Abstract
Introduction
Biliary atresia (BA) is a unique neonatal disease resulting from inflammatory and fibrosing obstruction of the extrahepatic biliary tree. Previous studies demonstrated the critical role of innate immunity and the Th1 response with activated inflammatory cells and over-expressed cytokines in the pathogenesis of BA. Myeloid differentiation factor 88 (MyD88) is a critical adaptor molecule which has been shown to play a crucial role in immunity. We investigated the role of MyD88 in the inflammatory response and development of cholangiopathy in murine BA.
Methods
MyD88 knockout (MyD88−/−) and wild-type (WT) BALB/c pups were injected with RRV or saline on day 1 of life. Mice were monitored for clinical symptoms of BA, including jaundice, acholic stools, and bilirubinuria and mortality. Liver and extrahepatic bile ducts were harvested for histologic evaluation, quantification of viral content, determination of cytokine expression and detection of inflammatory cells.
Results
RRV infection produced symptoms in 100% of both the MyD88−/− and WT pups with survival of 18% of WT and 0% of MyD88−/− mice. Histological analysis demonstrated bile duct obstruction in both MyD88−/− and WT mice. Viral titers obtained 7 days post infection and expression of IFN-γ and TNF-α at day 3, 5, 8 or 12 days post infection revealed no significant differences between WT and MyD88−/− mice. Flow cytometry demonstrated similar levels of activated CD8+ T cells and NK cells.
Conclusions
Pathogenesis of murine BA is independent of the MyD88 signaling inflammatory pathway, suggesting alternative mechanisms to be crucial in the induction of the model.
INTRODUCTION
Biliary atresiea (BA) is a unique neonatal disease resulting from inflammatory and fibrosing obstruction of the extrahepatic biliary ducts. Despite early surgical intervention with Kasai portoenterostomy, patients frequently progress to cirrhosis and end-stage liver disease. As a result, BA continues to be the leading indication for pediatric liver transplantation.1 Although the underlying etiology remains under investigation, viral infection of the bile duct resulting in subsequent immune mediated inflammation is one proposed mechanism. Evidence for this hypothesis includes patient-based investigations, which have demonstrated reovirus2, cytomegalovirus3–5, human papillomavirus6, Epstein-Barr virus7, and rotavirus8 in livers of infants with BA.
In order to gain further understanding of BA and advance treatment options, a murine model has been developed.9 Obstructive cholangiopathy with symptomatology that mirrors human disease can be elicited in the BALB/c mouse through intraperitoneal injection of rhesus rotavirus (RRV), a double stranded RNA virus of the Reoviridae family.10 Previously, our group demonstrated co-localization of RRV in biliary epithelial cells (BECs), resulting in a mortality rate of 81%.11 These mice exhibit symptoms of bile duct obstruction, including jaundice, bilirubinuria, and acholic stools. Ongoing research employing the RRV murine model has investigated the pathophysiological function of dsRNA viruses and the immune response on the pathogenesis of BA.
The role of the immune system and the resulting inflammatory response as a cause of biliary obstruction is an important focus of research in the field of BA. The innate immune response is instrumental in the recognition of conserved structures present on pathogenic microorganisms. These structures, pathogen-associated molecular patterns (PAMPs), are recognized by Toll-like receptors (TLRs). TLRs are transmembrane receptors that are widely expressed on immune cells and lead to the activation of the innate immune response through the intracellular adaptor molecule myeloid differentiation factor 88 (MyD88).12 MyD88 plays a central role in the propagation of the inflammatory pathway. MyD88 is also involved in adaptive immunity, a response that is antigen-specific and characterized by antibody-productive B-lymphocytes and the activation of cytotoxic T-lymphocytes. Previously, impaired interferon gamma (IFN-γ) production from CD4+ T cells was noted in MyD88-deficient mice, suggesting that the Th1 response is also regulated by MyD88 signaling pathways.13
The precise involvement of the immune system in the development of biliary atresia remains to be defined. Previous studies have demonstrated activation of the innate immune response as a pathway to the inflammation seen in BA.14 Furthermore, Harada et al. demonstrated that innate immune response was sustained after initiation in this model, thus a potential cause of ongoing cholangiopathy after the viral insult has been cleared.15 Additional studies have demonstrated that BA results in a Th1 immune profile with over-activated inflammatory cells and cytokines16,17 while others have discovered a link between BA and activation of the autoimmune response.18 Since TLRs are responsible for the induction of both the innate and adaptive systems and MyD88 is central in their activation, we hypothesized that MyD88 may play a role in BA pathogenesis.
MATERIALS AND METHODS
Cells, viruses, and animals
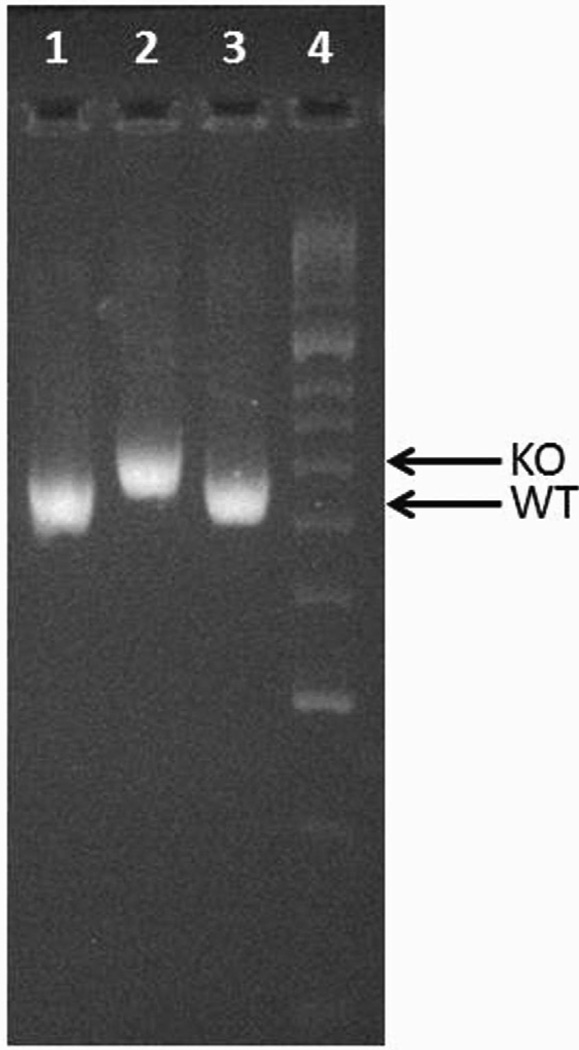
MA104 cells (Bio Whittaker, Walkersville, MD) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Cellgro) supplemented with 10% fetal bovine serum (FBS) (Gibco/BRL, Gaithersburg, MD), 0.01% penicillin-streptomycin (Gibco/BRL), 0.01% L-glutamine (Gibco/BRL), and 0.005% amphotericin B (Cellgro). Rhesus rotavirus (RRV), a simian strain of genotype G3P[3] (kindly provided by H. Greenberg, Stanford University, Palo Alto, CA) was used. Breeding pairs of BALB/c mice (Harlan Labs, Indianapolis, IN) and MyD88 Knockout mice (MyD88−/−) (a generous gift from Dr. Alegre, University of Chicago, Chicago, IL) were kept in micro isolator cages in a virus-free environment with free access to sterilized chow and water. The mice were bred, and pups in litters of 4 or more were used. MyD88 knockout mice were confirmed by PCR (Figure 1) as previously described.19
Figure 1. PCR for confirming MyD88 wild-type (WT) or knockout (KO) mice.
PCR was carried out according to the previously published method.19 The higher molecular weight band represents the KO (Well 2) and the lower molecular weight band represents the WT mice (Wells 1 and 3).
Viral inoculation of newborn mice for phenotypic characterization
Newborn pups were injected intraperitoneally (i.p.) with RRV at a dose of 1.5 × 106 focus-forming units (FFU) per mouse within 24 h of birth. Saline-injected pups served as controls. Overall survival rates and clinical signs of hepatobiliary injury, including jaundice in non-fur-covered skin, acholic stools, and bilirubinuria were recorded. The presence of bilirubin in the urine was detected quantitatively using commercially available urine dipsticks (Bayer Co., Elkhart, IN). A subset of injected mice was sacrificed 10 days post injection, the liver and extrahepatic biliary tract were harvested, preserved in formalin, and analyzed histologically as previously described.11 For another subset of mice harvested at 7 days after inoculation, the extrahepatic biliary tract was weighed (wet weight) and homogenized in Earle’s balanced salt solution (EBSS). Tissue samples were analyzed for the presence of infectious rotavirus by a focus-forming assay (FFA), and quantities of virus were reported as FFU per milliliter per milligram (wet weight) of tissue as described previously.11
Histologic assessment of biliary and hepatic injury
Ten days post injection with RRV or saline, the extrahepatic biliary trees and livers from mice were microdissected and preserved in formalin. After being embedded in paraffin, samples were sectioned at 5 µm serially along the length of the sample. Sections were allowed to dry overnight, deparaffinized at 60°C for 30 minutes, and stained with Hemotoxlin and Eosin (H&E) using standard techniques. All sections were analyzed using an Olympus BX51 microscope and photographed with an Olympus digital camera DP71 (Olympus, Center Valley, PA, USA).
Focus-forming assay
Tissue samples of bile ducts and livers were analyzed for the presence of infectious rotavirus by fluorescent focus-forming viral titration assays as described previously.20
RNA isolation and real time PCR for mRNA expression of cytokines
Subsets of previously injected mice were sacrificed at 3, 5, 8, and 12 days post-injection. Livers were harvested and total RNA from the tissues was extracted using the Direct-zol RNA MiniPrep (Zymo Research, Irvine, CA) according to manufacturer’s instructions. cDNA pools were generated using standard reagents (Invitrogen) and mRNA expression for IFN-γ and TNF-α relative to glyceraladehyde-3-phosphate dehydrogenase (GAPDH) was quantified by real-time PCR using SYBR Green on a Mx-3000 Multiplex Quantitiative PCR (Stratagene, La Jolla, Ca) as previously described.21 The murine primers used for PCR were as follows: IFN-γ: sense, 5’-GGCTGTCCCTGAAAGAAAGC-3’, antisense, 5’-GAGCGAGTTATTTGTCATTCGG-3’; TNF-α: sense, 5’-AAGGGAGAGTGGTCAGGTTGCC-3’, antisense, 5’-CCTCAGGGAAGAGTCTGGAAAGG-3’; GAPDH: sense, 5’-TACACTGAGGACCAGGTTGT-3’, antisense, 5’-CAAAGTTGTCATTGAGAGCA-3’.
Flow Cytometry
Livers were harvested from pups 7 days post injection and pooled two per sample in RPMI+2%FBS. Samples were then minced, aspirated through an 18g needle three times, and passed through a 40 nm filter. Following centrifugation at 2000 RPM, a 33% Percoll gradient (GE Lifesciences, Uppsala, Sweden) was used to purify the mononuclear cells. Pellets were then treated with red blood cell lysis buffer, washed two times, and suspended in 1XPBS+1%FBS. 1×106 cells were added to each well of a 96 well v-bottom plate, treated with FC blocker (BD Bioscience, San Jose, CA) and incubated at 4°C for 30 min. A panel of 1:100 dilution of anti-CD3, CD8, CD69 or CD49b (eBioscience, San Diego, CA) was next added for 30 mins and incubated at 4°C. Cells were washed twice and filtered through a 40 µm cell strainer into a tube containing 4% paraformaldehyde to fix cells. Samples were read on the Accuri C6 flow cytometry machine (BD Bioscience, San Jose, CA) and analyzed using FlowJo Software (Tree Star, Inc., Ashland, OR).
Statistical analysis
Assessment of symptom development and mortality rates following rotavirus inoculation was based on experimental groups of at least 12 pups. Findings were expressed as percentage of survival and percentage of pups expressing at least two symptoms. These results were analyzed using a Log-Rank test and a Fisher’s exact test, respectively. Each subset utilized for the FFAs consisted of at least 5 pups. The results of these continuous variables were expressed as arithmetic means ± standard errors and were analyzed using Student’s t test and by analysis of variance (ANOVA) with post hoc testing as appropriate. A P value of less than 0.05 was considered significant.
RESULTS
Symptoms and survival of MyD88−/− versus WT mice in the murine model of BA were analogous
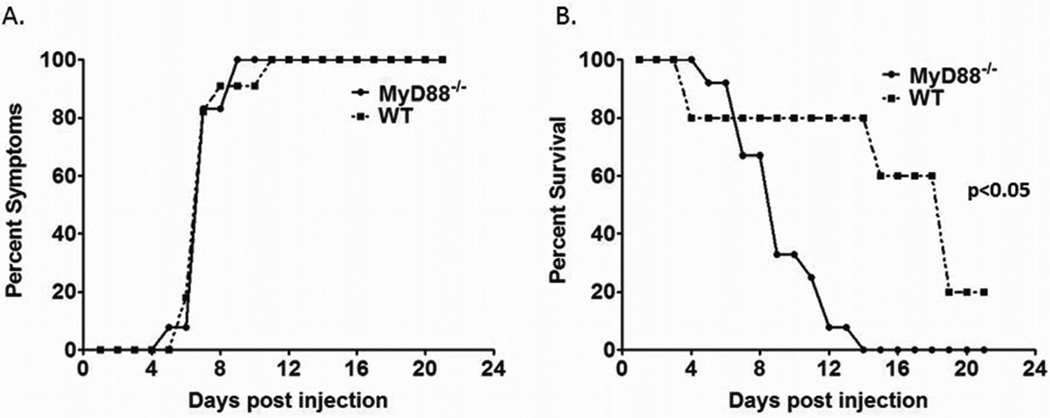
One hundred percent of both WT and MyD88−/− pups injected with RRV manifested symptoms of biliary obstruction. Symptomatology included jaundice, acholic stools, and bilirubinuria. Symptoms were detected at and beyond Day of Life (DOL) 7 in the MyD88−/− group. All pups demonstrated jaundice, acholic stools, and moderate to large bilirubinuria. This is similar to the findings of WT mice injected with RRV, where the phenotype was elicited in the entire group between DOL 5 and 12 (Figure 2A).
Figure 2. Symptoms and mortality after RRV infection in MyD88−/− and WT mice.
The symptoms and mortality were monitored for 21 days after RRV or saline challenge. (A) Both MyD88−/− and WT mice exhibited symptoms of BA with no significant difference between the two. (B) However, the mortality rate of MyD88−/− mice infected with RRV was significantly different from WT infected mice (p<0.05).
Mortality rates, monitored for 21 days, for WT pups infected with RRV was 82% (n=21) in comparison to 100% for the MyD88−/− mice injected with RRV (n=16) (Figure 2B). All surviving mice cleared the previously noted symptoms of biliary obstruction. The MyD88−/− mice were noted to expire at an earlier time point compared to WT mice, which was statistically significant (p<0.05).
Histologic assessment of liver and extrahepatic bile ducts of WT and MyD88−/− are identical
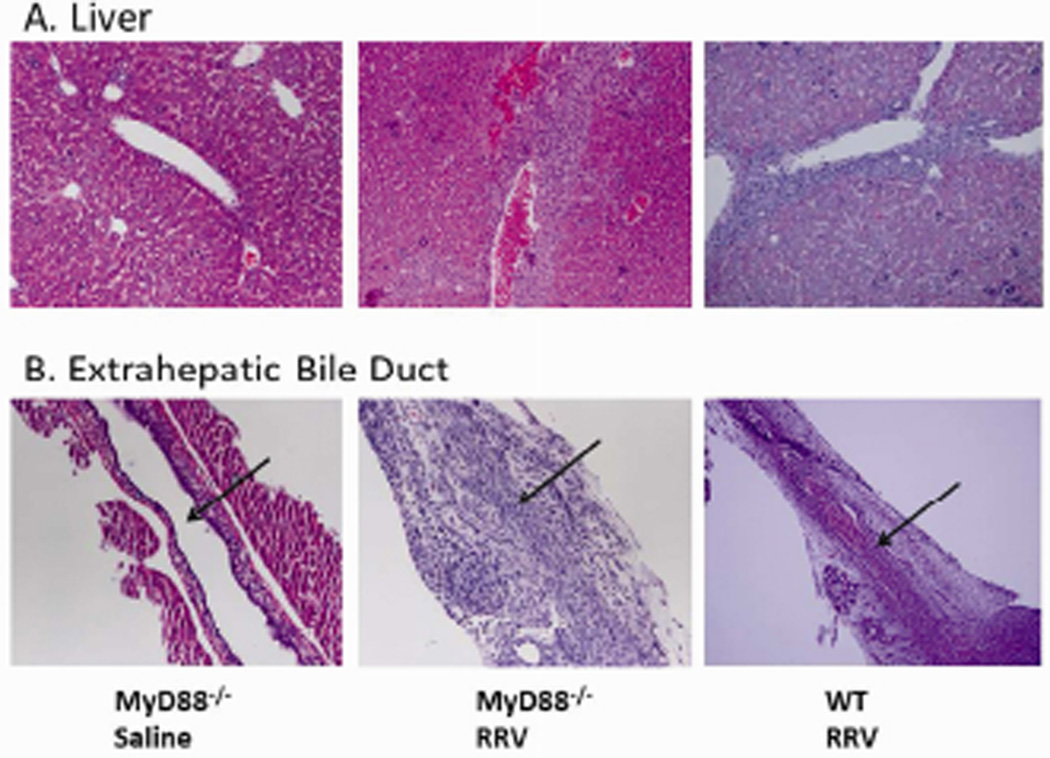
After observing symptoms of BA consistent with biliary obstruction, histology was performed to evaluate the ductal architecture and for liver damage. The livers and extrahepatic bile ducts from WT and MyD88−/− mice were harvested from a subset of mice 10 days after inoculation with RRV and histologically analyzed. Evaluation of the livers from both groups demonstrated extensive infiltration of inflammatory cells within the area of the portal tract (Figure 3A). Extrahepatic bile ducts examined from the two types of mice were indistinguishable; both revealed periductal inflammatory infiltration, epithelial sloughing, stromal proliferation, and lumen obstruction (Figure 3B). The histologic appearance of the livers and extrahepatic bile ducts of WT and MyD88−/− mice was consistent with the phenotypic characteristics seen prior to organ harvest.
Figure 3. Histological evaluation of the extrahepatic bile duct and liver following RRV infection.
The livers and extrahepatic bile ducts were harvested from MyD88−/− and WT mice 10 days post inoculation with RRV or saline and stained with H&E. Both strains showed accumulation of inflammatory cells in the livers around the portal tract (A). RRV injection led to obstruction of the lumens of the extrahepatic bile ducts in both MyD88−/− and WT mice (B). Arrows indicate bile duct lumen. Magnification: Liver 20×, Extrahepatic bile duct 40×.
Detection of infectious rotavirus in the liver and extrahepatic bile ducts is similar
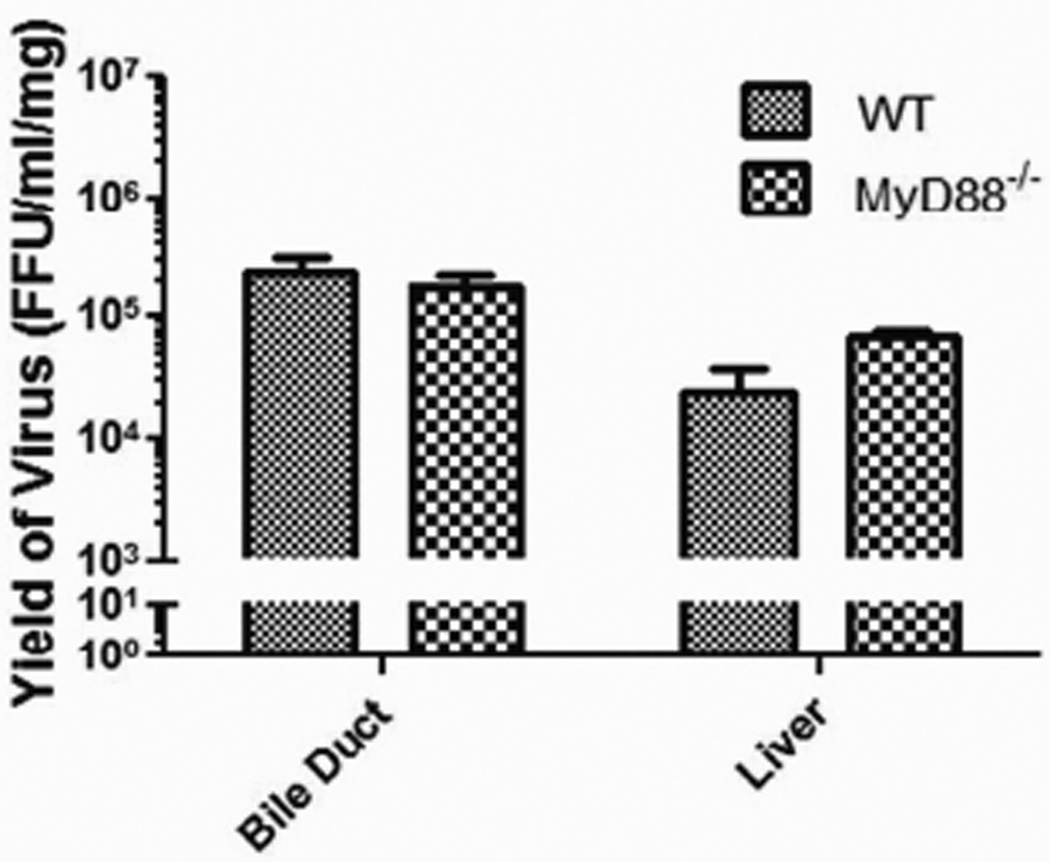
To determine if a difference in the quantity of virus in the liver and extrahepatic bile ducts of WT versus MyD88−/− mice existed after inoculation, viral titers were measured. The livers and extrahepatic bile ducts from WT and MyD88−/− mice were harvested from a subset of mice 7 days after infection with RRV, as previous studies have shown 7 days post infection to have maximum RRV titers.11 The viral titer of the livers from MyD88−/− mice (6.9±0.7×104 FFU/ml) was similar to that of livers from WT mice (2.5±0.6×104 FFU/ml) (p=0.476) (Figure 4). Analogously, the viral titer of the extrahepatic bile ducts was also similar in MyD88−/− mice (1.8±0.4×105 FFU/ml) compared to WT mice (2.4±0.4×105 FFU/ml) (p=0.394) (Figure 4). The amount of virus localized to the liver and extrahepatic bile ducts detected on day 7 was not affected by the absence of the MyD88.
Figure 4. Virus titers in the extrahepatic bile duct and liver.
Focus forming assay carried out on the extrahepatic bile duct and liver samples from MyD88−/− and WT mice harvested 7 days post infection with RRV revealed no difference in viral titers between the two mouse strains.
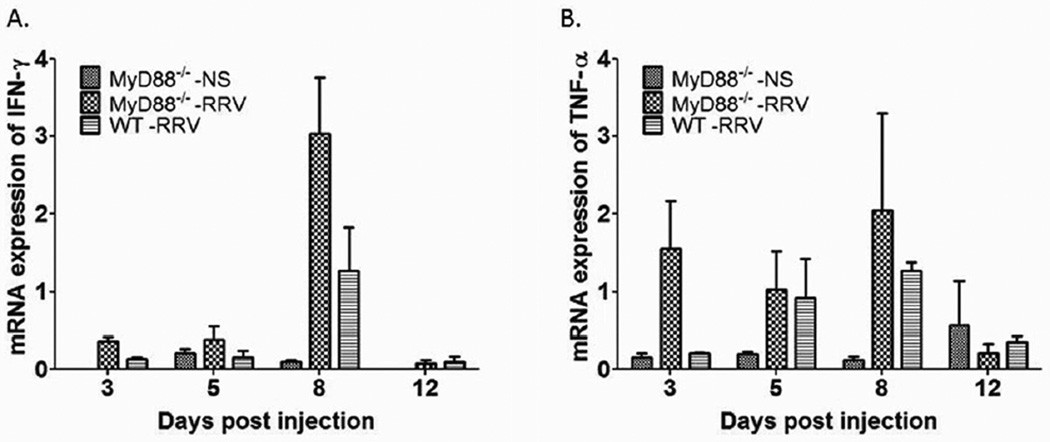
Expression of interferon gamma (INF-γ) and tumor necrosis factor alpha (TNF-α) are unchanged between the two groups
The inflammatory response has been demonstrated to play a key role in the pathogenesis of BA. To evaluate the inflammatory profile, expression of IFN-γ and TNF-α were determined in liver harvested at day 3, 5, 8 and 12 after inoculation. No significant difference in expression levels were detected in RRV infected MyD88−/− mice versus WT mice (Figure 5).
Figure 5. Cytokine mRNA expression after RRV challenge.
mRNA isolated from the livers of newborn mice injected with RRV and harvested at 3, 5, 8, and 12 days post-injection showed increased expression of IFN-γ (A) and TNF-α (B) which were not significantly different at any time point between MyD88−/− and WT mic
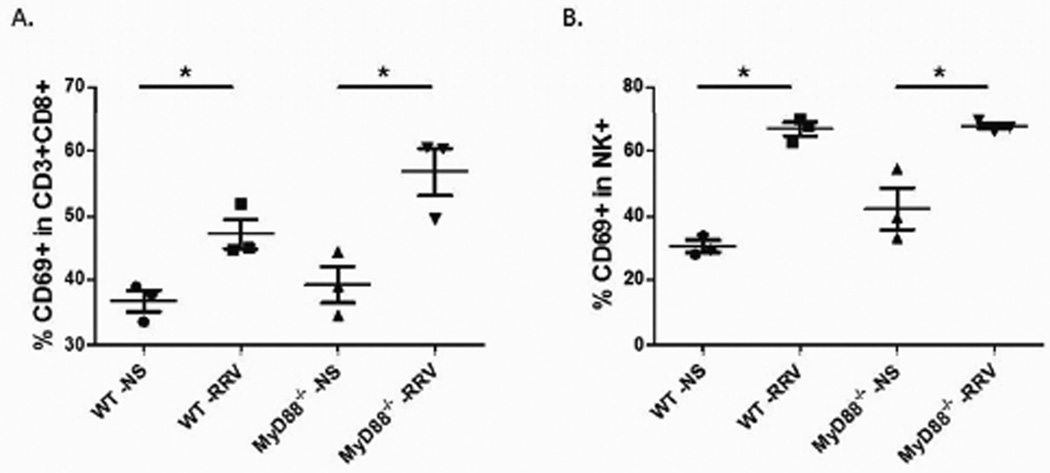
Flow cytometry demonstrates equal immune cell activation
It has previously been shown that increased numbers of activated CD8+ T cells and NK cells are found in the livers of RRV-infected mice at the time of bile duct obstruction (Day 7 post infection), thus implicating them as effector cells in the development of BA. We sought to determine if RRV infection in MyD88−/− mice induced a similar inflammatory response. MyD88−/− and WT mice were inoculated with RRV; saline injected mice served as negative controls. MyD88−/− mice inoculated with RRV demonstrated a significant increase in the percentage of CD8+ T cell and NK cell activation when compared to those injected with saline (47.1% versus 36.8% (p=0.02) and 67.6% versus 42.3% (p=0.02), respectively) (Figure 6). WT mice inoculated with RRV also demonstrate a significant increase in the percentage of CD8+ and NK cell activation when compared to saline injected mice (56.8% versus 39.3% (p=0.02) and 66.7% versus 30.3% (p<0.01), respectively). The percent increase in CD8+ T cells and NK cells did not differ significantly between the MyD88−/− and WT mice injected with RRV (p>0.05). The absence of MyD88 did not result in a decrease in activation of the inflammatory cells.
Figure 6. Analysis of activated CD8+ T cells and NK cells by flow cytometry.
CD8+ T cells and NK cells were isolated from livers of pups 7 days post infection. Both MyD88−/− and WT mice demonstrated a significant increase in CD8+ T cells (A) and NK cells (B) activation over saline control, but there was no significant difference in activation when compared between the two strains (*= p<0.05).
DISCUSSION
Although the etiology of BA is still unknown, viral infection, chronic inflammatory and autoimmune-mediated bile duct injury postulated as mechanisms in its pathogenesis.22,23 A number of studies have identified lymphocyte infiltrates surrounding the intrahepatic and extrahepatic bile ducts in patients with BA.21,24,25 Recently, the intricacies of the immune response have been a focus of research in hopes of determining the underlying pathogenesis of BA, including the innate response and the involvement of an autoimmune component. Innate immune cells and bile duct epithelial cells express pattern recognition receptors (PRRs), which recognize PAMPs on infected cells. This interaction leads to pathogen and even host cell death following activation and progression of the resulting inflammatory cascade.26 One hypothesis is that unregulated TLR signaling may lead to chronic inflammation and the development of obstructive cholangiopathy. TLR dependent activation of autoimmunity is a proposed mechanism, due to the potential for recognition of self-peptides.27
TLRs are central regulators during infection and inflammatory disease functioning as detectors of a variety of invading pathogens. The subtypes of TLR recognize and bind to different PAMPs. Viral PAMPs are detected by several TLRs: TLR3 detects dsRNA like RRV while TLR7 and TLR8 detect ssRNA.28 Several studies have been performed to evaluate upregulation of TLRs in the setting of BA. In humans with the disease, increased expression of TLR3, TLR7, and TLR8 has been demonstrated.29,30
Ligand binding to TLRs triggers recruitment of the adaptor molecule MyD88, which subsequently recruits the downstream signaling molecule known as IL-1R-associated protein kinase (IRAK).12 Activation of IRAK leads to a series of downstream signaling cascades that activate nuclear factor-kB (NF-kB), mitogen-activated protein kinase (MAPK), and other regulators of gene expression, leading to trans-activation of several proinflammatory cytokine genes.29,31 RRV infection of cholangiocytes has been shown to increase MAPK signaling, thus supporting the integral nature of this pathway in the pathogenesis of BA; how this pathway applies to the in vivo murine model remains unclear.32 Previous studies have confirmed the importance of MyD88 in inflammatory pathways, while others have examined the specific role of MyD88 in various liver diseases. Seki et al33 demonstrated a high mortality in MyD88-deficient mice infected with L. monocytogenes with simultaneous decrease in the normal level of proinflammatory cytokine production. In the setting of hepatic fibrosis induced by bile duct ligation, MyD88 was found to be a critical component.34 Our study is the first to investigate the role of MyD88 in the pathogenesis of BA.
Despite the central role of MyD88 in the propagation of most of the inflammatory pathways, the genetic loss of functional MyD88 did not affect the ability of RRV to induce BA in the murine model. Following RRV infection, MyD88−/− mice developed symptoms of jaundice, bilirubinuria, and acholic stools by 7 days of life, similar to the RRV inoculated WT mice. None of the MyD88−/− mice survived the infection while 18% of the WT mice survived. Interestingly, at a standard dose, the MyD88−/− mice experienced a significantly higher mortality rate as opposed to the anticipated protective affect against BA. This quicker death suggests that the absence of MyD88 may make the mice more susceptible to a viral infection. Both the pervasive presence of symptoms and ultimate demise of the mice lacking the MyD88 protein suggest that this protein is not necessary for the underlying inflammatory response that leads to the phenotype and mortality of BA. To evaluate for any difference occurring at the level of the tissue, histology was performed on the liver and extrahepatic bile ducts of both the MyD88−/− and WT mice. Again, no differences were observed, as both subsets demonstrated extensive infiltration of inflammatory cells within the area of the portal tract in the MyD88−/− and WT mice livers. Furthermore, periductal inflammation, epithelial sloughing, stromal proliferation, and luminal obstruction was seen in both MyD88−/− and WT extrahepatic bile ducts. Findings from the liver and extrahepatic bile duct histology demonstrate substantial inflammation in both the MyD88−/− and WT mice, indicating that MyD88 is not necessary for the inflammatory response leading to the development of BA.
In the murine model of BA, expression of inflammatory cytokines and immune cell activation parallels increased viral replication. In this study, we found that MyD88−/− mice had the same level of RRV replication as that seen in WT mice. Similarly, the inflammatory profile of MyD88−/− mirrored that of WT mice inoculated with RRV. Levels of IFN-γ and TNF-α were detected in liver and extrahepatic ducts at comparable quantities. Using flow cytometry to detect CD8+ T cells and NK cells, the inflammatory responses of MyD88−/− and WT mice were compared. When MyD88−/− and WT mice are inoculated with RRV, a significant increase in the percentage of CD8+ T cell and NK cell activation occurs when compared to control mice injected with saline. Comparing the activation of MyD88−/− and WT mice, however, there was no significant difference in CD8+ T cell and NK cell activation. Therefore, the absence of MyD88 did not affect the quantity of virus present at 7 days post infection nor did it result in decreased activation of the inflammatory cascade or downstream inflammatory response. The results of this study demonstrate that the pathogenesis of BA in this model was independent of the MyD88 signaling inflammatory pathway.
Activation of an alternative inflammatory pathway may be involved in the obstructive cholangiopathy seen in BA. One such pathway employs TLR3, which functions independent of MyD88.35 When TLR3 is activated by viral dsRNA produced by an infected cell, the Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF) signaling pathway is activated independent of MyD88 recruitment.36 TLR3 has been shown to induce NFκB over-expression, DC maturation, and expression of type I interferon (IFN-α and β), leading to antiviral and immune-stimulatory responses.37 Harada et al14 studied the involvement of TLR3 in the pathogenesis of BA and demonstrated TLR3 expression in extrahepatic bile ducts of patients. Consistent with TLR3 signaling, stimulation with a synthetic analog of viral dsRNA induced activation of transcription factors such as NFκB and production of IFN-β, as well as up-regulated the expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which was found to enhance apoptosis of biliary epithelial cells.14 In a separate study, Harada et al15 examined whether BECs demonstrated tolerance of the innate immune response to dsRNA given the previously established transient nature of viral infections in the setting of BA. In this study, cultured BECs failed to show innate immune tolerance to a dsRNA synthetic analog and the biliary epithelial innate immune response continued after clearance of the dsRNA virus in human BECs.24 While these investigations implicate TLR3 in the pathogenesis of BA, the unique immune response of the BECs may play a more complex role in the development of obstructive cholangiopathy.
In conclusion, although MyD88 plays a highly conserved roll in both innate and adaptive immune responses, the development of BA can occur in its absence in the murine model. Although not protective against the development of obstruction, a more rapid mortality occurred in the MyD88 knockout; thus, the absence of the protein may play a role in increased susceptibility to viral infection and identifies an area necessitating further investigation. In addition, the sustained induction of the innate response without the development of tolerance as well as the role of other inflammatory cells, such as dendritic cells, natural killer cells and neutrophils, are areas of ongoing research in further understanding BA.
Acknowledgments
GMT supported in part by NIH R01DK-091566
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 2.Tyler KL, Sokol RJ, Oberhaus SM, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 3.Fjaer RB, Bruu AL, Nordbo SA. Extrahepatic bile duct atresia and viral involvement. Pediatric transplantation. 2005;9:68–73. doi: 10.1111/j.1399-3046.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 4.Fischler B, Ehrnst A, Forsgren M, Orvell C, Nemeth A. The viral association of neonatal cholestasis in Sweden: a possible link between cytomegalovirus infection and extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1998;27:57–64. doi: 10.1097/00005176-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 5.De Tommaso AM, Andrade PD, Costa SC, Escanhoela CA, Hessel G. High frequency of human cytomegalovirus DNA in the liver of infants with extrahepatic neonatal cholestasis. BMC Infect Dis. 2005;5:108. doi: 10.1186/1471-2334-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drut R, Drut RM, Gomez MA, Cueto Rua E, Lojo MM. Presence of human papillomavirus in extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1998;27:530–535. doi: 10.1097/00005176-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Mahjoub F, Shahsiah R, Ardalan FA, et al. Detection of Epstein Barr virus by chromogenic in situ hybridization in cases of extra-hepatic biliary atresia. Diagnostic pathology. 2008;3:19. doi: 10.1186/1746-1596-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174:8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatric research. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Coots A, Donnelly B, Mohanty SK, McNeal M, Sestak K, Tiao G. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. The Journal of surgical research. 2012;177:275–281. doi: 10.1016/j.jss.2012.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen SR, Jafri M, Donnelly B, et al. Effect of rotavirus strain on the murine model of biliary atresia. J Virol. 2007;81:1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toubi E, Shoenfeld Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity. 2004;37:183–188. doi: 10.1080/08916930410001704944. [DOI] [PubMed] [Google Scholar]
- 13.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. The EMBO journal. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada K, Sato Y, Itatsu K, et al. Innate immune response to double-stranded RNA in biliary epithelial cells is associated with the pathogenesis of biliary atresia. Hepatology. 2007;46:1146–1154. doi: 10.1002/hep.21797. [DOI] [PubMed] [Google Scholar]
- 15.Harada K, Sato Y, Isse K, Ikeda H, Nakanuma Y. Induction of innate immune response and absence of subsequent tolerance to dsRNA in biliary epithelial cells relate to the pathogenesis of biliary atresia. Liver international : official journal of the International Association for the Study of the Liver. 2008;28:614–621. doi: 10.1111/j.1478-3231.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 16.Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology. 2006;44:1231–1239. doi: 10.1002/hep.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 20.McNeal MM, Broome RL, Ward RL. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology. 1994;204:642–650. doi: 10.1006/viro.1994.1579. [DOI] [PubMed] [Google Scholar]
- 21.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. The Journal of clinical investigation. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9:646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 23.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. Journal of pediatric surgery. 1995;30:515–518. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 25.Bezerra JA, Tiao G, Ryckman FC, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 26.Feldman AG, Mack CL. Biliary atresia: cellular dynamics and immune dysregulation. Seminars in pediatric surgery. 2012;21:192–200. doi: 10.1053/j.sempedsurg.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nature reviews Immunology. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang JH, Chou MH, Wu CL, Du YY. Implication of innate immunity in the pathogenesis of biliary atresia. Chang Gung medical journal. 2006;29:240–250. [PubMed] [Google Scholar]
- 29.Saito T, Hishiki T, Terui K, et al. Toll-like receptor mRNA expression in liver tissue from patients with biliary atresia. Journal of pediatric gastroenterology and nutrition. 2011;53:620–626. doi: 10.1097/MPG.0b013e3182307c9c. [DOI] [PubMed] [Google Scholar]
- 30.Huang YH, Chou MH, Du YY, et al. Expression of toll-like receptors and type 1 interferon specific protein MxA in biliary atresia. Laboratory investigation; a journal of technical methods and pathology. 2007;87:66–74. doi: 10.1038/labinvest.3700490. [DOI] [PubMed] [Google Scholar]
- 31.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Jafri M, Donnelly B, McNeal M, Ward R, Tiao G. MAPK signaling contributes to rotaviral-induced cholangiocyte injury and viral replication. Surgery. 2007;142:192–201. doi: 10.1016/j.surg.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Seki E, Tsutsui H, Tsuji NM, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 34.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature medicine. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 36.Arancibia SA, Beltran CJ, Aguirre IM, et al. Toll-like receptors are key participants in innate immune responses. Biological research. 2007;40:97–112. doi: 10.4067/s0716-97602007000200001. [DOI] [PubMed] [Google Scholar]
- 37.Chu WM, Ostertag D, Li ZW, et al. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]