Summary
Nucleotide oligomerization and binding domain (NOD)-like receptors (NLRs) are a major constituent of the cytosolic innate immune sensing machinery and participate in a wide array of pathways including nuclear factor κB (NF-κB), mitogen-activated protein kinase (MAPK), inflammasome, and type I interferon (IFN) signaling. NLRs have known roles in autoimmune, autoinflammatory, and infectious diseases. With respect to virus infection, NLRP3 is the most extensively studied NLR, including mechanisms of activation and inhibition. Furthermore, the importance of NLRP3 in both innate and adaptive immunity has been demonstrated. In comparison to NLRP3, the roles of other NLRs during virus infection are only just emerging. NLRC2 is an important activator of innate antiviral signaling and was recently found to mitigate inflammation during virus infection through autophagy. Finally, functions for NLRX1 in immune modulation and reactive oxygen species (ROS) production require further examination and the importance of NLRC5 as a transactivator of major histocompatibility complex (MHC) class I and antigen presentation is currently developing. In this review, we discuss current knowledge pertaining to viruses and NLRs as well as areas of potential research, which will help advance the study of NLR biology during virus infection.
Keywords: NOD-like receptors, inflammasomes, viruses, inflammation, caspase-1, interferons
Introduction
To respond rapidly to infection or cellular damage that is brought on by metabolic or cellular stress, vertebrates express germline-encoded pattern-recognition receptors (PRRs) that recognize unique microbial or viral components known as pathogen-associated molecular patterns (PAMPs), such as bacterial cell wall components or uncapped viral RNA (1). Alternatively, these cells also express PRRs that detect damage-associated molecular patterns (DAMPs), which are byproducts of pathogen invasion or sterile cellular damage such as reactive oxygen species (ROS) or ATP release (2). Five main classes of PRRs have been described: C-type lectin receptors (CLRs), Toll-like receptors (TLRs), retinoic acid inducible gene-I (RIG-I)-like receptors (RLRs), nucleotide oligomerization and binding domain (NOD)-like receptors (NLRs), and the Pyrin-HIN (PYHIN) domain containing family (including AIM2). CLRs and TLRs are expressed on the cell surface and in endosomes, whereas NLRs, RLRs, and PYHINs sense PAMPS and DAMPS in intracellular compartments (3-5).
NLRs comprise a large receptor family of more than 20 members and are characterized by a conserved NOD motif (6, 7). Notably, NLRs may be the most evolutionarily ancient PRR family, as the domain structure of NLRs is similar to some plant disease-resistance (R) genes, which trigger the hypersensitive response and prevent dissemination of infection with fungal, viral, parasitic, and insect pathogens in plants (8). Structurally, NLRs have one of several amino-terminal protein–protein interaction domains such as the caspase recruitment domain (CARD), pyrin domain (PYD), and baculovirus inhibitor repeat (BIR) domain. This is followed by an intermediary NOD domain, which is an ATP binding domain required for self-oligomerization. Finally, the carboxy-terminus consists of a varying number of leucine-rich repeat (LRR) motifs with the proposed function of detecting PAMPs and DAMPs, thus leading to NLR activation. Although current models suggest that PAMPs or DAMPs are sensed by the LRRs of NLRs, only a few NLRs have any demonstrated affinity for their PAMPs or DAMPs (9). Once activated, however, NLRs expose the N-terminal effector domains to induce the recruitment and activation of CARD and PYD-containing effector molecules.
NLRs have a wide range of effector and activation domains and consequently activate multiple signaling pathways. For example, the prototypical NLRs, NLRC1 and NLRC2, interact with receptor-interacting protein kinase 2 (RIPK2) (also known as RICK or RIP2) to induce nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling (10). One of the most studied and well-known roles for NLRs is in inflammasome activation. Inflammasomes consist of an inflammatory caspase, caspase-1, the adapter protein ASC, and can be activated by NLRP1, NLRP3, or NLRC4 (11-13). Inflammasome activation results in caspase-1 mediated cleavage of pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into their active forms and can trigger a proinflammatory form of cell death known as pyroptosis (11). However, not all NLRs are proinflammatory. NLRP12 and NLRP6 have been shown to downregulate NF-κB signaling subsequent to TLR activation (14-16). NLRP12 has also been reported to regulate alternative NF-κB signaling downstream of TNF family receptors like CD40 (17). Finally, NLRC5 and CIITA play roles in the regulation of major histocompatibility complex (MHC) class I and II antigen presentation (18, 19). Clearly, NLRs are not just sensors of infectious disease but participate in a diverse set of immune signaling pathways and functions.
Our understanding of the crucial roles that NLRs play in mounting protective antiviral responses and the molecular mechanisms used by viral pathogens to evade them has progressed remarkably in the past few years (20). Depending on the nature of the infectious agent, particular NLRs and inflammasome complexes become activated to elicit tailored immune responses. Here, we review our findings on the role of NLRP3 and NLRC2 in inflammasome activation and regulation in response to viral infection and how these findings relate to the current body of knowledge. We also review the role of NLRX1 and NLRC5 in the regulation of inflammation and antigen presentation during viral infection.
NLRP3 inflammasome
Our first evidence for the involvement of NLR inflammasomes in viral infection came from a study in which sendai virus (SeV) and influenza A virus (IAV) were shown to stimulate caspase-1 activation and downstream production of IL-1β and IL-18 through NLRP3 (21). Since our first report, the critical role for the NLRP3 inflammasome during viral infection has been demonstrated by the sheer number and diversity of viruses that activate this pathway. These include RNA viruses from the families Orthomyxoviridae, Paramyxioviridae, Rhabdoviridae, Picornaviridae, and Flaviviridae, and DNA viruses from the families Poxviridae, Herpesviridae, and Adenoviridae (Table 1). Despite the many viruses that have been shown to activate the NLRP3 inflammasome, there are many gaps in our knowledge regarding the mechanisms by which these viruses activate NLRP3 and the in vivo relevance of the NLRP3 inflammasome.
Table 1.
NLRP3 activating viruses
| Virus Group | Virus Family | Virus species | Mode of NLRP3 activation | References |
|---|---|---|---|---|
| (-)ssRNA | Orthomyxoviridae | Influenza A virus | ROS, M2 viroporin, vRNA, Cathepsin B | (12, 21-23, 25, 35, 39, 47, 66) |
| Paramyxioviridae | RSV | ROS, K+ efflux, SH viroporin | (41, 43) | |
| Sendai virus | ?? | (21) | ||
| Measles virus | ?? | (37) | ||
| Rhabdoviridae | VSV | ?? | (38) | |
| Rabies virus | ?? | (27) | ||
| (+)ssRNA | Picornaviridae | EMCV | 2B viroporin | (34, 38, 40) |
| Polio virus | 2B viroporin | (40) | ||
| Enterovirus 71 | 2B viroporin | (40) | ||
| Flaviviridae | HCV | ROS | (42) | |
| West Nile virus | CLEC5A, Syk kinase | (49-51) | ||
| JEV | ROS, K+ efflux | (44) | ||
| dsDNA | Poxviridae | Myxoma virus | Cathepsin B, ROS | (26) |
| Vaccinia virus | ?? | (28) | ||
| Herpesviridae | VZV | ?? | (52) | |
| HSV | ?? | (91) | ||
| Adenoviridae | Adenovirus 5 | vDNA, Endosome rupture, ROS | (45, 46, 92) |
The common upstream activation mechanism for NLRP3 in the field of NLR biology is still not known, and based on the wide variety of viruses that activate NLRP3, there must be an equally broad ranging mechanism to explain NLRP3 activation. We initially reported that RNA viruses activate NLRP3 in a viral RNA (vRNA) mediated manner (21). Since this initial discovery, multiple laboratoriess have demonstrated that vRNA or synthetic analogues can activate the NLRP3 inflammasome. For example, transfection of human and mouse cell lines or primary human macrophages with single stranded RNA (ssRNA) or double stranded RNA (dsRNA) analogues such as polyinosinic–polycytidylic acid [poly(I:C)] activates NLRP3 (22-24). Similarly, purified dsRNA from rotavirus or brome mosaic virus and ssRNA of IAV also activate NLRP3 (21, 22, 25). In vivo administration of poly(I:C) or purified IAV ssRNA in mice also resulted in IL-1β secretion and inflammation through NLRP3 (12, 21, 22). However, a direct interaction between NLRP3 and vRNA has not been established. Therefore, two possible roles for vRNA in NLRP3 inflammasome activation exist. First, vRNA is sensed by TLRs or RLRs leading to the upregulation of pro-IL-1β and NLRP3 through NF-κB-mediated signaling pathways (25-28). This then primes the NLRP3 inflammasome for activation (Fig. 1). NLRP3 then senses other signals to activate the inflammasome. We discuss what these other signals may be below. Another possibility for the role of vRNA in NLRP3 activation was recently presented through the RNA sensing kinase PKR. PKR recognizes the presence of vRNA in cells and through its kinase activity shuts down protein translation to prevent virus replication by phosphorylation of the translation initiation factor eIF2α (29, 30). However, Lu et al. (31) showed that PKR can also regulate NLRP3 inflammasome activation in response to multiple stimuli, including poly(I:C). PKR was found to directly interact with several inflammasomes including NLRP3. Although autophosphorylation of PKR was required for inflammasome activation, phosphorylation of NLRP3 or other inflammasome components was not detected (31). The role of PKR in inflammasome activation was reported in another study where a mutant form of IAV, which lacked the inhibitory protein NS1, resulted in increased inflammasome activation. Treatment of NS1 mutant IAV infected cells with a PKR inhibitor 2-amminopurine, resulted in diminished inflammasome activation (32). However, the exact role for PKR in this publication was not examined in further detail. Finally, a recent publication by He et al. (33) found no role for PKR during inflammasome activation. Although they did not examine inflammasome activation during virus infection specifically, they treated cells with a wide variety of known NLRP3 activators and found no significant changes in inflammasome activation or IL-1β production in two different PKR deficient mouse lines. The reason for different findings by these investigators is unclear. Therefore, further investigation into the role of PKR for inflammasome activation during viral infection is required. In conclusion, regardless of the pathway, the role of vRNA in NLRP3 inflammasome activation appears to be through an indirect mechanism and not an interaction of vRNA with NLRP3.
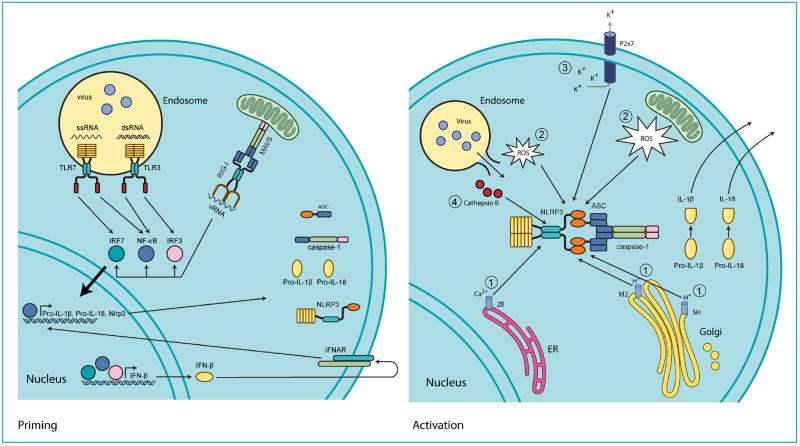
Fig. 1. Mechanisms of virus-mediated NLRP3 activation.
(Priming) During virus infection, viral genomic RNA (vRNA) consisting of either single stranded RNA (ssRNA) or double stranded RNA (dsRNA) is recognized by endosomal (TLR3 or TLR7) or cytoplasmic (RIG-I) pattern recognition receptors. This activates the transcription factor NF-κB and induces the production of inflammasome components and substrates like NLRP3, pro-IL-1β, and pro-IL-18, thus ‘priming’ the inflammasome for activation. Similarly, transcription factors IRF3 and IRF7 induce the expression or type I interferons, like IFN-α and IFN-β, which then feedback through the type I IFN receptor (IFNAR) to induce production of the same components and substrates NLRP3, pro-IL-1β and pro-IL-18. (Activation) Following priming, the NLRP3 inflammasome is assembled and activated in response to virus-induced damage-associated molecular patterns (DAMPs). Four main sources of DAMPs exist. (i) Virus encoded viroporins like M2, SH, and 2B allow for ion leakage from intracellular organelles into the cytosol. (ii) The generation of reactive oxygen species (ROS) resulting from PRR signaling, ER and mitochondrial stress, or virus-induced damage to endosomes. (iii) The P2x7 ion channel is opened in response to extracellular ATP from damaged or necrotic cells allowing for potassium efflux. (iv) During entry, virus infection damages endosomes and releases proteases such as cathepsin B into the cytosol.
Another role for vRNA in inflammasome activation may also come through the formation of alternative inflammasomes. With vesicular stomatitis virus (VSV), Poeck et al. (34) proposed that RIG-I senses vRNA and, in addition to interacting with the antiviral adapter MAVS (IPS1, VISA, CARDIF), may actually interact with ASC and caspase-1 directly to trigger inflammasome activation in an NLRP3-independent manner. In this same study, the authors found that encephalomyocarditis virus (EMCV), which utilizes another RLR, MDA5, still required NLRP3 for inflammasome activation (34). Another group recently reported that RIG-I interacts with caspase-1 and ASC and regulates inflammasome activation in primary normal human bronchial epithelial cells (NHBEs) derived from multiple donors and infected with IAV (35). Furthermore, this group determined that RIG-I also regulates the production of pro-IL-1β and NLRP3 transcript levels in NHBEs, suggesting that RIG-I may play a dual role in priming and activation of the inflammasome (Fig. 1). Finally, in NHBEs, both NLRP3 and RIG-I were required for inflammasome activation suggesting that RIG-I may cooperate with NLRP3 in inflammasome activation or that multiple distinct inflammasomes may form during IAV infection in human cells (35). However, other viruses that are known to activate RIG-I for IFN-α/β responses do not require RIG-I for inflammasome activation but do require NLRP3. Measles virus has been shown to activate both RIG-I and MDA5 for IFN-α/β production (36). However, measles virus requires only NLRP3 for inflammasome activation (37). This would indicate that RIG-I inflammasomes only form in response to certain viral infections. However, Rajan et al. (38) reported that RIG-I was not required for inflammasome activation during VSV infection but instead found that NLRP3 was required. This is in contrast to the initial finding by Poeck et al. (34). One main difference between these two reports was the need to prime macrophages before infection to detect IL-1β production. The study by Rajan et al. (38) required the use of priming with Pam3 (tripalmitoyl) to observe production of IL-1β. It is possible that by priming these cells, the response to VSV was diverted to the NLRP3 inflammasome, whereas infection without priming results in RIG-I inflammasome activation. The role of RIG-I in inflammasome activation is further complicated as IFN-α/β have also been shown to regulate NLRP3 activation (25). As a result, deletion or knockdown of RIG-I may have indirect effects on NLRP3 inflammasome activation (35). To completely verify the role or RIG-I in inflammasome activation, a targeted mutation of RIG-I or ASC/caspase-1, which only affects RIG-I binding to the inflammasome, will need to be examined. This must first be proceeded by molecular and biochemical experiments that can identify the interaction sites for RIG-I and inflammasome components.
As vRNA indirectly activates NLRP3, the question that remains is what is the final trigger leading to NLRP3 dimerization and inflammasome activation during viral infection? Indeed, NLRP3 activation is one of the main questions in the field of NLR biology. Multiple lines of evidence suggest that changes in cytoplasmic ions result in NLRP3 activation by viruses. IAV appears to activate NLRP3 as a result of H+ ion flux mediated by the virus M2 ion channel (39). The M2 protein enables H+ release from acidified Golgi and triggers activation of the NLRP3 inflammasome. Following priming, expression of the M2 protein alone activated the NLRP3 inflammasome (39). More recently, the pore-forming protein from other viruses has been shown to regulate NLRP3 inflammasome activation. The 2B protein from several picornaviruses, including EMCV, poliovirus and enterovirus 71 were shown to induce NLRP3 cytoplasmic relocalization and inflammasome activation in an intracellular Ca2+ mediated manner (40). Finally, another group published that the SH viroporin protein from respiratory syncytial virus (RSV) is responsible for NLRP3 activation and this likely occurs through ion leakage from the Golgi during infection (41). A final study failed to find a role for the HCV viroporin, but in this study the expression of HCV p7 viroporin was not accompanied by priming through the use of TLR ligands, partly due to the fact that the cells used for hepatitis C virus (HCV) infection have multiple defects in TLR and RLR signaling (42). The fact that viroporin proteins from five different viruses are capable of NLRP3 activation strongly indicates that this is a common mechanism of virus sensing by the NLRP3 inflammasome (Fig. 1).
In addition to the leakage of ions from intracellular compartments, the production of ROS ions is another common constituent of virus mediated NLRP3 activation. The treatment of virus-infected cells or mice with ROS scavengers like N-acetyl-L-cysteine (NAC) or (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC) have been shown to inhibit IAV mediated NLRP3 activation (22). Another study found that treatment of HCV-infected cells with another ROS scavenger pyrrolidine dithiocarbamat (PDTC) also inhibited NLRP3 inflammasome activation (42). Finally, RSV and Japanese encephalitis virus (JEV) have both been found to induce NLRP3 activation through K+ efflux from the cell and ROS production as treatment with K+ buffered medium or the ROS inhibitors APDC or diphenyleneiodonium chloride (DPI) inhibited NLRP3 inflammasome activation (43, 44). One final source of ion flux, which activates the NLRP3 inflammasome during viral infection, may result from endosomal destabilization. In the case of adenovirus infection, the mechanism of virus entry into the cytoplasm appears to result in the rupture of endosomes and subsequent NLRP3 activation (45, 46) (Fig. 1). The role for the NLRP3 inflammasome in vivo has been demonstrated for only a few viruses. The most extensively studied of these is IAV infection. At least four separate publications have examined the role for NLRP3 in vivo during IAV infection. The first of these reports described a role for NLRP3 in vitro and in the alveolar spaces of the lung, particularly in macrophages and dendritic cells, but did not observe an overall role for NLRP3 in morbidity and mortality to IAV infection (25). This initial paper reported a role for caspase-1 and ASC in the recruitment of macrophages, dendritic cells, natural killer cells, and neutrophils into the lungs. Viral clearance from the lungs was impaired, as were adaptive immune responses to IAV for caspase-1 and ASC-deficient mice. This included defective antibody isotype class switching and reduced numbers of CD4+ and CD8+ T cells with reduced IFN-γ production (25). Shortly after this publication, we and another group published that NLRP3-deficiency plays a more pronounced role in vivo during IAV infection (12, 22). Deletion of NLRP3, caspase-1, or the adapter ASC resulted in increased mortality and decreased infiltration of neutrophils and monocytes to the lungs in addition to decreases in IL-1β and IL-18 levels in the bronchi alveolar lavage fluid or serum (12, 22). However, neither of these publications reported a role for the NLRP3 inflammasome in adaptive immune responses. In the case of virus burden, one report showed increased viral burden in Nlrp3−/− mice on day 7 after infection (22), whereas we observed no difference at day 6 after infection (12). In addition to a potential role for the NLRP3 inflammasome in adaptive immunity and virus clearance, we demonstrated the importance of NLRP3 in the resolution of inflammation and the proper repair of lung damage as Nlrp3−/− mice had increased amounts of collagen deposition in the lungs and increased necrosis, suggesting defective wound repair (12). A fourth publication addressing the role of NLRP3 in aging during IAV infection was recently published (47). Although aging is known to result in a waning immune response, and plays a particularly important role during IAV infection (48), the exact signaling pathways responsible for waning immunity are not well understood. Stout-Delgado et al. (47) demonstrated that elderly mice have reduced secretion of active IL-1β during IAV infection, despite normal or elevated pro-IL-1β mRNA levels. Elderly mice had reduced expression of NLRP3, ASC, and caspase-1 and reduced caspase-1 activation. This impaired inflammasome activation could be rescued in vitro and in vivo if the mice or cells were infected with IAV and treated with the NLRP3 activator nigericin (47). Although these four reports differ in some of their outcomes, the consensus is that the NLRP3 inflammasome is required for innate immune functions including IL-1β, IL-18, and cellular infiltration during IAV infection. Some of the differences in these reports may be explained by the timing of the experiments (days 5, 6, 7, 8, 10 or 11 after infection), which were not uniform within all of the groups. Furthermore, the infectious doses used were different among the groups (10 PFU-1000 PFU for PR/8 IAV and 106 PFU for HKx31 IAV).
In addition to IAV, the NLRP3 inflammasome was recently shown to play an important role in protection of mice from West Nile virus (WNV) infection (49). Specifically, they showed that IL-1 signaling plays a particularly important role in regulating antiviral responses in the central nervous system (CNS). In Il1r−/− mice, WNV replicated to higher titers in the CNS. Initially, IL-1 signaling was required for cytokine production and immune cell infiltrates at day 6 after infection. However, at later time points, Il1r−/− mice had increased cytokine production and cellular infiltrates and increased neuronal damage, presumably due to increased viral load (49). The importance of inflammasome activation during WNV infection was also confirmed by another group using ASC-deficient mice with similar findings including diminished serum cytokine production but enhanced inflammation and virus replication in the central nervous system at later time points and increased mortality in ASC-deficient mice (50). Interestingly, another group reported that WNV infection activates the NLRP3 inflammasome through the viral glycoprotein CLEC5A binding to C-type lectin receptors on the cell and signaling through the adaptor Syk kinase (51). It is unclear whether this is required for priming the inflammasome or if CLEC5A can directly activate NLRP3 through Syk kinase activation.
The in vivo relevance for NLRP3 with other viruses is only correlative. During varicella zoster virus infection in skin allografts, there is an increased expression of NLRP3 (52). Finally, susceptibility to human immunodeficiency virus (HIV) infection in human patients was associated with several polymorphisms in the Nlrp3 gene (53, 54). However, the functional significance of the polymorphisms needs to be examined, but one of the major difficulties in examining the inflammasome in HIV infection is the lack of a transgenic animal model.
It is clear that the NLRP3 inflammasome plays an important role during infection with a wide variety of viruses. However, much research remains to be done, especially on the in vivo significance of NLRP3. Additionally, the exact mechanism by which viruses and pathogens in general activate NLRP3 will require a concerted effort from biochemical, structural, and molecular aspects. This information would greatly advance our understanding of this ubiquitously important mediator of inflammation allowing for the design of specific inhibitors or adjuvants, which can make use of the NLRP3 inflammasome. Finally, it was discovered after many of these publications that caspase-1−/− mice are actually deficient in both caspase-1 and caspase-11 (55). Although caspase-11 appears to be important for the activation of caspase-1 in response to some bacterial infections (55), the individual roles of caspase-1 and caspase-11 in viral infection should be examined.
NLRC2, a key regulator of viral inflammation
Much of our knowledge regarding the function of NLRC2 is centered on its ability to sense the bacterial cell wall molecule murymyl dipeptide (MDP), which is a constituent of peptidoglycan. Activation of NLRC2 by MDP recruits RIPK2 through CARD-CARD domain interactions. RIPK2 then binds and activates IKKγ and TAK1, resulting in the degradation of the NF-κB inhibitor IκBα and the subsequent nuclear localization of NF-κB (56, 57). NLRC2 also activates MAPKs (p38, ERK, and JNK) (58, 59) through a RIPK2-CARD9 mediated pathway (60). Furthermore, NLRC1 and NLRC2 have been shown to control the induction of autophagy, a cellular process where cytoplasmic membranes engulf proteins and organelles slated for recycling. NLRC1 and NLRC2 are also important for the killing of some bacteria in an autophagy-like process known as zenophagy (10, 61). Finally, NLRC2 has been shown to induce a type I interferon (IFN-α and IFN-β), response to Mycobacterium tuberculosis (MTB) (62). Here, the authors found that recognition of unique peptidoglycans from MTB by NLRC2 resulted in activation of a RIPK2 and IRF5 mediated signaling cascade with type I IFN production. Although NLRC2 has been studied extensively during bacterial infection, its role during viral infection is just emerging.
One study examined the role of NLRC2 during viral infection using a luciferase reporter assay designed to detect activation of the transcription factor IRF3, which is involved in the production of type I IFNs (63). Transfection of cells with synthetic ssRNA and viral ssRNA genomes were found to activate IRF3 in a NLRC2 and MAVS-dependent manner. The important physiological function of NLRC2 in antiviral defense was evident from decreased type I IFN responses, and increased RSV pathogenesis, lung disease and virus susceptibility in deficient mice (63). Similarly, macrophages and mice lacking NLRC2 had reduced IRF3 phosphorylation and diminished production of type I IFNs in response to IAV and parainfluenza viruses (63). Furthermore, Nlrc2−/− cells were deficient in their ability to inhibit VSV replication (63). This work suggests that NLRC2 functions as a viral PRR that is important for the production of type I IFNs in response to RNA viruses and demonstrates that NLRC2 can trigger multiple signaling pathways in response to cytoplasmic pathogens. Furthermore, RSV infection was shown to result in NLRC2 relocalization to mitochondria, where it binds to MAVS (63) (Fig. 2). It should be noted, however, that NLRC2 was not specifically shown to bind ssRNA and only to be required for ssRNA induction of type I IFNs. How NLRC2 is capable of recognizing the presence of both bacterial peptidoglycan and vRNA and the biochemical mechanism that links MAVS to IRF3 activation during NLRC2 signaling require further analysis.
Fig. 2. NLRC2 regulates pro- and anti-inflammatory signaling.
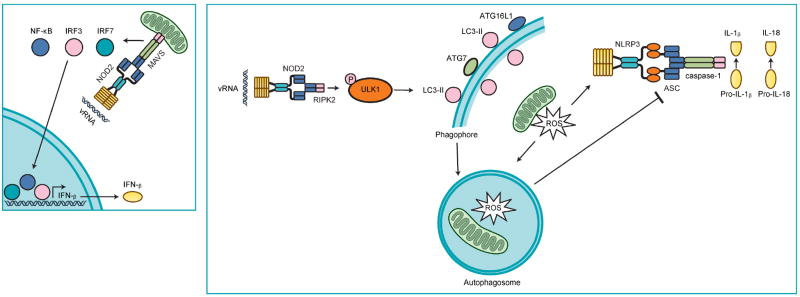
NLRC2 activation occurs during viral infection in response to vRNA and results in the interaction of NLRC2 and MAVS. Activation of MAVS triggers nuclear localization of transcription factors NF-κB, IRF3, and IRF7 and the subsequent production of IFN-β and other proinflammatory cytokines and chemokines. In the absence of NLRC2-MAVS signaling, viruses replicate to higher titers and induce more lung damage. In addition, vRNA mediated activation of NLRC2 results in interaction with RIPK2. RIPK2 then induces the phosphorylation of the autophagy protein ULK1 at Ser555. ULK1 then orchestrates the induction of a specific form of autophagy, known as mitophagy, that engulfs and degrades ROS producing damaged mitochondria. In the absence of NLRC2-RIPK2-mediated mitophagy, damaged mitochondria accumulate in the cell and further enhance NLRP3 inflammasome activation and lung inflammation. Thus, NLRC2 is an important inducer of antiviral signaling through MAVS as well as regulation of inflammasome activation through RIPK2.
NLRC2 has also been implicated in another antiviral pathway through an interaction with 2’-5’-oligoadenylate synthetase type 2 (OAS2) (64). Activation of OAS2 is required for the activation of RNAse L, which degrades viral and cellular RNA and inhibits virus replication (65). The interaction between NLRC2 and OAS2 positively regulated the enzymatic activity of OAS2 in response to poly(I:C), and enhanced the endonuclease activity of RNAse L, thus inhibiting virus replication (64).
Our own work has shown that NLRC2 is required for the regulation of inflammation during viral infection (66). We demonstrated that in response to IAV infection, NLRC2 interacts with the adapter RIPK2. However, this does not lead to NF-κB and MAPK activation as it does during bacterial infection. Instead, this virus induced interaction of NLRC2 and RIPK2 results in the induction of autophagy. In this context, a specific form of autophagy known as mitophagy, where autophagosomes engulf and degrade mitochondria, was required for the removal of damaged mitochondria from IAV-infected cells. In the absence of NLRC2 or RIPK2, more ROS was produced by the presence of more damaged mitochondria and resulted in enhanced NLRP3 inflammasome activation and particularly increased IL-18 production. The result of inflammasome dysregulation in our model was a rapid hyperinflammatory response characterized by neutrophilia and exacerbated NK and CD8+ T-cell responses in the lungs of mice with enhanced mortality (66). Our results demonstrate that an NLRC2-RIPK2 interaction is important during viral infection but in the context of modulating inflammation instead of inducing it. In all, NLRC2 appears to be a central regulator of inflammation during viral infection, controlling both pro- and anti-inflammatory events (Fig. 2).
The developing role for NLRX1
There are many reports examining the importance of NLRX1 during viral infection. NLRX1 was initially characterized as a mitochondrially targeted NLR that binds to the antiviral signaling adapter protein MAVS and suppresses MAVS-mediated activation of NF-κB and IRF3 transcription factors during infection with SeV, sindbis virus, or treatment with vRNA analogues resulting in the regulation of IFN-β, IL-6, TNF-α, and other proinflammatory cytokines (67). This initial report utilized overexpression of NLRX1, luciferase reporter assays, siRNA knockdown, and other molecular approaches to characterize the role of NLRX1 with MAVS. Another group reported that overexpression of NLRX1 actually generated several artifacts which lead to reduced signal in luciferase reporter assays, suggesting that NLRX1 may not be involved in MAVS signaling (68). However, Nlrx1−/− mice were subsequently generated and their responses to viral and non-viral stimuli examined. In vivo, Allen et al. (69) demonstrated that NLRX1 is critical for controlling lung inflammation to IAV infection or intranasal delivery of LPS. They further demonstrated that Nlrx1−/− mice have enhanced production of IFN-β and IL-6 with more cellular infiltrates in the lung. They also examined the responses of embryonic fibroblasts from Nlrx1−/− mice infected with multiple viruses including simian virus 5 (SV5), EMCV, SeV, VSV, IAV or treated with transfected poly(I:C). These experiments demonstrated that viruses, which activate RIG-I, resulted in enhanced production of IFN-β in the absence of NLRX1. However, EMCV, which activates MDA5, induced IFN-β levels similar to WT cells, suggesting that NLRX1 regulated RIG-I and MAVS interactions specifically. This report also demonstrated a role for NLRX1 in regulating TLR signaling after LPS stimulation through interactions with TRAF3 and TRAF6 (69). Although this finding was simultaneously confirmed by another group (70), one question that arises is how NLRX1, which is localized to the mitochondria, could affect TLR-mediated NF-κB signaling from the cell membrane? Recently, this group showed that NLRX1 mediates its effects through an interaction with mitochondrial Tu translation elongation factor (TUFM). Lei et al. (71) demonstrated that NLRX1 interacts with TUFM and that TUFM also interacts with MAVS to inhibit IFN-β production. In addition, Lei et al. (71) reported that NLRX1 and TUFM were required for the efficient induction of autophagy in response to VSV infection. TUFM was able to interact with autophagy proteins ATG5, ATG12, and ATG16L1. Finally, they demonstrated that both reduced autophagy and increased IFN-β production in NLRX1 or TUFM-deficient cells contributed to inhibition of VSV replication (71).
Although several publications by the same group indicate a role for NLRX1 in antiviral signaling, a second group has published several papers that indicate NLRX1 is important for regulating ROS production. Tattoli et al. (72) also found that NLRX1 localizes to the mitochondria but reported that NLRX1 overexpression resulted in increased ROS production. Following overexpression of NLRX1 and stimulation with TNF-α, Shigella flexneri, and poly(I:C), this group observed that NF-κB and JNK activation were increased in conjunction with increased ROS (72). Subsequently, this group published that NLRX1 has an N-terminal localization sequence for the mitochondrial matrix (73). Using multiple fractionation, colocalization, and trypsin-based protection assays, they determined that NLRX1 is localized to the mitochondrial matrix and found that NLRX1 interacts with UQCRC2, a member of the electron transport chain complex III (73). The discovery that NLRX1 can interact with the electron transport chain provides a potential mechanism by which NLRX1 induces ROS production. Finally, this group generated their own Nlrx1−/− mice and found no role for NLRX1 in the regulation of MAVS or any other inflammatory genes following virus infection (74). Interestingly, a third group found that NLRX1 interacts with UQCRC2 but they did not detect any differences in NF-κB or JNK activation in Nlrx1−/− mice or cells. They were also unable to detect any role for NLRX1 in MAVS signaling (75). A fourth group has published that NLRX1 regulates ROS production during infection with the bacteria Chlamydia trachomatis, and knockdown of NLRX1 results in reduced ROS and reduced growth of C. trachomatis. In this model, NLRX1 knockdown diminished rather than enhanced IFN-β production (76), which is in contrast to the reports during viral infection discussed above (67, 69).
A fifth group recently solved the crystal structure for NLRX1 and used several biochemical experiments to demonstrate that NLRX1 can bind directly to ssRNA and dsRNA (77). The fact that NLRX1 can bind directly to RNA would support a role for this protein in innate antiviral signaling, possibly through MAVS. In addition, this group found that NLRX1 was required for ROS production (77). These findings raise additional questions. If NLRX1 is in the mitochondrial matrix and activates ROS production, then how does vRNA enter the matrix to engage NLRX1 and activate its function? Finally, a polymorphism in Nlrx1 that is associated with increased risk of chronic hepatitis B virus (HBV) infection was recently reported, suggesting that NLRX1 may have some role in viral infection in human patients (78). However, the functional significance of this polymorphism has not been examined. The one aspect of NLRX1 that is consistent among these groups is its mitochondrial localization, although where it resides in the mitochondria is under debate. The role for NLRX1 during infection and inflammation is also not clear. Individual laboratories consistently produce different results, making it difficult to draw any overall conclusions. Clearly, there is a need for other research groups to weigh in on the role of NLRX1 during viral infection, as well as other infectious disease or immune models.
NLRC5 and antigen presentation
NLRC5 is the last NLR that has been extensively examined in the context of virus infection. NLRC5 was initially reported to negatively regulate RIG-I and MDA5 activation of MAVS and subsequent production of IFN-β during VSV infection or intracellular treatment with poly(I:C) (79). This inhibitory function was reportedly the result of outcompeting MAVS for interaction with RIG-I or MDA5, thus blocking MAVS signaling (79). This same group subsequently generated Nlrc5−/− mice. Tong et al. (80) demonstrated that NLRC5 is upregulated following IFN-β or IFN-γ treatment in a STAT1-dependent signaling manner. They also reported that macrophages and embryonic fibroblasts from Nlrc5−/− mice produce increased IFN-α/β in responses to VSV infection. However, in vivo infection with VSV only resulted in higher IFN-β levels at very early time points (6 h), and there was no significant difference in virus replication or survival of Nlrc5−/− mice (80). These results indicate that although NLRC5 may be able to regulate IFN-β, its physiological role in this pathway may be minimal or may only be important under certain conditions. However, two other groups reported that NLRC5 knockdown in multiple cell lines and primary human fibroblasts results in a reduction in IFN-α/β after infection with SeV or cytomegalovirus (CMV), suggesting that NLRC5 may actually potentiate antiviral signaling (81, 82). Finally, three additional groups have generated Nlrc5−/− mice and reported that antiviral and pro-inflammatory signaling are normal under all conditions including TLR and RLR stimulation, Newcastle disease virus infection, HSV infection, SeV infection, and infection with several different bacteria as well (83-85). The fact that all four in vivo reports found little or no role for NLRC5 in regulating IFN levels, virus replication or pathology suggest that this is not the main function of NLRC5. In contrast, NLRC5 was reported to play an important role in the production if MHC class I and antigen-processing and presentation genes in vivo by multiple groups, regardless of whether these groups observed any function for NLRC5 in IFN production (80, 84, 85). Finally, all reports thus far agree that NLRC5 is expressed mainly in hematopoietic cells and is induced in response to IFNs. The role for NLRC5 as a MHC class I transactivator seems to be greatest for T cells, B cells, and NK cells, but also plays some role in macrophages and DCs (84, 85). Mechanistically, NLRC5 is localized to the nucleus following activation of cells and binds to MHC class I promoters to activate transcription (18), likely through the regulation of chromatin remodeling (85). In all, NLRC5 appears to be a bona fide MHC class I transactivator. Although NLRC5 may also regulate antiviral signaling, this will require further examination. In the future, it will be of interest to see how important NLRC5 is for the CD8+ T-cell responses to virus infection, as this has yet to be examined in detail. For additional information on the role of NLRC5, there are several excellent reviews published recently that focus solely on NLRC5 (86, 87).
Clinical relevance
With regards to humans, as mentioned earlier, genome-wide association studies conducted for HIV and HBV lead to the detection of polymorphisms in the Nlrp3 (53, 54) or Nlrx1 genes, respectively, in human patients (78), which were associated with increased susceptibility to infection. In addition, many of the reports above utilized primary human cells, thus indicating that these NLRs are functionally relevant in human cells. Recently, a report demonstrated that IL-1β, NLRP3, caspase-1, NLRC2, and RIPK2 were all elevated during infection with IAV (88). These proteins were elevated in subjects who presented with clinical signs of IAV infection but were unaltered or diminished in asymptomatic subjects. Intriguingly, superoxide dismutase (SOD1) and the serine/threonine kinase 25 (STK25), both proteins associated with protection during oxidative stress, were reduced in symptomatic subjects and enhanced in asymptomatic subjects (88). As ROS has a role in NLRP3 inflammasome activation, these data may indicate that asymptomatic subjects are able to inhibit ROS production and reduce inflammasome activation. Alternatively, these subjects may more effectively inhibit virus replication or virus-induced cellular damage, which leads to less NLRP3 inflammasome activation. These findings not only indicate that NLR-mediated responses are triggered by IAV infection but that they play a role in the development or prevention of clinical symptoms during IAV infection (89). In further support of the clinical relevance of NLRs during IAV infection, another group found that NHBE cells from asthmatic patients infected with IAV produced higher levels of IL-1β and other pro-inflammatory cytokines and had increased expression of caspase-1, NLRC2, RIPK2, and NLRC5 (90). These findings also indicate that alterations in NLR regulation are partially responsible for the increased inflammation and susceptibility of asthmatics to IAV infection.
Although the relevance during IAV infection is evident, the human clinical relevance of NLRs during viral infection in general is dramatically lacking. In fact most of the clinical relevance of NLRs is associated with autoimmune or autoinflammatory diseases. NLRC2 polymorphisms are known to play a role in inflammatory bowel disease and Blau’s syndrome, whereas NLRP3 polymorphisms are associated with gouty arthritis and periodic fever syndrome. This spectrum of diseases includes familial cold autoinflammatory syndrome (FCAS) and Muckle-Wells syndrome (MWS) among others. What is not known is whether these same patients are more prone or more resistant to infections with viruses or other pathogens. Examination of the clinical relevance of NLRs will not only help predict susceptibility to infection but will also improve our understanding of the role these proteins play in the immune system and has enormous potential for translation into vaccine adjuvants or immunotherapies for infections, cancer, and other diseases.
Concluding remarks
The role of the NLRP3 inflammasome during viral infections is clearly of seminal importance (Fig. 1 and Table 1). Not only is the lack of inflammasome activation a cause for morbidity and mortality, but the overt activation of the NLRP3 inflammasome also results in immune mediated pathology and death. NLRC2 is also important for its roles in the induction of IFN-α/β and antiviral responses. However, our most recent findings indicate that NLRC2 serves as a critical regulator of the NLRP3 inflammasome during viral infection. Therefore, NLRC2 plays an apical role in virus infection by inducing productive antiviral signaling and at the same time limiting inflammasome activation to only that which is needed to resolve the infection (Fig. 2). Additional NLR family members NLRC5 and NLRX1 also play roles during virus infection. However, NLRX1 in particular needs to be examined in greater detail to determine what role it plays. NLRC5 appears to be important for MHC-I expression and will likely play an important role in the generation of cytotoxic T lymphocyte responses during viral infection. However, the role for NLRC5 as a modulator of antiviral signaling and inflammation in vivo appears to be dispensable. Finally, the importance of other NLR family members in virus infection is significantly lacking. Future research into the role of other NLRs will also help this field to progress. NLRP6-, NLRP12-, and NLRP10-deficient mice have all been generated but have yet to be examined in the context of virus infection.
Acknowledgments
We thank John Lukens for helpful editing of the manuscript and Tim Hammond for help in making and designing the figures. This work was supported by grants to T.-D.K. from the National Institutes of Health through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award Number AR056296), the National Institute of Allergy and Infectious Disease (Award Number AI101935), and the National Cancer Institute (Award Number CA163507). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also funded by the American Lebanese Syrian Associated Charities to T.-D.K.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 2012;3:414. doi: 10.3389/fimmu.2012.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 5.Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1:226–232. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Harton JA, Linhoff MW, Zhang J, Ting JP. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol. 2002;169:4088–4093. doi: 10.4049/jimmunol.169.8.4088. [DOI] [PubMed] [Google Scholar]
- 7.Inohara N, Nunez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20:6473–6481. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 8.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 9.Monie TP. NLR activation takes a direct route. Trends Biochem Sci. 2013;38:131–139. doi: 10.1016/j.tibs.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Anand PK, et al. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2011;286:42981–42991. doi: 10.1074/jbc.M111.310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185:3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masters SL, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaki MH, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand PK, Kanneganti TD. Targeting NLRP6 to enhance immunity against bacterial infections. Future Microbiol. 2012;7:1239–1242. doi: 10.2217/fmb.12.94. [DOI] [PubMed] [Google Scholar]
- 16.Anand PK, et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen IC, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meissner TB, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci USA. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo J, Rowe M. Herpesviruses placating the unwilling host: manipulation of the MHC class II antigen presentation pathway. Viruses. 2012;4:1335–1353. doi: 10.3390/v4081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupfer CR, Kanneganti TD. The role of inflammasome modulation in virulence. Virulence. 2012;3:262–270. doi: 10.4161/viru.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 22.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rintahaka J, Lietzen N, Ohman T, Nyman TA, Matikainen S. Recognition of cytoplasmic RNA results in cathepsin-dependent inflammasome activation and apoptosis in human macrophages. J Immunol. 2011;186:3085–3092. doi: 10.4049/jimmunol.1002051. [DOI] [PubMed] [Google Scholar]
- 24.Rajan JV, Warren SE, Miao EA, Aderem A. Activation of the NLRP3 inflammasome by intracellular poly I:C. FEBS Lett. 2010;584:4627–4632. doi: 10.1016/j.febslet.2010.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman MM, McFadden G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-kappaB innate response pathways. J Virol. 2011;85:12505–12517. doi: 10.1128/JVI.00410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence TM, Hudacek AW, de Zoete MR, Flavell RA, Schnell MJ. Rabies Virus is Recognized by the NLRP3 Inflammasome and Activates IL-1beta Release in Murine Dendritic Cells. J Virol. 2013;87:5848–5857. doi: 10.1128/JVI.00203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delaloye J, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Dey M, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stasakova J, et al. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J Gen Virol. 2005;86:185–195. doi: 10.1099/vir.0.80422-0. [DOI] [PubMed] [Google Scholar]
- 33.He Y, Franchi L, Nunez G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur J Immunol. 2013;43:1147–1152. doi: 10.1002/eji.201243187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 35.Pothlichet J, et al. Type I IFN Triggers RIG-I/TLR3/NLRP3-dependent Inflammasome Activation in Influenza A Virus Infected Cells. PLoS Pathog. 2013;9:e1003256. doi: 10.1371/journal.ppat.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikegame S, Takeda M, Ohno S, Nakatsu Y, Nakanishi Y, Yanagi Y. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. J Virol. 2010;84:372–379. doi: 10.1128/JVI.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komune N, Ichinohe T, Ito M, Yanagi Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1beta secretion. J Virol. 2011;85:13019–13026. doi: 10.1128/JVI.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajan JV, Rodriguez D, Miao EA, Aderem A. The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J Virol. 2011;85:4167–4172. doi: 10.1128/JVI.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito M, Yanagi Y, Ichinohe T. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS Pathog. 2012;8:e1002857. doi: 10.1371/journal.ppat.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin SH: a viral recognition pathway used by the host to signal inflammasome activation. Thorax. 2013;68:66–75. doi: 10.1136/thoraxjnl-2012-202182. [DOI] [PubMed] [Google Scholar]
- 42.Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. Hepatitis C virus activates interleukin-1beta via caspase-1-inflammasome complex. J Gen Virol. 2012;93:235–246. doi: 10.1099/vir.0.034033-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Segovia J, et al. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaushik DK, Gupta M, Kumawat KL, Basu A. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS One. 2012;7:e32270. doi: 10.1371/journal.pone.0032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barlan AU, Danthi P, Wiethoff CM. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology. 2011;412:306–314. doi: 10.1016/j.virol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012;188:2815–2824. doi: 10.4049/jimmunol.1103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valkenburg SA, et al. Early priming minimizes the age-related immune compromise of CD8(+) T cell diversity and function. PLoS Pathog. 2012;8:e1002544. doi: 10.1371/journal.ppat.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos HJ, et al. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar M, et al. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J Virol. 2013;87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu MF, et al. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood. 2013;121:95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]
- 52.Nour AM, Reichelt M, Ku CC, Ho MY, Heineman TC, Arvin AM. Varicella-zoster virus infection triggers formation of an interleukin-1beta (IL-1beta)-processing inflammasome complex. J Biol Chem. 2011;286:17921–17933. doi: 10.1074/jbc.M110.210575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pontillo A, Brandao LA, Guimaraes RL, Segat L, Athanasakis E, Crovella S. A 3’UTR SNP in NLRP3 gene is associated with susceptibility to HIV-1 infection. J Acquired Immun Def Syndr. 2010;54:236–240. doi: 10.1097/QAI.0b013e3181dd17d4. [DOI] [PubMed] [Google Scholar]
- 54.Pontillo A, Oshiro TM, Girardelli M, Kamada AJ, Crovella S, Duarte AJ. Polymorphisms in inflammasome’ genes and susceptibility to HIV-1 infection. J Acquired Immun Def Syndr. 2012;59:121–125. doi: 10.1097/QAI.0b013e3182392ebe. [DOI] [PubMed] [Google Scholar]
- 55.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 56.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 57.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girardin SE, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JH, et al. RICK/RIP2 Mediates Innate Immune Responses Induced through Nod1 and Nod2 but Not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 60.Hsu YM, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 61.Travassos LH, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 62.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabbah A, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dugan JW, et al. Nucleotide oligomerization domain-2 interacts with 2’-5’-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol Immunol. 2009;47:560–566. doi: 10.1016/j.molimm.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hovanessian AG. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2’-5’oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 2007;18:351–361. doi: 10.1016/j.cytogfr.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Lupfer C, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 68.Ling A, et al. Post-transcriptional inhibition of luciferase reporter assays by the Nod-like receptor proteins NLRX1 and NLRC3. J Biol Chem. 2012;287:28705–28716. doi: 10.1074/jbc.M111.333146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allen IC, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia X, et al. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei Y, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–946. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tattoli I, et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009;122:3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soares F, Tattoli I, Wortzman ME, Arnoult D, Philpott DJ, Girardin SE. NLRX1 does not inhibit MAVS-dependent antiviral signalling. Innate immunity. 2012 doi: 10.1177/1753425912467383. [DOI] [PubMed] [Google Scholar]
- 75.Rebsamen M, Vazquez J, Tardivel A, Guarda G, Curran J, Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdul-Sater AA, et al. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J Biol Chem. 2010;285:41637–41645. doi: 10.1074/jbc.M110.137885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong M, Yoon SI, Wilson IA. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity. 2012;36:337–347. doi: 10.1016/j.immuni.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Q, et al. Rare inborn errors associated with chronic hepatitis B virus infection. Hepatology. 2012;56:1661–1670. doi: 10.1002/hep.25850. [DOI] [PubMed] [Google Scholar]
- 79.Cui J, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tong Y, Cui J, Li Q, Zou J, Wang HY, Wang RF. Enhanced TLR-induced NF-kappaB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. 2012;22:822–835. doi: 10.1038/cr.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neerincx A, et al. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J Biol Chem. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuenzel S, et al. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol. 2010;184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 83.Kumar H, et al. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J Immunol. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- 84.Biswas A, Meissner TB, Kawai T, Kobayashi KS. Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J Immunol. 2012;189:516–520. doi: 10.4049/jimmunol.1200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robbins GR, Truax AD, Davis BK, Zhang L, Brickey WJ, Ting JP. Regulation of class I major histocompatibility complex (MHC) by nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins. J Biol Chem. 2012;287:24294–24303. doi: 10.1074/jbc.M112.364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- 87.Lamkanfi M, Kanneganti TD. Regulation of immune pathways by the NOD-like receptor NLRC5. Immunobiology. 2012;217:13–16. doi: 10.1016/j.imbio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y, et al. Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza a infection. PLoS Genet. 2011;7:e1002234. doi: 10.1371/journal.pgen.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bortz E, Garcia-Sastre A. Predicting the pathogenesis of influenza from genomic response: a step toward early diagnosis. Genome Med. 2011;3:67. doi: 10.1186/gm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bauer RN, et al. Influenza enhances caspase-1 in bronchial epithelial cells from asthmatic volunteers and is associated with pathogenesis. J Allergy Clin Immunol. 2012;130:958–967. e914. doi: 10.1016/j.jaci.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson KE, Chikoti L, Chandran B. HSV-1 Infection Induces Activation and Subsequent Inhibition of the IFI16 and NLRP3 Inflammasomes. J Virol. 2013;87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]