Abstract
Purpose
The purpose of the current study was to assess the effect of newly synthesized Curcumin analogs on COX-2 protein by molecular docking studies and by assessments of the effect of one such analog (CDF) on nuclear factor NF-κB and PGE2. In addition, we have determined the pharmacokinetics and tissue distribution of CDF in mice compared to Curcumin.
Methods
Molecular docking on COX-2 protein was assessed by standard computer modeling studies. PGE2 assay in conditioned media was done utilizing high sensitivity immunoassay kit following manufacturer’s instructions, while NF-κB was done by routine EMSA. Serum pharmacokinetics and tissue distribution studies were carried out using the validated high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods.
Results
The molecular docking showed that fluorocurcumin analogs do not introduce any major steric changes compared to the parent Curcumin molecule, which was consistent with down-regulation of NF-κB and reduced PGE2 levels in cells treated with CDF. Pharmacokinetic parameters revealed that CDF had better retention and bioavailability and that the concentration of CDF in the pancreas tissue was 10-fold higher compared to Curcumin.
Conclusion
Our observations clearly suggest that the bioavailability of CDF is much superior compared to Curcumin, suggesting that CDF would be clinically useful.
Keywords: COX-2, curcumin, fluorocurcumin, pharmacokinetics
INTRODUCTION
Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States; an estimated 37,000 new cases and almost equal number of deaths occurred in 2008 (1). The high mortality rate is in large part due to the high incidence of metastatic disease at initial diagnosis, the aggressive nature of the tumor, and the lack of effective systemic therapies. As a result, the disease-free survival time even after complete resection of the tumor and adjuvant administration of gemcitabine is less than a year (2). Since the administration of cytotoxic therapeutic agents has been found to be inadequate, there is a clear need for the development of new and novel chemotherapeutics, as well as targeted agents for the treatment of pancreatic cancer, because there is no curative therapy for this deadly disease.
There have been many reports linking inflammatory mediators including cytokines (e.g. TNF-alpha, IL-6, IL-8 and interferon-gamma (3–5)), transcription factors like NF-κB (6), and pro-inflammatory enzymes like Cyclooxygenase, as well as lipooxygenase isoforms (7, 8), with the development and progression of pancreatic cancer. The expression of COX-2 has been found to be increased in a variety of malignancies including pancreatic cancer (8-11). It has been well-established that COX-2-mediated synthesis of prostaglandins (PGE2) favors the growth of tumor cells by stimulating proliferation and angiogenesis (12). The COX-2 expression is regulated in part by transcriptional mechanism mediated by the transcription factor NF-κB, suggesting that inactivation of NF-κB pathway could also inhibit pancreatic cancer progression (13). This requirement is amicably met by Curcumin (diferuloylmethane) which is a naturally occurring active polyphenolic yellow pigment obtainable from the rhizomes of perennial herb Curcuma longa (14-23). In addition, the biological effects of Curcumin appear to be pleiotropic (15), suggesting the importance of Curcumin as a preventive and/or therapeutic agent against human malignancies. Most importantly, Curcumin has been reported to be very safe because it does not cause any adverse effects, even up to doses as high as 8 gm per day in humans, and no development of resistance against Curcumin has been reported (24). However, the bioavailability of Curcumin is a major concern limiting its therapeutic utility, since as much as 75% of Curcumin gets excreted in the feces, indicating its poor absorption in the gut (25). Piperine, a known inhibitor of hepatic and intestinal glucuronidation has been shown to increase the bioavailability of Curcumin (26,27). In addition, different drug delivery systems, including liposomes, micelles, phospholipid complexes, and nanoparticles, have also been employed to improve Curcumin’s bioavailability with disappointing and unacceptable results (27-32).
Since the chemical structure of Curcumin plays a crucial role in its biological activity, it is anticipated that enhanced absorption of Curcumin without loss in its activity can be achieved by preparing its appropriate analogs (33). Further studies have reported that cyclopentanone and cyclohexanone analogs have antibacterial properties indicating that of heteroaryl, and long chain substituents may enhance the activity of these compounds (34,35). More recently, pyrazolic and isoxaxolic analogs of Curcumin have also been prepared and evaluated for their neuroprotective activity (36). Another strategy employed to improve biological activity of Curcumin is through metal complexation (37), and some enhancement in anticancer activity has been reported by Kuttan et al. (38).
In our group, we have addressed the problem of slowing down the rapid metabolism of Curcumin by preparing its Knoevenagel condensates and their metal complexes, which were found to be more potent as anticancer agents than Curcumin, suggesting that such an approach may yield desirable analogs (39). Recently, we have explored the effects of introducing bioisosteric fluoro substitution in the Knoevenagel condensates and their corresponding Schiff bases (40) with the anticipation that the higher metabolic stability of C–F bond (than C–H or C–OH bonds) would slow down the metabolic breakdown of Curcumin, yielding an improved pharmacokinetic profile. We also found that amongst such analogs, CDF was superior in inhibiting the proteosome and cell growth and in inducing apoptosis (40). Based on these encouraging results, we have conducted studies to confirm the superiority of the new analog in the inactivation of NF-κB and one of its downstream targets, COX-2, through molecular modeling and corresponding bioassays. Most importantly, here we report, for the first time, the pharmacokinetic and tissue distribution of CDF in mice compared to Curcumin which clearly show that CDF is highly bioavailable, and especially accumulates in the pancreas, suggesting that CDF would be a better anti-tumor agent for the prevention and/or treatment of pancreatic cancer as well as other human malignancies.
EXPERIMENTAL DESIGN, MATERIALS AND METHODS
Cell Culture and Reagents
The human pancreatic carcinoma cell lines BxPC-3 and MIA PaCa-2 were obtained from American Type Culture Collection (Manassas, VA). The cell lines were maintained in continuous exponential growth by twice-a-week passaging in Dulbecco-modified Eagle’s medium (DMEM; Life Technologies, Inc., Gaithesburg, MD) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 10 mg/ml streptomycin. CDF and Curcumin were dissolved in DMSO at 10 mM/L concentration, aliquoted and stored at −20ºC and diluted to the desired concentration before use by media.
Materials
Curcumin and other chemicals required for synthesis of its analogs were obtained from Sigma-Aldrich (St. Louis, MO). Fluorocurcumin analogs were synthesized by a method that was described earlier by our laboratory (40).
Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared from treated samples, and EMSA was performed by incubating 10µg of nuclear extract with IRDye™–700 labeled NF-κB oligonucleotide as described earlier by Banerjee et al. (41). The DNA-protein complex formed was visualized by Odyssey Infrared Imaging System using Odyssey Software Release 1.1.
PGE2 Immunoassay for Quantitation of Prostaglandin E2
For determination of PGE2 levels, the BxPC-3 and MIAPaCa-2 cells were either untreated or treated with CDF (1 or 4 μM) and/or curcumin (1 or 4 μM) for 24 h. The conditioned medium was collected and analyzed for PGE2 concentration according to manufacturer’s protocol using PGE2 high sensitivity immunoassay kit (R & D Systems, Minneapolis, MN). The optical density was measured at 450 nm and concentration of PGE2 was calculated from the standard curve. Results are expressed as PGE2 in pg/106 cells. Statistical comparison between treatment groups and untreated control was assessed by paired t-test.
Docking Studies
All calculations were performed using Auto Dock 3.05 software. The crystal structure of COX-2 protein was obtained from PDB ID (6COX). The active site of the enzyme was defined to include residues ALA562, GLU 346, GLN 350 within 0.65A radius to any of the inhibitor atoms. The Auto Dock 3.05 program, which is an automated docking program, was used to dock all seven fluorocurcumin analogs, as well as parent Curcumin molecule in the active site of the COX-2 enzyme. For each compound, the most stable docking model was selected based upon conformation of best scored predicted by the Auto Dock scoring function. The compounds were energy minimized with a MMFF94 force-field until the gradient convergence value of 0.05 kcal/mol was reached using distance-dependence dielectric function (ε=4r).
Experimental Animals
Seven-to-eight-week-old female ICR-SCID mice were purchased from Taconic Farms (Germantown, NY). The mice were housed and maintained under sterile conditions and were used in accordance with Animal Care and Use Guidelines of Wayne State University. Mice received Lab Diet 5021 (Purina Mills, Inc., Richmond, IN).
Pharmacokinetics and Tissue Distribution Studies
The pharmacokinetics and tissue distribution of Curcumin and CDF were examined in female mice. The mice were randomly divided into two groups, each with 18 mice. One group was given a single dose of Curcumin (250 mg/kg) diluted in 0.1 ml volume of sesame oil by intragastric intubation, and the other was similarly administered with a single dose of CDF (250 mg/kg). Blood and tissue samples were harvested before initiation of treatment (0 h) and at 1, 2, 4, 6, 8, 12, 16, and 24 h following the intragastric administration. At each time-point, two mice were euthanized and ~200 μL blood was collected by cardiac puncture, and tissues (i.e., liver, lung, kidney, heart, pancreas, and colon) were harvested, washed free of blood with PBS, blotted dry, weighed, and stored at −80°C until analysis. The collected blood samples were allowed to clot and were centrifuged, and serum was separated and stored at −80°C until analysis.
Bioanalytical Assay
Curcumin and CDF in mouse serum and tissue samples were determined using the validated high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods as described below. The tissue samples were homogenized in 5 volumes of ice-cold 0.9% saline using VDI 12 homogenizer (VWR, USA). A 100 μL aliquot of serum or 250 μL aliquot of tissue homogenate was spiked with 500 μL (for serum) or 1 mL (for tissue) ethyl acetate (containing zileuton as internal standard 50 ng/mL). The mixture was vortex-mixed and centrifuged at 14,000 rpm for 5 min, and the top layer was transferred and evaporated to dryness under a stream of nitrogen in a water bath at 50°C±5°C. The residue was reconstituted in 100 μL of methanol/water containing 0.45% formic acid (70:30, v/v), and the mixture was centrifuged at 14,000 rpm for 5 min. One-hundred micro liters of the supernatant were injected into the HPLC and separated on a Waters XTerra MS column (2.1×50 mm, 3.5 μm i.d.) with a mobile phase consisting of methanol/water containing 0.45% formic acid (70:30, v/v) at a flow rate of 0.2 mL/min. The column effluent was monitored using a Waters Quattro Micro™ triple quadruple mass-spectrometric detector equipped with electrospray ionization source (Milford, MA, USA). Curcumin and CDF were monitored in the negative ionization mode at the transition of m/z, 367.1→148.8 and 491.1→216.9, respectively. The internal standard zileuton was monitored in the positive mode at the transition of m/z, 237.1→160.8. The calibration curves for Curcumin and CDF were constructed over the concentration range of 5 to 2,000 and 5 to 10,000 ng/mL, respectively, for the serum and tissue samples. The intra- and inter-day precision and accuracies of the assay was <15%.
Pharmacokinetic Data Analysis
Serum pharmacokinetic parameters were estimated using non-compartmental analysis with WinNonlin version 5.2 (Pharsight Corporation, Cary, NC). The maximum serum concentration (Cmax) and the time of occurrence for maximum concentration (Tmax) were obtained by visual inspection of the serum concentration-time curve after the drug administration. The total area under the serum concentration-time curve from time zero to the last measurable time point (AUClast) was calculated using the linear and logarithmic trapezoidal method for ascending and descending serum concentrations, respectively.
RESULTS AND DISCUSSION
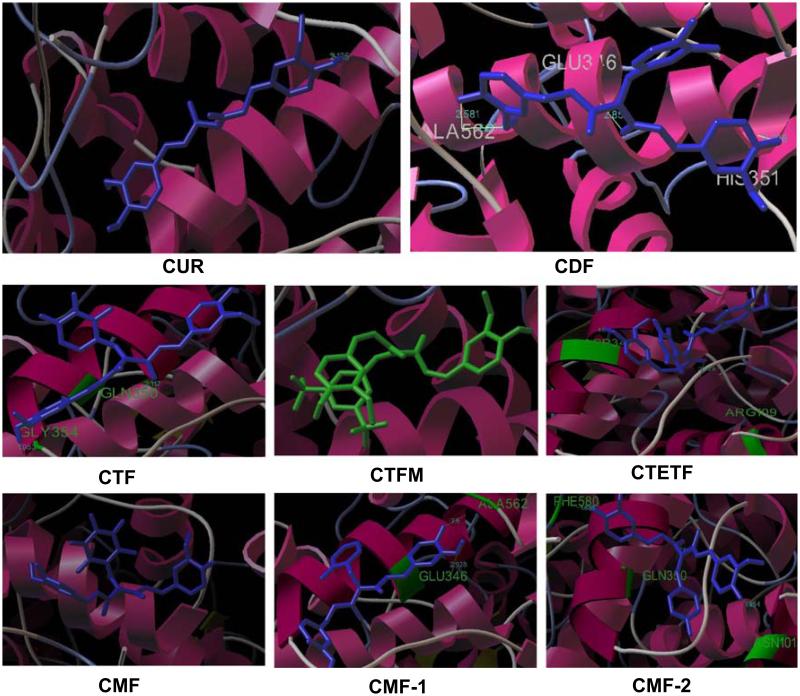
Since antioxidant property of Curcumin is a very crucial descriptor of most of its biological activities, we initially evaluated the antioxidant potential of all fluorocurcumin analogs synthesized presently using DPPH radical scavenging assay, which demonstrated significant antioxidant activity for CDF compound showing the lowest IC50 value (0.05µM) (data not shown), which was lower than parent Curcumin molecule (35µM). These results are consistent with published data by Sompran et al. (42). Since CDF also exhibited low IC50 value for the anti-proliferative activity against COX-2 positive BxPC-3 pancreatic cancer cells by MTT assay in our earlier study (40), we were prompted to perform molecular docking studies to understand ligand-protein interactions and COX-2 selectivity of the new analogs. All fluorocurcumin analogs were found to dock into the active site of COX-2, confirming that fluoro substitution does not introduce any major steric changes in the parent Curcumin molecule except to allow more hydrogen bonding interactions (Fig. 1 and Table I). The binding energies of these analogs were in the range −2.6 to −8.56 kcal/mol compared to Curcumin’s −5.71 kcal/mol. The lower interaction energy observed for CDF analog rationalizes the tighter binding of this compound in the active site of COX-2 than other analogs. In our docking experiments, Curcumin showed only one H-bonding interaction with ALA562. On the other hand the most potent fluoro analog, viz. CDF exhibits four H-bonding interactions involving residues GLU 346, PHE 580, ASN101 and GLN 350, respectively. All other compounds (except CTFM and CPF) exhibited a maximum of two H-bonding interactions, wherein the residue ALA562 is being common with parent Curcumin. Favorable van der Waals interactions between styryl carbon atoms and the hydrophobic residues such as GLU 346(3.01A), SER 353(3.13A), between methoxy group of CDF and HIS 351, ALA 582 contributed to stabilize the ligand-enzyme complexes (Fig. 1). The lower liposolubility observed for the CDF analog suggests that it should have slower metabolism with enhanced pharmacokinetic profile, which was further confirmed as presented below.
Fig. 1.
Binding of fluorocurcumin analogs into the active site of COX-2 as assessed by computer modeling studies.
Table I.
Docking Results and Consensus Scores of Fluorocurcumin Analogs
| Molecules | Docking Energy (kcal/mol) | Binding Energy (kcal/mol) | No. of H Bonds | H-bonding Residues | Log P |
|---|---|---|---|---|---|
| CUR | −7.78 | −5.71 | 1 | ALA 562 | 6.330 |
| CDF | −9.93 | −7.91 | 4 | GLU 346 | 4.321 |
| PHE 580 | |||||
| ASN 101 | |||||
| GLN 350 | |||||
| CTF | −10.31 | −7.83 | 2 | GYL 354 | 4.280 |
| GLN 360 | |||||
| CTFM | −9.5 | −6.15 | – | – | 4.160 |
| CTETF | −9.27 | −7.36 | 2 | ALA 562 | 4.700 |
| GLN 192 | |||||
| CPF | −10.61 | −8.56 | – | – | 4.606 |
| CMF-1 | −6.84 | −2.61 | 2 | ASP 347 | 5.102 |
| AGN 109 | |||||
| CMF-2 | −9.63 | −7.17 | 2 | ALA 562 | 5.288 |
| GLU 346 |
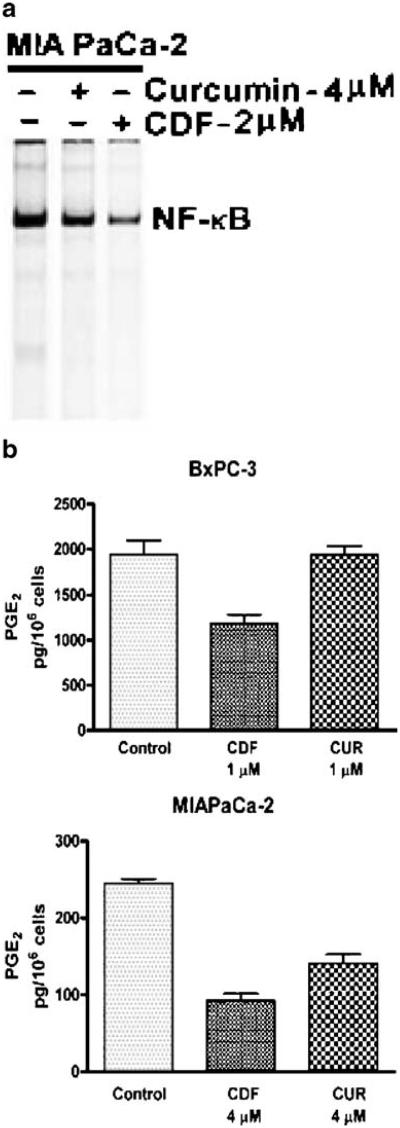
Since we found that CDF docks into the active site of COX-2, which is transcriptionally regulated by NF-κB, we believed that similar to Curcumin, CDF may also have an effect on the nuclear transcription factor NF-κB and also COX-2 activity, which was assessed by measuring the effects of CDF on NF-κB DNA-binding activity in MIAPaCa-2 cells, and PGE2 production in both MIAPaCa-2 and BxPC-3 pancreatic cancer cells. The efficacy of CDF was compared with Curcumin. PGE2 assay was performed using COX-2 over-expressing BxPC-3 and MIAPaCa-2 cells using CDF (1 or 4 μM) compared to similar concentration of Curcumin (1 or 4 μM). As widely reported, Curcumin caused down-regulation of NF-κB, but the effect was more pronounced with a low concentration of CDF. Additionally, we found that both drugs caused a significant decrease in the PGE2 levels in MIAPaCa-2 cells with a p value of 0.0076 with Curcumin and 0.0024 with CDF (Fig. 2B). However, in BxPC-3 cells, we found a significant decrease in PGE2 level only in CDF-treated cells (p=0.0268), but such results were not found with Curcumin treatment (Fig. 2B; p=0.9628). These results clearly suggest that CDF is a better target of COX-2, resulting in a greater inhibition of PGE2 production in both the cell lines relative to Curcumin.
Fig. 2.
(A) Electrophoretic mobility shift assay for NF-κB DNA binding activity in MIAPaCa-2 cells exposed to Curcumin and CDF at indicated concentrations; (B) PGE2 activity in conditioned medium derived from CDF- and Curcumin-treated BxPC-3 and MIAPaCa-2 pancreatic cancer cells. A significant reduction in PGE2 level was observed in BxPC-3 cells treated with CDF (P=0.0268), but no significant change in PGE2 level was noted when cells were treated with Curcumin (P=0.9628). In MIAPaCa-2 cells, a substantial reduction in PGE2 level was observed with both CDF (P=0.0024), and Curcumin (P=0.0076), but the effect was still better with CDF compared to Curcumin.
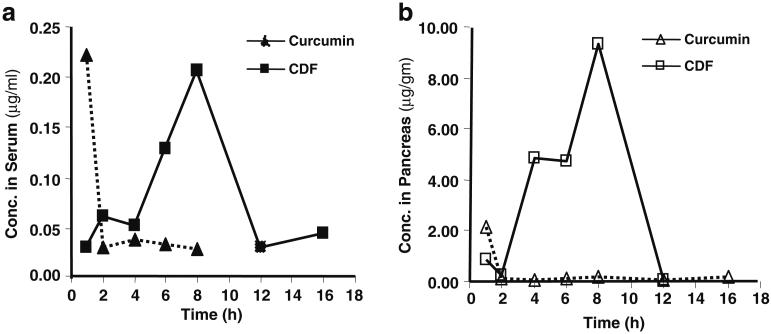
The concentration and time profiles of Curcumin and CDF in serum and pancreas tissue following a single dose oral administration (250 mg/kg) in female ICR-SCID mice are depicted in Fig. 3A and 3B. Pharmacokinetic parameters for Curcumin and CDF are summarized in Table II. Following a single oral dosing of 250 mg/kg in mice, Curcumin achieved the maximum serum concentration (Cmax) of 0.22 μg/mL at 1 h, after which serum concentration of Curcumin declined rapidly and was undetectable after 8 h (below the lower limit of 5 ng/mL) (Fig. 2A). The AUClast was estimated as 0.44 μg/mL*h (Table II). Compared to Curcumin, CDF achieved a similar Cmax (0.21 μg/mL) with a relatively slow oral absorption with Tmax of 8 h; however, CDF had 2.7-fold higher systemic drug level than Curcumin (AUClast, 1.22 vs. 0.44 μg/mL*h; Table II).
Fig. 3.
Concentration vs. time profiling of Curcumin and CDF in mice serum (A) and pancreas (B) following single intragastric administration (250 mg/kg) in mice. Each point represents the mean concentration from two mice.
Table II.
Comparative Pharmacokinetic Analysis of Curcumin and CDF in Serum and Pancreas Following a Single Intragastric Administration (250 mg/kg) in Mice. Data are Expressed as the Mean from Two Mice
| Serum |
Pancreas |
|||
|---|---|---|---|---|
| Curcumin | CDF | Curcumin | CDF | |
| Tmax (h) | 1.0 | 8.0 | 1.0 | 8.0 |
| Cmax (μg/mL for serum, μg/g for pancreas) | 0.22 | 0.21 | 2.15 | 9.35 |
| Tlast (h) | 8.0 | 16.0 | 16.0 | 12.0 |
| Clast (μg/mL for serum, μg/g for pancreas) | 0.03 | 0.04 | 0.20 | 0.04 |
| AUClast (h*μg/mL for serum, h*μg/g for pancreas) | 0.44 | 1.22 | 3.46 | 36.56 |
Cmax maximum serum concentration, Tmax the time to achieve maximum concentration, Clast last measurable concentration, Tlast the time for the last measurable concentration, AUClast total area under the serum concentration-time curve from time zero to the last measurable time point
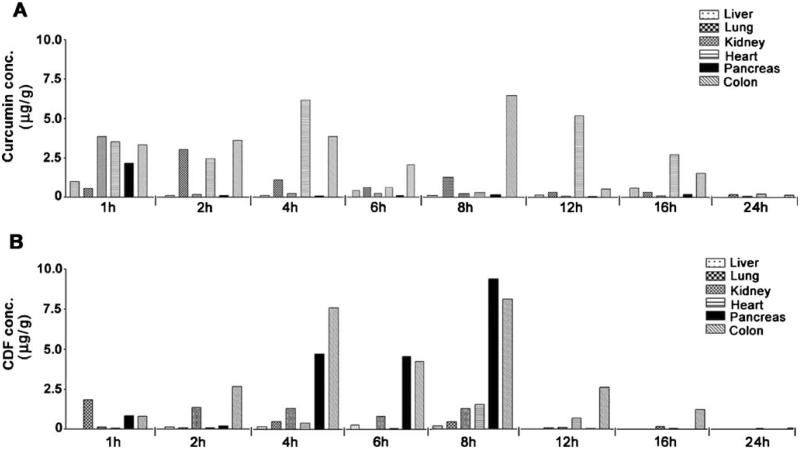
The distribution of Curcumin and CDF following single dose administration of 250 mg/kg body weight in mice is presented in Fig. 4A and 4B. As shown, both Curcumin and CDF were detectable in all tissues tested, including liver, lung, kidney, heart, pancreas, and colon. However, Curcumin and CDF were detectable at high concentrations in colon after oral administration. Interestingly, Curcumin was found to be present mainly in heart and lung, while CDF accumulated preferentially in the pancreas (Figs. 3B and 4B). Moreover, consistent with serum concentration vs. time profile, Curcumin and CDF also achieved the maximum concentration in pancreas at 1 and 8 h, respectively, after oral administration (Fig. 3B and 4B). The Cmax and AUClast of CDF in pancreas were 4.3-and 10.6-fold those for Curcumin, respectively (Table II), suggesting that CDF has a better bioavailability profile, especially in pancreas tissue. This observation further suggests that CDF would show better anti-tumor activity against pancreatic cancer, which is being currently tested in our laboratory.
Fig. 4.
Concentration vs. time profiling of Curcumin (A) and CDF (B) in mouse tissues following single intragastric administration (250 mg/kg) in mice. Each point represents the mean concentration from two mice.
Curcumin has been demonstrated to have a low oral bioavailability in animals and humans perhaps because of its rapid secretion as conjugates (28). Consistent with previous findings, we also observed very low serum levels of Curcumin after oral administration in mice. Following a single oral dose of 250 mg/kg in mice, Curcumin achieved the Cmax of 0.22 μg/mL at 1 h, after which Curcumin serum concentration declined rapidly and was undetectable after 8 h (Fig. 3A and 4A). It is important to note that our results were similar for the two animals. Collectively, our results are also consistent with a previously reported mouse study in which oral administration of 1 g/kg body weight of Curcumin resulted in a Cmax of 0.22 μg/mL at 1 h, and the serum concentrations then declined below the detection limit by 6 h (43). It has been postulated that poor water solubility and extensive first-pass intestinal and hepatic metabolism are attributable, for a large part, to the low oral bioavailability of Curcumin (43,44). In contrast, CDF, which is an analog of Curcumin, exhibited enhanced bioavailability, which could be due in part to its low excretion rate as conjugates, although further in-depth investigations are needed in this area. Interestingly, oral administration of CDF produced 2.7-fold more increase in systemic drug level than Curcumin (AUClast, 1.22 vs. 0.44 μg/mL*h; Table II), although the underlying mechanisms by which CDF shows superior bioavailability need further investigations.
Consistent with our objective, improved water solubility of CDF may account, at least in part, for its enhanced oral bioavailability. The water solubility of CDF was determined to be 8.4-fold higher than Curcumin (data not shown). Both Curcumin and CDF were found to be present, albeit in different concentrations, in almost all tissues tested, including liver, lung, kidney, heart, pancreas and colon. Of interest, CDF was accumulated preferably in pancreas (Figs. 3B and 4B). The Cmax (at 8 h) of CDF achieved in pancreas was 44.5-fold higher than in serum (Figs. 3 and 4, and Table II). Furthermore, relative to Curcumin, the concentration of CDF in pancreas was 10.6-fold higher (Table II). The mechanism for this observed preferential accumulation of CDF in pancreas remains to be determined. However, due to its high accumulation in pancreas, we speculate that CDF may be a good drug candidate for either inclusion in the prevention strategy of pancreatic cancer or for the treatment of pancreatic cancer in conjunction with cytotoxic agents similar to those reported for Curcumin (45-48). Therefore, CDF could become an ideal chemosensitizer in restituting sensitivity of pancreatic cancer to cytotoxic drugs, which must be tested in future studies for optimizing a successful treatment regimen for human pancreatic cancer.
In conclusion, here we have presented evidence in support of a superior, sustainable, and biologically active analog of Curcumin that could prove highly valuable in the future, not only in chemoprevention research in populations at risk of developing cancer, but also in an effective arsenal for the treatment of pancreatic and other human cancers either alone or in combination with conventional therapeutics. Moreover, in view of its preferential accumulation in the pancreas and its ability to down-regulate NF-κB, as well as reduce the levels of PGE2 concentration, it could further be exploited for molecular-targeted therapy against COX-2 over-expressing tumors including pancreatic cancer.
Footnotes
Subhash Padhye and Sanjeev Banerjee contributed equally.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. 2008. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 3.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 4.Friess H, Guo XZ, Nan BC, Kleeff O, Buchler MW. Growth factors and cytokines in pancreatic carcinogenesis. Ann N Y Acad Sci. 1999;880:110–21. doi: 10.1111/j.1749-6632.1999.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 5.Le X, Shi Q, Wang B, Xiong Q, Qian C, Peng Z, et al. Molecular regulation of constitutive expression of interleukin-8 in human pancreatic adenocarcinoma. J Interferon Cytokine Res. 2000;20:935–46. doi: 10.1089/10799900050198372. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333–8. doi: 10.1002/1097-0142(20010115)91:2<333::aid-cncr1006>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–90. [PubMed] [Google Scholar]
- 9.Hermanova M, Trna J, Nenutil R, Dite P, Kala Z. Expression of COX-2 is associated with accumulation of p53 in pancreatic cancer: analysis of COX-2 and p53 expression in premalignant and malignant ductal pancreatic lesions. Eur J Gastroenterol Hepatol. 2008;20:732–9. doi: 10.1097/MEG.0b013e3282f945fb. [DOI] [PubMed] [Google Scholar]
- 10.Matsubayashi H, Infante JR, Winter J, Klein AP, Schulick R, Hruban R, et al. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–75. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar FH, Adsule S, Li Y, Padhye S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev Med Chem. 2007;7:599–608. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]
- 12.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–6. [PubMed] [Google Scholar]
- 13.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001:480–481. doi: 10.1016/s0027-5107(01)00183-x. 243–68. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N YAcad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 16.Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:5940–7. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 17.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 18.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–92. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009 doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma C, Kaur J, Shishodia S, Aggarwal BB, Ralhan R. Curcumin down regulates smokeless tobacco-induced NF-kappaB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology. 2006;228:1–15. doi: 10.1016/j.tox.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–79. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 24.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 25.Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharm Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 28.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 29.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, et al. Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–55. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–12. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 33.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 34.Liang G, Yang S, Zhou H, Shao L, Huang K, Xiao J, et al. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur J Med Chem. 2009;44:915–9. doi: 10.1016/j.ejmech.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Liang G, Yang S, Jiang L, Zhao Y, Shao L, Xiao J, et al. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull. 2008;56:162–7. doi: 10.1248/cpb.56.162. (Tokyo) [DOI] [PubMed] [Google Scholar]
- 36.Poma P, Notarbartolo M, Labbozzetta M, Maurici A, Carina V, Alaimo A, et al. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: analysis of the possible molecular basis. Int J Mol Med. 2007;20:329–35. [PubMed] [Google Scholar]
- 37.Anand P, Thomas SG, Kunnumakkara AB, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 38.John VD, Kuttan G, Krishnankutty K. Anti-tumour studies of metal chelates of synthetic curcuminoids. J Exp Clin Cancer Res. 2002;21:219–24. [PubMed] [Google Scholar]
- 39.Zambare AP, Jamadar A, Padhye S, Kulkarni VM. Copper conjugates of Knoevenagel condesates of Curcumin and their Schiff base derivatives: synthesis, spectroscopy, magnetism, EPR and electrochemistry. Synth React Inorg, Metal-Org and NanoMetal Chemistry. 2007;37:19–27. [Google Scholar]
- 40.Padhye S, Yang H, Jamadar A, Cui QC, Chavan D, Dominiak K, et al. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm Res. 2009;26:1874–80. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee S, Wang Z, Kong D, Sarkar FH. 3, 3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 2009;69:5592–600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74–8. doi: 10.1248/bpb.30.74. [DOI] [PubMed] [Google Scholar]
- 43.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–94. [PubMed] [Google Scholar]
- 44.Ravindranath V, Chandrasekhara N. Metabolism of curcumin– studies with [3H]curcumin. Toxicology. 1981;22:337–44. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 45.Giri B, Gomes A, Sengupta R, Banerjee S, Nautiyal J, Sarkar FH, et al. Curcumin synergizes the growth inhibitory properties of Indian toad (Bufo melanostictus Schneider) skin-derived factor (BM-ANF1) in HCT-116 colon cancer cells. Anticancer Res. 2009;29:395–401. [PubMed] [Google Scholar]
- 46.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 47.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 48.Sung B, Kunnumakkara AB, Sethi G, Anand P, Guha S, Aggarwal BB. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol Cancer Ther. 2009;8:959–70. doi: 10.1158/1535-7163.MCT-08-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]