Abstract
Hepatocellular carcinoma (HCC) cells often have hepatitis B virus (HBV)-DNA integration and can be targeted by HBV-specific T cells. The use of viral vectors to introduce exogenous HBV-specific T-cell receptors (TCR) on T cells to redirect their specificity is complex and expensive to implement in clinical trials. Moreover, it raises safety concerns related to insertional mutagenesis and potential toxicity of long-lived HBV-specific T cells in patients with persistent infection. To develop a more practical and safer approach to cell therapy of HCC, we used electroporation of mRNA encoding anti-HBV TCR. Approximately 80% of CD8+ T cells expressed functional HBV TCR 24 hours postelectroporation, an expression efficiency much higher than that obtained by retroviral transduction (~18%). Antigen-specific cytokine production of electroporated T cells was efficient within 72-hour period, after which the redirected T cells lost their HBV-specific function. Despite this transient functionality, the TCR-electroporated T cells efficiently prevented tumor seeding and suppressed the growth of established tumors in a xenograft model of HCC. Finally, we established a method for large-scale TCR mRNA electroporation that yielded large numbers of highly functional clinical-grade anti-HBV T cells. This method represents a practical approach to cell therapy of HCC and its inherently self-limiting toxicity suggests potential for application in other HBV-related pathologies.
Keywords: hepatitis B virus, hepatocellular carcinoma, mRNA electroporation, TCR gene therapy
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide.1 In Asian countries, where chronic hepatitis B virus (HBV) infection is frequent, HBV is a major etiologic agent, accounting for at least 80% of HCC.2 HBV predisposes to the development of HCC by causing liver chronic inflammation but also by integrating into the genome of hepatocytes; a high frequency of HBV integrations has been observed in HBV-related HCC tumors3 resulting in the expression of HBV antigens on tumor cells.4,5
The therapeutic options for HCC are limited, particularly in patients with advanced disease, who almost invariably succumb to their disease.6 Tumors expressing viral peptides are attractive targets for cell therapies because their expression is limited to infected cells. In principle, it should be possible to isolate T lymphocytes with antiviral specificities from patients and expand them ex vivo before reinfusion,7,8 but this process is slow, laborious, and often unsuccessful. Moreover, we found that patients with HBV-related HCC, like those with only chronic hepatitis B, have a profound defect of HBV-specific T cells.9
Genetic modification of peripheral blood T cells with T-cell receptors (TCRs) can rapidly endow T cells with a defined antigen specificity10,11 and represents an attractive approach to cell therapy of tumors expressing viral peptides, like HBV-related HCC. Indeed, we recently demonstrated that T cells with redirected specificity toward HBV envelope antigens can recognize and lyse HCC lines with natural HBV-DNA integration.12 However, a specific concern regarding this approach is that HBV antigen expression is not exclusive to transformed hepatocytes; nontumor hepatocytes might also express HBV antigens and adoptive T-cell therapy could trigger severe liver damage.13,14,15 The traditional method to genetically engineer T cells, i.e., viral transduction, may compound this risk by producing T cells which permanently express anti-HBV specificity and may be difficult to eliminate once infused into patients. Moreover, viral vectors carry the risk of oncogene activation.16,17,18 Finally, the costs and regulatory requirements of implementing viral transduction in clinical trials further add to the complexity of implementing this form of cell therapy.
To overcome the limitations of current approaches to the cell therapy of HBV-related HCC, we determined whether effective anti-HBV T cells, transiently expressing anti-HBV TCR, could be generated by mRNA electroporation. We assessed receptor expression and functionality in T cells engineered by this method and tested their efficacy in killing HCC-like cells in vitro and in an animal model in relation to retrovirally transduced cells. Finally, we validated the suitability of a large-scale clinical-grade mRNA electroporation method to rapidly generate large numbers of anti-HBV redirected T cells for clinical infusion.
Results
Expression of TCR by mRNA electroporation
We prepared mRNA encoding the alpha and beta chains of the HBV s183-TCR and used electroporation to introduce it into activated T cells from five healthy donors. Expression of TCR was measured by pentamer staining and flow cytometry. As early as 6 hours after electroporation, 42 ± 23% of CD8+ T cells expressed the s183-TCR (Figure 1a,b). The highest TCR expression was measured at 24 hours postelectroporation, where 64–95% (mean 80.0%) of CD8+ T cells expressed the TCR (Figure 1a,b). TCR expression then gradually decreased and was not detectable after 72 hours (Figure 1b). The level of expression at 24 hours was much higher than that typically achieved by retroviral transduction (12–25% (mean 17.8%); n = 3). Mock electroporated activated T cells did not show any expression of TCR and as a negative control for functional assays, an irrelevant CMV pp65-TCR was also expressed on activated T cells by mRNA electroporation (Figure 1a).
Figure 1.

High level of TCR expression and polyfunctionality of mRNA electroporated T cells. (a) Dot plots from a representative HLA-A2-HBs183-191 pentamer staining in HBV s183-TCR mRNA electroporated T cells at 6, 24, and 72 hours postelectroporation and retrovirally transduced T cells at 72 hours. T cells that were mock-electroporated or electroporated with an irrelevant CMV pp65-TCR mRNA and mock-transduced served as negative controls. The percentages of pentamer+ cells out of CD8+ or CD8− cells are indicated. (b) Expression of TCR on electroporated CD8+ T cells and frequency of IFN-γ-producing CD8+ T cells after overnight coculture with s183 peptide-loaded T2 cells were determined at several time points as indicated. Results expressed as mean + SD (n = 5). (c) Dot plots from a representative activated T cells electroporated or retrovirally transduced with s183-TCR, after overnight coculture with s183 peptide-loaded T2 cells and stained for CD8 and IFN-γ (top row), TNF-α (middle row) and IL-2 (bottom row). Mock- and CMV pp65-TCR mRNA electroporated T cells cocultured with s183 peptide-loaded T2 cells served as negative controls. The percentages of cytokine-producing cells out of total T cells are indicated. (d) TNF-α and IL-2 production by IFN-γ+ T cells demonstrate polyfunctionality of electroporated T cells. (e) Cytokine co-expression subsets expressed as a percentage of total cytokine-producing electroporated or retrovirally transduced T cells. Mean for each group is shown. Single producers (blue slice), IFN-γ+, TNF-α+ or IL-2+; double producers (red slice), IFN-γ+ TNF-α+, IFN-γ+ IL-2+ or IL-2+ TNF-α+; triple producers (green slice), IFN-γ+ IL-2+ TNF-α+.
Comparison of signaling capacity, cytotoxicity, and phenotype of TCR expressed by electroporation or retroviral transduction
We tested electroporated T cells for their capacity to produce cytokines in response to s183-191 peptide-loaded T2 cells (a TAP-deficient human lymphoblastoid cell line) at regular intervals from 6 to 120 hours. The highest level of IFN-γ was produced at 24 hours postelectroporation, concomitant with the peak of s183-TCR expression (Figure 1b). At maximal TCR expression, not all pentamer+ CD8+ T cells produced IFN-γ (Figure 1b) in contrast to retrovirally transduced T cells where ≥98% of pentamer+ CD8+ T cells produced IFN-γ (Table 1). Importantly, while s183-TCR expression in electroporated T cells became undetectable after 72 hours, ~20% of CD8+ T cells still produced IFN-γ. Mock- or CMV pp65-TCR mRNA electroporated T cells did not produce any cytokines in response to s183-191 peptide-loaded T2 cells while the s183-TCR mRNA electroporated CD8 and CD4 T cells showed a level of polyfunctionality superior to the s183-TCR retrovirally transduced T cells and are able to efficiently produce IL-2 (Figure 1c,d, and Table 1). Stimulation of either s183-TCR electroporated or retrovirally transduced T cells with T2 cells loaded with irrelevant peptide did not produce any cytokines (not shown). About 36% of cytokine-producing electroporated T cells co-expressed all three cytokines (IFN-γ, TNF-α, and IL-2) in contrast to only 13% of cytokine-producing retrovirally transduced T cells (Figure 1e). Furthermore, the quantity of Th1 (IFN-γ, TNF-α, and IL-2), Th2 (IL-13), and IL-17 cytokines (with the exception of IL-4) that could be detected in supernatants of stimulated electroporated T cells was equal or higher than that produced by retrovirally transduced T cells (Supplementary Figure S1 online).
Table 1. Comparison of TCR expression and function of mRNA electroporated vs. retrovirally transduced T cells.

We then determined if the signaling capacity of TCR expressed by mRNA electroporation was similar to that of TCR expressed by retroviral transduction. Activated T cells from the same healthy donors were either electroporated or retrovirally transduced with s183-TCR and then tested for sensitivity to specific T-cell activation. We found that the functional avidity of electroporated and retrovirally transduced T cells was similar to the original HBV-specific CD8 T-cell clone from which the TCR was cloned, and activation could be observed at 100 fg/ml to 1 μg/ml (Figure 2a). Therefore, functional HBV-specific s183-TCR can be introduced with high efficiency into activated T cells by mRNA electroporation.
Figure 2.

Similar signaling capacity and cytotocixity of mRNA electroporated and retrovirally transduced T cells. (a) Sensitivity of T-cell activation using T cells produced by mRNA electroporation compared with retroviral transduction. Results are displayed as percentage of maximum IFN-γ response obtained from intracellular cytokine staining. (b) Dose dependent lysis of HepG2-env targets (solid symbols and line) or HepG2-core targets (open symbols and dotted line) by electroporated T cells (triangle) compared with retrovirally transduced T cells (circle). Results are displayed as mean of triplicate measurements + SD. (c) Expression of perforin (left panel) and granzyme (right panel) on a representative activated T cells electroporated (red histograms) or retrovirally transduced (blue histograms) with s183-TCR, after 5 hours coculture with peptide-loaded T2 cells. Coculture with unpulsed T2 cells served as negative control (gray histograms). MFI of perforin and granzyme are indicated. (d) mRNA electroporated T cells have TCM-like phenotype. Phenotype of total lymphocytes (left panel), CD8+ pentamer+ T cells (middle panel) and CD4+ Vb3+ T cells (right panel) in s183-TCR mRNA electroporated T cells (red shaded area) compared with retrovirally transduced T cells (blue shaded area). The percentages of CD45RA+/- and CD62L+/- cells within total lymphocytes or the gated CD8+ pentamer+ or CD4+ Vb3+ populations were determined by FACS. Cells were classified into different subsets: naive (CD45RA+CD62L+), TCM (CD45RA−CD62L+), TEM (CD45RA−CD62L−) and terminally differentiated EM (CD45RA+CD62L−). Results expressed as mean + SD (n = 3).
To test their cytotoxic ability, TCR-redirected T cells were cocultured with the HCC cell line HepG2, which constitutively expressed HBV surface antigen (HepG2-env) and luciferase or HBV core antigen (HepG2-core) expressing luciferase at various E:T ratios for 24 hours and cytotoxicity was measured by quantifying luminescence in remaining target cells after coculture with effectors (CD8+/pentamer+ cells). Coculture with HepG2-core cells triggered minimal lysis regardless of the transduction method used. In contrast, HepG2-env cells were specifically lysed by both types of engineered T cells (Figure 2b). In fact, the cytolytic activity of electroporated T cells was slightly higher in vitro than that of retrovirally transduced T cells, with maximum lysis at 1:5 E:T ratio. We then analyzed the expression of cytolytic mediators perforin and granzyme produced by the engineered T cells, and found no difference in perforin expression but a higher expression of granzyme in electroporated T cells (Figure 2c).
We also determined whether mRNA transfection affected T-cell immunophenotype. More than 80% of the electroporated lymphocytes from three healthy donors were central memory-like T cells (TCM), while retrovirally transduced lymphocytes comprised of 60% TCM and 34% effector memory (TEM) cells. Out of antigen-specific T cells, more than 80% of electroporated pentamer+ CD8 were TCM, 1.5-fold higher than that of retrovirally transduced T cells (Figure 2d). The phenotype of Vb3+ CD4+ T cells was similar with both transduction methods, with the majority being TCM cells.
TCR mRNA electroporated T cells have anti-HCC activity in tumor-bearing mice
To further test the antitumor effect of T cells redirected against HBV, we first established a mouse xenograft tumor model using HepG2 cells constitutively expressing HBV surface antigen and luciferase (HepG2-env) injected in the spleen of NOD-SCID-IL2RGnull mice. Tumor growth was monitored by bioluminescence imaging. In initial studies, we used retrovirally transduced cells to determine the number of TCR-expressing T cells required for effective antitumor activity and whether both CD4 and CD8 cells were needed, as previously reported.8,19,20,21 We found that 3 × 106 pentamer+ CD8 plus 3 × 105 pentamer+ CD4 was most effective and eliminated tumors in 4 days in all four mice tested (Figure 3a,b), whereas infusion of CD8 alone cleared tumor inefficiently: HCC-like cells were still present in the splenic pulp by histologic examination (Supplementary Figure S2a online). Infusion of CD4 cells alone led to only a slight retardation in tumor growth compared with control mice treated with mock-transduced T cells. Moreover, in mice that completely eliminated tumors, we also observed that antitumor T cells specifically trafficked to the tumor site that expressed the target antigen and effect tumor destruction (Figure 3c). This was also confirmed by histopathological analysis of hematoxylin and eosin stained spleen sections (Supplementary Figure S2b online).
Figure 3.

Efficient tumor clearance requires both CD8 cytotoxic and CD4 helper TCR-retrovirally transduced T cells. (a) One million HepG2-env tumor cells were inoculated by intrasplenic injection in NSG mice (n = 22). Ten days after tumor inoculation, mice were treated with 3 × 106 CD8 + 3 × 105 CD4 (n = 4), 3 × 106 CD8 (n = 4), 1.5 × 106 CD8 (n = 4), 1.5 × 106 CD4 (n = 3), or 3 × 105 CD4 (n = 4) s183-TCR transduced T cells injected i.v. Mice treated with 3 × 106 mock transduced T cells served as controls (n = 3). In all experiments, tumor size was monitored by bioluminescence imaging and results are displayed as average radiance (p/s/cm2/sr) of each mouse (colored lines) and the mean of each group (in black bold line). Control group (grey shaded area) is plotted as average radiance (p/s/cm2/sr) of the mean + SD. (b) Tumors were significantly eliminated in mice treated with total (3 × 106 CD8 + 3 × 105 CD4, n = 10) (P < 0.05) compared with 3 × 106 CD8 alone (n = 10) or 3 × 105 CD4 alone (n = 6) s183-TCR transduced T cells. Results from two independent experiments. (c) TissueFAXS staining of spleen tissue from mice treated with total s183-TCR transduced T cells at day 1 after adoptive cell transfer. Figure shows DAPI staining (blue), HepG2-env tumor cells expressing GFP (green) and CD8 (red, bottom panel; isotype control, top panel). Multiple CD8 T cells were detected in the tumor (white arrows) and in multiple fields. Two representative fields shown at 40× magnification.
We then used the same model to test the effect of T cells electroporated with anti-HBV TCR mRNA. Tumor-bearing mice were given s183-TCR mRNA electroporated T cells, consisting of 3 × 106 pentamer+ CD8 plus pentamer+ CD4; one infusion of T cells every 3 days. Three infusions of electroporated T cells blocked tumor growth and maintained stable disease (Figure 4). Of note, when therapy was interrupted after three infusions, the tumor grew, further demonstrating the antitumor effect of T-cell therapy.
Figure 4.
Multiple infusions of activated mRNA electroporated T cells control tumor growth and maintained stable disease. One million HepG2-env tumor cells were inoculated by intrasplenic injection in NSG mice (n = 11). Nine days after tumor inoculation, mice were treated with three doses of 3 × 106 activated s183-TCR mRNA electroporated T cells per dose, (n = 4, red line) injected i.v once every 3 days. Mice treated with 3 × 106 mock-electroporated T cells served as controls (n = 3, grey shaded area). Tumor size was monitored by bioluminescence imaging and plotted as average radiance (p/s/cm2/sr) of the mean + SD.
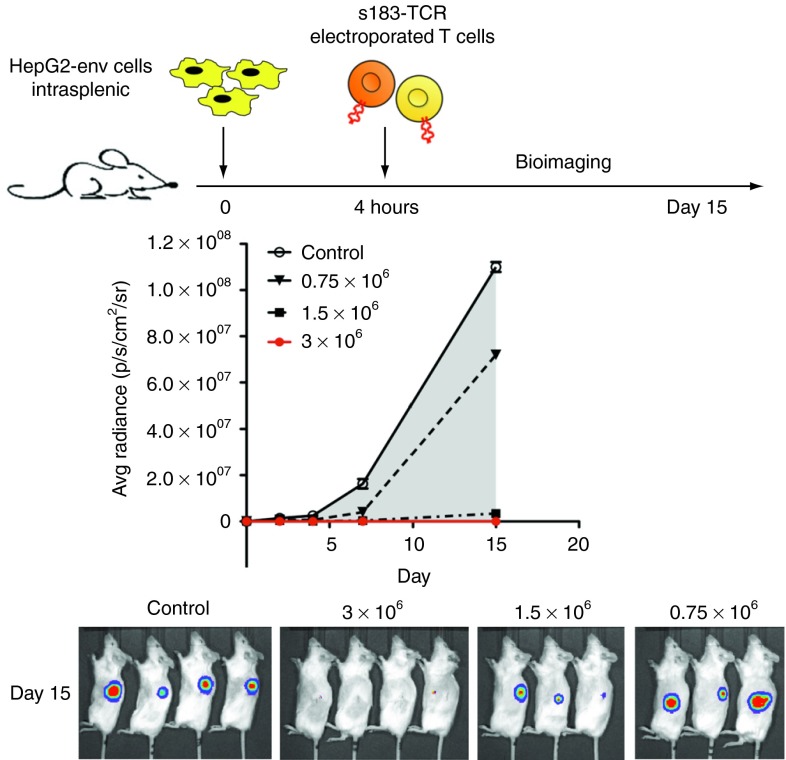
To further test the in vivo antitumor efficacy, we tested whether electroporated T cells could prevent HCC engraftments in NSG mice. Mice were inoculated with one million HepG2-env, and graded numbers of s183-TCR mRNA electroporated T cells were injected intravenously 4 hours later. In mice, receiving three million pentamer+ T cells, tumor seeding and growth was completely prevented, while lower numbers of T cells slowed tumor development in comparison with mice receiving similar numbers of mock-electroporated T cells (Figure 5).
Figure 5.
Prevention of HCC tumor cells seeding by mRNA electroporated T cells. One million HepG2-env tumor cells were inoculated by intrasplenic injection in NSG mice (n = 14). Four hours later, mice (n = 4 or 3 per group) were treated with graded doses (0.75, 1.5, 3 × 106 pentamer+ CD8) of s183-TCR mRNA electroporated T cells injected i.v. Mice treated with 3 × 106 mock-electroporated T cells served as controls (grey shaded area). Tumor size was monitored by bioluminescence imaging and plotted as average radiance (p/s/cm2/sr) of the mean + SD.
Development of clinical-grade, large-scale method to express anti-HBV TCR in T cells
The above results indicated that mRNA electroporation could be used to express functional anti-HBV TCR in T cells, which then demonstrate antitumor activity in vivo. To adapt this technology to large-scale conditions that can be used in HCC patients, we used a current good manufacturing practice (cGMP)-compliant electroporator. We transfected 2 × 108 activated T cells in a cGMP environment. Twenty-four hours after electroporation, 40% of CD8+ T cells were pentamer+. In total, we obtained 7 × 107 antigen-specific T cells (Figure 6). These cells produced IFN-γ, TNF-α, and IL-2 after HBV-specific stimulation. Overall, functionality was similar to that of T cells electroporated in the small-scale, research laboratory setting. Thus, it is possible to generate large batches of clinical-grade, functional antigen-specific T cells.
Figure 6.

High level of TCR expression and multifunctionality of mRNA electroporated T cells produced in large-scale, clinical-grade conditions. A schematic illustrating cell numbers, efficiency, yield, and functionality of laboratory-grade (top row) vs. clinical-grade (bottom row) electroporation of T cells. Dot plot of CD8 and HLA-A2-HBs183-191 pentamer staining in s183-TCR mRNA electroporated T cells at 24 hours postelectroporation. Bar charts show the frequency of IFN-γ, TNF-α, and IL-2 producing cells out of CD8 or CD4 electroporated T cells.
Discussion
The results of this study demonstrate the anti-HCC potential of T cells transiently expressing anti-HBV TCR by mRNA electroporation. The number of cells expressing the receptors 24 hours after electroporation was much higher than that achieved by retroviral transduction. Furthermore, TCR electroporation endowed antigen-specific functionality in approximately half of the TCR-expressing cells; they secreted multiple cytokines in response to HBV-specific peptide stimulation and effectively killed tumor cells expressing HBV antigen in vitro. The anti-HBV TCR mRNA electroporated cells demonstrated their functionality also in vivo by preventing the growth of human HCC-like cells in immunodeficient mice. Expression of the receptor and antigen-specific functionality of the T cells after electroporation was transient and became undetectable after 72 hours. Hence, multiple infusions of electroporated T cells may be necessary to achieve significant clinical effects. We do not regard this requirement as a major limitation because the transduction method is straightforward and requires only a few hours even when performed in a large-scale cGMP setting.
Immunotherapy using redirected T cells have so far favored strategies that lead to production of T cells stably expressing the introduced TCR as long-term persistence of antigen-specific T cells22,23 and establishment of memory T cells24,25,26 might have a higher therapeutic efficacy. A concern, however, is that T cells stably expressing transduced TCR have the potential to lead to chronic autoimmunity due to autoreactivity caused by mispairing of introduced and endogenous TCR chains.27 In the context of HCC, long-term immunity against HBV-infected hepatocytes may also be counterproductive and induce or exacerbate hepatitis. Because of the transient TCR expression resulting from mRNA electroporation, these issues should be less worrisome. Finally, with mRNA electroporation, there is no risk of insertional mutagenesis associated with the use of integrating viral vectors.16,17,18
Contrary to the results of cytotoxicity in vitro, where electroporated cells appeared to be more potent than retrovirally transduced cells, the latter were more efficient in vivo: one single injection could eradicate HCC-like cells in our HCC xenograft mouse model while three infusions of electroporated cells were required to suppress tumor cell growth but were not sufficient to fully eliminate them. These results were consistent with previous work performed with chimeric antigen receptor-electroporated T cells28 and are likely due to the transient nature of TCR expression after electroporation. It is possible that higher doses of T cells and/or a more intense administration schedule could have been more effective. Nevertheless the potential protective efficacy of our TCR-electroporated T cells was demonstrated by the fact that one single infusion of them was sufficient to prevent engraftment of HCC-like cells in our mouse model. We think that these data are particularly important since HCC therapy relies mainly on liver transplantation,6,29 and HCC recurrence in transplant patients frequently occurs due to the seeding of HCC cells often carrying HBV integrations30 in the newly transplanted normal liver or in extra-hepatic locations. The most important limitations of our model are that HCC-like cells seed and expand preferentially in the spleen and not in the liver, and these cells are the only cells that express HBsAg (the model does not have HBsAg-positive normal hepatocytes). In this regard, the model might somewhat recapitulate the scenario occurring after liver transplantation in chronic HBV patients with HCC. Conceivably, mRNA electroporated T cells expressing anti-HBV TCR might become a potential postoperative immunotherapy intervention to block the dissemination of HBV-expressing tumor cells. Whether a single TCR specificity will be sufficient in clinical practice to block HCC seeding is unclear. We have also already produced TCRs specific for multiple HBV epitopes and HLA-restrictions12 that can be used to adoptively transfer T cells of multiple HBV-specificities. The flexibility of mRNA TCR electroporation might permit an easier production of multispecific TCR-redirected T cells.
Indeed, the successful TCR expression in a large number of cells using cGMP-compliant procedures provides proof-of-principle that this approach can be translated into clinical application. The regulatory requirements associated with mRNA electroporation should be considerably less demanding than those controlling the production and clinical use of viral vectors. Moreover, the effort and cost required for mRNA production are significantly lesser than those needed to produce clinical-grade viral supernatant.31,32 The electroporation procedure can be accomplished within hours,33 at an estimated cost of less than $30,000 per patient. This is in contrast to retroviral transduction, whereby a batch of cGMP-grade retroviral supernatant for treating a patient with HBsAg expressing HCC metastasis costs at least four-times more (H. Stauss and A. Bertoletti, Manuscript in Preparation) and require lengthy preparation and testing, in addition to several days of transduction.
In summary, the TCR mRNA electroporation method described here should facilitate the clinical application of anti-HBV TCR-mediated cell therapy of HCC. Its practical features and their reduced half-life also suggest potential for other uses, such as attempts to boost antiviral T-cell therapy in patients with chronic hepatitis B. Finally, it should be possible to adapt the method to TCR recognizing other viral peptides, like Epstein–Barr virus, for treatment of other virally-associated malignancies.
Materials and methods
Cells. HepG2-core or HepG2-env expressing luciferase were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 20 mmol/l HEPES, 0.5 mmol/l sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, MeM amino acids, Glutamax, MeM nonessential amino acids, (Invitrogen, Carlsbad, CA), and 2 μg/ml puromycin (BD Biosciences, San Jose, CA) was added to maintain selection. T2 cells were cultured in RPMI 1640 supplemented as described above. Phoenix cells were maintained in Iscove's Modified Dulbecco's Medium, 10% fetal bovine serum, 25 mmol/l HEPES, Glutamax, and 5 μg/ml plasmocin.
Peripheral blood mononuclear cells were collected under informed consent from healthy donors. Peripheral blood mononuclear cells were stimulated with 600 IU/ml interleukin-2 (rIL-2; R&D Systems, Minneapolis, MN) and 50 ng/ml anti-CD3 (OKT-3; eBioscience, San Diego, CA) in AIM-V 2% human AB serum for 48 h, and used for retroviral transduction. T cells used for mRNA electroporation were activated using the same conditions for 8 days, and rIL-2 was increased to 1,000 IU/ml 1 day before electroporation.
Retroviral transduction, expansion, and isolation of CD8+ TCR-transduced T cells. The HBV s183-91-specific TCR (s183-TCR) Vα34 and Vβ3 chains were cloned and inserted into vector MP-71. Retrovirus was produced and bulk T cells were transduced as previously described;12 72 hours after transduction, expression of TCR transgenes was analyzed by flow cytometry analysis. Bulk transduced T cells were expanded and restimulated using 5 × 105 TCR-transduced cells, 2 × 105 irradiated (4,000 rads) T2 cells pulsed with 1 μg/ml HBs183-91 peptide (FLLTRILTI), 1.8 × 106 irradiated (2,500 rads) peripheral blood mononuclear cells as feeders, and grown in AIM-V 2% human AB serum + 100 IU/ml rIL-2. Mock-transduced T cells were expanded with 1 μg/ml phytohemagglutinin (Sigma–Aldrich, St Louis, MO) and irradiated feeders. Cells were cultured and used for adoptive cell transfer experiments 11 days later. Expanded bulk TCR-transduced T cells were analysed for CD8 and pentamer expression before adoptive cell transfer. In some experiments, expanded bulk TCR-transduced T cells were subjected to magnetic bead selection for negative isolation of CD8 T cells using a CD4 microbeads kit (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's instructions. The purity of the cell population ranged from 90 to 95% as estimated by FACS analysis.
Flow cytometry. Antibodies for cell surface staining were obtained from BD Biosciences (anti-human CD8-PE-Cy7, CD4-PECy7, CD8-V500, CD45RA-APC, CD62L-PECy7), eBioscience (anti-human CD45RO-eFluor650), Beckman Coulter (anti-TCR Vβ3-FITC), Proimmune (HLA-A201-HBs183-91-PE pentamer) and Gijs Grotenbreg (HLA-A1101-CMVpp65-PE tetramer). Antibodies for intracellular cytokine staining were obtained from BD Biosciences (anti-human IFN-γ-APC, TNF-α-Alexa488, IL-2-PE, Granzyme-APC) and Diaclone (anti-human perforin-FITC). Intracellular cytokine staining was performed by fixing and permeabilizing cells with cytofix/cytoperm (BD Biosciences). Flow cytometry was performed using FACS Canto or LSRII (BD Biosciences) flow cytometers and data was analyzed with the FACS Diva program (BD Biosciences).
Production of s183-191 TCR mRNA and electroporation procedures. We derived the TCR construct from a pUC57-s183cys b2Aa vector that we had previously made, and subcloned it into the pVAX1 vector. The plasmid was propagated purified from E. coli using the One Shot Top10 E. coli kit (Invitrogen), purified using QIAGEN Endo Free Plasmid Maxi Kit (QIAGEN, Valencia, CA), and linearized using the XbaI restriction enzyme. The linearized DNA was used to produce the TCR mRNA using the mMESSAGE mMACHINE T7 Ultra kit (Ambion, Austin, TX) or T7 mScript Standard mRNA Production System (Cellscript, Madison, WI); T7 RNA polymerase was added to start transcription; RNA was capped with Anti-Reverse Cap Analog (ARCA). Then, poly(A)-tail was added by E. coli Poly(A) Polymerase and ATP. The resulting product was concentrated by lithium chloride precipitation and re-dissolved in buffer.
For electroporation with the nucleofector device II (Lonza, Cologne, Germany), 10 × 106 peripheral blood mononuclear cells activated for 8 days with 600 IU/ml rIL-2 and 50 ng/ml OKT-3 in AIM-V 2% human AB serum as described above were suspended in 100 μl of Cell Line Nucleofector Solution V (Lonza) and TCR mRNA was added at 200–400 μg/ml. The mixture was placed in a certified cuvette (Lonza) and electroporated using program “X-001”. After electroporation, cells were resuspended in AIM-V 2% human AB serum + 100 IU/ml rIL-2, and cultured at 37 °C and 5% CO2 until analysis. Large-scale electroporation was performed following cGMP guidelines. 2.0 × 108 activated T cells were suspended in 5 ml electroporation buffer (MaxCyte, Gaithersburg, MD); s183-TCR mRNA was added at 200 μg/ml. The mixture was placed in a MaxCyte flow chamber, electroporated according to the “flow NK#2” program. After electroporation, cells were placed at 37 °C, 5% CO2 for 20 minutes, and then resuspended in AIM-V 2% human AB serum + 100 IU/ml rIL-2 and cultured at 37 °C and 5% CO2 until analysis.
Functional and cytotoxicity studies. HLA-A2+ T2 cells were pulsed with various concentrations (1 fg/ml–1 μg/ml) of s183-91 peptide for 1 hour at 106 cells/ml and then washed. Retrovirally transduced T cells or mRNA electroporated T cells were cocultured with peptide-loaded T2 cells for 5 hours in the presence of 10 μg/ml brefeldin A, and stained for CD8 and IFN-γ as previously described. Results were displayed as percent of maximum CD8+ IFN-γ response.
For the functional profile, retrovirally transduced or electroporated T cells were incubated with s183-91 peptide-loaded T2 cells (1 μg/ml peptide) overnight in the presence of 2 μg/ml brefeldin A and stained for CD8, IFN-γ, TNF-α, and IL-2.
HepG2-core or HepG2-env expressing luciferase were plated overnight in 96-well flat bottom plate to permit adherence. Cells were washed with HBSS (Invitrogen) and cocultured with effector retrovirally transduced T cells (CD8+/pentamer+) or electroporated T cells at various effector: target (E: T) ratios in triplicates in AIM-V supplemented with 2% human AB serum for 24 hours. Cytotoxicity was measured by quantifying luciferase expression in remaining target cells. Briefly, culture medium was discarded and 100 μl of Steady-Glo reagent (Promega, Madison, WI) was added to each well and incubated for 5 minutes to allow cell lysis. Luminescence was measured with a microplate reader (Tecan, Männedorf, Switzerland). Target cells without effectors were used as a reference for maximum luminescence. Results were expressed as % lysis = 100% − (luminescence remaining after lysis/maximum luminescence)% and calculated as mean of triplicate measurements ± SD.
In vivo xenograft tumor model and adoptive cell transfer. NOD-SCID-IL2RGnull (NSG) mice were bred in-house and kept under specific pathogen-free conditions. Animal experiments performed were approved by the Institutional Animal Care and Use Committee (IACUC) of A*STAR (Authorization No. IACUC 090491) in accordance with the guidelines of the Agri-Food and Veterinary Authority and the National Advisory Committee for Laboratory Animal Research of Singapore.
Eight- to ten-week-old NSG mice were inoculated with 1.0 × 106 HepG2-env expressing luciferase per mouse in the spleen, following a protocol previously described.34,35 Tumor was allowed to grow for 10 days. In vitro expanded retrovirally transduced T cells or electroporated T cells were resuspended in 100 μl PBS and injected intravenously. Tumor growth progression was monitored by in vivo imaging (IVIS; Xenogen, Alameda, CA). Mice were anesthetized, injected subcutaneously with 100 μl of D-luciferin potassium salt (Caliper Life Sciences) (5 mg/ml in PBS) and, 2 minutes later, bioluminescence images were acquired. Mice with no tumors on day 0 before tumor inoculation were injected with luciferin and imaged for background subtraction. Bioluminescence in the tumor was quantified using Living Imaging 3.0 software and expressed as average radiance units (p/s/cm2/sr).
TissueFAXS analysis and histopathology. Spleen tissues were embedded in Tissue-Tek OCT medium (Sakura, Torrance, CA) in cryomolds, snap frozen and stored at −80 °C. They were sectioned into 5 μm thick and mounted on polylysine slides for fixation and staining. Sections were fixed with 4% paraformaldehyde and then blocked with 5% normal goat serum for 30 minutes at room temperature then incubated with mouse monoclonal anti-human CD8 (Dako, Denmark) overnight at 4 °C, followed by AlexaFluor647-conjugated goat anti-mouse antibody (Invitrogen). Nuclei were stained with ProLong® Gold antifade reagent with DAPI (Invitrogen). Images were acquired using TissueFAXS (TissueGnostics GmbH) and TissueFAXS slides software. Histology work was performed by the Advanced Molecular Pathology Laboratory, IMCB, A*STAR, Singapore.
Statistical analysis. Statistical analysis was performed in GraphPad Prism (Graph-Pad Software Inc). For comparisons involving more than two groups, statistical significance was determined using the Kruksal–Wallis test with Dunn's post-test for multiple comparisons with P < 0.05 taken as evidence of a significant difference.
SUPPLEMENTARY MATERIAL Figure S1. Cytokines produced in supernatants by electroporated T cells compared to retrovirally transduced T cells after overnight coculture with T2 cells (unstim) or s183 peptide-loaded T2 cells (stim). Figure S2. Histopathological analysis of of hematoxylin and eosin stained spleen sections.
Acknowledgments
We thank the staff at the Advanced Molecular Pathology Laboratory, IMCB, A*STAR, Singapore for histological services and Gijs Grotenbreg at the National University of Singapore for synthesizing the HLA-A1101-CMVpp65-PE tetramer. This work was supported by the Agency for Science, Technology and Research (A*Star), Singapore. The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Supplementary Material
Cytokines produced in supernatants by electroporated T cells compared to retrovirally transduced T cells after overnight coculture with T2 cells (unstim) or s183 peptide-loaded T2 cells (stim).
Histopathological analysis of of hematoxylin and eosin stained spleen sections.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765–769. doi: 10.1038/ng.2295. [DOI] [PubMed] [Google Scholar]
- Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Edman JC, Gray P, Valenzuela P, Rall LB, Rutter WJ. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980;286:535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood. 2005;105:1898–1904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Gehring AJ, Ho ZZ, Tan AT, Aung MO, Lee KH, Tan KC, et al. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2009;137:682–690. doi: 10.1053/j.gastro.2009.04.045. [DOI] [PubMed] [Google Scholar]
- June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29:550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55:103–110. doi: 10.1016/j.jhep.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, et al. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A, Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection. Curr Opin Microbiol. 2000;3:387–392. doi: 10.1016/s1369-5274(00)00109-0. [DOI] [PubMed] [Google Scholar]
- Marshall E. Gene therapy a suspect in leukemia-like disease. Science. 2002;298:2002–2003. doi: 10.1126/science.298.5591.34. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia A, Bonini C, Magnani Z, Urbinati F, Sartori D, Muraro S, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker L, Klenerman P, Zinkernagel RM, Ehl S. Exhaustion of cytotoxic T cells during adoptive immunotherapy of virus carrier mice can be prevented by B cells or CD4+ T cells. Eur J Immunol. 2002;32:374–382. doi: 10.1002/1521-4141(200202)32:2<374::AID-IMMU374>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenen MM, de Boer R, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci USA. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008;134:1890–9; quiz 2155. doi: 10.1053/j.gastro.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Rabinovich PM, Komarovskaya ME, Ye ZJ, Imai C, Campana D, Bahceci E, et al. Synthetic messenger RNA as a tool for gene therapy. Hum Gene Ther. 2006;17:1027–1035. doi: 10.1089/hum.2006.17.1027. [DOI] [PubMed] [Google Scholar]
- Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P, et al. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy. 2012;14:830–840. doi: 10.3109/14653249.2012.671519. [DOI] [PubMed] [Google Scholar]
- Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–154. doi: 10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E, Guettier C, Kayibanda M, Le Rond S, Crain-Denoyelle AM, Marchiol C, et al. Regression of established liver tumor induced by monoepitopic peptide-based immunotherapy. J Immunol. 2004;173:4882–4888. doi: 10.4049/jimmunol.173.8.4882. [DOI] [PubMed] [Google Scholar]
- Rénia L, Rodrigues MM, Nussenzweig V. Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. Immunology. 1994;82:164–168. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytokines produced in supernatants by electroporated T cells compared to retrovirally transduced T cells after overnight coculture with T2 cells (unstim) or s183 peptide-loaded T2 cells (stim).
Histopathological analysis of of hematoxylin and eosin stained spleen sections.