Abstract
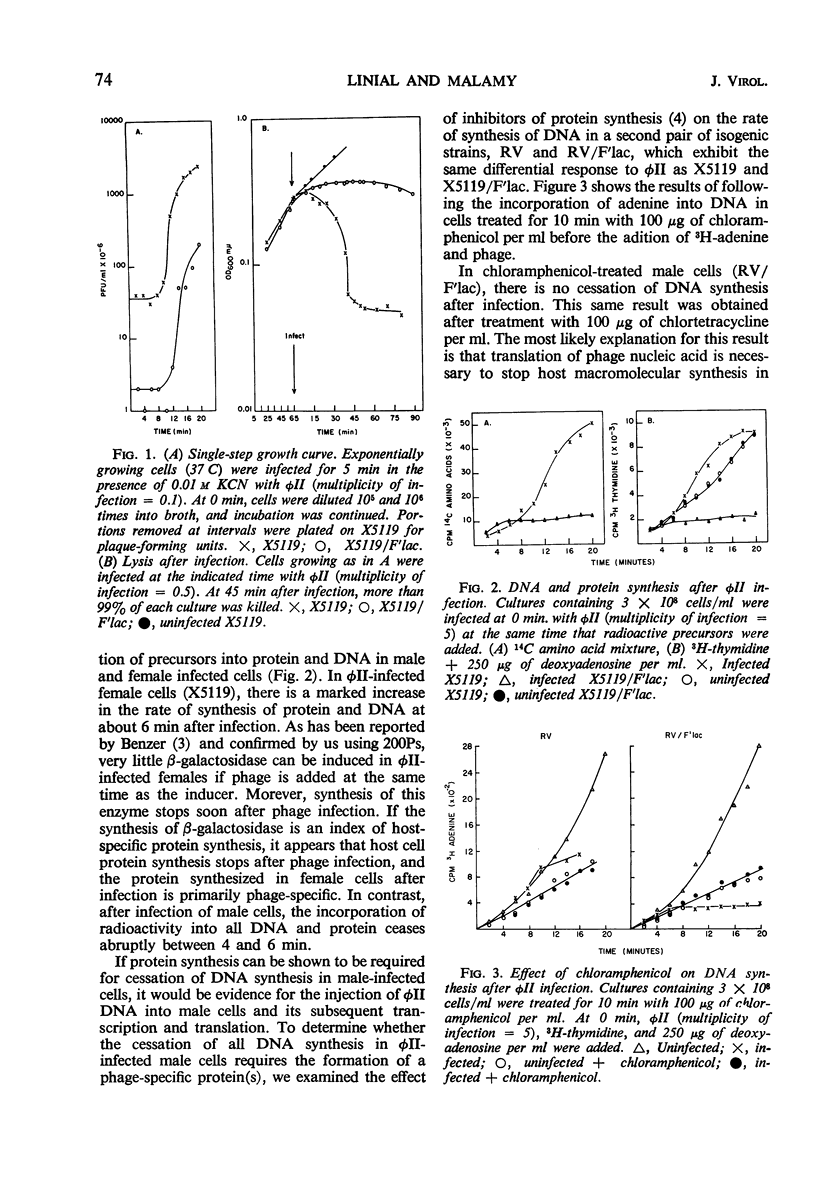
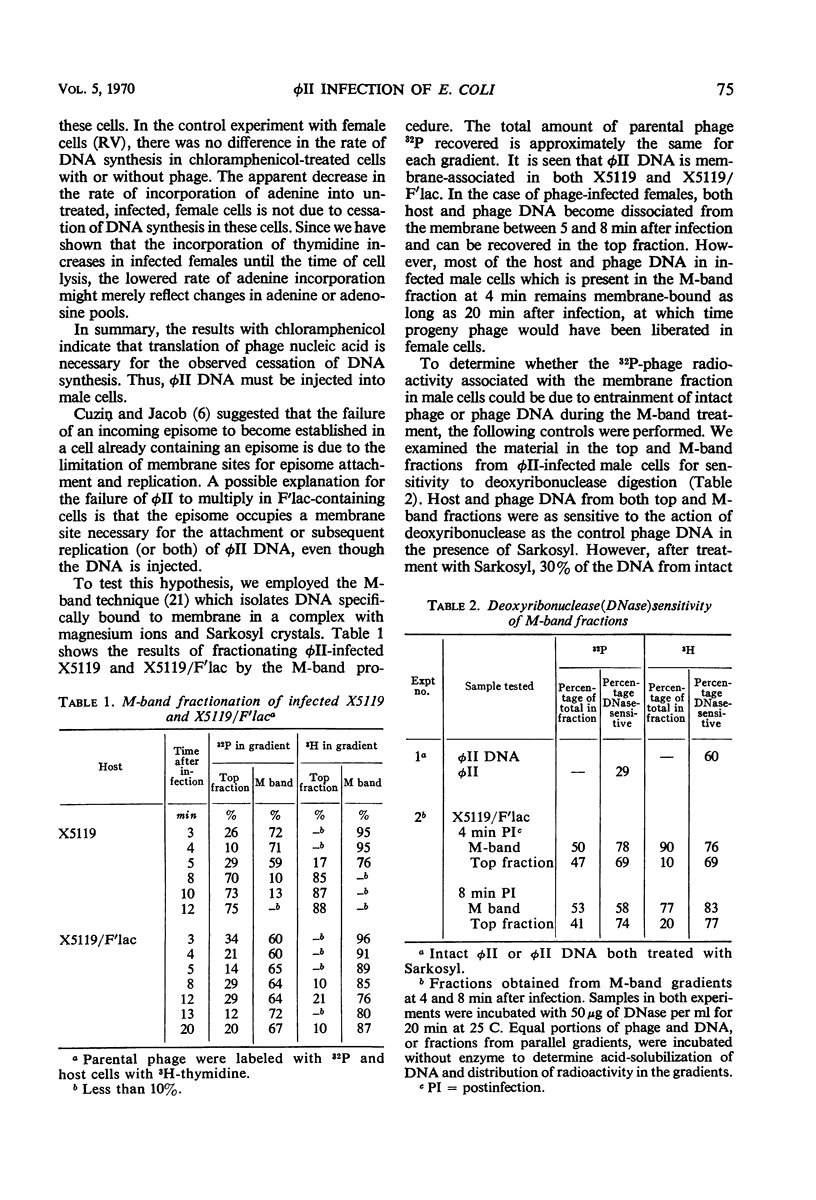
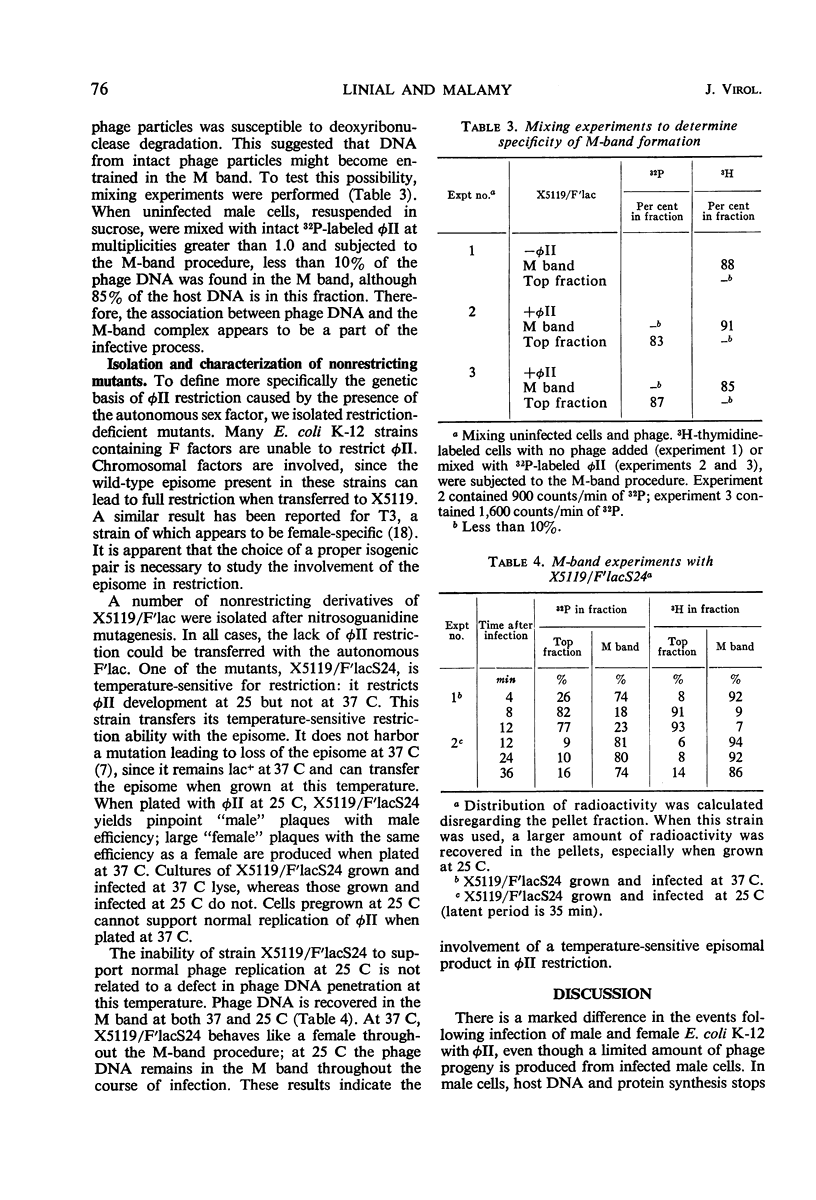
We studied the course of infection of the female-specific bacteriophage φII in male and female cells isogenic except for the presence of the substituted sex factor, F′lac. Both male and female cells are killed by φII; however, only limited phage replication occurs in male cells. Host macromolecular synthesis stops abruptly at 4 to 6 min after infection of male cells, and synthesis of phage components cannot be detected. Experiments with chloramphenicol indicate that phage deoxyribonucleic acid (DNA) penetrates into male cells, since protein synthesis after infection is required to stop synthesis of DNA in males. Phage DNA becomes membrane-associated in both female and male cells. In male cells, parental phage DNA does not dissociate from the membrane during the latent period as is the case with females, indicating a block in phage DNA replication. Isolation of nonrestricting F′lac mutations indicates involvement of a specific episome product in φII restriction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZER S. Induced synthesis of enzymes in bacteria analyzed at the cellular level. Biochim Biophys Acta. 1953 Jul;11(3):383–395. doi: 10.1016/0006-3002(53)90057-2. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Existence chez Escherichia coli d'une unité génétique de ségrétion formée de différents réplicons. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 17;260(20):5411–5414. [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- Cuzin F. Un bactériophage spécifique du type sexuel F- d'Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jun 14;260(24):6482–6485. [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. Assay for the Killing Properties of T2 Bacteriophage and Their "Ghosts". J Bacteriol. 1965 Sep;90(3):724–728. doi: 10.1128/jb.90.3.724-728.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart C. F., Tremblay G. Y., Daniels M. J., Schaechter M. DNA replication studied by a new method for the isolation of cell membrane-DNA complexes. Cold Spring Harb Symp Quant Biol. 1968;33:707–710. doi: 10.1101/sqb.1968.033.01.079. [DOI] [PubMed] [Google Scholar]
- FRANKEL F. R. An unusual DNA extracted from bacteria infected with phage T2. Proc Natl Acad Sci U S A. 1963 Mar 15;49:366–372. doi: 10.1073/pnas.49.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L. Comparison of the action of colicins E1 and K on Escherichia coli with the effects of abortive infection by virulent bacteriophages. J Bacteriol. 1969 Jan;97(1):78–82. doi: 10.1128/jb.97.1.78-82.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Smith H. S., Miovic M., Pylkas L. Effect of prophage W on the propagation of bacteriophages T2 and T4. J Virol. 1968 Nov;2(11):1339–1345. doi: 10.1128/jvi.2.11.1339-1345.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I. Abortive infection of Escherichia coli strain W by T2 bacteriophage. J Mol Biol. 1968 Oct 14;37(1):131–149. doi: 10.1016/0022-2836(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I., Pylkas L., Lederberg S. Abortive infection of Shigella dysenteriae P2 by T2 bacteriophage. J Virol. 1969 Aug;4(2):162–168. doi: 10.1128/jvi.4.2.162-168.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- WATANABE T., OKADA M. NEW TYPE OF SEX FACTOR-SPECIFIC BACTERIOPHAGE OF ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:727–736. doi: 10.1128/jb.87.3.727-736.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



