin Infect Dis. 2002;34:1025–1028. doi: 10.1086/339446. [DOI] [PubMed] [Google Scholar]
- 35.Crump JA, Morrissey AB, Ramadhani HO, Njau BN, Maro VP, Reller LB. Controlled comparison of BacT/ALERT MB system, manual MYCO/F LYTIC procedure, and ISOLATOR 10 system for detection of Mycobacterium tuberculosis bacteremia. J Clin Microbiol. 2011;49:3054–7. doi: 10.1128/JCM.01035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crump JA, Ramadhani HO, Morrissey AB, et al. Bacteremic disseminated tuberculosis in sub-Saharan Africa: a prospective cohort study. Clin Infect Dis. 2012;55:242–50. doi: 10.1093/cid/cis409. [DOI] [PMC free article] [PubMed] [Google Scholar]