Abstract
Cachexia is a serious complication of many chronic diseases, such as congestive heart failure (CHF) and chronic kidney disease (CKD). Many factors are involved in the development of cachexia, and there is increasing evidence that angiotensin II (Ang II), the main effector molecule of the renin-angiotensin system (RAS), plays an important role in this process. Patients with advanced CHF or CKD often have increased Ang II levels and cachexia, and angiotensin-converting enzyme (ACE) inhibitor treatment improves weight loss. In rodent models, an increase in systemic Ang II leads to weight loss through increased protein breakdown, reduced protein synthesis in skeletal muscle and decreased appetite. Ang II activates the ubiquitin-proteasome system via generation of reactive oxygen species and via inhibition of the insulin-like growth factor-1 signaling pathway. Furthermore, Ang II inhibits 5′ AMP-activated protein kinase (AMPK) activity and disrupts normal energy balance. Ang II also increases cytokines and circulating hormones such as tumor necrosis factor-α, interleukin-6, serum amyloid-A, glucocorticoids and myostatin, which regulate muscle protein synthesis and degradation. Ang II acts on hypothalamic neurons to regulate orexigenic/anorexigenic neuropeptides, such as neuropeptide-Y, orexin and corticotropin-releasing hormone, leading to reduced appetite. Also, Ang II may regulate skeletal muscle regenerative processes. Several clinical studies have indicated that blockade of Ang II signaling via ACE inhibitors or Ang II type 1 receptor blockers prevents weight loss and improves muscle strength. Thus the RAS is a promising target for the treatment of muscle atrophy in patients with CHF and CKD.
Keywords: angiotensin II, skeletal muscle, cachexia, congestive heart failure
Introduction
Angiotensin II (Ang II), the main effector molecule of the renin-angiotensin system (RAS), has multiple physiological actions including regulation of blood pressure and salt/water balance through a variety of effects on the central nervous system, the adrenal gland, the vasculature and the kidney. Furthermore, the RAS plays an important role in the pathogenesis of all stages of cardiovascular disease, ranging from early endothelial dysfunction to target-organ damage, congestive heart failure (CHF), renal or cerebrovascular disease (Werner et al. 2010). Patients with advanced cardiovascular or renovascular disease, such as CHF or end-stage renal disease (ESRD), often have cachexia, which independently worsens outcome (Tan and Fearon 2008; Werner et al. 2010). These patients often have increased Ang II levels (Masson et al. 1998; Roig et al. 2000; Anker et al. 2003), and ACE inhibitor treatment improves weight loss in CHF patients (Anker et al. 2003) and AT1 receptor blockade prevents skeletal muscle atrophy in a rat model of CHF (Dalla Libera et al. 2001), suggesting that Ang II could play an important role in the development of cachexia. Interestingly, an ACE inhibitor attenuated weight loss in a mouse model of cancer cachexia (Sanders et al. 2005), which suggests that Ang II signaling may be important not only in patients with CHF and ESRD, but also in cancer cachexia. In this review, we address the relationship between the RAS and skeletal muscle atrophy, the cellular and molecular mechanisms underlying this process, and its physiological and clinical importance.
Ang II increases protein degradation
Brink et al. first demonstrated that Ang II infusion in the rat caused a significant loss of body weight through a reduction of food intake and increased proteolysis in skeletal muscle (Brink et al. 1996). These effects were completely prevented by the AT1 receptor blocker losartan but not by the anti-hypertensive drug hydralazine, showing that Ang II causes muscle wasting via an AT1 receptor dependent mechanism independent of blood pressure increase. Ang II infusion causes an increase of protein breakdown and a decrease in IGF-1 signaling, which is the main anabolic pathway in skeletal muscle (Brink et al. 2001). A small component of the muscle wasting may be due to lower levels of protein synthesis, as synthesis rate was lower in Ang II-infused rats, but the difference was not statistically significant (Brink et al. 2001). Ang II-induced protein degradation was prevented by the proteasome inhibitor MG132, but not by lysosomal or calcium-activated protease inhibition, indicating that Ang II induces protein breakdown via the ubiquitin-proteasome system (UPS).
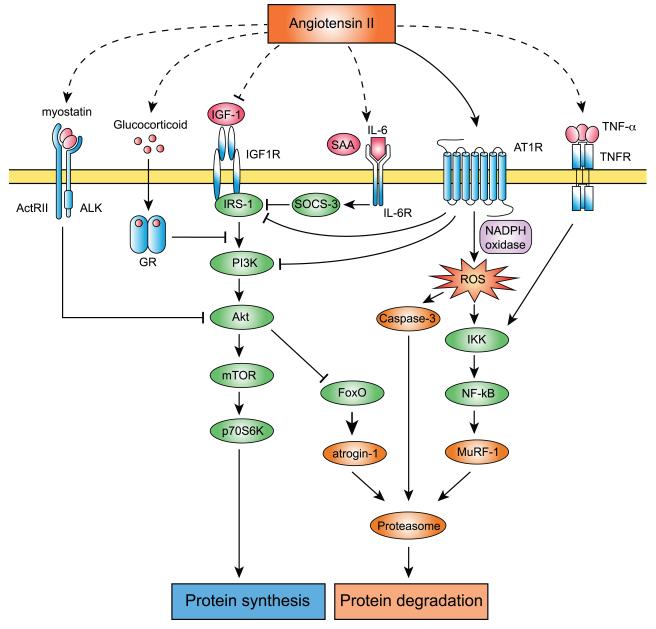
Studies of many different models of muscle wasting have indicated that accelerated proteolysis via the UPS is the principle cause of muscle atrophy induced in several types of cachexia, such as fasting, metabolic acidosis, disuse, sepsis and diabetes (Ventadour and Attaix 2006). Muscle fiber atrophy in conditions leading to cachexia may be fiber-type specific. Thus, type I fibers are more sensitive to inactivity, microgravity and denervation-induced atrophy, whereas type II fibers are more vulnerable to cancer cachexia, diabetes, CHF and ageing (Wang and Pessin 2013). The UPS degrades the major contractile skeletal muscle proteins and the activation of the UPS is responsible for progression of muscle wasting, whereas the other proteolytic enzymes act upstream (m-calpain, cathepsin L and/or caspase-3) and downstream (tripeptidyl-peptidase II and aminopeptidases) of the UPS for the complete breakdown of the myofibrillar proteins. Proteins that are subject to be broken down are marked for degradation by covalent linkage of a chain of ubiquitin molecules to an internal lysine on the protein and subsequently degraded by the 26S proteasome. This process is regulated by a series of enzymes, E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme and E3 ubiquitin ligase. Ubiquitin monomers are activated and linked to E1, transferred to E2, and interact with one of several hundred E3 to be transferred to the substrate protein. The ubiquitin-marked proteins are degraded by the 26S proteasome complex. The 26S proteasome complex is formed by a 20S core catalytic complex and one or two 19S regulatory complexes in charge of substrate recognition. The muscle specific E3 ubiquitin ligases atrogin-1/MAFbx and muscle RING finger-1 (MuRF-1) have been identified as genes strongly upregulated in different atrophy models (Bodine et al. 2001a). Overexpression of atrogin-1/MAFbx in cultured myotubes caused atrophy, whereas denervation-induced muscle atrophy is partially prevented in atrogin-1/MAFbx and MuRF-1 deficient animals (Bodine et al. 2001a). These data show that atrogin-1/MAFbx and MuRF-1 are critical regulators of the UPS and muscle atrophy. However, although Atrogin-1/MAFbx expression has been extensively used as a marker of skeletal muscle atrophy in many studies, it is of note that recent studies showed that such changes do not necessarily reflect alterations in muscle proteolysis per se as previously believed (Attaix and Baracos 2010). Myosin heavy chain (MHC) (Clarke et al. 2007) and myofibrillar proteins (Cohen et al. 2009) have been identified as substrates of MuRF-1, indicating that MuRF-1 is involved in muscle protein breakdown in atrophying muscle. On the other hand, the only proteins identified so far as a substrate of Atrogin-1/MAFbx is MyoD (Tintignac et al. 2005; Lagirand-Cantaloube et al. 2009) and eukaryotic translation initiation factor subunit F (eIF3-f) (Lagirand-Cantaloube et al. 2008; Csibi et al. 2009; 2010), which regulate muscle differentiation and protein synthesis, respectively. These data suggest that MuRF-1 is associated with muscle proteolysis, whereas Atrogin-1/MAFbx may be more related to protein synthesis. Also, it has been shown that the expression of multiple proteasome components are increased in different atrophy models (fasting, cancer cachexia, diabetes and uremia), including the 20S core complex (Psma1, Psma5, PSmb3 and Psmb4) and the 19S regulatory complex (Psmc4, Psmd8 and Psmd11) (Lecker et al. 2004). In Ang II-induced muscle wasting, expression of atrogin-1/MAFbx and MuRF-1, levels of ubiquitin-conjugated proteins and 20S proteasome activity are robustly increased (Song et al. 2005; Yoshida et al. 2010; Semprun-Prieto et al. 2011). These data indicate that Ang II activates the UPS in skeletal muscle by increasing expression of UPS components and by increasing 20S proteasome activity (Figure 1).
Figure 1. Molecules and signaling pathways involved in Ang II-induced skeletal muscle atrophy.
Ang II increases ROS in skeletal muscle via AT1R and NADPH oxidase dependent mechanisms. Increased ROS activates caspase-3 and the NF-κB pathway, which result in activation of the UPS and protein degradation. Ang II also suppresses IGF-1 signaling via serine phosphorylation of IRS-1 and PI3K, resulting in decreased protein synthesis. Local production of IGF-1 is decreased by Ang II and protein synthesis is attenuated. Systemic Ang II increase causes elevation of TNF-α, IL-6, SAA, glucocorticoid and myostatin. TNF-α induces protein degradation via activation of the NF-κB pathway. IL-6 and SAA synergistically activate SOCS-3, and prevent IGF-1 signaling. GR competitively prevents IRS-1 and PI3K association. Myostatin inhibits Akt phosphorylation, which results in decreased protein synthesis and increased protein degradation.
Insulin-like growth factor-1 (IGF-1) and Ang II interaction
Various protective factors are reported to help maintain muscle integrity (Tatsumi 2010). Among these factors, insulin-like growth factor-I (IGF-1) modulates muscle size via autocrine and paracrine signals by directly stimulating protein anabolism in myofibers and by the activation of satellite cell proliferation (Sandri 2008). Briefly, IGF-1 binds to the tyrosine kinase IGF-1 receptor (IGF1R) and its binding can be modulated by IGF binding proteins. Activated IGF1R recruits the insulin receptor substrate-1 (IRS-1), which results in the activation of at least two signaling pathways; the Ras-Raf-MEK-ERK pathway and the PI3K-Akt pathway. These two signaling pathways have been shown to exert opposing effects on muscle cell hypertrophy. The PI3K-Akt pathway inhibits Ras-Raf-MEK-ERK signaling in differentiated myotubes (Rommel et al. 1999), indicating that the PI3K-Akt pathway is dominant in skeletal muscle. Activation of the PI3K/Akt pathway induces muscle hypertrophy by stimulating translation via regulation of GSK and mTOR kinases (Bodine et al. 2001b). Decreased activity of the IGF-1/PI3K/Akt signaling pathway can lead to muscle atrophy (Bodine et al. 2001b). Inhibition of PI3K and expression of dominant-negative Akt reduce the mean size of myotubes in culture (Rommel et al. 2001). Muscles from mice deficient for Akt1 and Akt2 are small (Peng et al. 2003) and, conversely, activation of Akt in rat muscle can prevent denervation atrophy (Bodine et al. 2001b; Pallafacchina et al. 2002). Overexpression of IGF-1 under the control of a skeletal muscle specific promoter caused muscle cell differentiation and myofiber hypertrophy in two transgenic mouse lines (Coleman et al. 1995; Musaro et al. 2001). Furthermore, targeted IGF-1 transgene expression or gene transfer in hindlimb muscles reverses muscle wasting in various rodent models of muscle atrophy including Duchenne muscular dystrophy (Barton et al. 2002), crush injury (Rabinovsky et al. 2003), dexamethasone injection (Schakman et al. 2005), cast immobilization (Stevens-Lapsley et al. 2010) and Ang II infusion (Song et al. 2005). Ang II infusion in rats reduced circulating and skeletal muscle IGF-1 and IGF-1 binding proteins IGFBP-2 and IGFBP-3 (Brink et al. 1996; 2001). Furthermore, Ang II disrupts IGF-1 signaling at multiple levels. It has been shown that Ang II inhibits insulin/IGF-1 signaling via serine phosphorylation of IRS-1 and PI3K (Folli et al. 1997; Velloso et al. 2006), and Song et al. showed an increase of IRS-1 Ser307 phosphorylation in gastrocnemius muscle of Ang II-infused mice (Song et al. 2005). Also, the increase in glucocorticoids in response to Ang II may lead to binding of the glucocorticoid receptor (GR) to PI3K with resulting decreased binding to IRS-1 (Hu et al. 2009). Whereas systemic IGF-1 infusion does not rescue Ang II-induced muscle wasting, skeletal muscle targeted overexpression of IGF-1 reverses the effect of Ang II through Akt/FoxO activation and inhibition of E3 ubiquitin ligase atrogin-1/MAFbx expression (Song et al. 2005; Werner et al. 2010; Yoshida et al. 2010). Interestingly, although Ang II rapidly increases both atrogin-1/MAFbx and MuRF-1 expression, activation of the IGF-1/Akt/FoxO pathway prevents only the increase in atrogin-1/MAFbx. This is unusual since in most catabolic conditions atrogin-1/MAFbx and MuRF-1 are coordinately regulated (Lecker 2003; Attaix et al. 2005; Schakman et al. 2005), although only MuRF-1 expression is increased in NF-κB mediated muscle atrophy (Cai et al. 2004). Of note, progressive muscle atrophy in advanced stages of chronic heart failure in humans correlates with low serum levels and reduced local expression of IGF-1 (Hambrecht et al. 2002; Schulze et al. 2003). In a mouse model of myocardial infarction, there was marked skeletal muscle atrophy, which was associated with activation of FoxO transcription factors, a robust increase of atrogin-1/MAFbx expression and enhanced proteasome activity. Furthermore, skeletal muscle-specific overexpression of IGF-1 prevented the muscle atrophy in these animals. Importantly, it has been shown that plasma Ang II level is increased in these animal models (Jin et al. 2004; Lütken et al. 2009), suggesting the involvement of increased Ang II in triggering muscle atrophy in the setting of chronic heart failure. In summary, Ang II and IGF-1 exert opposing effects on skeletal muscle (Figure 1). Ang II reduces circulating IGF-1 and disrupts IGF-1 signaling in skeletal muscle and the atrophic effect of Ang II on skeletal muscle can be prevented in animals by local overexpression of IGF-1.
Ang II and oxidative stress
Oxidative stress is a common hallmark of several pathological conditions including atherosclerosis, diabetes, cancer and CHF (Kaneto et al. 2010), and it has been shown that increased oxidative stress contributes to disuse muscle atrophy (Powers et al. 2012). Oxidative stress generally refers to the increased cellular production of reactive oxygen species (ROS), molecules or ions formed by the incomplete 1-electron reduction of oxygen. ROS are involved in the regulation of normal cell signaling and cell growth, proliferation and expansion of the extracellular matrix (Apel and Hirt 2004). ROS can be produced in almost any cell types, including skeletal muscle cells, by activation of xanthine oxidase, lipoxygenases, nitric oxide synthase, the mitochondrial respiratory chain and the NADPH oxidases. Several reports suggested that two oxidant systems, namely NADPH oxidase and mitochondria, are major sources of ROS in atrophied skeletal muscles (Muller et al. 2007; Whitehead et al. 2010). Whitehead et al. reported that NADPH oxidase expression and NADPH oxidase-derived ROS are increased in skeletal muscle of dystrophic mdx mice (Whitehead et al. 2010), and that an NADPH oxidase inhibitor attenuated the loss of muscle force. Muller et al. (Muller et al. 2007) reported the increase of mitochondrial ROS generation in three conditions of muscle atrophy, in aging, in CuZn-SOD deficient mice and in neurodegenerative disease. ROS production in these different atrophy models was highly correlated with the extent of muscle atrophy, suggesting that mitochondrial ROS production is a common factor in the mechanism of muscle atrophy. Oxidative stress induces proteolysis and atrophy via several different mechanisms: (1) calcium overload and activation of calcium-activated proteases such as calpain; (2) stimulation of the 20S proteasome system via activation of caspase-3; (3) activation of E3 ubiquitin ligases atrogin-1/MAFbx and MuRF-1.
Ang II infusion has been reported to increase ROS in rat skeletal muscle (Zhao et al. 2006). Also, Ang II enhanced NADPH oxidase activity and consequent ROS generation in L6 myotubes via an AT1R dependent mechanism (Wei et al. 2006). Ang II-induced proteolysis is prevented by the antioxidants butylated hydroxytoluene (BHT) and diphenyleneiodonium in myotubes (Russell et al. 2007). Furthermore, Semprun-Prieto et al. (Semprun-Prieto et al. 2011) demonstrated that Ang II-induced ROS production is blocked in NADPH oxidase subunit p47phox deficient mice (p47phox-/-) and by the NADPH oxidase inhibitor apocynin, and that Ang II-induced muscle wasting and increased 20S proteasome activity are attenuated in p47phox-/- mice. These data suggest that Ang II increases ROS generation via activation of NADPH oxidase, leading to muscle proteolysis (Figure 1). Ang II also increases mitochondrial ROS formation in endothelial cells (Pueyo et al. 2000), vascular smooth muscle cells and in rat aorta in vivo (Kimura et al. 2005), and it has been speculated that NADPH oxidase-induced ROS could directly stimulate the mitochondria (Kimura et al. 2005). However, mitochondria-targeted antioxidant Mito-TEMPO administration failed to prevent Ang II-induced muscle wasting, suggesting that mitochondrial derived ROS do not directly contribute to Ang II-induced muscle wasting (Tabony et al. 2011). Several skeletal muscle dystrophies are characterized by extensive fibrosis, which hinders the normal healing of tissue damage. Cabello-Verrugio et al. and Morales et al. demonstrated that Ang II-induced ROS activate a fibrotic response in C2C12 myoblasts. The ROS production is mediated by AT1R-p38MAPK-NOX signaling and a subsequent increase of TGF-β (Cabello-Verrugio et al. 2011; 2012; Morales et al. 2012). These data suggest that Ang II-induced fibrosis contributes to mechanisms of Ang II-induced muscle atrophy.
Effect of Ang II on AMPK and energy balance
Several metabolic pathways are altered in atrophying muscles and many genes required for ATP production and for the late steps of glycolysis were down-regulated in many forms of muscle wasting (Lecker et al. 2004). Although it has been well established that Ang II induces skeletal muscle atrophy, little is known about potential effects of Ang II on muscle metabolism and energy stores or about the potential link between these effects and Ang II-induced muscle wasting. Tabony et al. (Tabony et al. 2011) demonstrated that Ang II depletes skeletal muscle ATP content likely via induction of mitochondrial dysfunction. AMP-activated protein kinase (AMPK) is a serine/threonine kinase that acts as a sensor of cellular energy status. AMPK regulates several intracellular systems such as cellular uptake of glucose, fatty acid β-oxidation and mitochondrial biogenesis, and activates ATP synthesis when cellular energy status is depleted (high AMP:ATP ratio). Importantly, Ang II inhibits AMPK activation in skeletal muscle, likely via upregulation of the inactivating phosphatase PP2Ca, thereby preventing normalization of muscle energy balance. The AMPK activator AICAR reversed Ang II-induced inhibition of AMPK, leading to restoration of ATP levels and inhibition of the Ang II induced reduction in muscle mass. Interestingly, AICAR administration blocked Ang II-induced expression of E3 ubiquitin ligases atrogin-1/MAFbx and MuRF-1, providing a potential additional mechanism whereby AICAR treatment prevents Ang II-induced wasting. The effects of AMPK on FoxO transcription factors and on subsequent regulation of ubiquitin ligase expression are complex, since although AMPK can phosphorylate FoxO on multiple residues that are activating, AMPK also activates Akt which can inhibit FoxO activity (Leclerc et al. 2010). For instance, increased AMP levels, AMPK upregulation and muscle atrophy is observed in S6 kinase-1 (S6K1) deficient mice (Aguilar et al. 2007). In these mice, AMPK inhibition restores cell growth and sensitivity to nutrient signals. Also, it has been shown that AMPK-mediated phosphorylation of FoxO activates E3 ubiquitin ligase expression in C2C12 cells (NAKASHIMA and YAKABE 2007). However, in Ang II infused animals the net effect of AMPK activation by AICAR resulted in Akt activation and inhibitory phosphorylation of FoxO1, which could explain the ability of AICAR to abrogate Ang II-mediated upregulation of E3 ubiquitin ligases. These data suggest a therapeutic potential for AMPK activators in chronic wasting conditions with elevated Ang II levels.
Ang II reduces appetite
Decreased intake of calories is a major problem for many patients with advanced CHF and significantly contributes to the development of cardiac cachexia. Food intake is regulated by complex mechanisms via multiple factors including hypothalamic orexigenic/anorexigenic neuropeptides and circulating factors secreted from peripheral organs (e.g. adipose tissues and gastrointestinal tract).
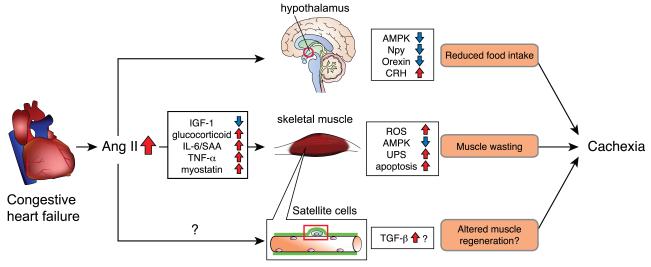
When Brink et al. first reported that Ang II-induced loss of body weight, the authors indicated that the loss of body weight was caused by two different mechanisms, increased catabolism of skeletal muscle and loss of food intake. Pair-feeding experiments, in which a group of animals received an identical amount of food as Ang II-infused animals, showed that the loss of body weight in Ang II infused animals was in large part (~80%) due to reduced food intake. Yamamoto et al. (Yamamoto et al. 2011) reported that Ang II type 1a receptor (AT1aR) deficient mice are hyperphagic and obese with increased adiposity. These mice showed decreased expression of the anorexigenic neuropeptide corticotropin-releasing hormone (CRH) in the hypothalamus and CRH administration suppressed food intake in AT1aR deficient mice. Furthermore, the CRH gene promoter was significantly transactivated via the cAMP-responsive element by Ang II. These data suggest that Ang II directly acts on hypothalamic neurons to regulate food intake via regulation of orexigenic/anorexigenic neuropeptides. Indeed, the AT1R is expressed in multiple hypothalamic neurons, including in the lateral hypothalamic area, paraventricular nucleus, retrochiasmatic area, and perifornical nucleus (Lenkei et al. 1997), whereas there is relatively minor expression of AT2R in the hypothalamus. Also, peripheral administration of the angiotensin-converting enzyme (ACE) inhibitor captopril was reported to decrease food intake, probably due to an increase of central Ang II, which was converted from increased Ang I (de Kloet et al. 2009). Furthermore, several studies have reported that chronic intracerebroventricular (icv) infusion of Ang II reduces food intake likely via regulation of orexigenic/anorexigenic neuropeptides in the hypothalamus. Porter et al. (Porter et al. 2003; Porter and Potratz 2004) reported that chronic icv infusion of Ang II in rats caused reduced food intake and increased CRH expression in the hypothalamus. De Kloet et al. (de Kloet et al. 2009) reported that chronic icv administration of Ang II in rats reduced food intake, which was associated with increased hypothalamic agouti-related protein (AgRP), proopiomelanocortin (POMC), thyrotropin-releasing hormone (TRH) and CRH. Yoshida et al. (Yoshida et al. 2012) demonstrated that systemic Ang II infusion caused a decrease of hypothalamic neuropeptide-Y (Npy) and orexin expression. Icv infusion of Ang II reduced food intake and Ang II reduced Npy and orexin expression in the hypothalamus cultured ex vivo in an AT1R dependent manner, suggesting that Ang II directly acts on hypothalamic neurons to regulate these orexigenic neuropeptides. Although all of these studies strongly suggest that the anorexigenic action of Ang II is mediated by hypothalamic orexigenic/anorexigenic neuropeptides, it is important to note that the changes in hypothalamic neuropeptide expression could be secondary to altered food intake and metabolism in response to Ang II administration. However, Yoshida et al. infusion rapidly reduced food intake (within 6hrs) and that this reduction was and orexin expression was also reduced rapidly, whereas levels of other orexigenic/anorexigenic neuropeptides were altered at later time points of analysis (e.g. 4 days). This was likely because of changes in appetite-regulating hormones secreted from peripheral organs. For instance, due to reduced food intake, Ang II infused animals have decreased fat mass, which results in a reduction in circulating leptin. Because leptin negatively regulates Npy expression, hypothalamic Npy expression is increased at the 4d time point after Ang II infusion. In summary, it is critical to determine early changes in hypothalamic neuropeptide expression to identify the initial targets of Ang II action, since many of the subsequent changes in neuropeptides are secondary to the reduction in food intake. The available data indicate that Ang II reduces food intake likely via rapid downregulation of Npy and orexin expression in the hypothalamus, and chronic Ang II elevation results in changes in orexigenic/anorexigenic neuropeptide expression such as CRH, TRH, POMC and AgRP in the hypothalamus (Figure 2).
Figure 2. Multiple mechanisms involved in Ang II-induced muscle wasting: potential mechanism of cardiac cachexia.
In heart failure patients, there is an increase of Ang II. Increased Ang II causes a reduction of IGF-1 and an increase in glucocorticoids, IL-6, SAA, TNF-α and myostatin, all of which result in muscle wasting. See figure 1 for detailed signaling pathways involved. Ang II also acts on hypothalamic neurons to reduce appetite, via regulation of orexigenic/anorexigenic neuropeptides such as Npy, Orexin and CRH. Ang II may regulate satellite cell function and prevent skeletal muscle regeneration. The combination of Ang II-induced muscle wasting, reduced food intake and altered muscle regeneration lead to the development of cachexia.
Ang II and Skeletal muscle regeneration
Skeletal muscle is capable of responding to injury by activating a well orchestrated regenerative response to repair damaged myofibers. Injury leads to activation and proliferation of mitotically quiescent mononuclear cells, satellite cells, which form myoblasts, terminally differentiate and fuse to form multinucleated myotubes (Yablonka-Reuveni and Day 2011). In adult skeletal muscle, satellite cell number and regenerative capacity remain nearly constant through multiple cycles of regeneration (McCroskery et al. 2003; Sacco et al. 2008). However, it is unknown whether normal regenerative responses involving activation of satellite cells occurred in the setting of Ang II-induced muscle catabolism. In fact, many clinical situations in which Ang II levels may be elevated are characterized by muscle wasting, such as CHF, ESRD, chronic obstructive pulmonary disease and cancer (Sanders et al. 2005; Argilés et al. 2008; Doehner et al. 2009; Haehling et al. 2009; Tisdale 2009). In these conditions little is known about the regenerative potential of skeletal muscle. Satellite cell apoptosis has been reported in different pathological conditions, including unloading, muscular dystrophy, ischemia and cancer cachexia. However, systemic quantitative studies on rates of apoptosis of satellite cells are lacking, and the response of satellite cells to pathophysiological conditions is poorly understood. Sarcopenia, namely muscle wasting secondary to aging, is one of the most characterized pathophysiological conditions in which satellite cell function may be altered. Although there is conflicting evidence of decreased satellite cell number in aged skeletal muscle (Collins et al. 2007; van der Meer et al. 2011), it is evident that aged satellite cells display reduced proliferative response and regenerative capacity (Conboy et al. 2003; 2005; Chakkalakal et al. 2012), and multiple mechanisms have been proposed. There is a decline in Notch activation in aged satellite cells and forced activation of Notch can rejuvenate aged satellite cells (Conboy and Rando 2002; Conboy et al. 2003). TGF-β expression is increased in both aged satellite cells and in myofibers and there is an increase of phospho-Smad3 (p-Smad3) in aged satellite cells (Carlson et al. 2008). Active-Notch and p-Smad3 competitively regulate CDK inhibitor (p15, p16, p21 and p27) transcriptional activation. Thus, an imbalance of Notch and p-Smad3 expression in aged muscle leads to increased CDK inhibitor expression, which leads to reduced satellite cell proliferation. Furthermore, FGF2 expression is increased in aged muscle and FGF2 acts on satellite cells to disrupt their quiescent state, resulting in their loss of self-renewing capacity (Chakkalakal et al. 2012). Loss of quiescence leads to satellite cell depletion and diminished regenerative capacity. In addition to reduced proliferative potential and self-renewal, aged satellite cells tend to convert from a myogenic to fibrogenic lineage in response to factors in the systemic environment, leading to an increase in tissue fibrosis (Brack et al. 2007). These data indicate that satellite cell function is negatively regulated by the old systemic and niche environment, suggesting that other pathophysiological conditions could have negative effects on satellite cells.
There is conflicting evidence for the effect of Ang II on skeletal muscle regeneration. Cohn et al. (Cohn et al. 2007) first reported the effect of AT1R blockade using losartan in two mouse models of myopathy, fibrillin-1 deficient mice and dystrophin-deficient mdx mice. In both of these animals, losartan attenuated TGF-β-induced failure of muscle regeneration, suggesting that Ang II negatively regulated muscle regeneration through TGF-β signaling. Bedair et al. (Bedair et al. 2008) also reported that losartan administration improved muscle regeneration and decreased fibrosis after laceration induced injury. However, two other studies have proposed that Ang II may stimulate muscle regeneration. Johnston et al. (Johnston et al. 2010) reported that an ACE inhibitor or ablation of the AT1aR significantly impaired skeletal muscle regeneration. Ang II increased the chemotactic capacity of C2C12 cells and primary myoblasts, while AT1aR-/- myoblasts showed impaired migration. Murphy et al. (Murphy et al. 2012) reported that muscle strength and locomoter activity were enhanced in AT1aR-/- mice. On the other hand, muscle regenerative capacity following myotoxic injury was impaired in these animals, by impaired myoblast fusion, prolonged collagen infiltration and inflammation, and delayed expression of myogenic regulatory factors. Reasons for these discrepant results are unclear and future analysis of direct Ang II effects (e.g. systemic Ang II infusion and/or muscle/satellite cell-specific AT1R knockdown) on muscle regeneration is likely to provide more conclusive results.
Indirect actions of Ang II
There are two Ang II receptor subtypes, AT1 and AT2 receptors, and these receptors mediate the majority of Ang II’s effects (de Gasparo et al. 2000; Stegbauer and Coffman 2011). Although the simplest explanation of Ang II-induced skeletal muscle atrophy would be that Ang II directly acts on AT1 receptor expressed on the cell surface of myofibers and activates the UPS pathway, there are conflicting studies relating to Ang II receptor expression in skeletal muscle. Sanders et al. (Sanders et al. 2005) and Russell et al. (Russell et al. 2006) showed that Ang II induces protein breakdown in cultured mouse myotubes (C2C12 myoblasts induced to differentiate for ~4 days) through the UPS pathway. Morales demonstrated that Ang II induces a fibrotic response through p38MAPK phosphorylation, NOX-induced ROS and TGF-β1 increase in C2C12 myoblasts (Morales et al. 2012). Co-administration of losartan prevented these effects, suggesting that Ang II-induced protein breakdown and development of fibrosis was mediated via the AT1 receptor expressed in C2C12 myoblasts/myotubes. However, none of the reports above provided direct evidence of Ang II receptor expression in cultured cells or skeletal muscle myofibers in vivo. Qi et al. (Qi et al. 2005) showed AT1 receptor expression in human primary myoblasts and Johnston et al. (Johnston et al. 2011) showed AT1 and AT2 receptor expression in mouse primary myoblasts. However, skeletal muscle Ang II receptor expression decreases at the end of gestation to very low levels in differentiated muscle (Grady et al. 1991; Tsutsumi et al. 1991). Moreover, Zhang et al. (Zhang et al. 2009) demonstrated there are no detectable levels of AT1 and AT2 receptor expression in C2C12, L6 and mouse skeletal muscle. C2C12 myotubes did not respond to Ang II with an increase in intracellular calcium or phosphorylation of extracellular signal-regulated kinase (ERK), but Ang II stimulated a calcium spike in C2C12 myotubes infected with an adenovirus encoding the AT1 receptor. These discrepancies could be explained by the difference of cells, tissues and organisms used in these studies. It is of note that myoblasts, myotubes and mature myofibers could express different levels of Ang II receptors, as indicated by Johnston et al. (Johnston et al. 2011) and it is critical to carefully assess the expression in different cell types at different status of differentiation. In addition, extra attention needs to be paid to analyze the muscle microenvironment since AT1R and AT2R are expressed in the skeletal muscle microvasculature, and Ang II signaling regulates basal- and insulin-regulated microvascular perfusion (Chai et al. 2010; 2011). It is also important to address whether the effect of Ang II on skeletal muscle cells in vitro can be applied to an in vivo experimental setting. It has been demonstrated that other circulating hormones and cytokines are involved in Ang II-induced muscle wasting. Glucocorticoids are required to stimulate the UPS in acidosis, diabetes and starvation (Wing and Goldberg 1993; Miller et al. 1999; Mitch et al. 1999), and urinary corticosterone excretion was increased in Ang II-infused animals (Brink et al. 2001). It has been shown that glucocorticoid injection in rats activates the UPS and protein degradation (Auclair et al. 1997), and reduces skeletal muscle IGF-1 (Gayan-Ramirez et al. 1999). This glucocorticoid-induced muscle wasting is mediated through GR-mediated inhibition of insulin/IGF-1 signaling (Hu et al. 2009). In a mouse model of acute diabetes, glucocorticoids decreased IRS-1-associated PI3K activity in skeletal muscle and increased muscle atrophy. Surprisingly, GR competitively binds to PI3K and reduces its association with IRS-1, which leads to decreased insulin/IGF-1 signaling and activation of muscle protein degradation. Song et al. (Song et al. 2005) showed that the GR antagonist RU486 blunted Ang II-induced muscle wasting, suggesting that Ang II indirectly activates muscle breakdown through glucocorticoid signaling. The cytokine interleukin-6 (IL-6) has been linked to muscle wasting. Thus, injection of IL-6 into rodents induces muscle protein breakdown and IL-6 receptor antibody prevents muscle atrophy in mice that overexpress IL-6 (Goodman 1994; Fujita et al. 1996; Tsujinaka et al. 1996). Zhang et al. (Zhang et al. 2009) found that circulating IL-6 and serum amyloid-A (SAA) levels were significantly increased in Ang II-infused mice and IL-6 and SAA synergistically mediated muscle wasting. IL-6 and SAA act synergistically to increase SOCS3 expression in muscle, and the increase in SOCS3 leads to downregulation of IRS-1 and impaired insulin/IGF-1 signaling. Ang II decreases Akt phosphorylation, a response that triggers caspase-3 and UPS activation, but these effects of Ang II are inhibited in IL-6 deficient mice, and IL-6 deficient mice are protected from Ang II-induced muscle wasting. Myostatin is a cytokine of the TGF-β superfamily that acts as a potent inhibitor of muscle growth. Myostatin overexpression produces severe reduction in skeletal muscle weight in mice (Zimmers et al. 2002). It has been shown that myostatin signals through activin receptor II (ActRII)/activin receptor-like kinase (ALK) receptor complex and reduces Akt/mTOR/p70S6K signaling to inhibit protein synthesis and muscle differentiation (Trendelenburg et al. 2009; Fearon et al. 2012). Although myostatin is predominantly expressed in skeletal muscle, its expression is increased in the heart after myocardial infarction (Sharma et al. 1999; Shyu et al. 2006). Cardiac-specific deletion of myostatin prevented heart failure-induced skeletal muscle atrophy, and cardiac-specific myostatin overexpression caused muscle atrophy, indicating that myocardial-derived myostatin regulates skeletal muscle atrophy (Heineke et al. 2010). Interestingly, Ang II induced myostatin expression in rat cardiomyocytes through AT1R and p38 MAPK dependent signaling (Wang et al. 2008). These data suggest that Ang II induction of myostatin expression in cardiomyocytes could result in an increase in systemic myostatin and contribute to skeletal muscle wasting in CHF patients. In summary, multiple studies strongly suggest that the catabolic effects of Ang II on skeletal muscle in vivo are in large part mediated via intermediate molecules activated by Ang II, rather than by direct Ang II mediated activation of protein breakdown in skeletal muscle myofibers (Figure 1).
Therapeutic potential of Ang II signaling blockade
Mechanisms of sarcopenia and cachexia are undoubtedly complex (Glass and Roubenoff 2010; Narici and Maffulli 2010) and the potential contribution of the RAS in these processes is still unclear. However, some studies have suggested that Ang II may play a role in reducing muscle mass in the elderly (Onder et al. 2002; Di Bari et al. 2004; Sumukadas et al. 2007), and ACE inhibitors are reported to reduce the risk of weight loss in patients with CHF (Anker et al. 2003). In patients with CHF and CKD there is a 2- to 5-fold increase in plasma Ang II levels, in many cases even in the presence of ACE inhibitor therapy (Graziani et al. 1993; Masson et al. 1998; Roig et al. 2000; Simões e Silva et al. 2006). Roig et al. (Roig et al. 2000) reported that despite chronic enalapril or captopril therapy, 50% of patients with heart failure had increased plasma Ang II. Plasma renin activity, IL-6, New York Heart Association functional class III-IV, furosemide dose, lack of beta-blocker therapy and norepinephrine were univariately associated with increased Ang II. Furthermore, increased plasma Ang II, despite ACE inhibitor therapy, was a significant predictor of death or heart failure. Masson et al. (Masson et al. 1998) also reported that circulating Ang II and aldosterone levels were elevated in 7 out of 18 heart failure patients despite clinically satisfactory ACE inhibition. These studies have been complemented by studies assessing the effects of ACE inhibitor treatment on skeletal muscle function. Onder et al. (Onder et al. 2002) reported that ACE inhibitor treatment in aged women with hypertension resulted in a lower decline in muscle strength during the 3-year study period. Di Bari et al. (Di Bari et al. 2004) reported that ACE inhibitor treatment in an aged population resulted in larger lower extremity muscle mass compared to groups treated with other drugs (β-blockers, thiazides or calcium channel blockers). Coirault et al. (Coirault et al. 2001) reported that ACE inhibitor treatment in patients with chronic heart failure improved respiratory muscle strength. Interestingly, ACE inhibitor treated aged hypertensive (but without congestive heart failure) patients had significantly higher circulating IGFBP-3 (Onder et al. 2007). Also, ACE inhibitor treatment in aged subjects with high cardiovascular disease risk profile led to higher circulating IGF-1 and IGFBP-3 levels (Giovannini et al. 2010). These data may explain the beneficial effect of ACE inhibitors on muscle wasting, since IGF-1 prevents Ang II-induced muscle atrophy. Involvement of Ang II signaling has also been demonstrated in several different mouse models of myopathy. In an animal model of myocardial infarction, there was an increase of plasma Ang II (Jin et al. 2004; Lütken et al. 2009). Schulze et al. demonstrated that CHF in mice induces skeletal muscle wasting via increased protein ubiquitination and proteasome activity (Schulze et al. 2005). Cohn et al. (Cohn et al. 2007) showed that in the mouse model of Marfan syndrome and Duchenne muscular dystrophy, increased TGF-β activity leads to failed muscle regeneration, and losartan administration normalized muscle function and repair. Burks et al. (Burks et al. 2011) demonstrated that losartan administration improved muscle regeneration after cardiotoxin-induced skeletal muscle injury in aged mice via downregulation of TGF-β signaling. Interestingly, losartan improved disuse-induced skeletal muscle regeneration in aged mice not via downregulation of TGF-β signaling, but via activation of the IGF-1/Akt/mTOR pathway. Two groups have shown that losartan and its derivative (L-150809) administration in the mouse model of laminin α2-deficient congenital muscular dystrophy type 1A (MDC1A) improved muscle strength, fibrosis and regenerative capacity via suppression of TGF-β signaling (Elbaz et al. 2012; Meinen et al. 2012). In addition, Zoll et al. (Zoll et al. 2006) reported that ACE inhibitor treatment improved myocardial infarction-induced skeletal muscle mitochondrial dysfunction. These data (summarized in Table 1) strongly suggest that blockade of Ang II signaling is a promising target for the treatment of muscle atrophy in pathological conditions such as cardiac cachexia.
Table 1.
Therapeutic potential of ACE inhibitors and angiotensin receptor blockers: summary of experimental and clinical studies
| Clinical studies | ||||||
| n | group | Drug | Duration | Measurement | Effect | Ref. |
|
| ||||||
| 641 | Hypertensive women Avg age: 77-80 no CHF |
ACE inhibitor | N/A | 3-year rates of decline in knee extensor muscle strength and walking speed |
ACE inhibitor treated group had a lower 3-year decline in muscle strength and walking speed. |
Onder et al. 2002 |
| 2431 | Well-functioning older persons Avg age: 73.6 no CHF |
ACE inhibitor •-blocker thiazide calcium channel blocker |
N/A | Lower extremity muscle mass (LEMM) |
Larger LEMM in ACE inhibitor group. | Di Bari et al. 2004 |
| 130 | Older persons with problems in mobility or functional impairment Avg age: 78.7 |
ACE inhibitor (perindopril) |
20 weeks | 6min walking distance EuroQol 5D |
Improved 6min walking distance in ACE inhibitor group. Mean score of part 1 of the EuroQol 5D was maintained in ACE inhibitor group (decline in placebo group). |
Sumukadas et al. 2007 |
| 1929 | CHF patients Avg age: 60.2 |
ACE inhibitor (enalapril) |
35 months | Body weight changes | ACE inhibitor group had a lower risk of 6% or more weight loss than no treatment group. |
Anker et al. 2003 |
| 18 | CHF patients Avg age: 62.0 |
ACE inhibitor (perindopril) |
6 months | Maximum inspiratory pressur (PImax) and maxium expiratory pressure (PEmax) |
ACE inhibitor group had improved respiratory muscle strength (increase in PImax and PEmax). |
Coirault et al. 2001 |
| Animal studies | ||||||
| Animal model | Drug | Measurement | Effect | Ref. | ||
|
| ||||||
| Mouse model of Marfan syndrome (heterozygous mutation of fibrilin-1 gene, Fbn1C1039G/+) and Duchenne muscular dystrophy (mdx) |
ARB (losartan) |
TGF-β levels and muscle regeneration and function after CTX injury |
Losartan reduced TGF-• expression and improved skeletal muscle regenerative capacity and function. |
Cohn et al. 2007 | ||
| Aged mice (21 months old) |
ARB (losartan) |
TGF-β levels and muscle regeneration and function after CTX injury or immoblization |
Losartan improved muscle function after CTX injury through inhibition of TGF-β, whereas losartan prevents disuse atrophy through increase of IGF-1/Akt/mTOR pathway. |
Burks et al. 2011 | ||
| Mouse model for laminin-α2-deficient congenital muscular dystrophy (MDC1A, dyW/dyW) |
ARB (L-158809) |
Muscle regeneration after notexin injury and muscle locomoter activity and strength |
L-158809 suppressed TGF-β in dyW/dyW mouse skeletal muscle and reduced fibrosis and inflammation after injury. L-158809 improved locomoter activity and strength, and increased body wiigh in mice. |
Meinen et al. 2012 | ||
| Mouse model for laminin-α2-deficient congenital muscular dystrophy (MDC1A, dy2J/dy2J) |
ARB (losartan) |
TGF-β signaling and muscle strength | Losartan improved muscle strength and ameliorate muscle fibrosis. Losartan inhibited TGF-β signaling and MAPK signaling (p38MAPK, c-Jun and ERK1/2) in skeletal muscle |
Elbaz et al. 2012 | ||
| Rat model of myocardial infarction (left descending coronary artery ligation) |
ACE inhibitor (perindopril) |
Mitochondrial function and protein expression in skeletal muscle |
Perindopril prevented MI-induced reduction of mitochondrial function and mRNA expression of mitochondrial transcription factors. |
Zoll et al. 2006 | ||
Conclusions/Future prospects
Many patients with chronic conditions such as advanced CHF or ESRD have significant muscle wasting and there is increasing evidence that Ang II, which is often elevated in these conditions, plays a role. The mechanisms whereby Ang II induces muscle atrophy are complex (Figure 2) and include potential direct effects on skeletal muscle and indirect effects from intermediate molecules such as glucocorticoids, IL-6, TNF-α, and SAA (Figure 1). The net effect of Ang II is to disrupt insulin, IGF-1 and AMPK signaling, to reduce ATP levels and to increase protein degradation (via activation of the UPS) and apoptosis in skeletal muscle (Figure 1). In addition to its effects on skeletal muscle Ang II reduces hypothalamic Npy and orexin expression, thereby decreasing food intake (Figure 2). This central effect of Ang II plays an important contributory role to its ability to induce muscle wasting. Important areas for future study include understanding mechanisms whereby Ang II may regulate muscle regenerative processes. The effects of Ang II to induce wasting are mediated via the AT1R, thus potential therapeutic strategies include use of AT1R blockers or ACE inhibitors, but there are few prospective trials that have assessed efficacy of this strategy and some animal data have suggested that ACE inhibitors may reduce food intake possibly via an elevation of central Ang II. The ability of Ang II to disrupt IGF-1 signaling in skeletal muscle and the fact that forced overexpression of IGF-1 in muscle prevent Ang II induced atrophy provides a rationale to explore the potential use of IGF-1 in cachectic states. Furthermore the recent demonstration that Ang II interference with AMPK signaling is an important mechanism mediating Ang II catabolic effects on skeletal muscle raises the possibility that AMPK activators may have beneficial effects in chronic wasting conditions in which the RAS is activated.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute R01-HL070241, R01-HL080682, NIH/National Center for Research Resources P20-RR018766, the NIH/National Institute of General Medical Sciences P20-GM103514, P30-GM103337-Subproject #7921 and U54-GM104940.
Abbreviations
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- CHF
congestive heart failure
- ESRD
end-stage renal disease
- ACE
angiotensin-converting enzyme
- UPS
ubiquitin proteasome system
- ROS
reactive oxygen species
- IGF-1
insulin-like growth factor-1
- IRS-1
insulin receptor substarte
- PI3K
phosphoinositide 3-kinase
- mTOR
mammalian target of rapamycin
- MAPK
mitogen-activated kinase
- ERK
extracellular signal-regulted kinase
- IL-6
interleukin-6
- SAA
serum amyloid A
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- GR
glucocorticoid receptor
- AMPK
AMP-activated kinase
- AICAR
5-Aminoimidazole-4-carboxamide ribonucleotide
- Npy
neuropeptide Y
- AgRP
agouti-related protein
- CRH
corticotropin-releasing hormone
- TRH
thyrotropin-releasing hormone
- POMC
proopiomelanocortin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, et al. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell Metab. 2007 Jun;5(6):476–87. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Anker SD, Negassa A, Coats AJS, Afzal R, Poole-Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003 Mar 29;361(9363):1077–83. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Argilés JM, López-Soriano FJ, Busquets S. Novel approaches to the treatment of cachexia. Drug Discov Today. 2008 Jan;13(1-2):73–8. doi: 10.1016/j.drudis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Attaix D, Baracos VE. MAFbx/Atrogin-1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care. 2010 May;13(3):223–4. doi: 10.1097/MCO.0b013e328338b9a6. [DOI] [PubMed] [Google Scholar]
- Attaix D, Ventadour S, Codran A, Béchet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86. doi: 10.1042/EB0410173. [DOI] [PubMed] [Google Scholar]
- Auclair D, Garrel DR, Zerouala AC, Ferland LH. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am J Physiol Cell Physiol. 1997 Mar 1;271(3):C1007–16. doi: 10.1152/ajpcell.1997.272.3.C1007. [DOI] [PubMed] [Google Scholar]
- Barton ER, Morris L, Musarò A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002 Apr 1;157(1):137–48. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008 Aug;36(8):1548–54. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001a Nov 23;294(5547):1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001b Nov;3(11):1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007 Aug 10;317(5839):807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001 Apr;142(4):1489–96. doi: 10.1210/endo.142.4.8082. [DOI] [PubMed] [Google Scholar]
- Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996 Jun 1;97(11):2509–16. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, van Erp C, Simmers JL, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med. 2011 May 11;3(82):82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Acuña MJ, Morales MG, Becerra A, Simon F, Brandan E. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Comm. 2011 Jul;410(3):665–70. doi: 10.1016/j.bbrc.2011.06.051. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Cordova G, Salas JD. Angiotensin II: Role in Skeletal Muscle Atrophy. Curr Protein Pept Sci. 2012 Sep 1;13(6):560–9. doi: 10.2174/138920312803582933. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Melendez PA, Oh B-C, Lidov HGW, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004 Oct 15;119(2):285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008 Jul 24;454(7203):528–32. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011 Nov;60(11):2939–46. doi: 10.2337/db10-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, et al. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010 Feb;55(2):523–30. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012 Oct 18;490(7420):355–60. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007 Nov;6(5):376–85. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009 Jun 15;185(6):1083–95. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007 Feb;13(2):204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coirault C, Hagège A, Chemla D, Fratacci MD, Guérot C, Lecarpentier Y. Angiotensin-converting enzyme inhibitor therapy improves respiratory muscle strength in patients with heart failure. Chest. 2001 Jun;119(6):1755–60. doi: 10.1378/chest.119.6.1755. [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995 May 19;270(20):12109–16. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007 Apr;25(4):885–94. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003 Nov 28;302(5650):1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005 Feb 17;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002 Sep;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Csibi A, Cornille K, Leibovitch MP, Poupon A, Tintignac LA, Sanchez AMJ, et al. The translation regulatory subunit eIF3f controls the kinase-dependent mTOR signaling required for muscle differentiation and hypertrophy in mouse. PLoS ONE. 2010;5(2):e8994. doi: 10.1371/journal.pone.0008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibi A, Leibovitch MP, Cornille K, Tintignac LA, Leibovitch SA. MAFbx/Atrogin-1 controls the activity of the initiation factor eIF3-f in skeletal muscle atrophy by targeting multiple C-terminal lysines. J Biol Chem. 2009 Feb 13;284(7):4413–21. doi: 10.1074/jbc.M807641200. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L, Ravara B, Angelini A, Rossini K, Sandri M, Thiene G, et al. Beneficial effects on skeletal muscle of the angiotensin II type 1 receptor blocker irbesartan in experimental heart failure. Circulation. 2001 May 1;103(17):2195–200. doi: 10.1161/01.cir.103.17.2195. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000 Sep;52(3):415–72. [PubMed] [Google Scholar]
- de Kloet AD, Krause EG, Kim D-H, Sakai RR, Seeley RJ, Woods SC. The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis. Endocrinology. 2009 Sep;150(9):4114–23. doi: 10.1210/en.2009-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bari M, van de Poll-Franse LV, Onder G, Kritchevsky SB, Newman A, Harris TB, et al. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004 Jun;52(6):961–6. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- Doehner W, Haehling von S, Anker SD, Lainscak M. Neurohormonal activation and inflammation in chronic cardiopulmonary disease: a brief systematic review. Wien Klin Wochenschr. 2009 Jun;121(9-10):293–6. doi: 10.1007/s00508-009-1194-7. [DOI] [PubMed] [Google Scholar]
- Elbaz M, Yanay N, Aga-Mizrachi S, Brunschwig Z, Kassis I, Ettinger K, et al. Losartan, a therapeutic candidate in congenital muscular dystrophy: studies in the dy(2J) /dy(2J) mouse. Ann Neurol. 2012 May;71(5):699–708. doi: 10.1002/ana.22694. [DOI] [PubMed] [Google Scholar]
- Fearon KCH, Glass DJ, Guttridge DC. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. Elsevier. 2012 Aug;16(2):153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997 Nov 1;100(9):2158–69. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J, Tsujinaka T, Ebisui C, Yano M, Shiozaki H, Katsume A, et al. Role of interleukin-6 in skeletal muscle protein breakdown and cathepsin activity in vivo. Eur Surg Res. 1996;28(5):361–6. doi: 10.1159/000129477. [DOI] [PubMed] [Google Scholar]
- Gayan-Ramirez G, Vanderhoydonc F, Verhoeven G, Decramer M. Acute treatment with corticosteroids decreases IGF-1 and IGF-2 expression in the rat diaphragm and gastrocnemius. Am J Respir Crit Care Med. 1999 Jan;159(1):283–9. doi: 10.1164/ajrccm.159.1.9803021. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Cachexia Sarcopenia Muscle. 2010 May 5;14(6):457–60. doi: 10.1007/s12603-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann NY Acad Sci. 2010 Nov;1211:25–36. doi: 10.1111/j.1749-6632.2010.05809.x. [DOI] [PubMed] [Google Scholar]
- Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc. Soc. Exp. Biol. Med. 1994 Feb;205(2):182–5. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991 Sep;88(3):921–33. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani G, Badalamenti S, Del Bo A, Marabini M, Gazzano G, Como G, et al. Abnormal hemodynamics and elevated angiotensin II plasma levels in polydipsic patients on regular hemodialysis treatment. Kidney Int. 1993 Jul;44(1):107–14. doi: 10.1038/ki.1993.219. [DOI] [PubMed] [Google Scholar]
- Haehling von S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009 Mar;121(3):227–52. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Schulze PC, Gielen S, Linke A, Möbius-Winkler S, Yu J, et al. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002 Apr 3;39(7):1175–81. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010 Jan 26;121(3):419–25. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest. 2009 Sep 14;119(10):3059–69. doi: 10.1172/JCI38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Takai S, Sakaguchi M, Okamoto Y, Muramatsu M, Miyazaki M. An antiarrhythmic effect of a chymase inhibitor after myocardial infarction. J Pharmacol Exp Ther. 2004 May;309(2):490–7. doi: 10.1124/jpet.103.061465. [DOI] [PubMed] [Google Scholar]
- Johnston APW, Baker J, Bellamy LM, McKay BR, De Lisio M, Parise G. Regulation of muscle satellite cell activation and chemotaxis by angiotensin II. PLoS ONE. 2010;5(12):e15212. doi: 10.1371/journal.pone.0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston APW, Baker J, De Lisio M, Parise G. Skeletal muscle myoblasts possess a stretch-responsive local angiotensin signalling system. J Renin Angiotensin Aldosterone Syst. 2011 Jun;12(2):75–84. doi: 10.1177/1470320310381795. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka T-A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators of Inflammation. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Zhang G-X, Nishiyama A, Shokoji T, Yao L, Fan Y-Y, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005 Mar;45(3):438–44. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS ONE. 2009;4(3):e4973. doi: 10.1371/journal.pone.0004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, et al. The initiation factor eIF3-f is a major target for Atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 2008 Mar 20;27(8):1266–76. doi: 10.1038/emboj.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker SH. Ubiquitin-protein ligases in muscle wasting: multiple parallel pathways? Curr Opin Clin Nutr Metab Care. 2003 May;6(3):271–5. doi: 10.1097/01.mco.0000068963.34812.e5. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004 Jan;18(1):39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- Leclerc GM, Leclerc GJ, Fu G, Barredo JC. AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Signal. 2010;5:15. doi: 10.1186/1750-2187-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997 Oct;18(4):383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- Lütken SC, Kim SW, Jonassen T, Marples D, Knepper MA, Kwon T-H, et al. Changes of renal AQP2, ENaC, and NHE3 in experimentally induced heart failure: response to angiotensin II AT1 receptor blockade. Am J Physiol Renal Physiol. 2009 Dec;297(6):F1678–88. doi: 10.1152/ajprenal.00010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson S, Latini R, Bevilacqua M, Vago T, Sessa F, Torri M, et al. Within-patient variability of hormone and cytokine concentrations in heart failure. Pharmacol Res. 1998 Mar;37(3):213–7. doi: 10.1006/phrs.1998.0288. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003 Sep 15;162(6):1135–47. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinen S, Lin S, Rüegg MA. Angiotensin II type 1 receptor antagonists alleviate muscle pathology in the mouse model for laminin-alpha2-deficient congenital muscular dystrophy (MDC1A) Skeletal Muscle. 2012 Sep 3;2(1):18. doi: 10.1186/2044-5040-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Villalobos AR, Pritchard JB. Organic cation transport in rat choroid plexus cells studied by fluorescence microscopy. Am J Physiol. 1999 Apr;276(4 Pt 1):C955–68. doi: 10.1152/ajpcell.1999.276.4.C955. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999 May;276(5 Pt 1):C1132–8. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- Morales MG, Vazquez Y, Acuña MJ, Rivera JC, Simon F, Salas JD, et al. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol. 2012 Nov;44(11):1993–2002. doi: 10.1016/j.biocel.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1159–68. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- Murphy KT, Allen AM, Chee A, Naim T, Lynch GS. Disruption of muscle renin-angiotensin system in AT1a-/- mice enhances muscle function despite reducing muscle mass but compromises repair after injury. Am J Physiol Regul Integr Comp Physiol. 2012 Aug;303(3):R321–31. doi: 10.1152/ajpregu.00007.2012. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001 Feb;27(2):195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA K, YAKABE Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem. 2007 Jul;71(7):1650–6. doi: 10.1271/bbb.70057. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- Onder G, Liperoti R, Russo A, Capoluongo E, Minucci A, Lulli P, et al. Use of ACE inhibitors is associated with elevated levels of IGFBP-3 among hypertensive older adults: results from the IlSIRENTE study. Eur J Clin Pharmacol. 2007 Apr;63(4):389–95. doi: 10.1007/s00228-007-0262-z. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BWJH, Balkrishnan R, Fried LP, Chaves PHM, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002 Mar 16;359(9310):926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA. 2002 Jul 9;99(14):9213–8. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X-D, Xu P-Z, Chen M-L, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003 Jun 1;17(11):1352–65. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JP, Anderson JM, Robison RJ, Phillips AC. Effect of central angiotensin II on body weight gain in young rats. Brain Res. 2003 Jan 3;959(1):20–8. doi: 10.1016/s0006-8993(02)03676-4. [DOI] [PubMed] [Google Scholar]
- Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol. 2004 Aug;287(2):R422–8. doi: 10.1152/ajpregu.00537.2003. [DOI] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012 May;15(3):240–5. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2000 Mar;20(3):645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- Qi J-S, Minor LK, Smith C, Hu B, Yang J, Andrade-Gordon P, et al. Characterization of functional urotensin II receptors in human skeletal muscle myoblasts: comparison with angiotensin II receptors. Peptides. 2005 Apr;26(4):683–90. doi: 10.1016/j.peptides.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, et al. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003 Jan;17(1):53–5. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]
- Roig E, Perez-Villa F, Morales M, Jiménez W, Orús J, Heras M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000 Jan;21(1):53–7. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001 Nov;3(11):1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999 Nov 26;286(5445):1738–41. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Russell ST, ELEY H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal. 2007 Aug;19(8):1797–806. doi: 10.1016/j.cellsig.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Russell ST, Wyke SM, Tisdale MJ. Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal. 2006 Jul;18(7):1087–96. doi: 10.1016/j.cellsig.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008 Nov 27;456(7221):502–6. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005 Aug 22;93(4):425–34. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008 Jun;23:160–70. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, et al. Insulin-like growth factor-I gene transfer by electroporation prevents skeletal muscle atrophy in glucocorticoid-treated rats. Endocrinology. 2005 Apr;146(4):1789–97. doi: 10.1210/en.2004-1594. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, et al. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005 Sep 2;97(5):418–26. doi: 10.1161/01.RES.0000179580.72375.c2. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Gielen S, Adams V, Linke A, Möbius-Winkler S, Erbs S, et al. Muscular levels of proinflammatory cytokines correlate with a reduced expression of insulinlike growth factor-I in chronic heart failure. Basic Res Cardiol. 2003 Jul;98(4):267–74. doi: 10.1007/s00395-003-0411-1. [DOI] [PubMed] [Google Scholar]
- Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, et al. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Comm. 2011 Jun 3;409(2):217–21. doi: 10.1016/j.bbrc.2011.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999 Jul;180(1):1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006 Oct;36(10):713–9. doi: 10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Simões e Silva AC, Diniz JSS, Pereira RM, Pinheiro SVB, Santos RAS. Circulating renin Angiotensin system in childhood chronic renal failure: marked increase of Angiotensin-(1-7) in end-stage renal disease. Pediatr Res. 2006 Dec;60(6):734–9. doi: 10.1203/01.pdr.0000246100.14061.bc. [DOI] [PubMed] [Google Scholar]
- Song Y-H, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005 Feb;115(2):451–8. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011 Jan;20(1):84–8. doi: 10.1097/MNH.0b013e3283414d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, Ye F, Liu M, Borst SE, Conover C, Yarasheski KE, et al. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab. 2010 Nov;299(5):E730–40. doi: 10.1152/ajpendo.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas D, Witham MD, Struthers AD, McMurdo MET. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007 Oct 9;177(8):867–74. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabony AM, Yoshida T, Galvez S, Higashi Y, Sukhanov S, Chandrasekar B, et al. Angiotensin II upregulates protein phosphatase 2Cα and inhibits AMP-activated protein kinase signaling and energy balance leading to skeletal muscle wasting. Hypertension. 2011 Oct;58(4):643–9. doi: 10.1161/HYPERTENSIONAHA.111.174839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008 Jul;11(4):400–7. doi: 10.1097/MCO.0b013e328300ecc1. [DOI] [PubMed] [Google Scholar]
- Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010 Feb;81(1):11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005 Jan 28;280(4):2847–56. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009 Apr;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009 Jun;296(6):C1258–70. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996 Jan 1;97(1):244–9. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K, Strömberg C, Viswanathan M, Saavedra JM. Angiotensin-II receptor subtypes in fetal tissue of the rat: autoradiography, guanine nucleotide sensitivity, and association with phosphoinositide hydrolysis. Endocrinology. 1991 Aug;129(2):1075–82. doi: 10.1210/endo-129-2-1075. [DOI] [PubMed] [Google Scholar]
- van der Meer SFT, Jaspers RT, Jones DA, Degens H. Time-course of changes in the myonuclear domain during denervation in young-adult and old rat gastrocnemius muscle. Muscle Nerve. 2011 Feb;43(2):212–22. doi: 10.1002/mus.21822. [DOI] [PubMed] [Google Scholar]
- Velloso LA, Folli F, Perego L, Saad MJA. The multi-faceted cross-talk between the insulin and angiotensin II signaling systems. Diabetes Metab Res Rev. 2006 Mar;22(2):98–107. doi: 10.1002/dmrr.611. [DOI] [PubMed] [Google Scholar]
- Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2006 Nov;18(6):631–5. doi: 10.1097/01.bor.0000245731.25383.de. [DOI] [PubMed] [Google Scholar]
- Wang B-W, Chang H, Kuan P, Shyu K-G. Angiotensin II activates myostatin expression in cultured rat neonatal cardiomyocytes via p38 MAP kinase and myocyte enhance factor 2 pathway. J Endocrinol. 2008 Apr;197(1):85–93. doi: 10.1677/JOE-07-0596. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pessin JE. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013 May;16(3):243–50. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GME, Clark SE, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006 Nov 17;281(46):35137–46. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- Werner C, Pöss J, Böhm M. Optimal antagonism of the Renin-Angiotensin-aldosterone system: do we need dual or triple therapy? Drugs. 2010 Jul 9;70(10):1215–30. doi: 10.2165/11537910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE. 2010;5(12):e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing SS, Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol. 1993 Apr;264(4 Pt 1):E668–76. doi: 10.1152/ajpendo.1993.264.4.E668. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Day K. Skeletal Muscle Stem Cells in the Spotlight: The Satellite Cell. Regenerating the Heart. Springer. 2011:173–200. [Google Scholar]
- Yamamoto R, Akazawa H, Fujihara H, Ozasa Y, Yasuda N, Ito K, et al. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J Biol Chem. 2011 Jun 17;286(24):21458–65. doi: 10.1074/jbc.M110.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P. IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1565–70. doi: 10.1152/ajpheart.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012 Mar;153(3):1411–20. doi: 10.1210/en.2011-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, et al. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009 Mar;20(3):604–12. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, et al. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension. 2006 Oct;48(4):637–43. doi: 10.1161/01.HYP.0000240347.51386.ea. [DOI] [PubMed] [Google Scholar]
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002 May 24;296(5572):1486–8. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- Zoll J, Monassier L, Garnier A, N’Guessan B, Mettauer B, Veksler V, et al. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol. 2006 Aug;101(2):385–91. doi: 10.1152/japplphysiol.01486.2005. [DOI] [PubMed] [Google Scholar]