Abstract
Objective
To (1) evaluate the effects of a single session of four non-pharmacological pain interventions, relative to a sham tDCS procedure, on pain and electroencephalogram- (EEG-) assessed brain oscillations, and (2) determine the extent to which procedure-related changes in pain intensity are associated with changes in brain oscillations.
Methods
30 individuals with spinal cord injury and chronic pain were given an EEG and administered measures of pain before and after five procedures (hypnosis, meditation, transcranial direct current stimulation [tDCS], and neurofeedback) and a control sham tDCS procedure.
Results
Each procedure was associated with a different pattern of changes in brain activity, and all active procedures were significantly different from the control procedure in at least three bandwidths. Very weak and mostly non-significant associations were found between changes in EEG-assessed brain activity and pain.
Conclusions
Different non-pharmacological pain treatments have distinctive effects on brain oscillation patterns. However, changes in EEG-assessed brain oscillations are not significantly associated with changes in pain, and therefore such changes do not appear useful for explaining the benefits of these treatments.
Significance
The results provide new findings regarding the unique effects of four non-pharmacological treatments on pain and brain activity.
Keywords: spinal cord injury, chronic pain, electroencephalography, brain states, non-pharmacological treatments
Introduction
Despite the significant advances in our scientific understanding of pain and its causes, chronic pain remains a significant health problem worldwide with profound negative impacts on individuals and society (Institute of Medicine Committee on Advancing Pain Research Care and Education, 2011; Nakamura et al., 2011; Reid et al., 2011; Schopflocher et al., 2011). Earlier models of pain popular in the 20th century and earlier focused on peripheral activity. However, research in the late 20th and early 21st centuries has established CNS structures as playing a key role in the development and experience of chronic pain (Apkarian et al.,2009; Jensen, 2010). We now know that while nociception (information about damage or potential damage sent to the CNS from the periphery) plays a role in some chronic pain problems, nociception is neither necessary nor sufficient for someone to experience pain. Rather, pain is now recognized to be the result of a complex interaction of activity in multiple cortical-subcortical neural networks and processes (Jensen, 2010). Research has also demonstrated central neural networks are plastic: ongoing activation of pain-related central networks can lead to changes in these networks, consolidating and thus facilitating pain processing even independent of peripheral neural activation (Gustin et al., 2012). This growing recognition of the importance of CNS among pain researchers and clinicians has contributed to a concomitant interest in interventions – many of them non-pharmacological – that directly or indirectly target cortical or subcortical activity as ways to manage pain (Moseley and Flor, 2012).
Interventions that target CNS activity include hypnosis, electroencephalographic (EEG) biofeedback (also known as neurofeedback), meditation training and practice, and transcranial direct current stimulation (tDCS). However, the extent to which these treatments operate via their effects on CNS activity and whether these CNS effects are similar or different across treatments has not yet been elucidated.
At its most basic, hypnosis can be viewed as having two components: (1) a hypnotic induction (“…initial suggestions for using one’s imagination”; p. 262, Green et al., 2005) followed by (2) “…suggestions for changes in subjective experience, alterations in perception, sensation, thought, or behavior” (p. 262, Green et al., 2005). Although treatment response to suggestions without a formal hypnotic induction is possible, research indicates that responses to suggestions are stronger when a hypnotic induction is part of the procedure (Derbyshire et al., 2004). As might be expected, hypnotic treatments for chronic pain usually include suggestions for experiencing reductions in pain intensity or increases in the ability to ignore pain. In addition, hypnotic pain treatment often also includes suggestions for changes in pain-related thoughts and behaviors (Jensen et al., 2011; Patterson and Jensen, 2003).
In neurofeedback, subjects are given direct information about their brain states – usually as measured by EEG – and asked to use this information to directly alter brain activity thought to be related to specific behaviors (e.g. pain; Jensen et al., 2007a; Sime, 2004).
Meditation may be the most difficult non-pharmacological intervention to define, given the many procedures and activities that have been described as meditation. However, most of these can be classified into two primary types: (1) “mindfulness” meditation (paying attention to one’s current experience in a nonjudgmental way; Carmody, 2009) and (2) “concentration” meditation (purposeful concentration on a single stimulus, such as one’s breathing or a single word or phrase; Dunn et al., 1999).
tDCS involves the application of weak electrical direct currents (1 – 2 mA) over the scalp using usually two electrodes – a positive anode electrode and a negative cathode electrode. In pain, the most common electrode montage consists of placing the anode electrode over the primary motor cortex and the cathode electrode over the supra-orbital area (Fregni et al., 2006). tDCS has been shown to provide a sufficient amount of electrical current to reach cortical areas and modify cortical excitability (Brunoni et al., 2012; Wagner et al., 2007).
Evidence supports the clinical efficacy of hypnosis for reducing chronic pain intensity (Jensen and Patterson, 2006; Patterson and Jensen, 2003). Preliminary evidence also supports the potential for neurofeedback (Caro and Winter, 2011; Kayiran et al., 2010), meditation practice (Marchand, 2012; Zeidan et al., 2011), and tDCS (Fenton et al., 2009; Fregni et al., 2006) for reducing chronic pain intensity. Importantly, research findings from a number of sources suggest the possibility that these treatments might be effective, at least in part, because they alter brain states. Moreover, these cortical changes may be reflected in oscillatory cortical electrical wave activity that can be measured by EEG. For example, both acute and chronic pain studies have shown reproducible changes of increased “fast” (beta [13 – 35 Hz]) brainwave activity thought to be related to active information processing, and reduced “slow” (mostly alpha [8– 12 Hz], but also in some studies, theta [4 – 7.5 Hz]) brain activity associated with subjective relaxation (e.g., Bromm and Lorenz, 1998; Chen et al., 1983). Interestingly, hypnosis and meditation have been shown to increase slow wave activity, especially theta activity (Crawford, 1990; Fell et al., 2010; Williams and Gruzelier, 2001). Further, neurofeedback for pain treatment commonly seeks to reduce fast wave and increase slower wave activity (Jensen et al., 2007a; Sime, 2004). Based on these findings, it would be reasonable to hypothesize that increases in slower wave activity (e.g., theta and alpha) and decreases of faster wave activity (e.g., beta) would be associated with reductions in pain intensity.
Although non-pharmacological interventions theoretically reduce pain by altering brain activity, no studies have performed head-to-head comparisons of the effects of these treatments on brain activity. Nor have any studies examined associations between cortical effects of different treatments and treatment-related changes in pain intensity in the same sample. Increased knowledge about the effects of these treatments on EEG and the associations between changes in EEG activity and changes in pain is important for a number of reasons. First, although each of these treatments appears on the surface to be different, it is possible that they share common underlying mechanisms for reducing pain. For example, it is possible that all of these treatments effectively reduce pain via altering activities associated with reduced fast wave and/or increased slow wave EEG activity. If this were found to be the case, then future research could determine which of these treatments (or which combination(s) of treatments) might work to produce the most profound and longest lasting effects on those EEG bandwidths that are most closely linked to pain relief. On the other hand, if each of these treatments has different effects on brain activity, this would suggest that they operate via different mechanisms. In this case, it is possible that treatments might at times act at odds with each other, and maximizing treatment efficacy may involve matching patients to treatments most effective for their particular condition. Alternatively, different mechanisms of action might be combined synergistically. Such an approach has been shown effective for the combination of noninvasive brain stimulation and antidepressants for the treatment of depression (Brunoni et al., in press).
Given these considerations, the primary purposes of this study were to: (1) determine the effects of a single session of hypnosis, neurofeedback, tDCS, and a concentration meditation procedure on EEG-assessed bandwidth activity, relative to a sham (placebo) tDCS procedure; (2) compare the efficacy of each procedure for reducing pain intensity in a sample of patients with chronic pain (specifically, in this case, patients with spinal cord injury [SCI] and chronic pain); and (3) identify the EEG-assessed bandwidth activity measures and scalp locations that are most strongly associated with improvements (reductions) in pain intensity in a sample of patients with chronic refractory pain. This last question is particularly important for establishing neuro-feedback protocols that require selection of specific scalp regions for training.
Based on previous research (e.g., Graffin et al., 1995; see also review by Crawford and Gruzelier, 1992), we hypothesized that hypnosis will result in increases in theta activity. Given that hypnosis’ effects on alpha activity have been inconsistent in previous research (Crawford and Gruzelier, 1992), and its effects on beta, delta and gamma have been very rarely studied, we did not have a priori hypotheses about the effects of hypnosis on bandwidth activity other than theta. For meditation, there is a fairly consistent finding that meditation practices are associated with increases in both theta and alpha band activity (Cahn and Polich, 2006); we therefore hypothesized significant increases in these oscillations following meditation. Based on previous research demonstrating that neurofeedback has effects on EEG activity consistent with training protocols for slower wave activity and weaker effects on faster beta activity (Egner, 2004; Egner and Gruzelier, 2004; Egner et al., 2002; Gevensleben et al., 2009; Kayiran et al., 2010), we hypothesized that a single-session neurofeedback protocol that reinforced more alpha activity and less beta activity would result in significant increases in alpha oscillations only. Given a tendency for tDCS to be associated with more fast wave (beta) and less slow wave (delta, theta, alpha) activity in areas that lie under the positive lead (Ardolino et al., 2005; Jacobson et al., 2012; Keeser et al., 2011; Maeoka et al., 2012), we hypothesized more beta and less delta, theta, and alpha activity following tDCS, especially in the anterior scalp regions where the positive lead was placed. Finally, we anticipated that the sham tDCS procedure would have no systematic effects on brain oscillations.
In terms of the effects of these interventions on pain intensity, we hypothesized based on previous research (e.g., Jensen et al., 2009) that a single session of hypnosis would result in significant decreases in pain intensity. We also predicted that the sham tDCS procedure would not be associated with significant pain reductions. Most efficacy research for meditation, neurofeedback and tDCS has studied response to several treatment sessions. Therefore, we anticipated a single session of each would likely result in modest decreases in pain intensity and correspondingly small effects on EEG parameters which may not be statistically significant (e.g., Buhle and Wager, 2010; Fregni et al., 2006; Jensen et al., 2007a).
Regarding the associations between changes in bandwidth activity and pain, and given research that has linked both alpha and beta to pain intensity (Jensen et al., 2008), we predicted that increases in alpha and decreases in beta activity would be associated with pain reduction. Finally, we did not have strong a priori hypotheses regarding the associations between changes in EEG activity at specific electrode sites and changes in pain. This is mostly because such site-specific analyses have been rarely done. However, based on the known effects of these treatments on EEG, we speculated some site-specific associations. For example, given that anodal (positive lead) tDCS appears to increase faster wave (beta) activity and meditation and hypnosis both increase slower wave (alpha and theta) activity, we speculated that pain reductions with tDCS might be associated with increases in beta as measured over the motor cortex (perhaps representing activation of inhibitory processes in subcortical areas such as thalamic areas), and that pain reductions with hypnosis and meditation might be associated with increases in slow wave activity above or near the sensory cortices (perhaps representing decreased activity in these areas associated with less processing of nociceptive information).
Methods
Participants
One hundred eighteen potential participants (patients with SCI identified from an existing patient registry or medical records) were screened for participation in the study. Inclusion criteria for study participation included: (1) age at least 18 years; (2) being at least 12 months post SCI; (3) daily SCI pain for at least 6 months; (4) average daily SCI pain of greater than 3 (on a 0–10 scale) over the prior week at screening; and (5) ability to read, write and understand English. Study exclusion criteria included: (1) history of seizure disorder; (2) significant brain injury or skull defect as determined during physical exam; (3) non-normative brain activity as detected by initial EEG assessment; (4) a medical condition unrelated to the SCI that could impact or otherwise interact with pain scores; (5) significant psychological or psychiatric disturbance (specifically, active suicidal ideation with intent or evidence of significant paranoia); and (6) hospitalization for psychiatric reasons in the past six months. In order to participate in each day’s procedures (which included an EEG assessment before and after each treatment), participants must, on that day: (1) report a current pain intensity level of at least 3 on a 0–10 scale and (2) if female, report that they are not experiencing menses.
Of the 54 individuals who were contacted and who met the study inclusion criteria, 18 declined participation for various reasons (e.g. concerns over the tDCS procedure, lack of transportation, family emergency, or did not respond after the initial screening where they were found eligible for the study). Of the 36 remaining participants, 3 were deemed ineligible during the initial medical screening evaluation, 1 was found to be sensitive to the EEG gel at the first EEG assessment and therefore chose not to continue participation, and the last one reported no pain at the baseline visit.
The remaining 31 individuals were randomly assigned to receive the five procedure conditions in one of five orders, using a Latin square design. All 31 participants received the hypnosis, neurofeedback, and meditation conditions (described below). However, one participant was not included in any of the analyses, because his EEG measures had an anomalous reading from one single electrode that was consistent across all treatment conditions. Another participant dropped out of the study before completing the tDCS and sham procedures. For other participants, some EEG measures within individual (but not all) treatment conditions were excluded when participants indicated that they wished to stop the procedure due to discomfort, that they had ingested medication that could affect EEG measures, or when the EEG readings themselves were unusable. Thus, the EEG and pain measures that comprise the analytic dataset include 30 participant treatment sessions from the meditation and neurofeedback conditions, 29 from the hypnosis condition, 28 from the tDCS condition, and 27 from the sham tDCS treatment conditions. Study participant demographic and pain type information are presented in Table 1. All of the study procedures were approved by the University of Washington Institutional Review board, and all participants signed informed consent forms prior to participation.
Table 1.
Study participant descriptive information.
| Variable | Range or number | Mean or percent |
|---|---|---|
| Age in years | 22, 77 | 49.16 |
| Sex | ||
| Men | 22 | 73% |
| Women | 8 | 27% |
| Ethnicity | ||
| White | 25 | 83% |
| Black | 1 | 3% |
| Asian | 1 | 3% |
| Hispanic | 2 | 7% |
| More than one race* | 1 | 3% |
| Highest education level | ||
| Some high school | 1 | 3% |
| High school or GED | 3 | 10% |
| Some college | 11 | 37% |
| College graduate | 12 | 40% |
| Graduate school | 3 | 10% |
| Marital status | ||
| Married | 8 | 27% |
| Divorced | 5 | 17% |
| Unmarried, living with partner | 3 | 10% |
| Never married | 14 | 47% |
| Pain type | ||
| Neuropathic | 11 | 37% |
| Nociceptive | 2 | 7% |
| Mixed | 17 | 57% |
| ASIA Impairment Score* | ||
| Level 1 | 17 | 59% |
| Level 2 | 5 | 17% |
| Level 3 | 2 | 7% |
| Level 4 | 5 | 17% |
Note: GED = Graduate Equivalency Degree
One subject described himself as White and American Indian
One subject’s sensation deficit was not clearly attributable to SCI, so the evaluating physician was unable to assign level by clinical exam, although the SCI diagnosis was confirmed by radiographical exam.
Medical Screening Evaluation and baseline assessment
At an initial (baseline) visit, the study physician performed a medical evaluation to confirm eligibility and ensure that the pain problem (or any other medical problem) did not require immediate medical attention. The evaluation also included the use of a standardized protocol to classify the participants’ pain as neuropathic or nociceptive (Cardenas et al., 2002). The subject’s most significant pain locations were recorded. The medical evaluation also included an ASIA impairment assessment (Kirshblum, 2011; Marino, 2003).
Measures
Pain intensity
Pain intensity experienced during the 10 minute EEG assessment procedures before and after each procedure was assessed using a series of 0–10 Numerical Rating Scales (NRS) with 0 = “No pain sensation” and 10 = “The most intense pain sensation imaginable”. Current, least, worst and average pain (for the latter three intensity domains, pain “in the past five minutes”) were assessed five minutes and then again 10 minutes after the start of the EEG assessment. Thus, eight 0–10 pain intensity ratings were obtained for each EEG assessment. These eight ratings were averaged to compute a single composite score of characteristic pain intensity experienced during the EEG assessments. A great deal of evidence supports the reliability and validity of 0–10 NRSs as measures of pain intensity (Jensen, 2001). Moreover, the use of composite pain scores is recommended as a way to increase measurement reliability and validity (Jensen et al., 1999).
Procedures
Day before and day of procedure screening
The day before each study visit, subjects were contacted via telephone and given recommendations (e.g., to wash their hair and not use hair products) to prepare for the EEG assessments and procedures. The morning of each visit, subjects were telephoned again and asked to report their current pain level to assess eligibility for participating that day. Women were also asked to confirm they were not experiencing menses.
EEG Recording
To prepare for the EEG recordings, each participant’s forehead and earlobes were prepped with Nuprep (Weaver and Company, Aurora, CO). Next, an electrode cap with pre-measured sites using the international 10/20 system was fit to each subject. Each electrode site was then filled with Electrogel (Electro-Cap International, Eaton, OH) and prepped to ensure impedance values between 3 and 5 kOhms between each electrode site and each ear individually, as well as between the ears. Electroencephalograph (EEG) data were digitally recorded using the WinEEG (Mitsar, St. Petersburg, Russia) acquisition software utilizing 19 electrodes referenced to A1 and A2 (linked ear montage) (Jasper, 1958). The signals were sampled at 250 Hz, amplified, and filtered using a bandpass of 0.3–27 Hz. EEG was recorded for 20 minutes (during 10 minutes eyes open and 10 minutes eyes closed conditions) at an initial evaluation session, and then again for 10 minutes (eyes closed) before and after each treatment procedure. A trained researcher monitored each subject during the recording and ensured that the subject remained awake.
The initial baseline EEG assessment was used to both habituate the subject to the EEG procedures and to screen for potential seizure or abnormal brain activity. All subjects were asked to engage in standardized cognitive tasks during the eyes closed conditions (specifically, to view a beach scene and then to keep that image in mind for 10 minutes of eyes closed EEG) to help control for cognitive activity that might affect the EEG measures. The initial baseline EEG was reviewed within 72 hours of data collection by the study physician (who is a neurologist with expertise in electroencephalography) to determine if any abnormal brain activity was present which would prevent the subject from continuing with the five procedure visits.
Treatment conditions
Each treatment procedure session lasted 20 minutes.
tDCS
The ActivaDose constant current stimulator (ActivaTek, Salt Lake City, USA) was used to apply 2ma of stimulation for 20 minutes using a saline-soaked rubber sponge anode electrode (35 cm2). This device has a maximum output of 4 mA. The anode electrode was placed over the left central scalp overlying motor cortex (C3 in the EEG 10/20 system) in participants with bilateral or right-sided pain, and the cathode electrode was placed over the contralateral supraorbital area. The right primary motor cortex (C4) was stimulated if subjects reported predominant left sided pain. Of the 28 participants who received active tDCS, 22 (78.6%) received left motor cortex stimulation and 6 (21.4%) received right primary motor cortex (M1) stimulation.
Sham tDCS
Sham tDCS consisted of 10 seconds of 2 mA of direct stimulation using the ActivaDose (over the left or right motor cortex, as appropriate) which was gradually reduced to 0 mA after 10 seconds using the same placement of electrodes listed above. This method of blinding has been previously shown to effectively control for active tDCS; that is, individuals are not able to distinguish this procedure from active tDCS (Fregni et al., 2006a; Gandiga et al., 2006).
Hypnosis (HYP)
During the HYP procedure, participants listened to a recording of a standard hypnotic induction followed by five suggestions for reduced pain and suffering; specifically, suggestions were given for: (1) comfortable relaxation; (2) decreased negative affective response to pain; (3) pain reduction; (4) imagined analgesia; and (5) altered sensations. The suggestions were based on those demonstrated to be effective for reducing pain in persons with SCI (Jensen et al., 2009).
Neurofeedback (NF)
For NF, electrodes were placed over the temporal lobes bilaterally (at T3 and T4 in the EEG 10/20 system), on each earlobe (which were used as reference for each temporal placed active electrode), and a ground electrode was placed either on the mastoid behind the ear or close to the hairline above the forehead on men with a receding hairline. EEG activity was amplified using NeXus-4 (MindMedia B.V., The Netherlands) and Biotrace4 software (MindMedia B.V., The Netherlands) to provide participants with feedback. Contingencies were set such that increases in alpha activity (8–12 Hz) and decreases in high beta activity (18–30 Hz) were reinforced. T3 and T4 were selected as training sites because they were used in some studies that showed promising results (Jensen et al., 2007a; Sime, 2004), and alpha activity was reinforced and beta activity was suppressed because these oscillations have been found to be negatively and positively associated with pain intensity, respectively, in previous research (Bromm and Lorenz, 1998; Chen, 1993, 2001).
Meditation
Participants in the meditation condition were given standard “relaxation response” instructions in which they were asked to select a single neutral word to focus on (any preferred word if they had one or the word “one” if they had no preference) and repeat that word to themselves for the entire 20 minute session (Benson, 1975).
Data Analysis
The EEG data were exported to the EureKa! software (Congedo and Sherlin, 2010) and re-montaged to the average reference montage. The data were plotted and inspected to eliminate any artifact (e.g., evidence of eye blinks, eye movements, body movements). If one or more channels exhibited presence of artifact during any 4 second epoch, that entire epoch was removed. Among the remaining 4 second epochs EEG spectra for five bandwidths (delta, theta, alpha, beta, and gamma) were calculated from the first 2 minutes of artifact-free data (Prichep and John, 1992) with Fast Fourier Transform (FFT) with 1/32 seconds of overlapping window advancement factor. We did not apply any time domain tapering for frequency domain smoothing.
To test for global effects of treatment procedure on absolute EEG bandwidth activity, we constructed a multilevel linear model for each of the five bandwidths studied. Like repeated measures analysis of variance, multilevel models with fixed treatment effects allow for statistical hypothesis testing of differences in (marginal) means associated with each treatment condition. While both multilevel modeling and repeated measures analysis of variance can account for dependency induced by repeated measurements, the multilevel model is particularly well suited to data with multiple nested structures of dependency. For a more thorough exploration of multilevel modeling techniques, reviews are available (Gelman and Hill, 2007; Goldstein, 1994). In this study, dependency was expected from two distinct features of the data: (1) correlation between EEG measures within participants and (2) correlation between EEG measures within electrode sites. Because EEG measurements are simultaneously nested within both electrode and participant, and because electrode and participant are non-nested, a cross-classified multilevel model was chosen to account for the dependence induced by this clustering of data. This is critical to accurate probability estimation during statistical testing of the hypothesized effects of each procedure; that is, (1) hypnosis increases overall (i.e., EEG as measured from all of the 19 electrode sites) theta, (2) meditation increases overall theta and alpha, (3) NF increases in overall alpha, (4) tDCS increases in overall beta and decreases in overall delta, theta, and alpha, and (5) sham tDCS induces no change in EEG. Exploratory analyses were then performed to examine the effects of each procedure on bandwidths for which there were no specific a priori hypotheses, and to examine the effects of the procedures on EEG activity at each of the 19 electrode sites.
To test the hypotheses that hypnosis would result in significant pre- to post-session decreases in pain and that the sham tDCS procedure would not, and to explore the effects of a single session of meditation, neurofeedback and tDCS on pain intensity, we carried out a series of exploratory repeated measures t-tests. Next, we examined the hypothesized associations between pre- to post-session changes in pain intensity and changes in overall alpha and beta EEG activity by computing Pearson correlation coefficients. Finally, in exploratory analyses, we computed correlation coefficients between pre- to post-session changes in pain intensity and changes in overall delta, theta, and gamma activity in addition to correlations in all five bandwidths examined at each of the 19 electrode sites. All statistical analyses were carried out using STATA/IC 12.1 for Mac (StataCorp, College Station, TX, 1985–2011).
Results
Effects of the procedures on EEG activity
Overall (all electrodes) effects
Table 2 presents the means and standard errors of the pre- to post-session differences in absolute amplitude EEG bandwidth measures for each of the five procedures over all 19 electrodes. Every procedure is different from at least one other procedure in at least one of the five bandwidths; that is, each shows a different pattern of changes in brain oscillations. For example, relative to meditation, hypnosis resulted in more theta, more alpha, less beta, and more gamma activity. Relative to sham tDCS, tDCS resulted in more theta, less alpha, less beta, and less gamma.
Table 2.
Means and standard errors of the pre- to post-session changes in absolute amplitude by EEG bandwidth and treatment condition.
| Bandwidth | Hypnosis
|
Neurofeedback
|
Meditation
|
tDCS
|
Sham tDCS
|
Between- group Wald χ2 (df) |
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| Delta (2 – 3.5 Hz) | −.023a (.013) | −.003bc (.013) | −.018ab (.014) | −.001bd (.014) | .003cd (.014) | 12.89** (4) |
| Theta (4 – 7.5 Hz) | .045a (.013) | .016b (.013) | .017b (.013) | .074c (.013) | .053a (.013) | 96.79*** (4) |
| Alpha (8 – 12 Hz) | .095a (.021) | .035b (.021) | .064c (.021) | .043bc (.021) | .121d (.021) | 78.85*** (4) |
| Beta (13 – 21 Hz) | .003ab (.006) | −.007a (.006) | .017c (.006) | −.001a (.006) | .014bc (.007) | 25.25*** (4) |
| Gamma (32 – 45 Hz) | −.019a (.005) | −.002b (.005) | .001b (.005) | −.011a (.005) | .004b (.005) | 43.55*** (4) |
Note: tDCS = transcranial direct current stimulation; negative means indicate a pre-post session decrease and positive means indicate a pre-post session increase in absolute amplitude; bold face means are significantly different from zero, and means with different subscripts within each row are significantly different from one another.
p < .001,
p < .05.
Also, as hypothesized, participants who received hypnosis evidenced significant pre- to post-session increases in theta activity. As found in some studies, but not others, a significant increase in alpha activity with hypnosis was also observed in our sample. We also found evidence for significant decreases in gamma with hypnosis, a bandwidth rarely examined in other hypnosis EEG studies. Although an increase in alpha (consistent with the reinforcement protocol) was observed in those who received NF, this increase was not statistically significant. In fact, no significant change in any EEG bandwidth across all 19 electrodes was observed with NF training. Consistent with the study hypotheses, meditation was associated with significant increases in alpha. However, and inconsistent with the study hypotheses, meditation was not associated with significant increases in theta activity. Beta activity also increased significantly with the meditation procedure. The direction of the effects of the tDCS procedure were mostly opposite to those that were hypothesized. Instead of the hypothesized significant increase in beta activity and decrease in slower wave (delta, theta, alpha) activity, there was a very small (non-significant) decrease in beta and statistically significant increases in theta and alpha with tDCS. A statistically significant decrease was also observed for tDCS in the gamma bandwidth. Also contrary to the study hypotheses, sham tDCS showed significant changes in three bandwidths: increases in theta, alpha, and beta.
Effects associated with individual electrodes
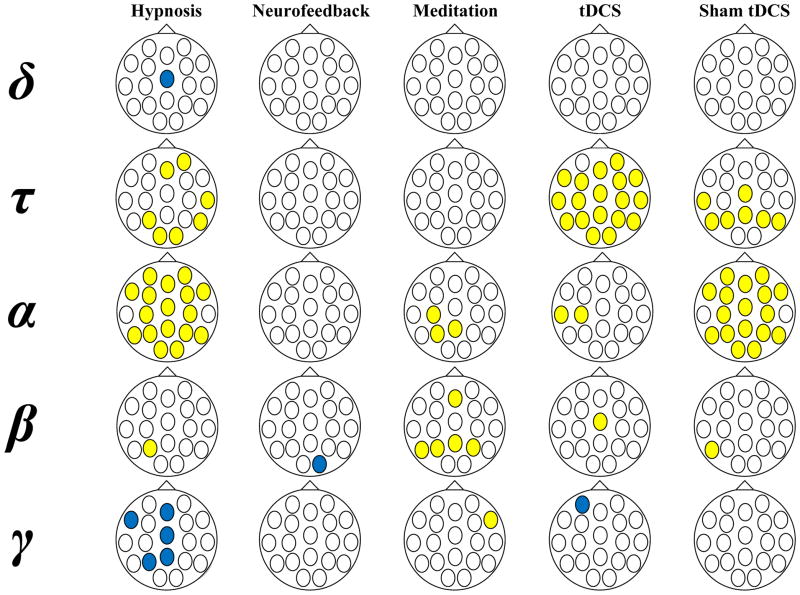
Figure 1 displays the results of each of the procedures on absolute amplitude EEG bandwidth activity at each electrode site. Significant increases in activity are indicated in yellow and significant decreases in blue. Because of the increased risk for Type I error entailed in multiple comparisons, these data should be interpreted very cautiously. However, different patterns of oscillation activity changes with each procedure appear to be present. For example, increases in theta associated with hypnosis appeared more in the left hemisphere, and at both frontal and posterior sites. The increases in alpha with hypnosis, on the other hand, appeared to be more diffuse. Decreases in gamma with hypnosis appeared mainly in central sites. The increases in alpha and beta with meditation were mostly in left parietal areas. The increases in theta with tDCS were diffuse, while alpha increased mostly in left temporal region. Finally, the increases alpha with sham tDCS were diffuse, while the sham tDCS-related increases in theta were mainly over parietal areas and beta over left parietal region.
Figure 1.
Pre- to post-session changes in absolute bandwidth at different electrode sites.
Effects of the procedures on pain intensity
Table 3 presents the means and standard deviations of characteristic pain intensity scores obtained during the EEG assessments before and after each of the procedures, as well as the results of the repeated measures t-tests examining the significance of the changes. As predicted, hypnosis resulted in significant pre- to post-session decreases in pain intensity, and sham tDCS did not. In addition we also observed that meditation led to statistically significant decreases in pain. Active tDCS and neurofeedback did not induce significant pre- to post-session changes in pain intensity. Moreover, the effect sizes of all of the five single session interventions were small – the two significant conditions induced an effect size of 0.25 (hypnosis) and 0.23 (meditation). Also, although the effect size of active tDCS was 1.6 times as large as the effects size of sham tDCS (0.13 vs. 0.08), it was too small to result in significant differences. Neurofeedback showed an effect size that was similar in magnitude to sham tDCS (0.10).
Table 3.
Means and standard deviations of pre- and post-procedure characteristic pain scores.
| Procedure | n | Pre-Procedure
|
Post-Procedure
|
t | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Sham tDCS | 27 | 4.39 | 2.07 | 4.23 | 2.02 | 0.71 |
| tDCS | 28 | 4.19 | 2.02 | 3.92 | 2.21 | 1.14 |
| Hypnosis | 29 | 4.27 | 2.08 | 3.74 | 2.16 | 3.34* |
| Neurofeedback | 30 | 4.61 | 1.93 | 4.41 | 2.09 | 1.44 |
| Meditation | 30 | 4.44 | 2.16 | 3.96 | 1.97 | 3.57* |
Note: tDCS = Transcranial direct current stimulation.
p < .01
Associations between changes in pain intensity and changes in EEG activity
Associations with EEG activity from all electrodes
Of the two correlation coefficients computed to test our specific hypotheses about the associations between mean changes in alpha and beta and change in characteristic pain intensity, both were negligible and non-significant. Likewise, in the three exploratory correlation analyses for the other three bandwidths, no statistically significant correlations were observed.
Associations with EEG activity from individual electrodes
Of the 95 additional exploratory correlation coefficients computed (five bandwidths X 19 electrode sites), only three were marginally statistically significant at (ps <0.05, not corrected for multiple comparisons). Delta activity at O2 (rs =−0.21) and delta activity at T6 (rs = −.20) and absolute gamma activity at O1 (r = −.18) were all negatively associated with changes in pain. None of the pre- to post-session changes in alpha, beta, or theta assessed at any electrode site was significantly associated with change in pain.
Discussion
The findings provide evidence for effects of hypnosis, neurofeedback, meditation, and tDCS on EEG-assessed brain activity, as well as support the pain reducing effects of a single session of hypnosis and meditation on pain. However, the findings do not support our hypothesis that changes in EEG activity and changes in pain intensity correlate following a single session of these treatments. In short, the findings confirm a number of previous observations about the effects of non-pharmacological pain treatments on brain activity, while raising important questions regarding the extent to which brain states reflect the experience of pain.
One consistent finding from previous research is the effect of hypnosis on increasing theta activity (Crawford and Gruzelier, 1992; Graffin et al., 1995). Hypnosis has also been shown to increase alpha activity in some studies (Crawford and Gruzelier, 1992). Our findings showing significant increases in both theta and alpha are generally consistent with this previous research. The current study further clarifies effects of hypnosis on additional delta, beta and gamma activities (which have not been commonly reported); specifically we found no significant effects of hypnosis on delta or beta, but did find significant decreases in gamma.
Smaller, but also statistically significant decreases in gamma were also observed for tDCS. Given that these changes in gamma represent new findings, replication is warranted before making much of the effect. Research suggests gamma activity reflects activity in domains of attention, communication between brain structures, and sensory processing (Jensen et al., 2007b; Salinas and Sejnowski, 2001), including the processing of pain (Zhang et al., 2012). This raises the intriguing possibility that some non-pharmacological treatments such as hypnosis might exert their analgesic effect, at least in part, by reducing or interrupting attentional processes or within-brain communication and thus effectively inducing “dissociation” among brain systems or structures required for the experience of pain. However, the lack of a significant linear association between changes in the gamma band activity and changes in pain intensity complicates our understanding of the relationship. At least two competing hypotheses may explain these discrepancies: (1) the association between changes in gamma and pain reductions may be non-linear (i.e., only a certain amount of change in gamma is needed to result in pain relief; more than this change has no additional benefit) or (2) the effects of hypnosis on gamma and the effects of hypnosis on pain are unrelated. More research is needed to examine these possibilities.
We predicted, based on previous research (Cahn and Polich, 2006), that the meditation procedure would result in significant increases in both theta and alpha activity. The hypothesis related to alpha activity was supported, while that related to theta activity was not. Moreover, we found a significant increase in beta activity with the meditation procedure used (mostly over the temporal lobes of both hemispheres) which is not consistent with previous research (Jacobs et al., 1996). We also found that the hypnosis procedure appears to have induced a greater amount of cortical slowing (e.g., more activity in the slower theta/alpha rhythms) than the meditation procedure, although both resulted in significant increases in alpha activity. Given that these two procedures were both associated with pain relief in our sample, a tempting conclusion might be that they were effective, at least in part, because of their effects on alpha activity. However, both tDCS and sham tDCS resulted in increases in alpha – in fact, the participants evidenced the largest increases in alpha following sham tDCS – yet neither tDCS nor sham tDCS were associated with significant decreases in pain. Moreover, we did not find any association between pain relief and pre- to post-session changes in alpha activity in the hypnosis condition. As a whole, the findings demonstrate that the hypnosis and meditation procedures used in this study reduce pain, but the findings do not provide evidence that pain reduction associated with a single session of these procedures can be explained by the EEG changes assessed
tDCS and sham tDCS also show unique patterns of EEG changes. tDCS shows greater theta increase than any other procedure and some alpha increase (but less than hypnosis or sham tDCS). The unexpected result of increased theta frequency after active tDCS may be due to homeostatic effects of the procedure, as patients with chronic pain tend to have increased baseline cortical excitability (Lefaucheur et al., 2006; Portilla et al., 2013). In terms of changes on EEG, sham tDCS appeared to be most similar to hypnosis, in that both resulted in large increases in alpha. However, despite the changes in EEG activity shown for both tDCS and sham tDCS, neither had a significant effect on pain intensity. Moreover, the significant effects of sham tDCS on EEG – in particular, alpha activity – were not expected. This may be due to the effects of simply sitting quietly with one’s eyes closed (most of the participants closed their eyes during the sham tDCS procedure) leading to specific changes in EEG (i.e., especially increases in alpha) that last for at least 30 minutes to an hour after the procedure. The fact that response to tDCS and sham tDCS differed significantly (with participants receiving tDCS showing significantly less increases in alpha but significantly more increases in theta, relative to sham tDCS) suggests that the differences found are reliable, and not due to random variation. At a minimum, the finding regarding the effects of sham tDCS on EEG argues for the importance of including control conditions in any study such as the present one that seeks to understand the effects of non-pharmacological treatments; one cannot assume that “doing nothing” will have no effect on EEG.
With regard to neurofeedback, there are not yet any clear standardized protocols for treating chronic pain; sites used include T3 and T4 (e.g., Jensen et al., 2007a; Sime, 2004), Cz (e.g., Caro and Winter, 2011), C4 (e.g., Kayiran et al., 2010), and mixed sites (e.g., Jensen et al., 2007a; Sime, 2004). Frequencies reinforced in the neurofeedback protocols have also varied, and include reinforcement of alpha (9 – 11 Hz; e.g., Gannon and Sternbach, 1971), 12–15 Hz (e.g., Caro and Winter, 2011; Kayiran et al., 2010), and mixed frequencies (e.g., Jensen et al., 2007a; Sime, 2004), although the majority of the protocols seek to suppress beta activity. Given this variability, we selected a protocol that would be generally consistent those used in previous studies. However, we elected to focus on reinforcing alpha and suppressing beta, given that pain has been shown to be linked to less alpha and more beta activity (Bromm and Lorenz, 1998; Chen, 1993, 2001). Still, it is possible that a protocol other than the one we used (e.g., one that aimed more directly at the somatosensory cortex) might have produced some effect on pain. More research is needed to compare the relative efficacy of different neurofeedback protocols.
An important aim of this study was to assess clinical effects of a single session of 4 different non-pharmacological procedures thought to impact pain. We found small effect sizes associated with all of the procedures (hypnosis, meditation, active tDCS, neurofeedback, and sham tDCS, 0.25, 0.23, 0.13, 0.10, and 0.08). Moreover, only the effects of hypnosis and meditation on pain were statistically significant. There are at least two possible reasons for the small effect sizes found. First, clinical administration of these procedures involves repeated sessions, so one session of the procedures would not necessarily be expected to have a profound effect on pain. Second, the patients in our sample had a relatively low baseline levels of pain intensity (as low as 3 on a 0–10 scale), and therefore floor effects may have limited the effect sizes. An important next step will be to examine the effects on both pain and brain oscillations of a full course of the treatments studied.
A primary question driving this research was whether the benefits of these non-pharmacological treatments on pain might be operating via similar effects on brain activity (e.g., by increasing slow wave activity, decreasing fast wave activity, or both). To our knowledge, this is the first study to address this question by making a head-to-head comparison of the effects of four different non-pharmacological treatments, relative to a sham condition, on both EEG activity and pain. The findings appear clear: each of the procedures has different effects on EEG, and their effects on pain intensity are not related to the EEG variables studied in any systematic (or similar) way. Thus, the findings indicate that when a single session of these non-pharmacological interventions impacts pain (as two did in this study), their effects are not reflected in a unified underlying pattern of EEG activity – at least in the EEG activity measures used here. Future studies need to address this same question for repeated sessions of treatment.
The study has a number of limitations that should be considered when interpreting the results. Of primary importance, and despite the fact that we had a number of a priori specific hypotheses, many of the analyses performed were exploratory. Therefore, there is a risk that some of our significant findings are due to type I error. Until further research is performed to determine which of the findings reported here replicate, the study results should be considered suggestive only. Second, the EEG assessments were performed roughly 20 to 30 minutes before and after but not during the treatment procedures. Thus, the EEG effects assessed and analyzed reflect the short-term (i.e., lingering) rather than immediate impact of the procedures on EEG activity. It is possible, even likely, that the immediate effects of the procedures on EEG might differ from their lingering effects. However, we also assessed pain intensity when we assessed EEG activity; if specific bandwidth activity measures were strongly associated with pain, or if changes in bandwidth activity were strongly associated with changes in pain, those associations should have emerged in our analyses.
Related to this issue, the study sample consisted of a group of individuals with spinal cord injury and chronic pain presenting with both neuropathic and mixed (neuropathic and nociceptive) pain problems. Moreover, 18 of 51 individuals who were identified as eligible to participate in the study declined participation. The extent to which the current findings generalize to individuals with SCI in general, or who have a specific type of pain (i.e., only neuropathic, only non-neuropathic pain, or even pain types within these larger categories, such as central vs. peripheral neuropathic pain), or to individuals with other chronic pain conditions, is not known. It might be possible, for example, that stronger associations might have emerged had the sample consisted only or primarily of individuals with neuropathic pain or nociceptive pain. It is also possible that EEG-assessed bandwidth activity measures may be more strongly linked to pain intensity in populations of patients with other pain diagnoses, such as headache, low back pain, or chronic widespread pain (e.g., fibromyalgia). Additional research is needed to test these possibilities. Likewise, as discussed previously, we were only able to compare the effects of a single administration of the treatment procedures; it is possible that effects of the treatments would have been greater had participants continued to be provided and to practice the interventions. A longitudinal examination of comparative effects of these procedures on both pain and cortical activity is warranted to evaluate this possibility.
It is also important to acknowledge the limitations of correlational analysis used when examining the relationship between changes in self-reported pain intensity (for which there was a single measure) and changes in EEG activity (for which there were multiple electrode measures). We acknowledge that using a summary estimate of EEG activity is less than ideal in that some information is lost in this process (i.e. the variance component). Nevertheless, the subsequent exploratory analysis was carried out at the level of electrode site where this no longer presents an issue and is of less concern given the largely null effects observed. The complexity of the multilevel model was also limited by the sample size of 30 subjects, and concerns with model stability prohibited the examination of more complex interactions.
Despite the study’s limitations, however, the findings advance our understanding of the effects of non-pharmacological pain interventions on brain activity as measured by EEG in this first (to our knowledge) head-to-head comparison of four treatments and a sham procedure. We found that each procedure showed particular effects on EEG activity, relative to a sham tDCS procedure. We also found that the sham procedure resulted in significant pre- to post-session changes in EEG, suggesting that simply sitting quietly for 20 minutes with one’s eyes closed may produce significant short-term changes in EEG. Therefore, such control procedures are necessary in this type of research. Finally, although the lack of association between changes in EEG activity and changes in pain can be viewed as a negative finding, this result is important, as it rules out systematic changes in brain oscillations (at least in the bandwidths assessed here) as mediating the analgesic effects of single session of these treatments. However it remains to be determined whether these findings will be replicated when repeated sessions of these treatments are administered.
Highlights.
This is the first study to examine, in the same sample, the effects of hypnosis, meditation, transcranial direct current stimulation (tDCS), and neurofeedback on pain and brain oscillations, relative to a control procedure.
Each procedure resulted in oscillation changes that differed from the control procedure and from each other, suggesting different modes of action on brain activity.
Changes in pain intensity associated with the procedures were not, however, significantly associated with changes in brain oscillations, suggesting that brain activity measures used in this study do not reflect pain intensity.
Acknowledgments
This research was supported by grant number R21 HD058049 from the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Medical Rehabilitation Research.
Footnotes
Conflict of Interest Statement
Leslie Sherlin is a principle owner of Nova Tech EEG, Inc., which provides QEEG analysis services and distributes QEEG equipment and analysis software tools. He is also the chief science officer for Neurotopia, Inc. where he develops hardware and software platforms for cognitive and neurofeedback training in athlete populations. Finally he is a provider of QEEG analysis and neurofeedback services and is a principle owner at Arizona Brain Performance Center. The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol. 2005;568:653–63. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. 1998;107:227–53. doi: 10.1016/s0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–95. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zando TA, de Oliveira JF, Goulaart A, et al. The Sertraline vs electrical current therapy for treating depression clinical study: Results from a factoraial, randomized, controlled trial. JAMA Psychiatry. doi: 10.1001/2013.jamapsychiatry.32. in press. [DOI] [PubMed] [Google Scholar]
- Buhle J, Wager TD. Does meditation training lead to enduring changes in the anticipation and experience of pain? Pain. 2010;150:382–3. doi: 10.1016/j.pain.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Turner JA, Warms CA, Marshall HM. Classification of chronic pain associated with spinal cord injuries. Arch Phys Med Rehab. 2002;83:1708–14. doi: 10.1053/apmr.2002.35651. [DOI] [PubMed] [Google Scholar]
- Carmody J. Evolving conceptions of mindfulness in clinical settings. J Cogn Psychother. 2009;23:270–80. [Google Scholar]
- Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl Psychophysiol Biofeedback. 2011;36:193–200. doi: 10.1007/s10484-011-9159-9. [DOI] [PubMed] [Google Scholar]
- Chen AC. Human brain measures of clinical pain: a review. II. Tomographic imagings. Pain. 1993;54:133–44. doi: 10.1016/0304-3959(93)90201-Y. [DOI] [PubMed] [Google Scholar]
- Chen AC. New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. Int J Psychophysiol. 2001;42:147–59. doi: 10.1016/s0167-8760(01)00163-5. [DOI] [PubMed] [Google Scholar]
- Chen AC, Dworkin SF, Drangsholt MT. Cortical power spectral analysis of acute pathophysiological pain. Int J Neurosci. 1983;18:269–78. doi: 10.3109/00207458308987371. [DOI] [PubMed] [Google Scholar]
- Congedo M, Sherlin L. EEG source analysis: methods and clinical implications. In: Coben R, Evans J, editors. Neurofeedback and neuromodulation techniques and applications. New York: Academic Press/Elsevier; pp. 25–46. [Google Scholar]
- Crawford HJ. Cognitive and psychophysiological correlates of hypnotic responsiveness and hypnosis. In: Brown DP, Fass ML, editors. Creative master in hypnosis and hypnoanalysis: A festschrift for Erika Fromm. Hillsdate, NJ: Erlbaum; 1990. pp. 155–68. [Google Scholar]
- Crawford HJ, Gruzelier JH. A midstream view of the neuropsychophysiology of hypnosis: Recent recearch and future directions. In: Fromm E, Nash MR, editors. Contemporary Hypnosis Research. Vol. 1992. New York: Guilford Press; 1992. pp. 227–66. [Google Scholar]
- Derbyshire SW, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Dunn BR, Hartigan JA, Mikulas WL. Concentration and mindfulness meditations: unique forms of consciousness? Appl Psychophysiol Biofeedback. 1999;24:147–65. doi: 10.1023/a:1023498629385. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. EEG biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related brain potentials. Clin Neurophysiol. 2004;115:131–9. doi: 10.1016/s1388-2457(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Egner T, Strawson E, Gruzelier JH. EEG signature and phenomenology of alpha/theta neurofeedback training versus mock feedback. Appl Psychophysiol Biofeedback. 2002;27:261–70. doi: 10.1023/a:1021063416558. [DOI] [PubMed] [Google Scholar]
- Egner T, Zech TF, Gruzelier JH. The effects of neurofeedback training on the spectral topography of the electroencephalogram. Clin Neurophysiol. 2004;115:2452–60. doi: 10.1016/j.clinph.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N, Haupt S. From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Med Hypotheses. 2010;75:218–24. doi: 10.1016/j.mehy.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Fenton BW, Palmieri PA, Boggio P, Fanning J, Fregni F. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimul. 2009;2:103–7. doi: 10.1016/j.brs.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Gannon L, Sternback RA. Alpha enhancement as a treatment for pain: A case study. J Behav Ther Exp Psychiatry. 1971;2:209–13. [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2007. [Google Scholar]
- Gevensleben H, Holl B, Albrecht B, Schlamp D, Kratz O, Studer P, et al. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int J Psychophysiol. 2009;74:149–57. doi: 10.1016/j.ijpsycho.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Goldstein H. Multilevel Cross-Classified Models. Soc Meth Res. 1994;22:364–75. [Google Scholar]
- Graffin NF, Ray WJ, Lundy R. EEG concomitants of hypnosis and hypnotic susceptibility. J Abnorm Psychol. 1995;104:123–31. doi: 10.1037//0021-843x.104.1.123. [DOI] [PubMed] [Google Scholar]
- Green JP, Barabasz AF, Barrett D, Montgomery GH. Forging ahead: the 2003 APA Division 30 definition of hypnosis. Int J Clin Exp Hypn. 2005;53:259–64. doi: 10.1080/00207140590961321. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32:14874–84. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Advancing Pain Research Care and Education. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- Jacobs GD, Benson H, Friedman R. Topographic EEG mapping of the relaxation response. Biofeedback Self Regul. 1996;21:121–9. doi: 10.1007/BF02284691. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Ezra A, Berger U, Lavidor M. Modulating oscillatory brain activity correlates of behavioral inhibition using transcranial direct current stimulation. Clin Neurophysiol. 2012;123:979–84. doi: 10.1016/j.clinph.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- Jensen M, Grierson C, Tracy-Smith V, Bacigalupi SC, Othermer S. Neurofeedback treatment for pain associated with Complex Regional Pain Syndrome Type I: A case series. J Neurother. 2007a;11:45–53. [Google Scholar]
- Jensen M, Hakimian S, Sherlin LH, Fregni F. New insights into non-pharmacological and noninvasive neuromodulatory approaches for the treatment of pain. J Pain. 2008;9:193–9. doi: 10.1016/j.jpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Jensen M, Patterson DR. Hypnotic treatment of chronic pain. J Behav Med. 2006;29:95–124. doi: 10.1007/s10865-005-9031-6. [DOI] [PubMed] [Google Scholar]
- Jensen M, Turner JA, Romano JM, Fisher L. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Jensen MP. A neuropsychological model of pain: research and clinical implications. J Pain. 2010;11:2–12. doi: 10.1016/j.jpain.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, et al. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. Int J Clin Exp Hypn. 2009;57:239–68. doi: 10.1080/00207140902881007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Ehde DM, Gertz KJ, Stoelb BL, Dillworth TM, Hirsh AT, et al. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2011;59:45–63. doi: 10.1080/00207144.2011.522892. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of pain assessment. Vol. 2001. New York: Guilford; 2001. pp. 15–34. [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007b;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamursel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl Psychophysiol Biofeedback. 2010;35:293–302. doi: 10.1007/s10484-010-9135-9. [DOI] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, et al. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage. 2011;55:644–57. doi: 10.1016/j.neuroimage.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011) J Spinal Cord Med. 2011;34:535– 46. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67:1568–74. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett. 2012;512:12–6. doi: 10.1016/j.neulet.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Marchand WR. Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and zen meditation for depression, anxiety, pain, and psychological distress. J Psychiatr Pract. 2012;18:233–52. doi: 10.1097/01.pra.0000416014.53215.86. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26:S50– S6. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair. 2012;26:646–52. doi: 10.1177/1545968311433209. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nishiwaki Y, Ushida T, Toyama Y. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci. 2011;16:424–32. doi: 10.1007/s00776-011-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DR, Jensen M. Hypnosis and clinical pain. Psychol Bull. 2003;29:495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- Portilla AS, Bravo GL, Miraval FK, Villamar MF, Schneider JC, Ryan CM, et al. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. J Burn Care Res. 2013;34:48–52. doi: 10.1097/BCR.0b013e3182700675. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER. QEEG profiles of psychiatric disorders. Brain Topogr. 1992;4:249–57. doi: 10.1007/BF01135562. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449–62. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–50. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag. 2011;16:445–50. doi: 10.1155/2011/876306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime A. Case study of trigeminal neuralgia using neurofeedback and peripheral biofeedback. J Neurother. 2004;8:59–71. [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35:1113–24. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Williams JD, Gruzelier JH. Differentiation of hypnosis and relaxation by analysis of narrow band theta and alpha frequencies. Int J Clin Exp Hypn. 2001;49:185–206. doi: 10.1080/00207140108410070. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–38. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]