Abstract
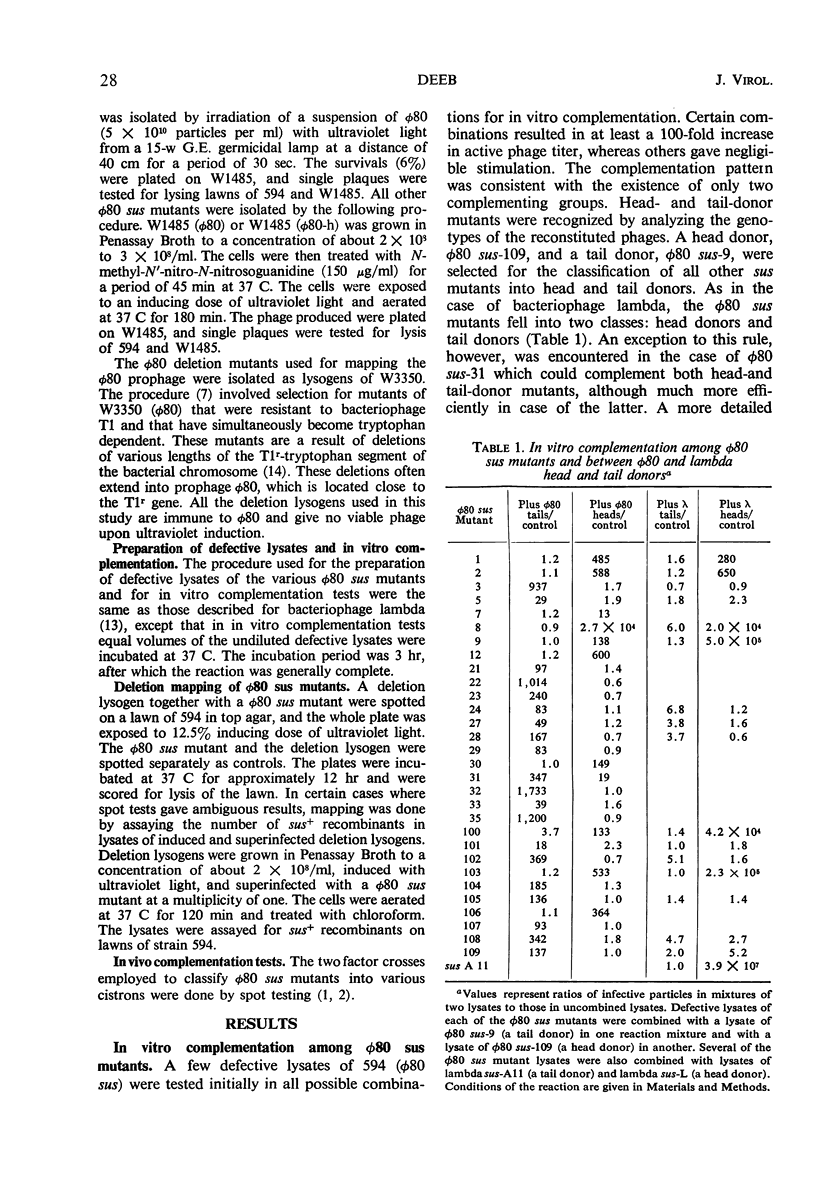
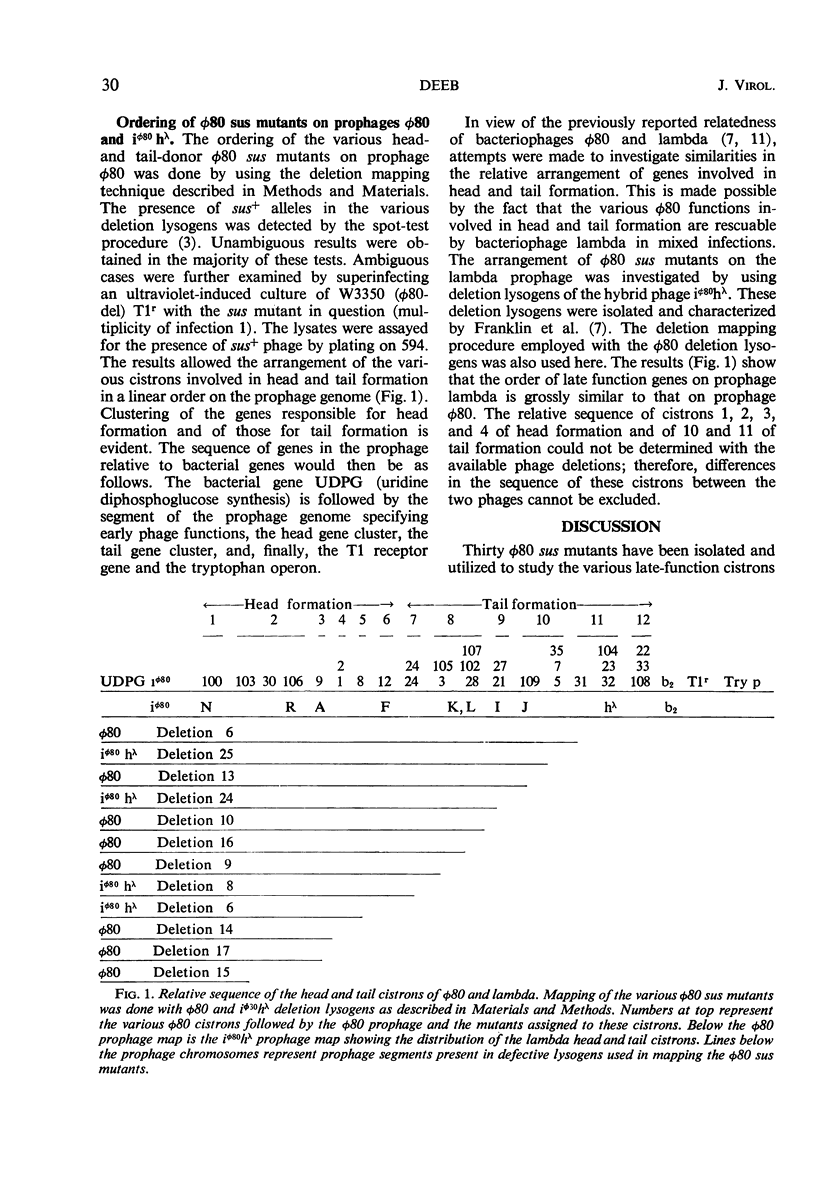
Suppressor-sensitive (sus) mutants of bacteriophage φ80 defective in late functions were classified, by means of in vitro assembly tests, into two complementation groups: head donors and tail donors. Each group of mutants was subdivided, by means of two-factor crosses, into six cistrons. Deletion mapping revealed clustering of tail and also of head cistrons. The two clusters were located in the left arm of vegetative φ80 (the tail specifying cluster being distal). In vitro cross complementation between φ80 and lambda sus mutants revealed that whereas lambda heads could quite efficiently bind φ80 tails to form viable phage, the union of φ80 heads and lambda tails was very much less efficient. Deletion mapping of the φ80 sus mutants, using both φ80 and iφ80hλ deletion lysogens indicated congruent gross gene arrangement in the two related bacteriophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Deeb S. S., Okamoto K., Hall B. D. Isolation and characterization of non-defective transducing elements of bacteriophage phi-80. Virology. 1967 Feb;31(2):289–295. doi: 10.1016/0042-6822(67)90173-0. [DOI] [PubMed] [Google Scholar]
- Dove W. F. Action of the lambda chromosome. I. Control of functions late in bacteriophage development. J Mol Biol. 1966 Aug;19(1):187–201. doi: 10.1016/s0022-2836(66)80060-8. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda-phage. 3. Mutations in cistron N affecting heat induction. Genetics. 1966 Sep;54(3):835–844. doi: 10.1093/genetics/54.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUSHIRO A. Specialized transduction of tryptophan markers in Escherichia coli K12 by bacteriophage phi-80. Virology. 1963 Apr;19:475–482. doi: 10.1016/0042-6822(63)90041-2. [DOI] [PubMed] [Google Scholar]
- SIGNER E. R. RECOMBINATION BETWEEN COLIPHAGES LAMBDA AND PHI-80. Virology. 1964 Apr;22:650–651. doi: 10.1016/0042-6822(64)90090-x. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]



