Abstract
A number of premotor and prefrontal brain areas have been recently shown to play a significant role in response selection in overt sentence production. These areas are anatomically connected to the basal ganglia, a set of subcortical structures that has been traditionally involved in response selection across behavioral domains. The putamen and the caudate, the two major inputs to the basal ganglia, have been shown to undertake motor- as well as non-motor-related selection operations in language processing. Here we investigate the role of these basal ganglia structures in sentence repetition and generation in healthy adults. Although sentence generation is known to activate prefrontal and premotor cortical areas that reciprocally connect with these two neostriatal structures, their specific contributions are not known. We present evidence suggesting that that the putamen undertakes articulation-related aspects across tasks, while the caudate selectively supports selection processes in sentence generation.
Keywords: response selection, sentence production, caudate nucleus, putamen, basal ganglia, fMRI
1. Introduction
A fundamental aspect of spoken language production is selection, both of linguistic units (e.g., words) that convey a particular meaning and of sequences of motor programs that instantiate these units as articulatory gestures. Constraints in response selection may vary dramatically across tasks. In contrast to word repetition, for instance, where the linguistic response is externally pre-selected, word generation involves internally imposed constraints in the selection of the correct response among competing alternatives (e.g., Crosson et al., 2001). Despite the importance of this process, its neural underpinnings have not been well integrated into current neurobiological models of language (see Tremblay & Small, 2011b for references and discussion). Recent evidence suggests that a number of cortical areas engaged in the production of words and oral motor gestures, including the pre-supplementary motor area (pre-SMA), the ventral premotor cortex, and the pars opercularis and triangularis of the inferior frontal gyrus, are involved in selecting motor and/or lexical responses during word (Tremblay & Gracco, 2009a, 2009b; Tremblay, Shiller, & Gracco, 2008) or sentence production (Tremblay and Small, 2011b). A fundamental property of these areas is their participation in multiple segregated frontal–basal-ganglionic–thalamic loops (e.g., Middleton & Strick, 2000). Each loop includes projections from the cerebral cortex, through the basal ganglia (BG), to the thalamus, and back to the cerebral cortex. The neostriatum, consisting of the caudate nucleus and putamen, receives the main input from the cerebral cortex to the BG: the putamen from motor and premotor cortices, while the caudate from various prefrontal structures (Hoover & Strick, 1999; Parent, 1990). The caudate and putamen each project to distinct segments of the medial globus pallidus, and, via projections to the thalamus, reach the cortical regions to which they are reciprocally connected. Both tract tracing studies in primates and non-invasive imaging in humans (e.g., resting-state functional connectivity, white matter tractography with diffusion tensor imaging) have shown that the pre-SMA as well as the dorsolateral and ventrolateral prefrontal cortices connect with the caudate head and the anterior putamen (i.e., associative cortico-striatal loop), while the motor and premotor cortices connect (primarily) with the posterior and dorsolateral anterior putamen (i.e., sensorimotor cortico-striatal loop) (e.g. Akkal, Dum, & Strick, 2007; Di Martino et al., 2011; Chan, Ryan, & Bever, 2011).
The differentiation of these cortico-cortical-BG-thalamic loops is strongly suggestive of relative functional specialization within the BG, promoting the idea that different aspects of language processing rely more on certain BG components than others. However, while involvement of the BG in language is well established (e.g., Chan et al., 2011; Ketteler, Kastrau, Vohn, & Huber, 2008), its role remains unclear. Selective BG lesions do not consistently replicate classical aphasic symptoms (Crosson, Benjamin, & Levy, 2007; Crosson & Haaland, 2003) and there is some thought that the resulting language deficits are more related to cortical hypoperfusion caused by the BG lesion than to the lesion per se (e.g., Hillis et al., 2002). Nevertheless, the architectural parallels among the different cortico-BG loops have suggested that the BG function in a unitary fashion across behavioral domains. Two popular proposals on the role of the BG are (i) action selection among competing alternatives (e.g., Jueptner & Weiller, 1998); and (ii) suppression of undesired actions and facilitation-initiation of desired ones (e.g., Gerfen, 1992). Studies on monolingual speakers have demonstrated BG involvement in the controlled process of syntactic integration (Friederici & Kotz, 2003; Friederici, Kotz, Werheid, Hein, & Yves von Cramon, 2003), while studies on bilingual speakers have highlighted the significance of the BG in second language comprehension and in the control of switching between languages (e.g., Abutalebi, Miozzo, & Cappa, 2000; Friederici, 2006; Lehtonen et al., 2005). The BG may thus play a role in cognitive control, assisting multi-level language processes by enhancing selected actions while suppressing competing ones (Crosson et al., 2003, 2007). Of particular relevance for sentence generation and production (Tremblay and Small, 2011b) is the recent finding implicating the left caudate in single word suppression (Ali, Green, Kherif, Devlin, & Price, 2009).
In the present study, we aim to build on these results that have demonstrated (i) the roles of the BG in enhancement and suppression during single word processing and in cognitive control during sentence processing, and (ii) the reciprocal connectivity of the BG with cortical areas involved in response selection in overt production of both single words and sentences. The question that we address is whether structures of the neostriatum (caudate and putamen) are involved in the production of larger strings of words, such as sentences, in the same fashion as that they participate in single word selection and, more broadly, action selection. To this aim, we compare neostriatal activation during sentence repetition (externally constrained selection) with that during sentence generation (volitional selection). Based on the above, we hypothesize, first, that the caudate nucleus would be more active during sentence generation than repetition, given its involvement in the prefrontal-associative loop and its significance in aspects of response selection, cognitive control, and semantics; and second, that the putamen would be similarly active in both repetition and generation, based on its involvement in the motor-attentional cortico-striatal loop.
2. MATERIALS AND METHODS
2.1. PARTICIPANTS
The present study represents a reanalysis of data collected previously (Tremblay and Small, 2011a) and here we briefly repeat the methods that are described fully in that paper. Twenty-one healthy righthanded (Oldfield, 1971) native speakers of English (mean: 25 ± 4.4 years of age; 10 males) with a mean of 15.4 years of education participated. All had normal hearing, as assessed by normal pure-tone thresholds and normal speech recognition scores (92.3% accuracy on the Northwestern University auditory test number 6). The study was approved by the Institutional Review Board of the Division of Biological Sciences of The University of Chicago.
2.2. Experimental Procedures
Participants completed 5 different tasks inside the scanner: (1) passive sentence listening, (2) passive picture observation, (3) sentence repetition, (4) sentence generation, and (5) passive observation of short action movies. The individual trials for each of these tasks were grouped together in separate runs and, within each of these runs, experimental trials were alternated with periods of “rest” during which participants were asked to relax. For each run, the order of the conditions and number of rest trials was optimized using OPTseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/). In the present article, we focus on the first four tasks.
A detailed description of stimulus construction and presentation may be found in Tremblay and Small (2011a, b). The sentence listening run consisted of 110 total trials, including 80 active auditory sentence listening trials (0.9 to 1.3 sec each) and 30 visual fixation control trials (crosshairs) in a pseudorandom sequence. Half of these sentences described manual object-directed actions and the other half described visual properties of the same set of objects. The sentence stimuli were presented while the MRI gradients were shut off, which ensured ease of auditory processing for participants (“sparse sampling” MRI acquisition (Gracco, Tremblay, & Pike, 2005)). The picture observation run involved 77 trials, consisting of 40 simple black-and-white line drawings and 37 visual fixation control trials (1 sec each). The pictures represented common man-made objects selected from the International Picture Norming Project corpus from the Center for Research in Language at the University of California San Diego (Bates et al., 2003; Szekely et al., 2003). Participants were instructed to attend to the pictures. The sentence repetition run consisted of 110 trials, including 80 auditory sentence trials (40 action, 40 object sentences) and 30 visual fixation control trials; participants were instructed to repeat the sentence. Both stimulus presentation and response occurred while the gradients were shut off for a 4.5 second period of silence. At the beginning of the silent interval, a ‘Go’ cue was presented, instructing participants to start repeating the sentence. All responses were recorded. The sentence generation run consisted of 108 trials. In the 80 active trials, participants were asked to generate sentences (40 action, 40 object), with 28 visual fixation trials pseudorandomly interspersed in the run. In each experimental trial, a picture was presented for one second and was followed, after 500ms, by a visual ‘Go’ cue instructing participants to start generating a sentence. All responses occurred while the MR gradients were shut off. The listening and picture observation tasks provided control conditions for the sentence repetition and sentence generation tasks, respectively.
2.3. IMAGE ACQUISITION AND ANALYSIS
2.3.1. Image acquisition
Functional data were collected on a 3T General Electric Signa HDx MRI scanner with EXCITE parallel acquisition capability. Subjects wore MR-compatible headphones and goggles (Nordic NeuroLabAudio/Visual system). 34 axial slices (3.125 mm × 3.125 mm × 3.6 mm, no gap, FOV=256 mm × 256 mm, matrix=64×64) were acquired in 1.5 sec using a multi-slice EPI sequence with parallel imaging (ASSET=2; TE=26 ms; FOV = 20 cm; 64×64 matrix; Flip angle: 73). A sparse image acquisition technique (Gracco, Tremblay, & Pike, 2005) was used for the three language tasks (sentence generation, repetition, and listening), to eliminate movement artifacts associated with speaking, and to ensure satisfactory audition. A silent period (1.5 sec for listening, 4.5 sec for repetition and generation) was interleaved between each volume acquisition. High-resolution T1-weighted volumes were also acquired for anatomical localization.
2.3.2. Time series pre-processing
We first segmented each individual’s high-resolution structural image, using the Freesurfer parcellation of white and grey matter (e.g. Dale, Fischl, & Sereno, 1999). The functional images were co-registered to each other and then to the structural volume (Saad et al., 2009), and the functional data were motion-corrected (within and across runs), de-spiked, and mean-normalized using AFNI (Cox, 1996). A linear least squares model was used to establish a fit to each time point of the hemodynamic response function for each condition. We modeled the entire trial duration (i.e., 6 s), which included stimulus presentation and speech production.
2.3.3. First level (subject) analysis
Event-related signals were deconvolved by linear interpolation, beginning at stimulus onset, and continuing for 12s, using AFNI’s tent function (i.e., a piecewise linear spline model). For sentence generation and sentence repetition, we examined the fit at two different time lags (0–6s, and 6–12s) to identify the time point showing the strongest hemodynamic response both across the brain as well as in all of our regions of interest (left and right caudate and putamen; see section 2.3.3 below). All subsequent analyses focused on the activation from the first 6 seconds post-stimulus onset. There were separate regressors for each of the experimental conditions (sentence generation, sentence repetition, sentence listening, picture observation), as well as for each of the six motion parameters (x, y, z, roll, pitch, yaw). To remove additional sources of spurious variance unlikely to represent signal of interest, we also included the regression signal from the lateral ventricles (Dick, Solodkin, & Small, 2010; Fox et al., 2005), which was identified using the automated subcortical segmentation from Freesurfer to mask the ventricles. Data were smoothed to achieve a target smoothing value of 3 mm using a Gaussian full width half maximum (FWHM) filter. Anatomical and functional data sets were then spatially normalized to the ICBM 452 template to compensate for inter-subject variability in structural and functional anatomy.
2.3.4. Whole-brain analyses
Whole-brain group analyses were performed using AFNI on the participants’ beta values resulting from the first-level analysis. As our objective was to compare the activation related to sentence generation and sentence repetition, we first subtracted the activation from baseline (sentence generation – picture observation; sentence repetition – sentence listening). Next, we examined the difference between sentence generation and sentence repetition. These subtraction-type analyses were complemented by a “conjunction” analysis (Nichols, Brett, Andersson, Wager, & Poline, 2005) to uncover brain regions jointly active across the two tasks. For each analysis, a permutation approach (Nichols & Holmes, 2002) was used to identify significant clusters of activated voxels, with an individual voxel threshold of p < .00005, corrected for multiple comparisons to achieve a family-wise error (FWE) rate of p < .01 (clusters ≥ 3 voxels, i.e. 105.5 µl)1.
2.3.5. Anatomical region of interest (ROI) analysis
An automated segmentation scheme implemented in FreeSurfer (Fischl et al. 2002) was used to parcellate the neostriatal structures of each individual participant. We focused on anatomical regions of interest, thus avoiding selection bias (e.g., Vul & Kanwisher (2009) for discussion). The four regions of interest (ROIs) were the left and right caudate and the left and right putamen. The beta values resulting from the first-level analysis of sentence generation and sentence repetition were averaged across all voxels within each ROI for each subject, and then entered in a 2 × 2 × 2 repeated measures ANOVA (task, ROI, hemisphere). One-sample two-tailed t-tests were used to examine whether the activation magnitude in each ROI was significantly different from zero for repetition and generation.
3. Results
3. 1. Whole neostriatum group analysis
3.1.1. Sentence Generation, Sentence Repetition, Sentence Generation ∩ Sentence Repetition
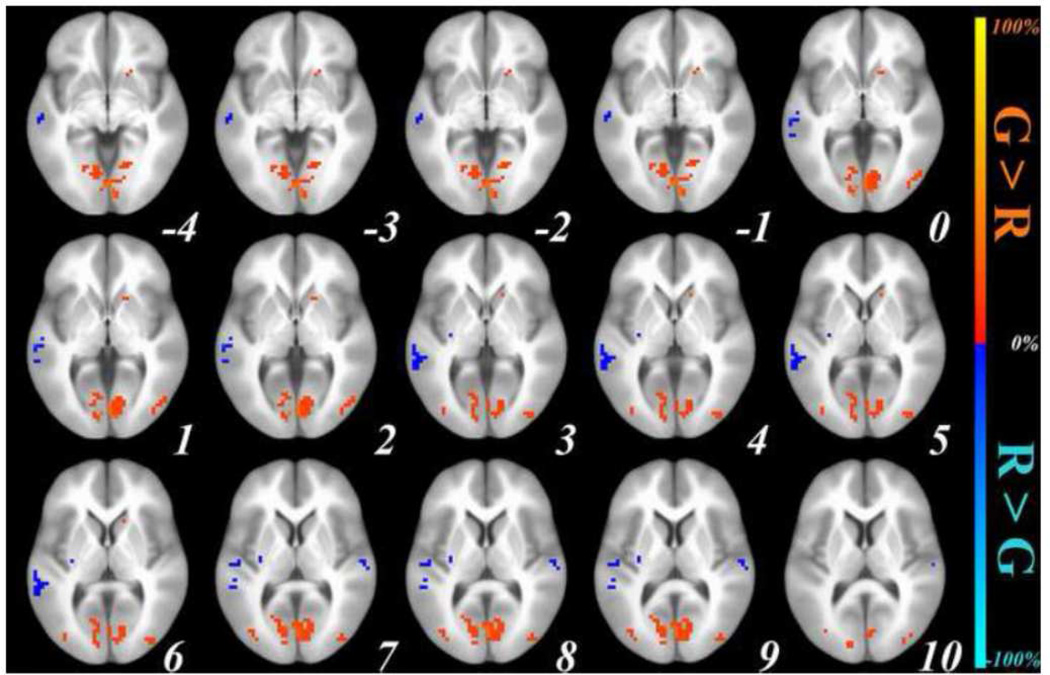
Comparing sentence generation with picture observation in the neostriatum demonstrated significant activation in both caudate nuclei (body-tail) and putamina (mid-anterior). Comparison of sentence repetition with sentence listening yielded significant bilateral putamen activation (mid-posterior). There was no overlap in caudate activation across the two tasks. Putaminal activations in both conditions were mainly seen in the median areas. Figure 1 below illustrates the brain areas jointly activated for sentence repetition and sentence generation, along with those activated exclusively for sentence generation and those exclusively for sentence repetition. An exhaustive list of all neostriatal regions is presented in Table 1. Each entry in the table represents a single cluster of activation; sometimes clusters span over more than one structure.
Figure 1.
Family-wise error-corrected (cluster size ≥ 3 contiguous voxels, corrected at p < .01) group-level (n = 21) neostriatal activations (signal % change, individual voxel threshold of p < .00005) for Sentence Generation (RED) and Sentence Repetition (BLUE) after subtracting their corresponding baseline activations, and for Sentence Generation ∩ Sentence Repetition (GREEN); top left to bottom right: axial slices in ICMB 452 space from z = −4 to z = 20. RAI orientation.
Table 1.
Family-wise error-corrected (cluster size ≥ 3 contiguous voxels, corrected at p < .01) group-level (n = 21) neostriatal activations (signal % change, individual voxel threshold of p < .00005) for Sentence Generation, Sentence Repetition and Sentence Generation ∩ Sentence Repetition. All coordinates are in ICMB 452 space. CM: Center of Mass; MI: Maximum Intensity; SEM: Standard Error of the Mean. RAI orientation.
| Task | Brain Structure | Hemi | volume (µl) | CM x |
CM y |
CM z |
MI x | MI y | MI z | Mean | SEM | MI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generate | brainstem | L,R | 15065.6 | 3.1 | 13.6 | 2.3 | 1.6 | 11.9 | 15.4 | .372 | .006 | .967 |
| thalamus | L,R | |||||||||||
| putamen (anterior-mid) | L,R | |||||||||||
| caudate (body-tail) | L,R | |||||||||||
| Repeat | putamen (mid) | L | 211.2 | 22.4 | −0.8 | 9.7 | 20.3 | −0.6 | 8.2 | .383 | .028 | .463 |
| R | 105.6 | −23.4 | 1.4 | 12.9 | −23.4 | 2.5 | 11.8 | .167 | .009 | .178 | ||
| putamen (posterior) | L | 105.6 | 25.6 | 6.7 | −2.6 | 26.6 | 8.8 | −2.6 | .337 | .014 | .363 | |
| R | 105.6 | −28.5 | 3.5 | −2.6 | −26.6 | 2.5 | −2.6 | .253 | .022 | .292 | ||
| Generate ∩ Repeat | putamen (mid) | L | 105.6 | 21.4 | −1.7 | 9.4 | ||||||
3.1.2. Sentence Generation versus Sentence Repetition
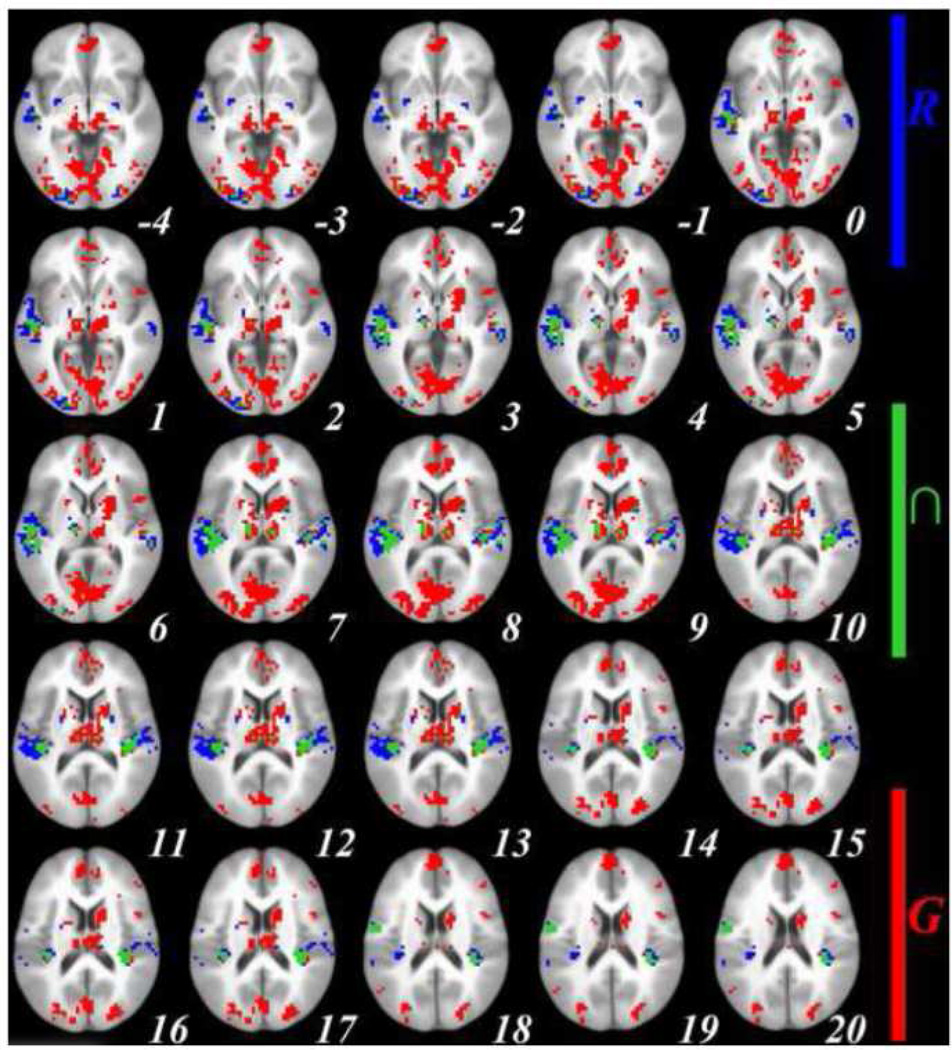
Next we compared sentence generation and sentence repetition after controlling for activation in their respective control conditions ([sentence generation – picture observation] – [sentence repetition – sentence listening]). As shown in Figure 2, activation in the left anterior putamen and caudate was found for sentence generation > sentence repetition, and in the right posterior putamen for sentence repetition > sentence generation. These results are detailed in table 2. To demonstrate that this pattern did not result from differences in control conditions, we conducted two supplementary analyses comparing sentence generation and sentence repetition directly before “subtracting” activation in their respective control conditions, as well as comparing activations in the control conditions (picture observation and sentence listening). Activations in the same striatal areas were observed when comparing sentence generation and sentence repetition before subtracting activations in their control conditions, while no striatal activations were observed when comparing the control conditions (see Supplementary Material).
Figure 2.
Family-wise error-corrected (cluster size ≥ 3 contiguous voxels, corrected at p < .01) group-level (n = 21) neostriatal activations (signal % change, individual voxel threshold of p < .00005) for Generation > Repetition (RED) and Repetition > Generation (BLUE), after subtracting baseline activations from each; top left to bottom right: axial slices in ICMB 452 space, from z = −4 to z = 10. RAI orientation.
Table 2.
Family-wise error-corrected (cluster size ≥ 3 contiguous voxels, corrected at p < .01) group-level (n = 21) neostriatal activations (signal % change, individual voxel threshold of p < .00005) for Sentence Generation > Sentence Repetition and Sentence Repetition > Sentence Generation. All coordinates are in ICMB 452 space. CM: Center of Mass; MI: Maximum Intensity; SEM: Standard Error of the Mean. RAI orientation.
| Comparison | Brain Structure | Hemi | volume (µl) | CM x | CM y | CM z | MI x | MI y | MI z | Mean | SEM | Max Int |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generate > Repeat | putamen (anterior); caudate (head) | L | 176 | 16.6 | −17.3 | 0.1 | 17.2 | −16.2 | 1 | 0.332 | 0.029 | 0.411 |
| Repeat > Generate | putamen (posterior) | R | 105.6 | −29.7 | 14 | 6.8 | −29.7 | 15 | 4.6 | −0.202 | 0.017 | −0.237 |
3.2. ROI analysis
The three-way omnibus ANOVA (Task, Hemisphere, ROI) yielded a main effect of Task [F(1,20) = 13.41, p = .002]2 across hemispheres [Task × Hemisphere: F(1,20) = 1.07, p = .3], along with a Task × ROI interaction [F(1,20) = 5.54, p = .029], also observed across hemispheres [Task × ROI × Hemisphere: F(1,20) = .82, p = .4]. However, the two ROIs did not differ in activation independent of the task [ROI: F(1,20) = 1.42, p = .2]. There was also a statistically insignificant effect of Hemisphere [F(1,20) = 4.06, p = .06] across ROIs and tasks [ROI × Hemisphere: F(1,20) = 1.06, p = .3] with a tendency for left ROIs to show stronger activation than right ROIs.
A two-way ANOVA on activation in the caudate nucleus replicated the main effect of Task [F(1,20) = 13.13, p = .002], and an ANOVA on putaminal activation was also significant [F(1,20) = 4.43, p = .048]. The two-way ANOVAs on the two tasks showed no effect of ROI in the sentence generation task [ROI: F(1,20) = .2, p = .7], but a significant main effect on the sentence repetition task [ROI: F(1,20) = 6.71, p = .02].
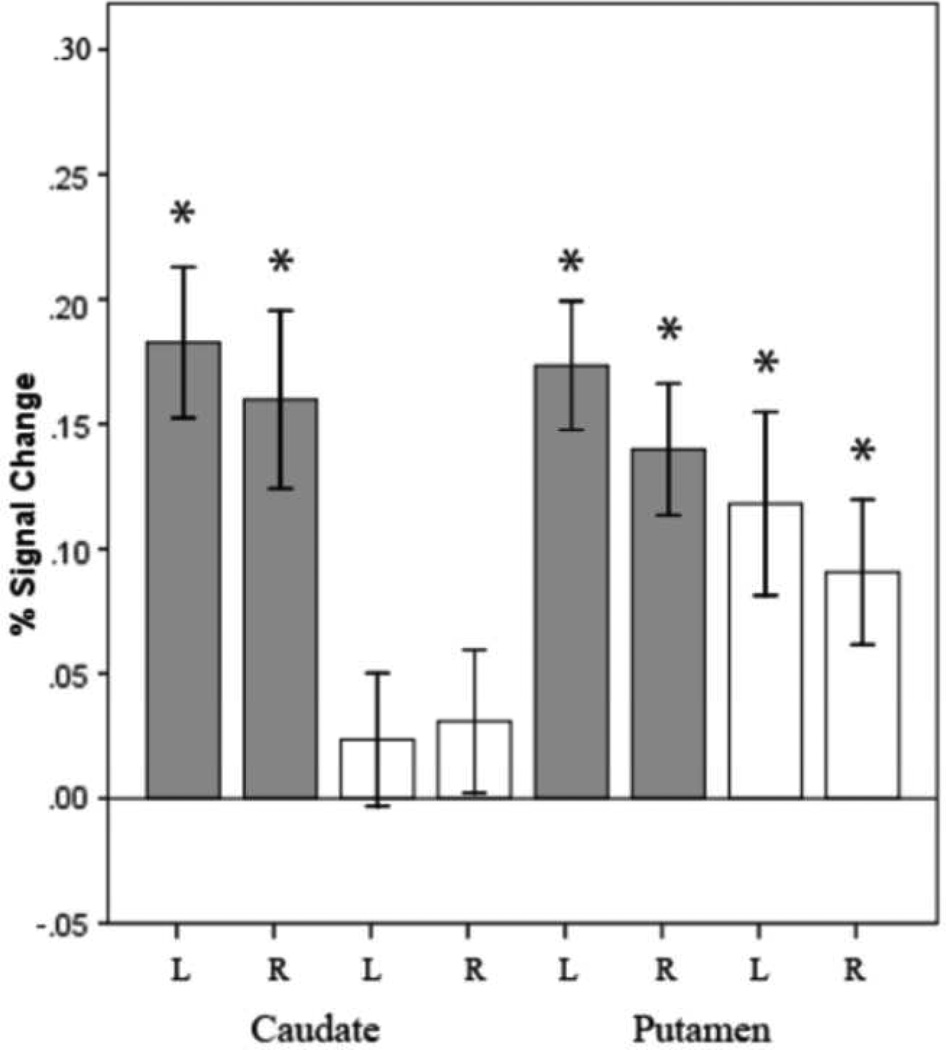
To examine these results further, we tested the activation level in each ROI against zero, using one-sample t-tests. Significant activation was found in the putamen across conditions [left putamen, sentence generation: t(1,20) = 6.72, p = .000002; repetition: t(1,20) = 3.21, p =.004; right putamen, sentence generation: t(1,20) =5.29, p = .00004; repetition: t(1,20) = 3.12, p =.005]. The caudate, in contrast, was selectively active for sentence generation [left caudate, sentence generation: t(1,20) = 6.04, p = .000007; repetition: t(1,20) = .89, p = .4; right caudate, sentence generation: t(1,20) = 4.48, p = .0002; repetition: t(1,20) = 1.08, p = .3].
We also performed a series of paired sample t-tests between sentence generation and sentence repetition in each ROI. Activation in both the left and the right caudate for sentence generation was significantly stronger than for sentence repetition [left caudate: t(1,20) = 4.16, p = .0005; right caudate: t(1,20) = 2.87, p = .009]. For the putamen, the same difference was significant only in the left hemisphere [left putamen: t(1,20) = 2.07, p = .05; right putamen: t(1,20) = 1.88, p = .08].
4. Discussion
The BG is a set of complex structures that play a fundamental role in many aspects of human behavior, including the planning and execution of action and cognition. Although the significance of the BG in language processing is widely accepted, their specific role remains elusive. Our objective was to examine the involvement of neostriatal structures in overt sentence production, and more specifically in response selection during sentence production. To this aim, we conducted a direct comparison of BG activation patterns across two different language tasks. Such direct comparisons are indeed rare because of the variability and reduced amplitude of BG activation compared to that of the cerebral cortex (see Crosson et al. (2003, 2007) for discussion). Despite these difficulties, we found differences across language tasks, both in the neostriatum as a whole and in four neostriatal regions of interest.
We predicted that both caudate and putamen would be involved in response selection in overt sentence production for four reasons: (i) they have well-established reciprocal connectivity with the cerebral cortex (e.g., Hoover & Strick, 1999; Parent, 1990; Middleton & Strick, 2000), a subset of which is involved in motor/lexical selection processes in single word as well as sentence production (Tremblay & Small, 2011a); (ii) they have a well-established role in overall motor response selection (e.g. Jueptner & Weiller, 1998; Gerfen, 1992); (iii) they have significant involvement in language processes of various types (e.g., Cross & Haaland, 2003; Crosson et al., 2007); and perhaps most importantly, (iv) they are heavily involved in single word selection processes (e.g., Ali et al., 2009; Price, Green, & von Studnitz, 1999; Abutalebi et al., 2008; van Heuven, Schriefers, Dijkstra, & Hagoort, 2008). Based on the connectivity of these two neostriatal regions, our specific predictions were that (1) the putamen, a structure largely embedded within the motor cortico-BG-thalamic-cortical loop, would show significant activation in both sentence generation and sentence repetition, and that (2) the caudate, an area predominantly involved in the associative loop, would be involved either selectively in sentence generation, or more strongly during generation compared to repetition. Our findings are discussed in the following paragraphs.
4.1. Putamen: sentence production
Our results demonstrate that the putamen was similarly active in sentence repetition and sentence generation. We thus suggest that this structure is involved in motor aspects of response selection present in both tasks. Importantly, this interpretation is coherent with evidence on the anatomical connectivity of the putamen and particularly its posterior parts, which connect reciprocally with motor and premotor cortical areas (e.g., Di Martino et al., 2011). Tremblay and Small (2011b) reported that the rostral and caudal parts of the left ventral premotor cortex are active in both sentence repetition and sentence generation, and also exhibit a significant task-related modulation. Indeed, in the present study, the putamen shows an identical pattern, reflecting its connection with the ventral premotor cortex within the ‘motor’ cortico-striatal loop.
4.2. Caudate nucleus: response selection
The strongest version of our prediction was indeed verified for the caudate, which showed no activation in sentence repetition. We interpreted this pattern as reflecting a role for the caudate in response selection during language production. Our findings are consistent with evidence on the anatomical connectivity of the caudate, and, in particular, the caudate head, which connects with the dorsolateral and ventrolateral prefrontal cortex (e.g., Di Martino et al., 2011). Tremblay and Small (2011b) reported that the left pars triangularis of the inferior frontal gyrus showed selective activation in sentence generation, in the same fashion as the caudate here, with which it is connected within the ‘associative’ corticostriatal loop. Moreover, the caudate head activation observed complements a growing body of evidence on the significance of this structure along with the inferior parietal lobule, pulvinar thalamic nuclei, cerebellar lobules, and the anterior cingulate in selection operations in language processing (see Ketteler & Ketteler (2010) as well as Lieberman (2001) for further discussion).3
An alternative interpretation is that this activation pattern reflects processing difficulty rather than selection demands. Indeed, sentence generation is more demanding than sentence repetition, requiring more attentional resources and increased error monitoring. It is therefore possible that the neostriatal modulation observed can be attributed to these cognitive processes (see Chan et al. (2011) for discussion) rather than to response selection. However, while participants made more errors in generation than repetition, representing 13.5% and 1.2% of all trials respectively (see Tremblay & Small, 2011a,b for the details), all such trials were removed from the present analysis. Furthermore, the sentence generation and repetition tasks did not differ on any online measures (i.e., sentence length, word length, accuracy). Moreover, structural priming was anticipated (and ultimately observed) in the generation task (see Pickering & Ferreira, 2008 for a recent review), originating from the sentence listening and sentence repetition task, and thus the sentences generated by participants were largely identical to those they had heard (see Tremblay and Small, 2011b, for details). Consequently, during sentence generation, participants chose what responses (words) to produce but kept the syntactic structure fairly constant, meaning that the main difference between the two tasks was demand on response selection rather than syntax. Given that sentence repetition poses far fewer semantic processing demands than generation, and since the caudate was not active in repetition, we suggest that the caudate may be selectively involved in semantic aspects of selection. Of note here is its demonstrated role in regulating semantic competition between words in different languages. For instance, caudate activation increases when translating rather than repeating words (Price, Green, & von Studnitz, 1999), when naming pictures in the first language in a bilingual as compared with a monolingual context (Abutalebi et al., 2008), or when making a lexical decision on a letter string in subjects’ second language when it is also a word with different semantics in their first (van Heuven, Schriefers, Dijkstra, & Hagoort, 2008).
Another interpretation is that the caudate was selectively active in sentence generation due to increased demands for cognitive control, i.e., the inhibition of inappropriate responses, and the release of an appropriate one from inhibition. Indeed, sentence generation also differs from sentence repetition in the degree of automaticity and cognitive control. This explanation would not commit the caudate to a particular level of language processing, but to a processing mode (automatic vs. controlled). In a study of lexical ambiguity resolution, for instance, Ketteler et al. (2008) implicated the caudate, along with a number of cerebral cortical areas in the regulation of pre-formulated language segments for motor programming and semantic verification. Similarly, Ali et al (2011) found caudate activation in a Stroop task, which they attributed to overcoming habitual or overlearned actions, irrespective of behavioral domain (e.g., Shadmehr & Holcomb, 1999; for a discussion of the significance of the BG in the automatization of language processing, see also Argyropoulos (2008) and references therein). In a study of intraoperative electrical stimulation on awake patients during brain surgery, stimulation of the caudate elicited perseveration, while stimulation of the anterior putamen elicited dysarthria/anarthria. The authors concluded that there are two separate BG systems involved in language, one mediated by the putamen and playing a motor role, and the other mediated by the caudate, plausibly involved in cognitive control (Gil-Robles, Gatignol, Capelle, Mitchell, and Duffau, 2005). In a sequential learning task, certain neurons in the monkey striatum are preferentially active for new sequences and others for older sequences, with the former localizing more to the “association” region of the caudate and the rostral putamen, and the latter in the “sensorimotor” region of the posterior putamen (Miyachi Hikosaka, & Lu, 2002). Further research would thus be required to dissociate between these two explanations.
5. Conclusion
The present study provides evidence for the involvement of the human neostriatum in response selection during overt sentence production. In particular, it extends our knowledge of the caudate by showing a role for this structure in response selection beyond the single-word to the sentence level. Further, our findings show that particular subcortical structures are involved in linguistic response selection in overt sentence production. A more refined segmentation of the neostriatum into anterior/posterior putamen and caudate head/body and tail should allow us to examine whether the patterns observed here were driven by particular areas within the caudate and the putamen4, and whether there are coactivations among specific neostriatal and cerebrocortical regions. Further research is required to clarify whether this involvement reflects domain-general processes of cognitive control in language, or to more specific semantic processes.
Supplementary Material
Figure 3.
Brain activity expressed as a percentage of signal change for sentence generation (grey) and sentence repetition (white) for the anatomical ROIs of the left and right caudate and putamen. The asterisk indicates that the statistics are significant (p ≤ .005). Error bars represent +/−1 Standard Error of the Mean.
Acknowledgments
The data were acquired when two of the authors (P.T., S.L.S.) were at The University of Chicago, and were analyzed at the University of Chicago and the University of California, Irvine by two of the authors (G.A., P.T.). The support of these institutions is acknowledged. The study was supported by National Institute of Deafness and other Communication Disorders (NIDCD) of the National Institutes of Health of the United States under Grant R01 DC003378. Their support is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The results yielded were not compromised by relaxing the individual voxel threshold at p < .0001, or by not applying cluster size correction at p < .00005.
To ensure that the effects observed could not be attributed to differences in SNR values across the brain, we calculated the mean SNR value per ROI per condition per subject. We entered these values in a similar 2 × 2 × 2 ANOVA for the experimental conditions (Task: sentence generation/repetition; ROI: caudate/putamen; Hemisphere: left/right), and another 2 × 2 × 2 ANOVA for the experimental baselines (Task: picture observation/sentence listening; ROI: caudate/putamen; Hemisphere: left/right). No Structure × Task interaction was observed, for either the experimental (F < 1) or baseline conditions (p > .1).
Information on cerebral cortical activations can be found in Tremblay and Small (2011a,b). No activation was observed here for the cerebellum or the thalamus.
It should be noted, however, that an analysis of the activations of the putamen and the caudate as two types of ROIs was not unmotivated. In their resting state functional connectivity analysis, di Martino et al. (2011) showed that their putamen seeds predicted activation in primary and secondary cortical motor areas, as supported by a direct comparison between the combination of their 3 caudate seeds and their 3 putamen seeds. Furthermore, even the anterior putamen, much like its posterior areas, has been often shown to play a motor-related role in language processing, as compared with the caudate (Gil-Robles et al. 2005; see main text).
References
- Abutalebi J, Miozzo A, Cappa SF. Do subcortical structures control language selection in bilinguals? Evidence from pathological language mixing. Neurocase. 2000;6:101–106. [Google Scholar]
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Khateb A. Language control and lexical competition in bilinguals: An event-related fMRI study. Cereb Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar Output. J Neurosci. 2007;27(40):10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Green DW, Kherif F, Devlin JT, Price CJ. The role of the left head of caudate in suppressing irrelevant words. J Cogn Neurosci. 2009;22(10):2369–2386. doi: 10.1162/jocn.2009.21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulos GP. The subcortical foundations of grammaticalization. In: Smith Andrew DM, Smith Kenny, Ferrer i Cancho Ramon., editors. The Evolution of Language: Proceedings of the 7th International Conference on the Evolution of Language. Singapore: World Scientific Press; 2008. pp. 10–17. [Google Scholar]
- Bates E, D’amico S, Jacobsen T, Szekely A, Andonova E, Devescovi A, Herron D, Lu CC, Pechmann T, Pleh C, Wicha N, Federmeier K, Gerdjikova I, Gutierrez G, Hung D, Hsu J, Iyer G, Kohnert K, Mehotcheva T, Orozco-Figueroa A, Tzeng A, Tzeng O. Timed picture naming in seven languages. Psychon. Bull. Rev. 2003;10:344–380. doi: 10.3758/bf03196494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Ryan L, Bever TG. Role of the striatum in language: Syntactic and conceptual sequencing. Brain Lang. 2011 doi: 10.1016/j.bandl.2011.11.005. in press. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benjamin M, Levy I. Role of the basal ganglia in language and semantics: Supporting cast. In: Hart J, Kraut MA, editors. Neural basis of semantic memory. Cambridge: Cambridge University Press; 2007. pp. 219–243. [Google Scholar]
- Crosson B, Haaland KY. Subcortical functions in cognition: Toward a consensus. J Int Neuropsychol Soc. 2003;9:1027–1030. [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Bacon Moore A, Wierenga CE, Gopinath K, Soltysik D, Bauer RM, Auerbach EJ, Gokcay D, Leonard CM, Briggsi RW. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. J Int Neuropsychol Soc. 2003;9:1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Crosson C, Sadek JR, Maron L, Gökcay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW. Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word Generation. Journal of Cognitive Neuroscience. 2001;13(2):272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dick AS, Solodkin A, Small SL. Neural development of networks for audiovisual speech comprehension. Brain Lang. 2010;114(2):101–114. doi: 10.1016/j.bandl.2009.08.005. [Comparative Study Research Support, N.I.H, Extramural]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage. 2003;20(Suppl. 1):S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What’s in control of language? Nat Neurosci. 2006;9:991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA, Werheid K, Hein G, von Cramon DY. Syntactic Comprehension in Parkinson’s Disease: Investigating Early Automatic and Late Integrational Processes Using Event-Related Brain Potentials. Neuropsychology. 2003;17(1):133–142. [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gil-Robles S, Gatignol P, Capelle L, Mitchell M-C, Duffau H. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76:940–946. doi: 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco VL, Tremblay P, Pike B. Imaging speech production using fMRI. Neuroimage. 2005;26(1):294–301. doi: 10.1016/j.neuroimage.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage. 2008;39(4):2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Ketteler S. Is schizophrenia “the price that Homo sapiens pays for language”? Subcortical language processing as the missing link between evolution and language disorder in psychosis – A neurolinguistic approach. Journal of Neurolinguistics. 2010;23(4):342–363. [Google Scholar]
- Lehtonen M, Laine M, Niemi J, Thomson T, Vorobyev VA, Hughdal K. Brain correlates of sentence translation in Finnish–Norwegian bilinguals. Neuroreport. 2005;16:607–610. doi: 10.1097/00001756-200504250-00018. [DOI] [PubMed] [Google Scholar]
- Lieberman P. Human Language and Our Reptilian Brain: The Subcortical Bases of Speech, Syntax, and Thought. Perspectives in Biology and Medicine. 2001;44(1):32–51. doi: 10.1353/pbm.2001.0011. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain ResRev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nichols T, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Pickering MJ, Ferreira VS. Structural Priming: A Critical Review Psychol Bull. 2008;134(3):427–459. doi: 10.1037/0033-2909.134.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R. A functional imaging study of translation and language switching. Brain. 1999;122:2221–2235. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Inhibitory control of competing motor memories. Exp Brain Res. 1999;126:235–251. doi: 10.1007/s002210050733. [DOI] [PubMed] [Google Scholar]
- Szekely A, D’amico S, Devescovi A, Federmeier K, Herron D, Iyer G, Jacobsen T, Bates E. Timed picture naming: extended norms and validation against previous studies. Behav. Res. MethodsInstrum. Comput. 2003;35:621–633. doi: 10.3758/bf03195542. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS) Brain Research. 2009a;1268:112–124. doi: 10.1016/j.brainres.2009.02.076. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Gracco VL. On the selection of words and oral motor responses: evidence of a response-independent fronto-parietal network. Cortex. 2009b;46(1):15–28. doi: 10.1016/j.cortex.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Shiller DM, Gracco VL. On the time-course and frequency selectivity of the EEG for different modes of response selection: evidence from speech production and keyboard pressing. Clin Neurophysiol. 2008;119(1):88–99. doi: 10.1016/j.clinph.2007.09.063. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Small SL. From language comprehension to action understanding and back again. Cereb Cortex. 2011a;21:1166–1177. doi: 10.1093/cercor/bhq189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Small SL. Motor response selection in overt sentence production: a functional MRI study. Frontiers in Language Sciences. 2011b doi: 10.3389/fpsyg.2011.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heuven WJB, Schriefers H, Dijkstra T, Hagoort P. Language conflict in the bilingual brain. Cereb Cortex. 2008;18:2706–2718. doi: 10.1093/cercor/bhn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Kanwisher N. Begging the question: the non-independence error in fMRI data analysisin. In: Hanson SB, Bunzl M, editors. Foundational Issues of Human Brain Mapping. Cambridge: MIT Press; 2009. pp. 71–92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.