Abstract
This study was conducted to determine whether CD4 T cell responses to citrullinated fibrinogen occur in patients with rheumatoid arthritis (RA), especially in HLA-DR4-positive subjects. Whole peripheral blood mononuclear cells (PBMCs) of RA patients and control subjects were stimulated with citrullinated fibrinogen peptides, and T-cell production of proliferation and proinflammatory cytokines, such as interferon-γ(IFN-γ) and interleukin-17A (IL-17A), were measured. In addition, CD4 T cells from RA patients were stimulated with the citrullinated fibrinogen peptide, Fib-α R84Cit, identified as a DRB1*0401-restricted T cell epitope in HLA-DR4 transgenic mice, and the degree of T cell activation was examined similarly. No proliferative responses to the citrullinated fibrinogen peptides were observed in whole PBMCs or CD4 T cells from RA patients. Furthermore, no increased production of IFN-γ or IL-17A was found in whole PBMCs or CD4 T cells stimulated with the citrullinated fibrinogen peptides, although these cells responded to recall antigen, a mixture of tetanus toxoid, purified protein derivative (PPD) from Mycobacterium tuberculosis, and Candida albicans. The results of this study indicate that anti-citrulline immunity in RA patients may be mediated by fibrinogen because there is no evidence of CD4 T cell-mediated immune responses to citrullinated fibrinogen peptides.
Keywords: Rheumatoid arthritis (RA), Citrullinated fibrinogen (cFBG), CD4 T cell, HLA-DR4, Interferon-γ(IFN-γ), Interleukin-17A (IL-17A)
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory polyarthritis characterized by cartilage destruction and bone erosion. Although RA has long been considered an autoimmune disease, there is no consensus regarding critical autoantigen involved in the pathogenesis of the disease (1,2). Recently, there is increasing evidence to support that immunity to citrullinated protein i.e., to the peptide post-translationally modified by the conversion of arginine to citrulline, is highly specific and even pathogenic for RA (3,4). Anticitrullinated protein antibodies (ACPA), such as anti-cyclic citrullinated peptide (CCP) antibodies, are useful serological markers for the diagnosis and prediction of development of RA (1,2). Recent studies have shown that the presence of anti-CCP antibodies correlates with the presence of specific human leukocyte antigen (HLA)-DRB1 alleles, known as "shared epitope" genes (3,5). Citrullinated peptides can bind with a higher affinity to major histocompatibility complex (MHC) containing the shared epitope and thereby lead to activation of CD4 T cells (6). This may provide a link between immune responses to citrullinated protein and CD4 T cells in the context of certain HLA molecules.
Several proteins, such as type II collagen, proteoglycan and fibrinogen, have been proposed as potential auto-antigens in RA model mice and RA patients (7). Fibrinogen, which has been shown to be citrullinated in synovial tissues of inflamed joints, is one of the most extensively studied auto-antigens in RA. A recent study has reported that citrullinated fibrinogen (cFBG) was detected in synovial fluids and may be a potential auto-antigen for ACPA in patients with RA (8). Indeed, experimental arthritis can be induced in HLA-DR4-IE transgenic mice which carry the RA-associated shared epitope, such as HLA-DRB1*0401, after immunization with cFBG (9). Furthermore, this study demonstrated the HLA-DR4-restricted T cell epitope within cFBG. Although there is relatively strong evidence to support that cFBG acts as an auto-antigen in RA-induced animals, CD4 T cell-mediated immune responses to cFBG have not yet been fully investigated in RA patients.
In this study, we investigated the possibility that cFBG peptides could be autoantigens in RA patients, and especially cFBG peptides (Fib-α R84Cit) within cFBG targeted by T cells in HLA-DR4-IE transgenic mice could be CD4 T-cell epitopes restricted to the susceptible HLA-DR allele in RA patients.
MATERIALS AND METHODS
Study subjects
All RA patients (n=51) who fulfilled the 1987 revised criteria for the classification of RA proposed by the American College of Rheumatology (formerly, the American Rheumatism Association) (10) were included in this study. The control group (n=17) consisted of patients with osteoarthritis, Behcet's disease, or other inflammatory diseases (e.g., fibromyalgia and systemic lupus erythematosus). Demographic and clinical characteristics of the subjects are summarized in Table I. The study was approved by the Institutional Review Board of Eulji University Hospital and Kangnam St. Mary's Hospital, and written informed consent was obtained from each participant (approval No. 08-11).
Table I.
The demographic and clinical characteristics of the study population
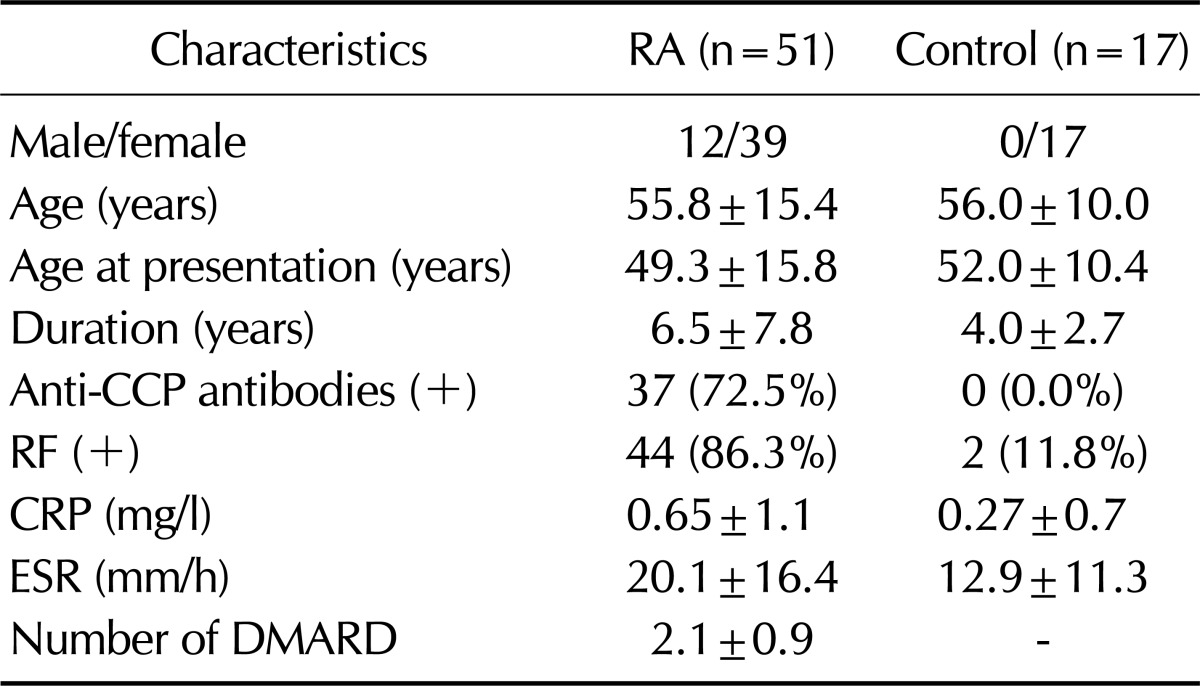
Data are mean±SD. Anti-CCP antibodies (+): (>5 U/ml), RF (+): (>18 IU/ml). CCP, cyclic citrullinated peptide; CRP, C reactive protein; ESR, Erythrocyte sedimentation rate.
Anti-CCP antibody and HLA-DRB1 genotyping
Anti-CCP antibody levels were assessed using the DIASTAT anti-CCP kit (MBL Co., Nagoya, Japan). According to the manufacturer's instruction, anti-CCP antibody was considered positive when its absorbance was higher than the cutoff value (5 U/ml). HLA-DRB1 genotyping was performed by the PCR-SSP (sequence specific primer) method (11). Each tube contained a primer mix consisting of allele- or group-specific primer pairs as well as a positive control primer that matched the non-allelic sequences. Specific amplification of the HLA-DRB1 gene was performed using 20 specific pairs of primers for HLA-DRB1.
Peptides
Citrullinated and non-citrullinated fibrinogen peptides were chemically synthesized (Peptron, Daejeon, Korea). High-performance liquid chromatography showed the purity of peptides is over 90%. Peptide sequences are shown in Table II, and the underlined arginine residue was substituted with citrulline.
Table II.
Sequences of fibrinogen peptides used in the study
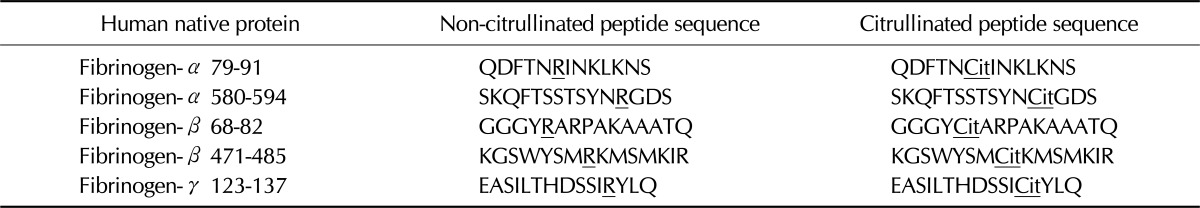
Arginine (R) converted to citrulline (Cit) was underlined.
Cell purification and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples by density-gradient centrifugation using lymphocyte separation medium (PAA Laboratories, Linz, Austria). PBMCs (1×106) were stimulated with either non-citrullinated or cFBG peptides (1, 10, and 30µg/ml for each) in RPMI 1640 medium supplemented with penicillin G (50 U/ml), streptomycin (50µg/ml), L-glutamine (2 mM), and 10% fetal bovine serum (WelGene, Seoul, Korea) at 37℃ in a humidified atmosphere of 5% CO2. For recall antigen responses, tetanus toxoid (5µg/ml; Calbiochem, San Diego, CA, USA), purified protein derivative (PPD) from Mycobacterium tuberculosis (5µg/ml; Statens Serum Institute, Copenhagen, Denmark), and Candida albicans (10µg/ml; Greer Laboratories, Lenoir, NC, USA) were used.
For Fib-α R84Cit stimulation, CD4 T cells and CD14-positive monocytes were positively isolated from PBMCs using magnetic cell sorting (autoMACSpro, Miltenyi Biotec Inc., Germany). CD4 T cells (5×105) were stimulated in the presence or absence of 50µg/ml non-citrullinated or cFBG peptides. CD14-positive cells (5×104) used as antigen-presenting cells (APCs) were irradiated in a dose of 3,000 rads.
In vitro proliferation and cytokine measurement
For cell proliferation, carboxyfluorescein succinimidyl ester (CFSE) labeling was performed (Invitrogen, Carlsbad, CA, USA). Briefly, whole PBMCs were labeled with 3µM CFSE before stimulation. After 4 days of culture, the levels of CFSE dilution indicating cell proliferation were measured within CD4 T cells by FACS analysis.
For Fib-α R84Cit peptide stimulation, cell pellets were collected, and proliferation was determined by using the BrdU proliferation ELISA assay kit (Roche diagnostics, Germany) according to the manufacturer's instruction. Briefly, the BrdU labeling solution was added to the wells and incubated for additional 24 hours. The amount of BrdU uptake was subsequently determined by fixation and incubation with an anti-BrdU antibody conjugated with peroxidase (POD), followed by colorimetric detection.
Supernatants from the wells were removed, and the levels of IFN-γ and IL-17A were measured using a standard sandwich ELISA according to the manufacturer's instructions (eBioscience, San Diego, CA, USA).
Statistical analysis
Statistical analysis (unpaired t test) was performed using GraphPad Prism Software V5.0 (GraphPad Software, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
Effect of cFBG peptide stimulation on PBMCs from RA patients
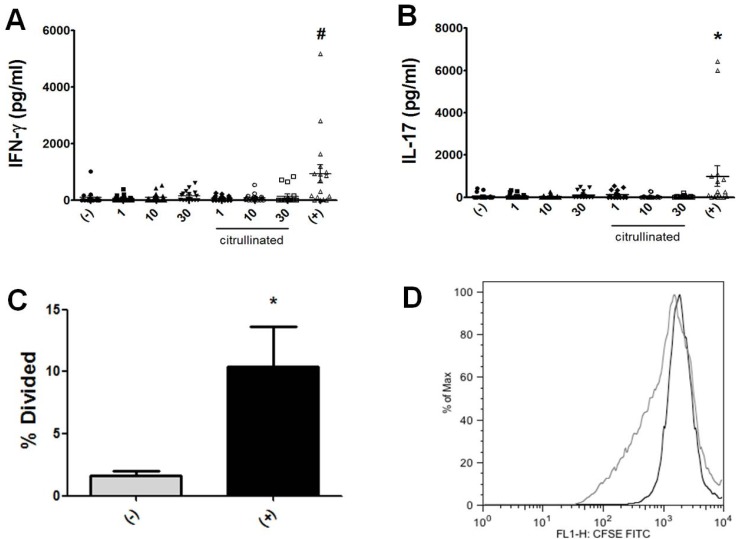
To determine whether antigen-specific T cell responses can be elicited by cFBG peptides, we first searched HLA DRB1*0401-restricted peptides from α, β and γ chains of fibrinogen by using a web- based program (Immune epitope database, National Institute of Allergy and Infectious Diseases [NIAID]). Peptides were selected according to their predicted affinity for DRB1*0401 as in a previous study (12). Because citrulline (Cit) is not accounted for in the predictive algorithm, the value of glutamine (Q) was substituted for arginine (R) when identifying T cell epitope candidates (Q has a same terminal side chain group with Cit), and each epitope sequences are shown in Table II. For cFBG peptide-specific stimulation, we combined 5 high-peptides at various concentrations and added 1 million PBMCs isolated from RA patients. After 4 days of culture, the levels of IFN-γ and IL-17 were measured by ELISA, which were not enhanced (Fig. 1A and B). For T-cell proliferation, we utilized CFSE labeling in which whole PBMCs were stained with CFSE and the degree of CFSE, dilution indicating cell proliferation was measured with FACS within the CD4 T-cell population. Similar to cytokine production, we could not detect T-cell proliferation stimulated with cFBG peptides (Fig. 1C and D). To check for T-cell activation, we performed T-cell proliferation and cytokine production assay in response to recall antigen which is a combination of tetanus toxoid, M. tuberculosis-PPD, and C. albicans. We detected CD4 T cells from RA patients that responded well to a panel of recall antigen (Fig. 1). For example, T cells responded to the recall antigen to produce IFN-γ (Fig. 1A), IL-17 (Fig. 1B) and to proliferate well (Fig. 1C and D). Therefore, these findings indicated that CD4 T cells from RA patients may be not specific for cFBG peptides, although they respond well to the recall antigen.
Figure 1.
Immune responses of PBMCs from RA patients to cFBG peptides. PBMCs from RA patients (n=17) were stimulated with either non-citrullinated or citrullinated fibrinogen (cFBG) peptides at indicated concentrations (µg/ml). IFN-γ secretion was quantified from culture supernatant of peptide-stimulated PBMCs (A). Similarly, IL-17 secretion was quantified from the supernatant (B). Cell proliferation was evaluated with a dilution of CFSE (C). Representative FACS results are shown (D) (un-stimulation in black [% division: 0.31] and recall antigen stimulation in gray line [% division: 16.5]). #p<0.01 compared with non-stimulation (-). *p<0.05 compared with non-stimulation. (+) represents a mixture of recall antigens.
Role of DRB1*0401-restricted cFBG peptide in stimulation of CD4 T cells
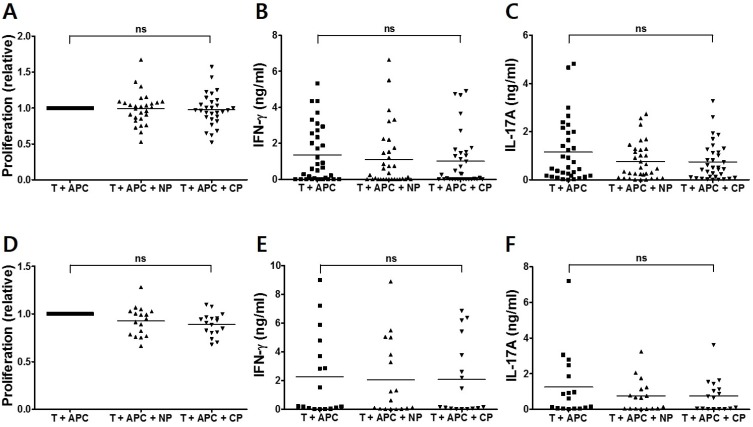
Next, we investigated the possibility that a specific cFBG peptide, Fib-α R84Cit, which is identified as a DRB1*0401-restricted T cell epitope in DR4 transgenic mice (9), can be recognized by CD4 T cells from RA patients. Thus, CD4 T cells from RA patients were incubated with either a non-citrullinated (Fib-α 79-91) or a cFBF peptide (Fib-α R84Cit), in the presence of CD14-positive monocytes as APCs. Significant proliferative responses to the cFBG peptide were not observed after in vitro stimulation with both Fib-α 79-91 and Fib-α R84Cit (Fig. 2A). We also examined cytokine production by stimulation with cFBG peptide. Neither IFN-γ nor IL-17A induction was observed in T cells stimulated with Fib-α R84Cit peptide (Fig. 2B and C). Similar to the results from RA patients, proliferation and cytokine production were not observed from CD4 T cells from control subjects (Fig. 2D~F). These results indicate that CD4 T cells may not respond to Fib-α R84Cit peptide regardless of the presence of RA.
Figure 2.
CD4 T-cell responses of PBMCs from RA patients and control subjects to Fib-α R84Cit peptide. CD4 T cells from RA patients (n=34) were stimulated with non-citrullinated (NP) and citrullinated (CP) fibrinogen peptides in the presence of APCs. Cell proliferation was quantified by the incorporation of BrdU. The degree of cell proliferation was compared between stimulation and non-stimulation, and values represent the degree of well proliferation in individual samples (A). IFN-γ (B) and IL-17A (C) secretion was quantified from each peptide-stimulated culture supernatant by ELISA. Similarly, T-cell proliferation (D), and IFN-γ (E) and IL-17A (F) production of CD4 T cells from control subjects (n=17) are shown. ns=not significant.
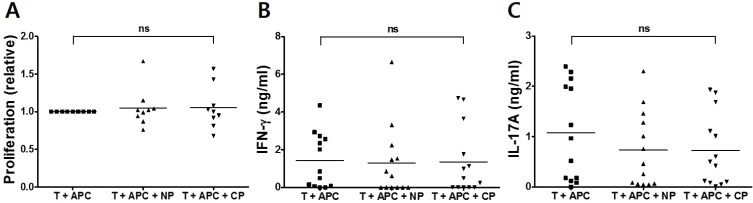
Anti-CCP antibodies are useful serological markers for the diagnosis of RA, and immune responses to citrullinated protein could be confined to RA patients who have a high titer of anti-CCP antibodies (3). Because we also used the DRB1*0401-restricted T-cell epitope within the cFBG to assess CD4 T cell-mediated immune responses, we assessed T-cell responses to anti-CCP antibody in HLA-DR4-positive patients. However, neither proliferative response nor IFN-γ and IL-17A secretion was observed when CD4 T cells were stimulated with the citrullinated peptide even in anti-CCP antibody-positive RA patients carrying HLA-DRB1*04 (Fig. 3). Again, to ensure that failure to detect significant responses in our study was not attributed to improper assay, we examined T-cell proliferation and cytokine production in response to the recall antigen. We were able to detect CD4 T cells from RA patients and healthy controls that responded well to the panel of recall antigens, including tetanus toxoid, M. tuberculosis-PPD, and C. albicans (data not shown). Therefore, these results demonstrated that significant proliferation and cytokine responses may not be induced by cFBG peptide stimulation of CD4 T cells from RA patients, although these CD4 T cells respond well to common recall antigens.
Figure 3.
CD4 T-cell responses of PBMCs from anti-CCP Ab/HLA-DRB1*04-positive RA patients to Fib-α R84Cit peptide. CD4 T cells from anti-CCP Ab/HLA-DRB1*04-positive RA patients (n=13) were stimulated with non-citrullinated (NP) and citrullinated (CP) fibrinogen peptides in the presence of APCs. Cell proliferation was quantified by the incorporation of BrdU (A). IFN-γ (B) and IL-17A (C) secretion was quantified from each peptide-stimulated culture supernatant by ELISA. ns=not significant.
DISCUSSION
In this study, we determined whether T-cell epitope within cFBG could act as an auto-antigen in RA. We detected neither T-cell proliferative response nor Th1 (IFN-γ) response by stimulation with cFBG peptides. Furthermore, Th17 (IL-17A) response against cFBG peptides, which may be more intimately associated with RA, was not enhanced in RA patients, either.
Genetic studies have shown that certain HLA-class II alleles, "shared epitopes" are the most important risk factor for RA (3). In addition, it has been shown that HLA-DRB1 shared epitopes only confer susceptibility to anti-CCP and antibody-positive RA and that they contribute to the production of ACPA (5,13). However, we failed to observe any significant responses in CD4 T cells from anti-CCP and HLA-DR4-positive RA patients.
It is not clear why our findings are different from those of an HLA-DR4 transgenic mouse RA model (9). The diversity and/or heterogeneity of human RA is a good possibility. Thus, it seems likely that multiple peptides, instead of a single peptide, successfully activate multiclonic cFBG-specific T cells. Indeed, fibrinogen has several sites that can be citrullinated (14). However, in our study, multiple cFBG peptide stimulation clearly showed that T cells were not activated. Furthermore, it should be pointed out that immunization with cFBG protein, which is citrullinated by peptidylarginine deaminase, induces arthritis in only less than half of cases even in HLA-DR4 transgenic mice (9). It has also been shown that immunization with Fib-α R84Cit peptide itself does not induce arthritis even if T-cell responses to Fib-α R84Cit are evident. In addition, recent studies have demonstrated that T cell-mediated immune responses are detected in response to different citrullinated proteins, such as citrullinated vimentin and/or citrullinated aggrecan in RA patients (15,16). Those studies also stated that the degree of immune responses to citrullinated proteins is not significant.
The difference in the T-cell receptor (TcR) repertoire between species is another possibility. A previous study has documented that cFBG peptide or Fib-α R84Cit is identified as DRB1*0401- restricted T-cell epitope in DR4 transgenic mice (9). However, it is apparent that the TcR repertoire of RA patients differs from that of HLA-DR4 transgenic mice and that T cells from RA patients may respond to citrullinated peptides other than Fib-α R84Cit.
In summary, our study demonstrates that T-cell responses to cFBG peptides may not be present in RA patients, suggesting that anti-citrulline immunity in RA patients is unlikely to be mediated by citrullinated fibrinogen.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, the Republic of Korea (A101151), the Korea Health 21 R&D Project, by the Ministry of Health and Welfare (01-PJ3-PG6-01GN09-003), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2010714).
Abbreviations
- RA
rheumatoid arthritis
- CD
cluster of differentiation
- PPD
purified protein derivative
- ACPA
anti-citrullinated protein antibodies
- CCP
cyclic citrullinated peptide
- cFBG
citrullinated fibrinogen
- Cit
citrulline
- HLA
human leukocyte antigen
- PBMC
peripheral blood mononuclear cell
- CFSE
carboxyfluorescein succinimidyl ester
Footnotes
The authors have no financial conflict of interest.
References
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 2.Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol. 2009;4:417–434. doi: 10.1146/annurev.pathol.4.110807.092254. [DOI] [PubMed] [Google Scholar]
- 3.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Holers VM. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–1121. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 6.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 7.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmström V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 8.Takizawa Y, Suzuki A, Sawada T, Ohsaka M, Inoue T, Yamada R, Yamamoto K. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann Rheum Dis. 2006;65:1013–1020. doi: 10.1136/ard.2005.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, Lee DM, Hueber W, Robinson WH, Cairns E. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205:967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Jordan F, McWhinnie AJ, Turner S, Gavira N, Calvert AA, Cleaver SA, Holman RH, Goldman JM, Madrigal JA. Comparison of HLA-DRB1 typing by DNA-RFLP, PCR-SSO and PCR-SSP methods and their application in providing matched unrelated donors for bone marrow transplantation. Tissue Antigens. 1995;45:103–110. doi: 10.1111/j.1399-0039.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 12.Hammer J, Bono E, Gallazzi F, Belunis C, Nagy Z, Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J Exp Med. 1994;180:2353–2358. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizinga TW, Amos CI, van der Helm-van Mil AH, Chen W, van Gaalen FA, Jawaheer D, Schreuder GM, Wener M, Breedveld FC, Ahmad N, Lum RF, de Vries RR, Gregersen PK, Toes RE, Criswell LA. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum. 2005;52:3433–3438. doi: 10.1002/art.21385. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama-Hamada M, Suzuki A, Kubota K, Takazawa T, Ohsaka M, Kawaida R, Ono M, Kasuya A, Furukawa H, Yamada R, Yamamoto K. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun. 2005;327:192–200. doi: 10.1016/j.bbrc.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 15.Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, Huizinga TW, de Vries RR, Toes RE, Ioan-Facsinay A. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:117–125. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 16.von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:143–149. doi: 10.1002/art.25064. [DOI] [PubMed] [Google Scholar]