Abstract
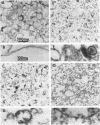
Membranes prepared from HEp-2 cells infected with herpes simplex virus and free from soluble proteins, virus, ribosomes, and other cellular constituents were solubilized and subjected to electrophoresis on acrylamide gels. The electropherograms showed the following. (i) The synthesis of host proteins and glycoproteins ceases after infection. However, the spectrum of host proteins in membranes remains unaltered. (ii) Between 4 and 22 hr postinfection, at least four glycoproteins are synthesized and bound to the smooth cytoplasmic membranes. On electrophoresis, these glycoproteins form two major and two minor bands in the gel and migrate with proteins ranging from 50,000 to 100,000 daltons in molecular weight. (iii) The same glycoproteins are present in all membranes fractionated by density and in partially purified virus. The implications of the data are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Duesberg P. H. Proteins of Newcastle disease virus and of the viral nucleocapsid. J Virol. 1969 Oct;4(4):388–393. doi: 10.1128/jvi.4.4.388-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Hagopian A., Eylar E. H. Cellular membranes: the isolation and characterization of the plasma and smooth membranes of HeLa cells. Arch Biochem Biophys. 1968 Oct;128(1):51–69. doi: 10.1016/0003-9861(68)90008-8. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- Clifford P., Singh S., Stjernswärd J., Klein G. Long-term survival of patients with Burkitt's lymphoma: an assessment of treatment and other factors which may relate to survival. Cancer Res. 1967 Dec;27(12):2578–2615. [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A. Observations on the mode of release of herpes virus from infected HeLa cells. J Cell Biol. 1962 Mar;12:589–597. doi: 10.1083/jcb.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kingsbury D. W. Separation of Newcastle disease virus proteins by polyacrylamide gel electrophoresis. Virology. 1969 Apr;37(4):597–604. doi: 10.1016/0042-6822(69)90277-3. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Teather C., Andrews H. Organizational difference of cell surface "hematoside" in normal and virally transformed cells. Biochem Biophys Res Commun. 1968 Nov 25;33(4):563–568. doi: 10.1016/0006-291x(68)90332-x. [DOI] [PubMed] [Google Scholar]
- Haslam E. A., Cheyne I. M., White D. O. The structural proteins of Newcastle disease virus. Virology. 1969 Sep;39(1):118–129. doi: 10.1016/0042-6822(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Klein G., Pearson G., Henle G., Henle W., Diehl V., Niederman J. C. Relation between Epstein-- Barr viral and cell membrane immunofluorescence in Burkitt tumor cells. II. Comparison of cells and sera from patients with Burkitt's lymphoma and infectious mononucleosis. J Exp Med. 1968 Nov 1;128(5):1021–1030. doi: 10.1084/jem.128.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Pearson G., Nadkarni J. S., Nadkarni J. J., Klein E., Henle G., Henle W., Clifford P. Relation between Epstein-Barr viral and cell membrane immunofluorescence of Burkitt tumor cells. I. Dependence of cell membrane immunofluorescence on presence of EB virus. J Exp Med. 1968 Nov 1;128(5):1011–1020. doi: 10.1084/jem.128.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- ROSAN R. C., NAHMIAS A. J., KIBRICK S., KERRIGAN J. A. STUDIES IN GLYCOPROTEIN PRODUCTION: INFECTION OF PRIMARY CULTURES OF HUMAN AMNION WITH HERPES SIMPLEX VIRUS. Exp Cell Res. 1964 Dec;36:611–624. doi: 10.1016/0014-4827(64)90317-9. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkey F. H., Erlandson R. A., Bailey R. B., Babcock V. I., Southam C. M. Virus biographies. II. Growth of herpes simplex virus in tissue culture. Exp Mol Pathol. 1967 Feb;6(1):39–67. doi: 10.1016/0014-4800(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Siegert R., Falke D. Elektronenmikroskopische Untersuchungen über die Entwicklung des Herpesvirus hominis in Kulturzellen. Arch Gesamte Virusforsch. 1966;19(2):230–249. [PubMed] [Google Scholar]
- Siminoff P., Menefee M. G. Normal and 5-bromodeoxyuridine-inhibited development of herpes simplex virus. An electron microscope study. Exp Cell Res. 1966 Nov-Dec;44(2):241–255. doi: 10.1016/0014-4827(66)90429-0. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. An improved procedure for H-3 and C-14 counting in acrylamide gels with a nonaqueous scintillation system. Anal Biochem. 1968 Oct 10;26(1):197–200. doi: 10.1016/0003-2697(68)90048-1. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. The proteins specified by herpes simplex virus. I. Time of synthesis, transfer into nuclei, and properties of proteins made in productively infected cells. Virology. 1968 Dec;36(4):545–555. doi: 10.1016/0042-6822(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Roizman B. Herpes simplex virus products in productive and abortive infection. 3. Differentiation of infectious virus derived from nucleus and cytoplasm with respect to stability and size. J Virol. 1968 Oct;2(10):979–985. doi: 10.1128/jvi.2.10.979-985.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]