Abstract
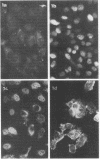
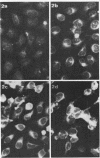
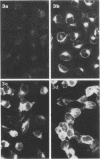
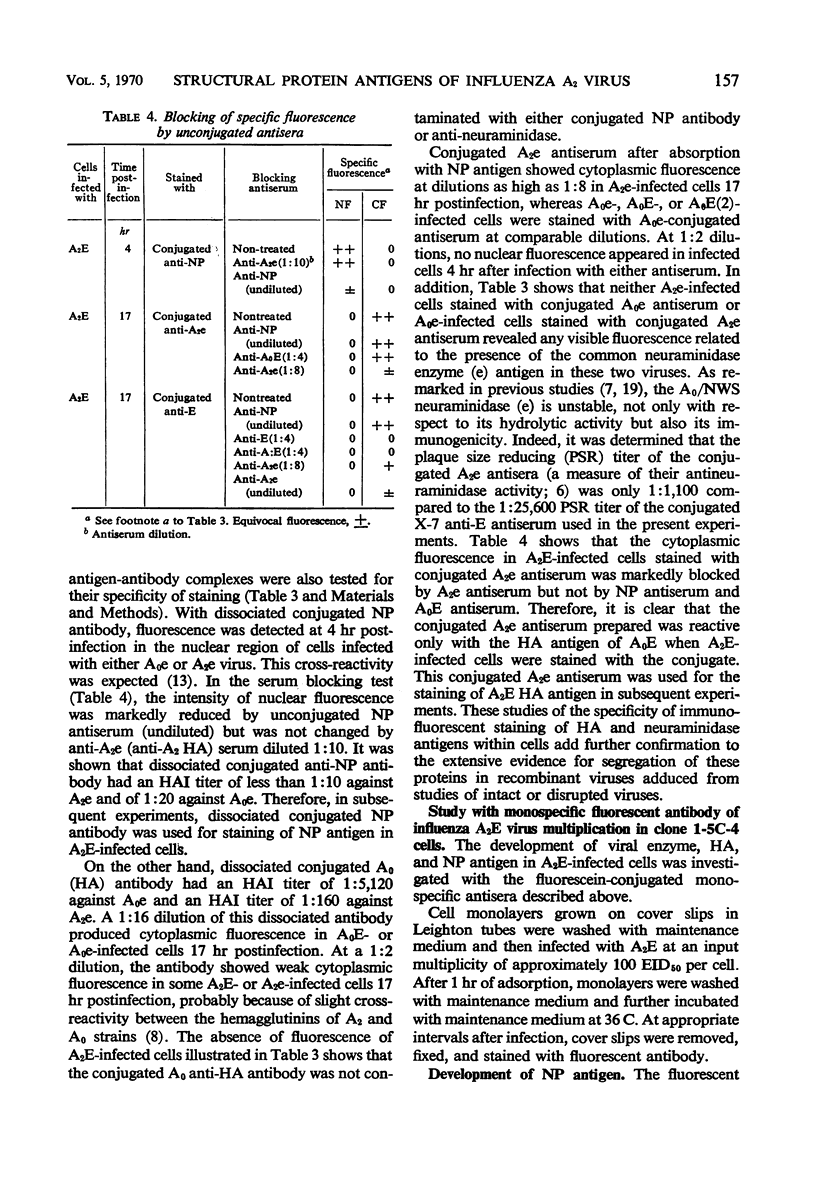
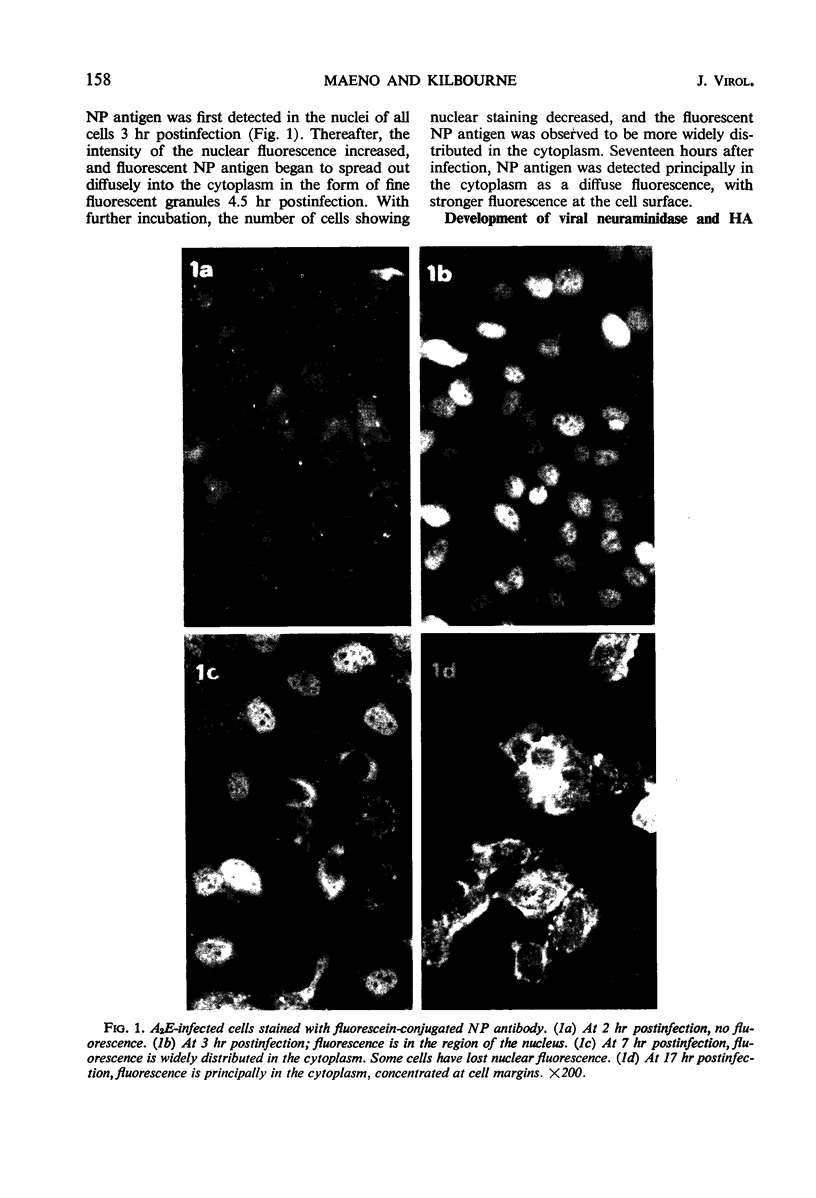
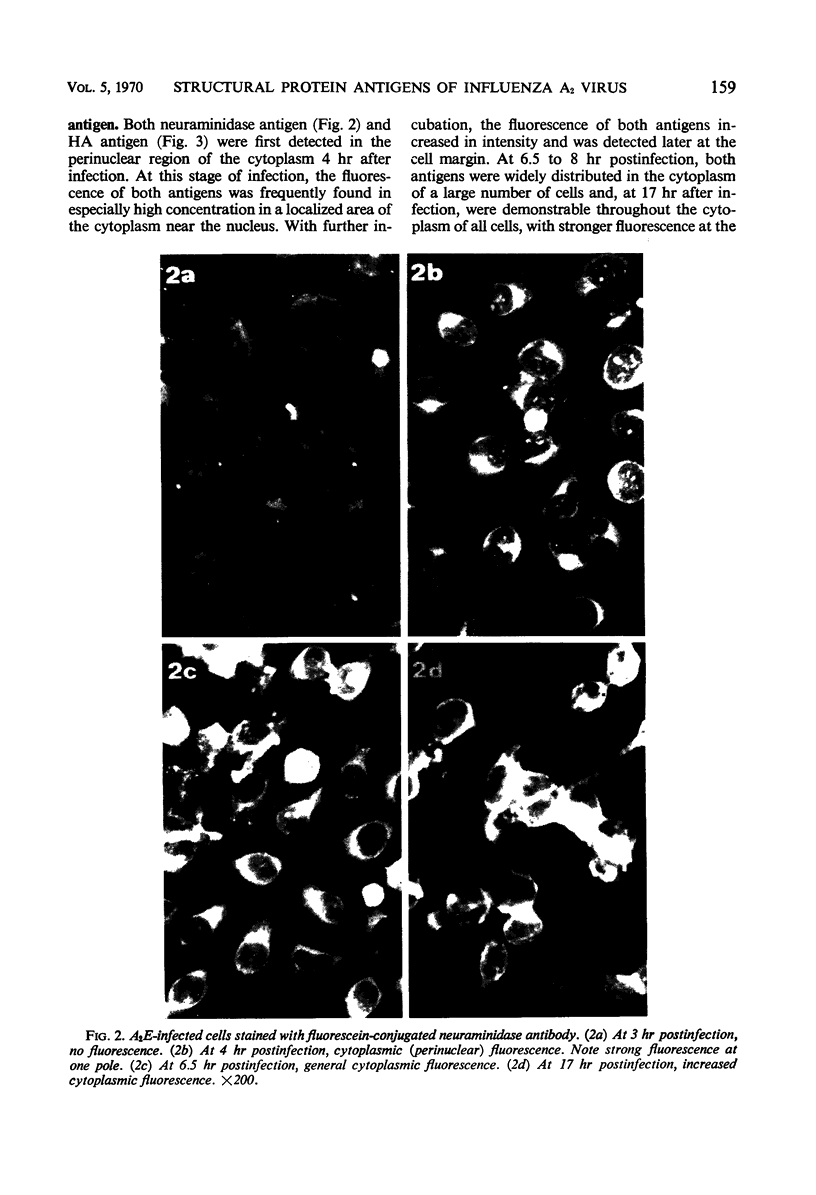
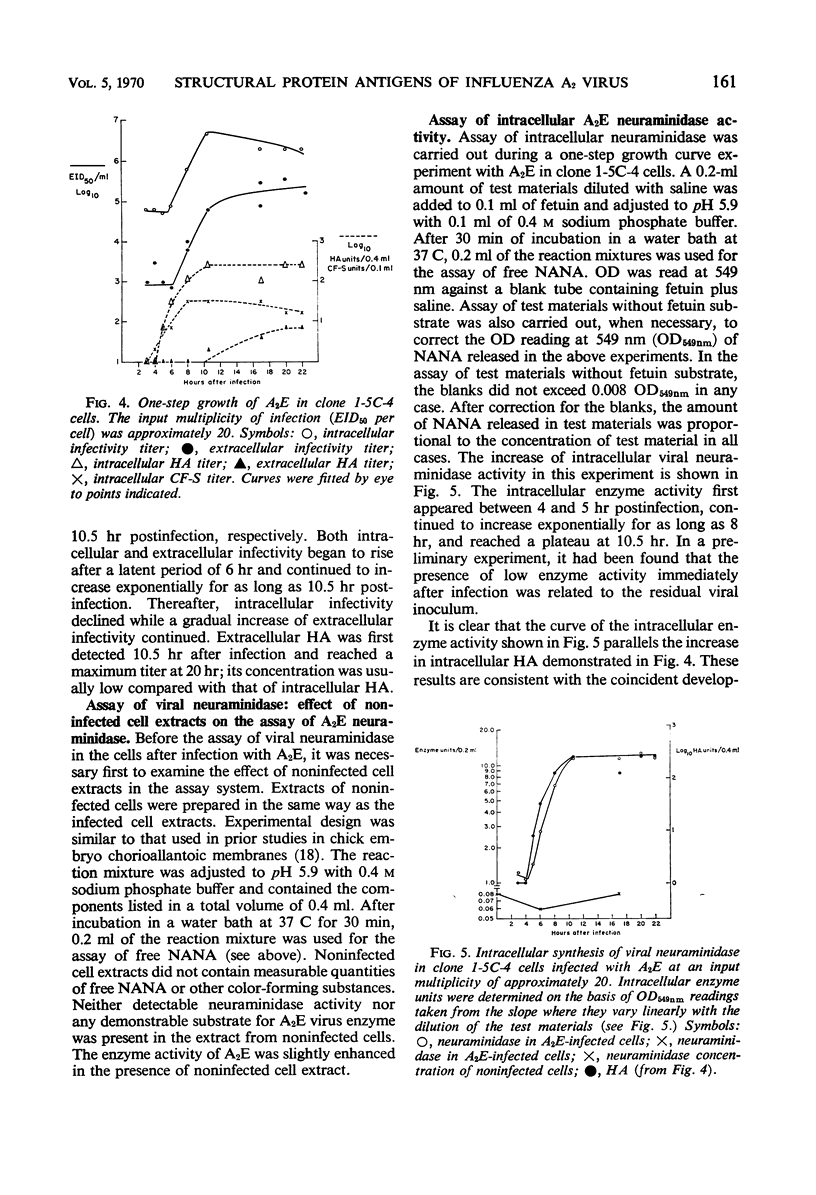
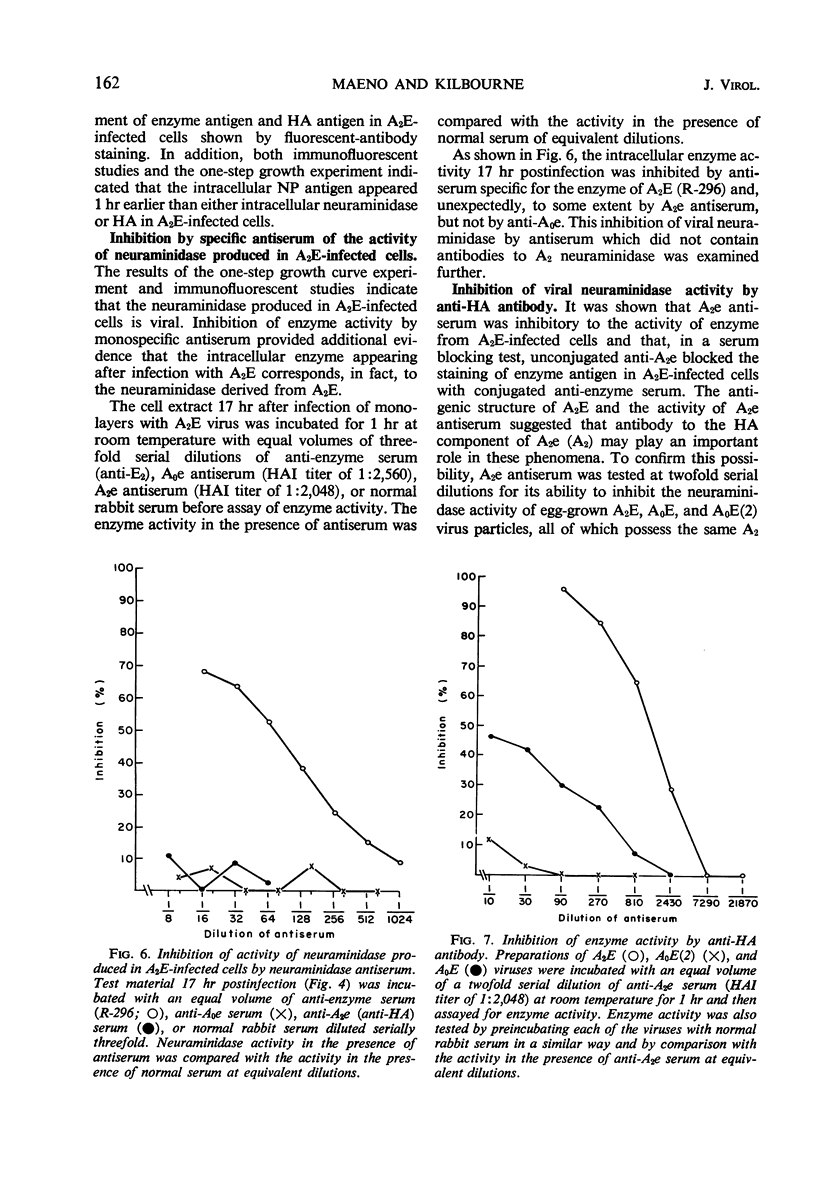
Specific antisera for hemagglutinin (HA) and neuraminidase antigens of influenza A2 virus (A2E) were produced through the segregation of the two proteins in reciprocal viral recombinants of A2E and A0e viruses. Gamma globulin fractions of these specific antisera and of antiserum specific for the nucleoprotein (NP) antigen of A0e virus were conjugated with fluorescein isothiocyanate and employed to follow the synthesis of the three structural proteins in clone 1-5C-4 human aneuploid cells, with parallel measurement of serological and biological activity of the antigens by other techniques. In this system, NP antigen appeared first (at 3 hr) in the cell nucleus, whereas HA and neuraminidase appeared coincidentally, at 4 hr after infection, in the cytoplasm. The initial detectability of biological or complement-fixing activity of the proteins coincided with their demonstrability as stainable antigens. Late in infection, all three antigens were detected at the cell surface. Antibody specific for HA partially blocked the intracellular staining of neuraminidase and inhibited the enzymatic activity of both extracted and intact extracellular virus. These observations suggest the close intracytoplasmic proximity of the two envelope antigens and perhaps their initial association in a larger protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREITENFELD P. M., SCHAFER W. The formation of fowl plague virus antigens in infected cells, as studied with fluorescent antibodies. Virology. 1957 Oct;4(2):328–345. doi: 10.1016/0042-6822(57)90067-3. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M., ROTT R., SCHAEFER W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960 Nov 1;112:765–782. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTERMANN O. A., HILLIS W. D., MOFFAT M. A. The development of soluble (S) and viral (V) antigens of influenza A virus in tissue culture as studied by the fluorescent antibody technique. 1. Studies employing a low multiplicity of infection in beef embryo kidney cells. Acta Pathol Microbiol Scand. 1960;50:398–408. doi: 10.1111/j.1699-0463.1960.tb01209.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahiel R. I., Kilbourne E. D. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus-antibody reactions. J Bacteriol. 1966 Nov;92(5):1521–1534. doi: 10.1128/jb.92.5.1521-1534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBER M. W., HENLE W. A comparison of influenza complement fixation antigens derived from allantoic fluids and membranes. J Immunol. 1950 Aug;65(2):229–244. [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D., Schulman J. L. The induction of broadened (multitypic) immunity with doubly antigenic influenza virus recombinants. Trans Assoc Am Physicians. 1965;78:323–333. [PubMed] [Google Scholar]
- LIU C. Studies of influenza infection in ferrets by means of fluorescein-labelled antibody. I. The pathogenesis and diagnosis of the disease. J Exp Med. 1955 Jun 1;101(6):665–676. doi: 10.1084/jem.101.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C. Studies on influenza infection in ferrets by means of fluorescein-labelled antibody. II. The role of soluble antigen in nuclear fluorescence and cross-reactions. J Exp Med. 1955 Jun 1;101(6):677–686. doi: 10.1084/jem.101.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- MAYRON L. W., ROBERT B., WINZLER R. J., RAFELSON M. E., Jr Studies on the neuraminidase of influenza virus. I. Separation and some properties of the enzyme from Asian and PR8 strains. Arch Biochem Biophys. 1961 Mar;92:475–483. doi: 10.1016/0003-9861(61)90387-3. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. III. Influenza virus. J Exp Med. 1956 Aug 1;104(2):171–182. doi: 10.1084/jem.104.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLL H., AOYAGI T., ORLANDO J. Intracellular synthesis of neuraminidase following infection of charioallantoic membranes with influenza virus. Virology. 1961 May;14:141–143. doi: 10.1016/0042-6822(61)90141-6. [DOI] [PubMed] [Google Scholar]
- Paniker C. K. Serological relationships between the neuraminidases in influenza viruses. J Gen Virol. 1968 May;2(3):385–394. doi: 10.1099/0022-1317-2-3-385. [DOI] [PubMed] [Google Scholar]
- SCHOLTISSEK C., ROTT R., HAUSEN P., HAUSEN H., SCHAEFER W. Conparative studies of RNA and protein synthesis with a myxovirus and a small polyhedral virus. Cold Spring Harb Symp Quant Biol. 1962;27:245–257. doi: 10.1101/sqb.1962.027.001.024. [DOI] [PubMed] [Google Scholar]
- SUGIURA A., KILBOURNE E. D. GENETIC STUDIES OF INFLUENZA VIRUSES. II. PLAQUE FORMATION BY INFLUENZA VIRUSES IN A CLONE OF A VARIANT HUMAN HETEROPLOID CELL LINE. Virology. 1965 Jul;26:478–488. doi: 10.1016/0042-6822(65)90010-3. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Khakpour M., Kilbourne E. D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968 Aug;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Kilbourne E. D. Independent variation in nature of hemagglutinin and neuraminidase antigens of influenza virus: distinctiveness of hemagglutinin antigen of Hong Kong-68 virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):326–333. doi: 10.1073/pnas.63.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H., Yamamoto H., Abe C. Differentiation of adenovirus 12 antigens in cultured cells with immunofluorescent analysis. Virology. 1967 Apr;31(4):748–752. doi: 10.1016/0042-6822(67)90213-9. [DOI] [PubMed] [Google Scholar]
- WONG S. C., KILBOURNE E. D. Changing viral susceptibility of a human cell line in continuous cultivation. I. Production of infective virus in a variant of the Chang conjunctival cell following infection with swine or N-WS influenza viruses. J Exp Med. 1961 Jan 1;113:95–110. doi: 10.1084/jem.113.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]