Introduction
Degenerative intervertebral disc (IVD) disease and associated chronic lower back pain constitute a major health problem with estimated costs in the U.S. of up to $50 billion yearly.1 Despite decades of research, no fundamental multidisciplinary understanding of the mechanism(s) of IVD degeneration has surfaced,2,3 and consequently, clinical therapies are still in the earliest stages of development.4 This debilitating disease is caused by cell-mediated functional tissue degradation in response to progressive structural failure.5 The IVD consists of the gel-like nucleus pulposus (NP) surrounded by the densely fibrous annulus fibrosus (AF). Disc degeneration is characterized by increased breakage of the existing NP matrix due to elevated expression of matrix metalloproteinases and inflammatory factors, and altered matrix production. In addition, cell apoptosis and formation of cell clusters, due to accelerated cell replication, can lead finally to cell senescence.2,6 Finally, the process extends to the AF, as a result of altered loading, and leads to microtrauma and pain.7 All these changes are mediated by disturbances in the function of cells residing in the disc.2,8 The disc as organ possesses a minimal capability for intrinsic regeneration2 probably due to a malfunction in early progenitors, repair cells, residing in the NP.
It is well known that progenitor cells maintain homeostasis within the tissue in which they reside and play a major role in regeneration following injury.9 In certain pathological conditions, such as osteoarthritis, motor neuron degeneration, and end-stage postinfarction cardiomyopathy, resident cells exhibit an altered capacity to proliferate and differentiate, which may eventually lead to a loss of tissue homeostasis and inability of tissue to self-regenerate.10–12
Previously we showed that progenitor cells exist in human degenerated discs and in healthy rat discs13 and are able to transdifferentiate into osteogenic, adipogenic, and chondrogenic lineages in vitro. Henriksson et al. documented the presence of mesenchymal stem cell (MSC) markers in the NP and AF of degenerated discs.14 Blanco and colleagues found that those cells in a degenerated human disc resemble to BM-derived MSCs isolated from the same patient.15
To date the effect of IVD degeneration on progenitors cells residing in the NP is not fully understood. Here we investigated the functionality of NP-derived cells from porcine degenerated discs (D-NP) and compared it to the functionality of cells isolated from healthy porcine discs (H-NP) obtained from the same animal. An annular injury approach, a well-established model, was utilized to induce degeneration in the porcine discs. In this model the injury is limited to the annulus fibrosus, resulting nucleus degeneration to progress along a more natural pathway.16 Although to date there is no ideal animal model for IVD degeneration, mini pig’s disc is preferred due to its high resemblance in nutritional and biomechanical aspects to the human disc.4 We sought to explore the effect of IVD degeneration on the abundance of cells in the IVD, their differentiation potential, and their ability to give rise to NP-like cells. We hypothesized that IVD degeneration affects proliferation rate of NP progenitor cells as well as their differentiation potential.
Materials and methods
Materials
All materials were supplied by Sigma Aldrich (USA) unless stated otherwise.
Animal studies
All animal procedures conformed to the requirements of the Animal Welfare Act, and were approved by the Institutional Animal Care and Use Committee (IACUC) of Cedars-Sinai Medical Center, Los Angeles, CA. Ten healthy female skeletally mature Yucatan miniature pigs (average age 1.5 years, 35–40 kg,) were included in this study. Under general anesthesia, NP degeneration was induced at three target levels (L1/L2, L2/L3, and L3/L4), via a posterolateral approach, using a surgical scalpel to create a 4-mm-deep incision through the AF, parallel to the endplate.17_ Intact discs in each animal served as controls. The animals were euthanized 7–8 weeks postsurgery. Degenerated and healthy discs, subcutaneous adipose tissue and costal BM were harvested for cells’ isolation or histological analysis.
Confirmation of IVD degeneration
Anesthetized pigs or harvested spines were subjected to MRI. High-resolution MR images were obtained at 24°C using a Verio 3T (Siemens Medical Solutions) equipped with a 3-tesla vertical wide-bore superconducting magnet. T1- and T2-weighted sagittal and axial images were obtained to evaluate signal intensity and the structures of punctured and control discs. Disc degeneration was classified as severe if T2-weighted sequences demonstrated >50% decrease in the signal intensity of the NP.18 To confirm IVD degeneration, paraffin-embedded sections of harvested discs were stained with Masson’s trichrome (MTC) as previously reported.19
NP cells isolation and expansion
Degenerated discs and healthy control discs were harvested from the same animal. NPs from healthy and degenerated discs were subjected to overnight enzymatic digestion using DMEM culture media containing 10% FBS, 2mM l-glutamine, 100U/ml penicillin/streptomycin (Invitrogen) supplemented with 1mM sodium pyruvate, 25mM HEPES, 2mg/ml collagenase type IV, and 0.15mg/ml hyaluronidase, at 37°C with stirring. Tissue debris was filtered using a nylon mesh (100µm). Trypan blue exclusion and an automated cell counter (Countess™, Invitrogen) were used to count viable cells. A portion of noncultured cells was designated for flow cytometry and a CFU-F assay. The remaining NP cells were plated at a density of 1.8×105 cells/cm2; the culture medium was changed at 72 hrs and thereafter every 3–4 days. At confluence the cells were trypsinized using 0.25% trypsin-EDTA (Invitrogen) and replated at a density of 5×103 cells/cm2 for expansion. The NP cells were expanded until the 3rd passage (p3). Porcine adipose-derived SCs (ASCs) and BM-derived MSCs (BM-MSCs) were isolated and cultured as previously reported.19,20
Colony-forming unit–fibroblast (CFU-F) assay
Freshly isolated NP cells were plated at a density of 1.1×105 cells/cm2 (n=3). After 14 days the cells were fixed with 4% formaldehyde, stained with hematoxylin (Pioneer Research Chemicals LTD, Essex, UK). Colonies of 20 cells or more were scored as CFU-Fs. The assay was performed separately with cells isolated from 3 different animals.
Flow Cytometry
NP cells freshly isolated from healthy and degenerated discs from 6 animals were analyzed for surface marker expression based on known MSC surface markers and with consideration of the limited availability of anti-pig antibodies. Cells were stained with mouse anti-human (with cross reactivity to pig) CD90, mouse anti-pig CD29 (BD Biosciences Pharmingen, San Diego, CA, USA) (n=6), and rat anti-pig CD44 (Fitzgerald Industries Intl., North Acton, MA USA) (n=4). Bonded primary antibodies were detected using the fluorochrome-conjugated secondary antibodies rat anti-mouse-PE (BD Biosciences Pharmingen) and donkey anti-rat PE (Imgenex Corp., San Diego, CA, USA) according to manufacturer recommendations. The cells were analyzed using LSR-II FACS (BD, Heidelberg, Germany), BD Diva and FCS express software. Nonspecific binding of secondary antibodies was quantified, and the fluorescent signal was subtracted from experimental group detection values.
Immunohistochemical (IHC) assay
An IHC assay was performed to detect and validate the expression of MSC markers on paraffin sections of healthy NP tissue by using a HISTOMOUSE-SP (broad spectrum) kit (Zymed Laboratories, San Francisco, CA, USA). Five-micron sections were deparaffinized and rehydrated. The antigens were retrieved enzymatically by incubation in TRIS-EDTA buffer (20 minutes, 95°C). Endogenous peroxidase activity was terminated by treatment with 0.1% H2O2. Slides were incubated overnight at 4°C with primary antibodies described at the FACS section. The slides were then rinsed in PBS, and incubated with a secondary biotin-conjugated antibody (Zymed, laboratories) (room temperature, 30 minutes) following by detection using the streptavidin-biotin-horseradish peroxidase complex. The slides were counterstained with hematoxylin, mounted with GVA, and visualized with the aid of light microscopy.
Cell proliferation
Cell proliferation in vitro was assessed using cell counts and the Trypan blue exclusion test. Cells were seeded at 4.75×103 cells/cm2 density (n=5) and grown for 4–6 days, trypsinized, and counted using the Countess™ device. Then the cells were reseeded at the same density and labeled as p2. This process was repeated until p6. Cell doublings were calculated as the number counted in each well divided by 2, divided by the initial seeded cell number and divided by the number of days in culture. The assay was repeated for cells from 4 different animals.
Differentiation assays
All differentiation assays were performed in 3 independent experiments using adherent cells derived from at least 3 different animals. All cells used in the differentiation experiments were expanded up to passage 3.
Osteogenic differentiation assay: To induce osteogenic differentiation, H-NP, D-NP cells and BM-MSCs were grown with osteogenic supplements as previously described.20 Cells were harvested on Day 0 and Day 14 postinduction and assessed for ALP activity (n=16 for H-NP cells, n=11 for D-NP cells, and n=12 for BM cells, each experiment was done using cells from 3 animals)20. Values were normalized for protein content, which was measured using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Von Kossa staining was performed to evaluate the cells’ calcium deposition. Cells were fixed in cold 10% formaldehyde, rinsed with distilled water, immersed in 2% silver nitrate solution, and exposed to bright light for 15 minutes. Culture plates were counterstained with 0.1% safranin-O (5 minutes, room temperature). Mineralization was captured using a light microscope.
Adipogenic differentiation assay: porcine adipose-derived mesenchymal stem cells (ASCs) and NP cells derived from healthy and degenerated discs were grown in the presence of adipogenic supplements as previously described (n=12 in total, experiment was done with cells from 3 different animals).20 Undifferentiated cells were harvested on Day 0. After 21 days of adipogenic induction the cells were stained with Oil-Red-O to confirm adipogenic differentiation13 and documented using microphotography. Oil-Red-O was eluted from the wells by incubation with 100% isopropanol for 15 minutes and read at the 500-nm wavelength using spectrophotometry. Optical density (OD) values were normalized to the protein content, quantified using the BCA assay.
Chondrogenic differentiation assay: To induce chondrogenic differentiation NP cells derived from healthy and degenerated discs and BM-MSCs were grown with chondrogenic supplements as previously described.8 Aliquots of 5×105 cells were seeded in Transwell™ filters (Corning B.V. Life Sciences, Schiphol-Rijk, The Netherlands). The medium was replaced every 2 days for up to 21 days. Negative control samples were harvested upon formation of disc-shaped cell aggregates on Day 3. Chondrogenic differentiation was assessed by quantification of sulfated glycosaminoglycans (sGAG) using a DMMB assay (n=10 in total, experiment was done with cells from 3 different animals).21
Differentiation toward NP-like cells
H-NPs, D-NPs and BM-MSCs were differentiated toward NP-like cells in hypoxic conditions. Cells were suspended in 1.2% low-viscosity sodium alginate in a 0.9% NaCl solution at a concentration of 2×106 cells/ml. The alginate-cell suspension was expelled through a 27-gauge needle into a solution of 102mM CaCl2, resulting in bead formation. The beads were incubated for 10 minutes in CaCl2 solution, then maintained in DMEM supplemented with 10 ng/ml transforming growth factor β1 (R&D Systems, MN), 100nmol/L dexamethasone, 50µg/ml ascorbate 2-phosphate, 100µg/ml sodium pyruvate, 40µg/ml proline, and ITS-plus, as previously described.8 Alginate beads were cultured in a hypoxia workstation (Biospherix Ltd.) at 2% O2 at 37°C for 7 or 21 days according to the assay. Control beads were harvested at Day 0 postinduction.
DMMB assay: Cell differentiation was assessed by quantification of sGAG using the DMMB assay modified to suit alginate-containing samples (n=15 in total)22 sGAG quantity was normalized to cell number based on cell counts. The assay was repeated for cells from 3 different animals in 3 independent experiments.
Chondrogenic gene expression analysis
RNA expression of chondrogenic genes was evaluated in fresh NP tissue and cultured p3 H-NP cells. RNA was extracted using TRIzol® reagent.19 The RNA was then retrotranscribed using random primers and reverse transcriptase (Promega Corp., Madison, WI, USA); and PCRs for aggrecan, collagen-IIa and SOX-9 and β-actin were performed with primers that were described else were.23 PCR reaction product was subjected to electrophoresis on a 2% agarose gel with 0.5 µg/ml EtBr. Quantitative RT-PCR was performed to estimate the degree of differentiation into NP-like cells cultured in alginate beads described earlier. The beads were harvested at Days 0 and 7 postinduction, Total RNA was extracted from the encapsulated cells by using a FastTrack MAG 96 mRNA Isolation Kit (Invitrogen) according to the manufacturer’s protocol. RNA was retrotranscribed using random primers and reverse transcriptase (Promega Corp) Quantitative RT-PCR for aggrecan, collagen-IIa and SOX-9 expression was performed with the aid of an ABI7500 Prism system (Applied Biosystems, Foster City, CA, USA) using a relative quantification method and Assay-on-Demand gene-expression assays (Applied Biosystems, Ss03374825_m1, Ss03373344_g1, and Ss03392406_m1, respectively). The expression of each gene was normalized to the house-keeping gene 18S (Hs99999901_s1) using the 2−ΔΔCt method24 and calibrated to its expression by H-NP cells on Day 0. RQ values shown in the figure represent gene expression/gene expression of H-NP cells at Day 0. The assays were performed in two independent experiments using cells derived from 2 different donors (n=10 in total).
Quantification of aggrecan: An enzyme-linked immunosorbent assay (ELISA) (Invitrogen) was performed to measure the amount of aggrecan secreted during the differentiation toward NP–like cells. Medium from NP cells cultured in alginate beads on Days 3 and Day 21 postinduction was collected. Values were normalized to manually counted cell number per alginate bead. Media from blank beads containing alginate alone were collected as well. This procedure was conducted according to the manufacturer’s protocol (n=4).
Statistical analysis
Assays were performed using cells obtained from 3 different animals (unless stated differently) and in separate sets of experiments. All mean values in Results and in figures are displayed with their standard errors. Statistical tests for significance were performed using a paired Student t-test where applicable. Longitudinal data analysis was conducted to compare proliferation in NP cells, taking into account the effect over time. A linear mixed-effects model was fit to the data, with average cell doubling/day/passage being the outcome variable. The model included a subject-specific random effect to take into account correlation of data from each individual. All tests were two-sided with a 0.05 significance level.
Results
IVD degeneration induction
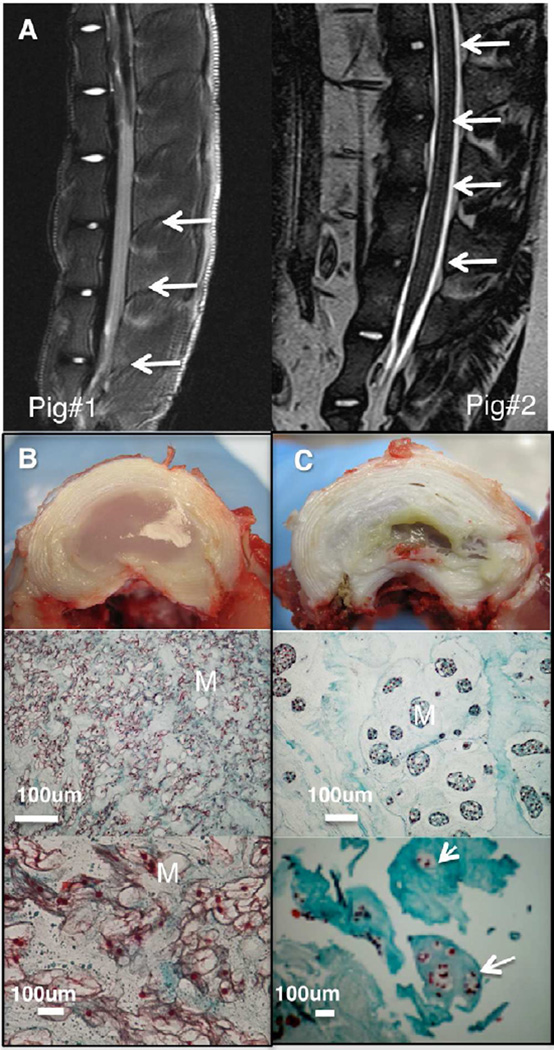
An annular puncture model was used to induce experimental IVD degeneration. There was a clear decrease in the intensity of the T2-weighted signal in MR images of punctured discs (Figure 1a) compared with MR images of adjacent healthy discs. Freshly isolated discs exhibited clear signs of degeneration. The degenerated disc lost its well-defined structure and border between the NP and the AF. The NP had shrunk extensively, and become yellowish and fibrous. In contrast, no sign of degeneration or inflammation was seen in healthy discs. Histological analysis showed an abnormal structure and matrix, with signs of hypertrophic cartilage and cell clusters typical of degenerated NP2 (Figure 1b–c).
Figure 1. IVD degeneration in a porcine model.
Degeneration was verified 8 weeks post AF puncture. T2 weighted MR images ex-vivo (left) and in-vivo (right) showing decrease in signal intensity in the degenerated discs (a) arrows indicate punctured-discs; microphotographs and histological analysis of NP tissue stained with MTC for healthy (b); and degenerated (c) discs, M indicates matrix, arrows indicate cell clusters; Microphotographs of the degenerated disc indicating that the disc has lost its well-defined structure and border between the NP and the AF. The NP (in the center of the disc) had shrunk extensively, and become yellowish and fibrous.
NP cells isolation and proliferation
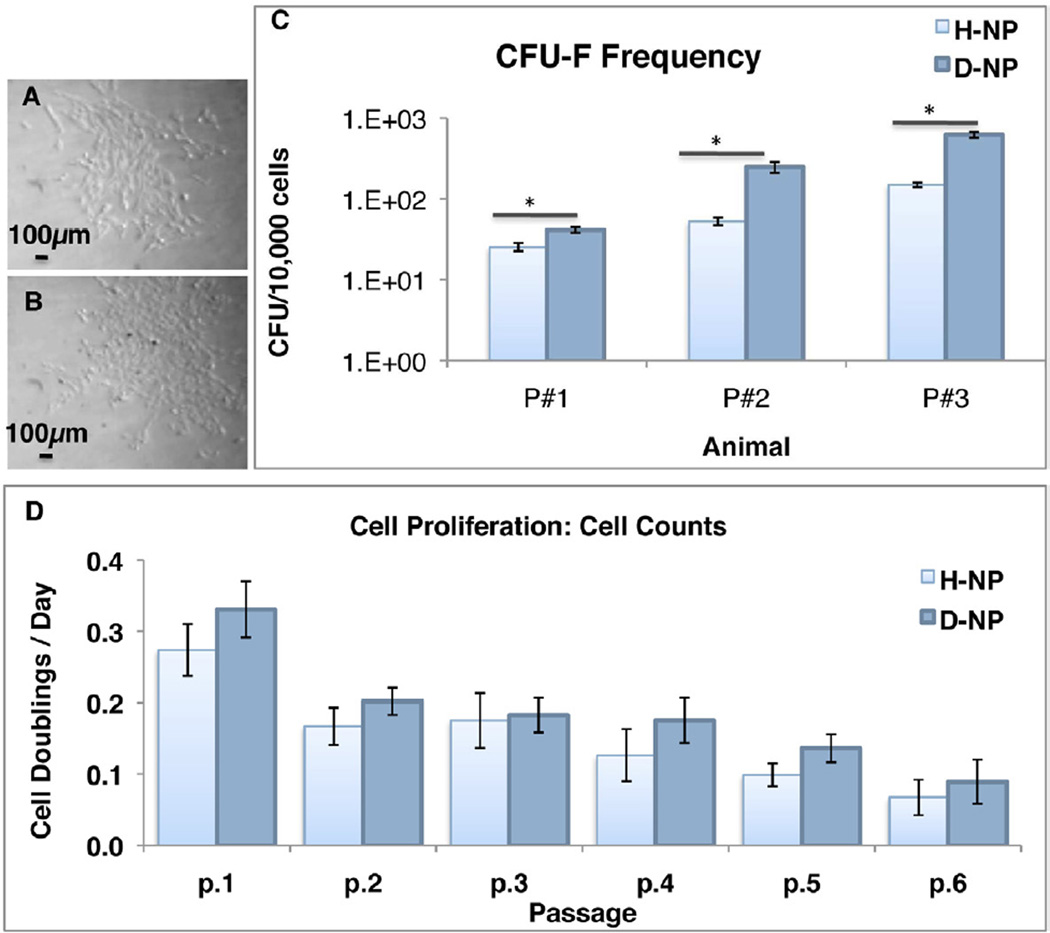
NP Cells were isolated from healthy and degenerated porcine discs for various analyses to explore the effect of degeneration on cells residing in the NP. Freshly isolated cells from both degenerated and healthy discs exhibited heterogeneous population of cells. After plastic adherence (p0), cells from both groups displayed spindle-shaped structure and colony formation typical to fibroblasts and progenitor cells of mesenchymal origin25 (Figure 2a–b). Healthy NPs contained 1.39±0.28×105 cells/NP (counted from 8 animals), whereas degenerated NPs had 1.61±0.47×105 cells/NP (n=8). Disc degeneration had no significant effect on the abundance of cells: however, the CFU assay showed that fresh (non cultured) D-NP cells had more CFUs than fresh (non cultured) H-NP cells. On average, D-NP cells produced 3.49 times more CFUs than H-NP cells (n=3, Figure 2c). We examined the degenerative effect on cell proliferation as well; the results indicated that there is a significant difference in proliferation between D-NP and H-NP cells (n=5 in 4 animals). Our cell counts showed that D-NP cell doublings/24hr were 4±1% higher than those in H-NP cells. The proliferation rate similarly decreased with passaging in both groups till they decline at passage 4–5 (Figure 2d).
Figure 2. NP cells proliferation.
Microphotographs of passage 0 H-NP (a) and D-NP (b) cells; CFU-F frequency of freshly isolated NP cells (n=3) (c); cell counts of NP cells represented as cell doublings/day (d), in longitudinal data analysis there is a significant difference in proliferation between D-NP and H-NP cells (n=5 in different animals). Bars indicate SE, * indicates p<0.05.
NP cells and MSC markers
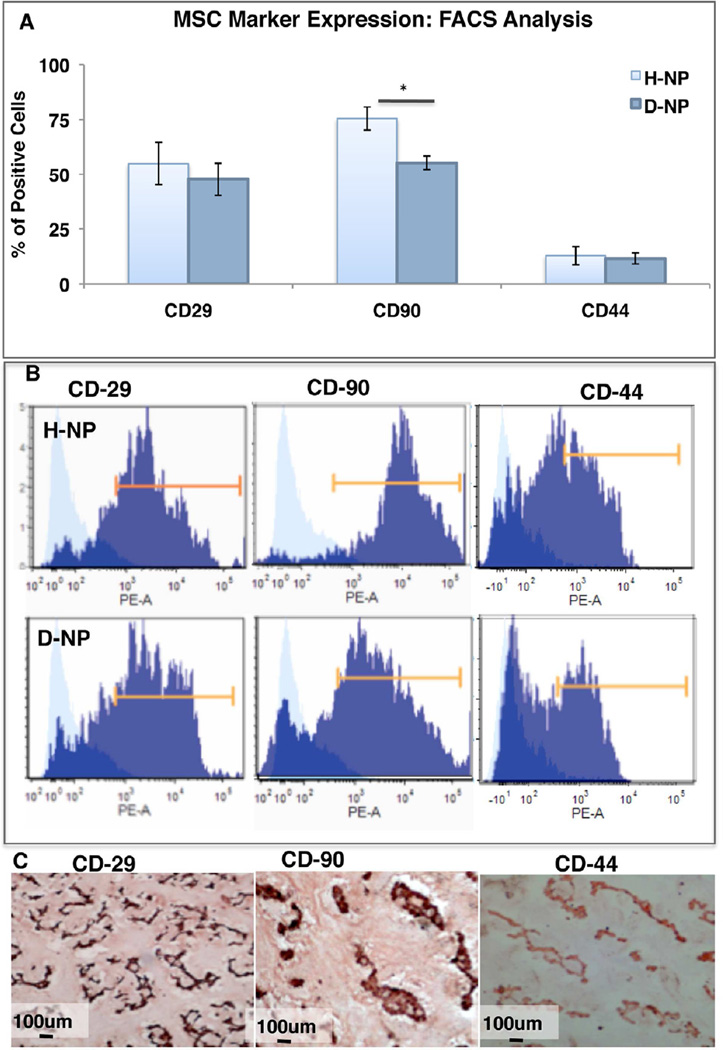
Freshly isolated, non-cultured D-NP and H-NP cells were subjected to FACS analysis to evaluate MSC marker expression. Both cell populations, derived from healthy or degenerated NP, had a high percentage of CD29-positive cells (54.93±7.68% and 47.70±7.57%, respectively; n=6). Expression of the CD90 surface marker in H-NP cells was significantly higher than that in D-NP cells (75.46±5.63% and 55.63±3.34%, respectively; n=6, p<0.05). The CD44-positive cell fraction was lower in both groups: 12.82±5.17 for H-NP cells and 11.6±2.92 for D-NP cells (n=4) (Figure 3a–b). When the FACS analysis was performed on cultured H-NP and D-NP cells displayed high expression (>90%) of all markers (data not shown). To validate the abundance of progenitor cells that express MSC surface markers among the NP-derived cells, an IHC analysis for these markers was performed in healthy NP tissue (Figure 3c). IHC analyses for CD29, CD90, and CD44 markers validated the results of the FACS analysis, showing high positive staining for each marker on the surface of the cells.
Figure 3. MSC markers expression.
FACS analysis of MSC markers expression in non-cultured NP-cells, Bars indicate SE, * indicates p<0.05 (a); representative histograms of FACS analysis of H-NP cells (top panel) and D-NP cells (lower panel) (b); IHC for MSC markers in healthy NP tissue (c).
NP cells differentiation potential
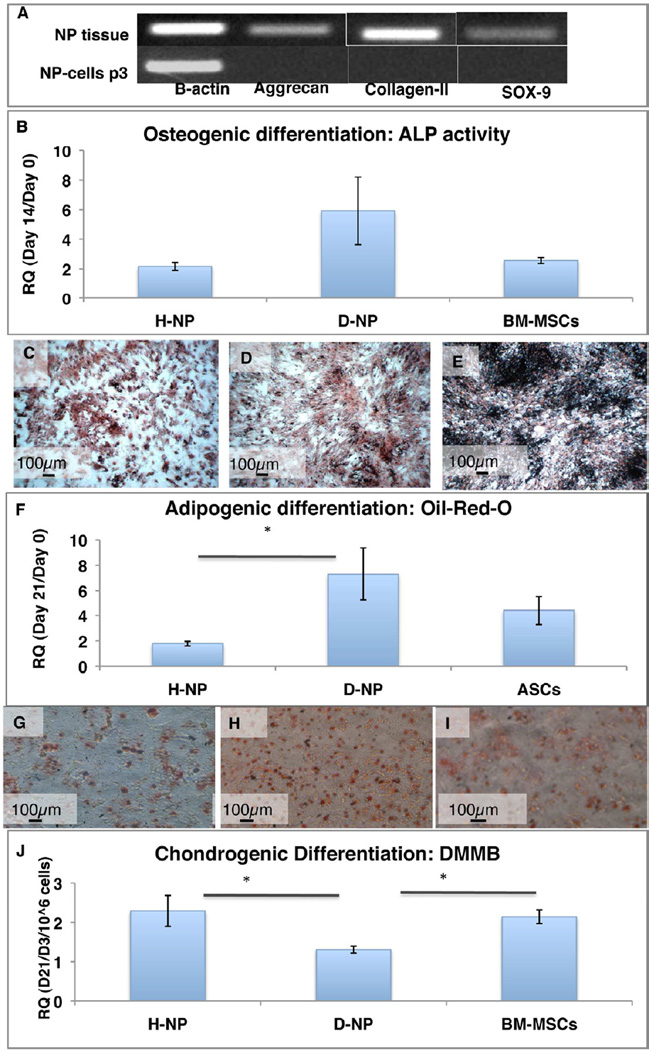
NP tissue and NP-isolated cells were tested for of the chondrogenic genes expression aggrecan, collagen–II, and SOX-9. PCR results demonstrated that only NP tissue–not NP cells–expressed these chondrogenic genes (Figure 4a).
Figure 4. Plastic adherent NP cells’ differentiation potential.
PCR for chondrogenic genes of NP tissue and NP-cells (a). Osteogenesis was estimated using ALP assay, normalized to BCA (n=11–16) RQ values of ALP/BCA (ALP Activity/ml/min/µg protein) Day 14/day 1 are shown.(b) and Von-Kossa staining (c–e). Adipogenesis was evaluated using Oil-red-O colorimetric assay normalized to BCA content (n=12) RQ values of Oil-red-O/BCA (OD/µg protein) Day 14/day 0 are shown (f) and Oil-red-O images of differentiated cells (g–i); Chondrogenesis was evaluated using DMMB assay normalized to cell number in each disc. (n=10) RQ values of GAG content/µl/106 cells of day 21/day 3 are shown (j). Bars - SE, * p<0.05.
H-NP and D-NP cells were successfully differentiated towards mesenchymal lineages. Undifferentiated cells (Day 0) had significantly lower values than differentiated cells in all groups. When cultured under osteogenic conditions, NP-derived cells from both groups showed similar potential to differentiate into the osteogenic lineage, demonstrated by ALP activity and Von Kossa staining. Relative quantity (RQ) values of ALP activity (Day 14 normalized to Day 0) showed no significant difference between groups (n=16 for H-NP cells, n=11 for D-NP cells, and n=12 for BM-MSC cells; p>0.05). Von-Kossa staining revealed clear signs of calcium deposition in all groups (Figure 4b–e).
Differentiation into the adipogenic lineage in vitro was assessed on Day 21 postinduction by using Oil-Red-O staining. The H-NP cells had a relative value of 1.78±0.18 (RQ Day 21/Day 0 of OD/µg protein), whereas the D-NP cells had a statistically higher value of 7.30±2.07 RQ (n=12; p<0.05, Figure 4f–i). To compare the chondrogenic potential of the two groups, H-NP and D-NP cells were seeded into Transwells™ in the presence of chondrogenic supplements. A quantitative DMMB assay showed that H-NP cells produced significantly higher levels of sGAG than D-NP cells, with a relative value of 4.58±0.78 (RQ normalized to Day 3 control/106 cells, n=10), as opposed to D-NP cells, which had a value of 2.62±0.18 (RQ normalized to Day 3 control/106 cells, n=10) in the DMMB assay. H-NP cells showed a similar differentiation potential to BM-MSCs (Figure 4j). We normalized the DMMB assay results obtained on Day 21 to Day 3 time point, since it's the earliest time when the cell clusters are being formed in vitro in Transwells™.
Differentiation of NP-derived cells back to NP-like cells
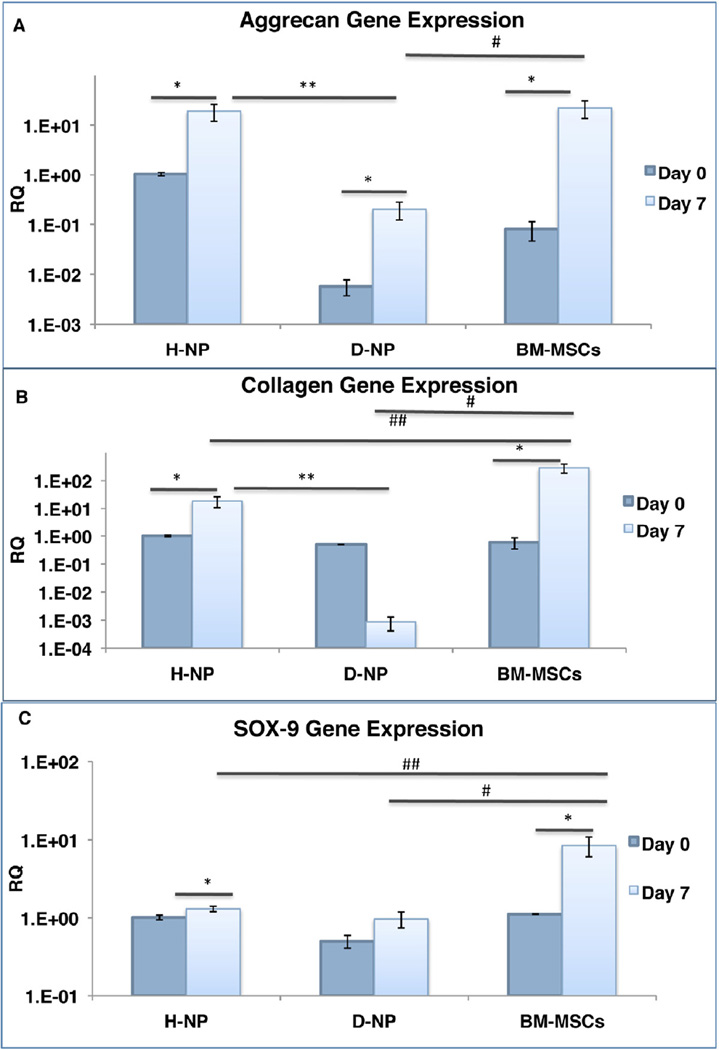
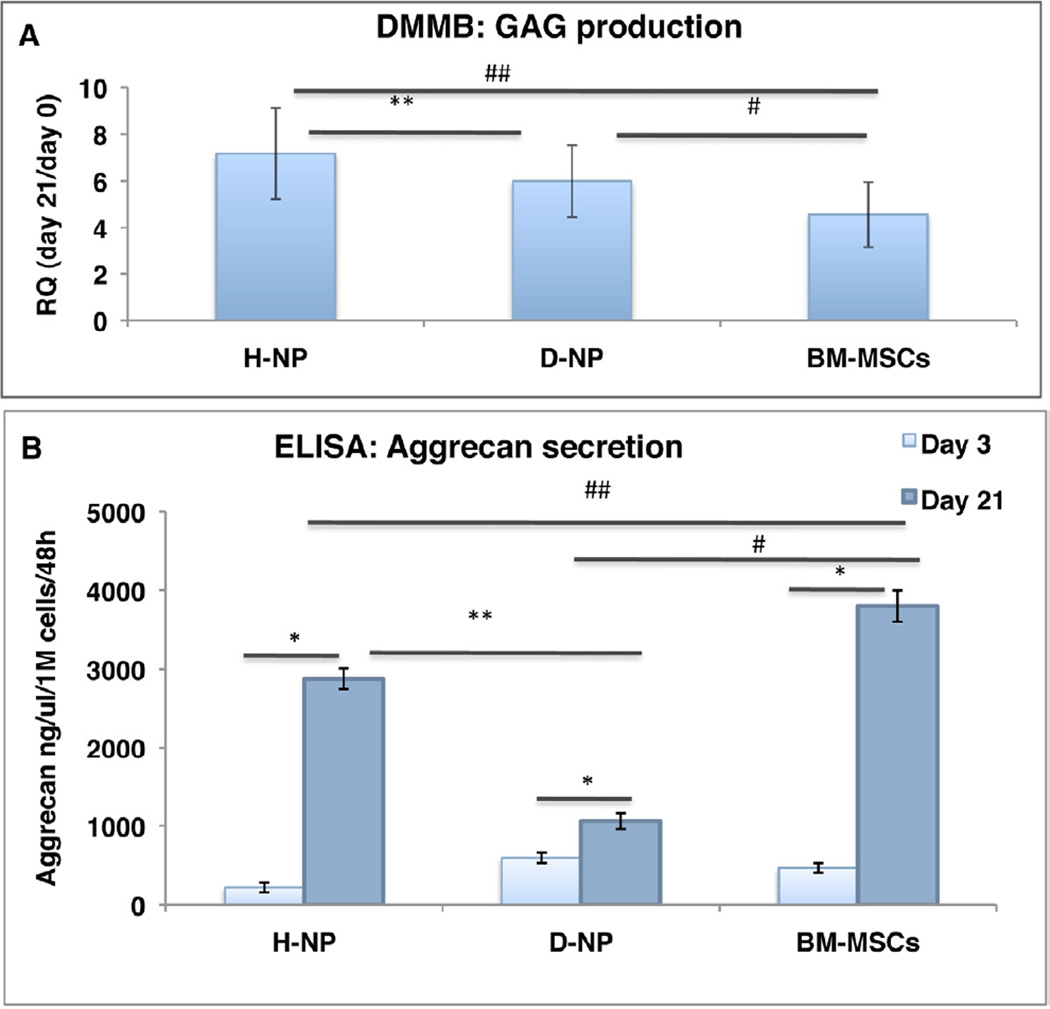
To achieve differentiation toward NP-like cells, the cells were encapsulated in alginate beads and cultured in a chemically defined medium and 2% hypoxic conditions. Overall, H-NP cells were found to be more responsive than D-NP cells to differentiation. Gene expression of collagen-II, aggrecan, and SOX-9 was evaluated on Day 7 and normalized to the level of expression in H-NP cells baseline (Day 0). H-NP and BM-MSCs differentiated cells exhibited significantly higher collagen-II, aggrecan and SOX-9 expression on Day 7, compared to undifferentiated cells within those groups. D-NP differentiated cells followed the same pattern, only in aggrecan gene expression. It was also evident there was significantly higher expression of collagen-II and aggrecan in the H-NP cells comparing to D-NP cells, whereas SOX-9 expression levels were similar in the two groups (n=10, Figure 5). In addition, when we compared the gene expression of differentiated H-NP cells and BM-MSCs we found similar aggrecan expression between the two groups, whereas gene expression in D-NP cells and BM-MSCs was significantly different. A DMMB assay for matrix secretion was conducted on alginate-encapsulated cells on Day 21 to further explore the differentiation potential of the two cell types (Figure 6a); the assay showed that H-NP cells produced 1.19 times more sGAG than D-NP cells (RQ values of Day 21/Day 0 of µg sGAG/µl/cell number, n=15 in total; p<0.05). The ELISA for secreted aggrecan showed that H-NP cells were significantly more efficient in secreting aggrecan into the culture media during the differentiation process, with a mean value of 2879±131ng aggrecan/ml/106 cells/48 hrs, (n=4), comparing to D-NP cells that secreted 1061±99ng aggrecan/ml/106 cells/48 hrs, (n=4). BM-MSCs, a reference group for this experiment, secreted 3798± 59ng aggrecan/ml/106 cells/48 hrs. (Figure 6b).
Figure 5. Differentiation towards NP-like cells: RNA expression.
Plastic adherent-NP cells and BM-MSCs cultured in alginate beads in hypoxia for 7 days. Gene expression (normalized to the expression of housekeeping gene 18S for each sample) of aggrecan (a); collagen-II (b); SOX-9 (c) (n=10).Gene expression is shown as RQ calibrated to the gene expression of H-NP at day 0.Bars indicate SE, *, ** (H-NP to D-NP), # (D-NP to BM-MSCs), ## (H-NP to BM-MSCs) indicate p<0.05.
Figure 6. Differentiation towards NP-like cells.
Plastic adherent-NP cells and BM-MSCs cultured in alginate beads in hypoxia for 21 days. DMMB assay for GAG production (n=15) sGAG quantity was normalized to cell number within the alginate beads. RQ values of sGAG/µl/cell number of day 21/day 0 are shown (a); ELISA for aggrecan secretion (n=4) Aggrecan content in culture media was normalized to cell number within the alginate beads(c). Bars indicate SE, *, ** (H-NP to D-NP), # (D-NP to BM-MSCs), ## (H-NP to BM-MSCs) indicate p<0.05.
Discussion
The goal of this study was to explore the effect of IVD degeneration on cells residing in the NP. We isolated total cell population from healthy and degenerate porcine NPs. D-NP cells demonstrated a significantly higher rate of proliferation and CFUs. Our data also indicate that NP-derived adherent cells can transdifferentiate into mesenchymal lineages; however, H-NP cells showed a significantly higher rate of chondrogenic differentiation and differentiation into NP-like cells than D-NP cells.
Our choice of a porcine model to study disc degeneration was based on the advantages it offers: large-volume discs; spinal anatomy similar to humans; and avoidance of age-related changes (all the pigs used in the study were 1.5–2 years old (skeletally mature) at the time of the research), low availability, and ethical restrictions.26,27 However some drawbacks exist in the porcine model, such as the limited commercial availability of anti-porcine antibodies28 and the presence of notochordal cells in adulthood. Notochordal cells, now believed to give rise to the entire population of cells in the NP and to play a role in matrix synthesis, persist in pigs and some other species into adulthood, whereas in humans they are said to disappear around the age of 10.29–31 Based on this assumption, there is an ongoing debate on the validity of animal models for disc degeneration.32,33 Risbud et al. suggested that when choosing an animal model for IVD study, the anatomical and mechanical characteristics of the spinal segment are more valid than concerns of possible notochordal cell presence.30 In addition, in this study we compared for the first time NP cells from healthy and degenerative discs isolated from the same animal. Any influence notochordal cells may have on our study should affect both cell populations.
Disc degeneration was successfully achieved using an annular injury approach, a well-established model in pigs.17 Although the annular injury does not produce degeneration identical to that observed clinically in humans, it enables systematic studies of IVD degeneration. Disc degeneration was evident on MRI, disc morphology, and histology. Unlike a previous study,2 we did not see any effect of degeneration on cell abundance in the disc, but we did see an effect on cell properties and differentiation potential.
We previously showed that progenitor cells can be isolated from human degenerated and healthy rat discs.13 NP cells from both degenerated and healthy discs showed a high frequency of CFUs, the ability to differentiate into the three mesenchymal lineages, and high expression of MSC markers in noncultured cells. These findings support the notion that the cells isolated from the discs include a population of progenitor cells that are able to proliferate, differentiate and potentially to contribute to disc regeneration process. Furthermore, the population of cells expressing MSC markers grew significantly higher with in vitro culturing, resembling other MSC-like populations.34 Interestingly, a significant difference between healthy and degenerative disc–derived cells was seen with respect to the CD90 antigen alone. Weismann et al. have reported that mechanical load can cause a decrease in CD90 expression in human MSCs.35 This can explain decreased expression in cells derived from a degenerate disc.
Our study shows that NP cells from degenerate discs have superior proliferation ability in vitro. This could be attributed to the fact that a different “chemical environment” with altered secretion of cytokines in the disc causes resident progenitor cells to proliferate further.36 Interestingly, a similar phenomenon was demonstrated12 with an increase in neural progenitors proliferation in the lumbar region of the adult spinal cord in response to motor neuron degeneration.
High expression of chondrogenic genes was found in NP tissue but not in cultured NP cells, indicating that the isolated NP cells are mainly progenitors, which are not fully differentiated after isolation, but bear the differentiation potential.
Recent publications support the notion that donor age and disease stage can alter progenitor cells’ differentiation potential.37,38 Our results show that the ability of H-NP cells to differentiate into the chondrogenic lineage and into NP-like cells is superior to that of D-NP cells displaying higher values of sGAG synthesis, aggrecan secretion, and RNA expression of aggrecan and collagen-II. Interestingly, SOX-9 expression was similar in both groups; this can be attributed to the fact that IVD degeneration has an effect that manifests in aggrecan and collagen-II expression, downstream to SOX-9 expression.39 Similar to our findings, Hegewald et al. showed that cells that had been harvested from herniated disc tissue and grown in a 3D culture system possess very limited regenerative potential compared to cells from NP tissue.27 In another study, although human degenerated disc-derived cells demonstrated lower collagen production than healthy disc-derived cells, their sGAG production remained similar.40 Interestingly, in addition to lower expression of collagen II and aggrecan in D-NP cells, the results showed that there additional downregulation in collagen II expression in D-NP cells from Day 0 to day 7 of differentiation. Only several studies have evaluated the degenerative effect on stem or progenitor cells residing in other degenerative tissues. Studies that examined the effect of osteoarthritis and rheumatoid arthritis on isolated MSCs and chondrocytes correlate to our findings; a significant reduction in chondrogenic activity was seen compared to that in cells from nonosteoarthritic patients.41–43 However, it should be noted that there are reports that found no correlation between osteoarthritis etiology and stem cells’ potential.44,45
With regard to osteogenic and adipogenic lineages, D-NP cells showed a higher differentiation potential. However, statistically significant results were seen only in the adipogenic differentiation experiments. Differentiation toward chondrogenic and NP-like lineages, which are induced in similar pathways,46,47 may explain the superiority of H-NP cells over D-NP cells in these two lineages. We believe that although cells from both groups can be defined as “progenitor cells,” they are in different stages of their differentiation toward the NP fate.
Our findings suggest that IVD degeneration has a clear effect on resident cells in the NP in general, and on the progenitor population in particular. We believe that D-NP cells are not responsible for degeneration, but they provide evidence of the response of cells populating the IVD to the degenerative process. Nevertheless, the decrease in differentiation potential in these cells might account partially for the tissue’s lack of ability to regenerate itself and reverse the degenerative process. Based on our findings, H-NP cells and BM-MSCs can be used for IVD regeneration, because they can differentiate successfully toward an NP-like fate. On the other hand, to successfully differentiate degenerated discs-derived cells, additional factors such as gene therapy or proper scaffolds should be employed. For example, Wallach and Le Maitre demonstrated that overexpressing TMP-1 or cartilage-derived morphogenetic protein genes could increase matrix synthesis in cells from degenerated discs.48,49
We have conducted a methodical comparison of cells derived from healthy and degenerated porcine discs and have shown that disc degeneration induced with an abnormal matrix has a clear effect on proliferation and differentiation potentials of progenitor cells residing in the IVD. Thus D-NP cells clearly are in the middle of the differentiation and tissue regenerative process; however, this process is not sufficient to facilitate full regeneration of the disc and reverse the course of degeneration. However, the reason for the insufficient ability of this response to prevent and reverse the degenerative process remains to be elucidated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schafer J, O'Connor D, Feinglass S, Salive M. Medicare Evidence Development and Coverage Advisory Committee Meeting on lumbar fusion surgery for treatment of chronic back pain from degenerative disc disease. Spine (Phila Pa 1976) 2007 Oct 15;32(22):2403–2404. doi: 10.1097/BRS.0b013e3181573841. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C-Q, Wang L-M, Jiang L-S, Dai L-Y. The cell biology of intervertebral disc aging and degeneration. Ageing Research Reviews. 2007;2(6):247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Freemont AJ, Hoyland JA. Morphology, mechanisms and pathology of musculoskeletal ageing. J. Pathology. 2007;211(2):252–259. doi: 10.1002/path.2097. [DOI] [PubMed] [Google Scholar]
- 4.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010 Feb;16(1):147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006 Aug 15;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 6.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009 Jan;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 8.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004 Dec 1;29(23):2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 9.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000 Jan 7;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 10.Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6(5):R422–R432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi L, Ke Y, Luo C, et al. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006 Jan;24(1):34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007 Nov 1;32(23):2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 14.Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009 Oct 1;34(21):2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 15.Blanco JF, Graciani IF, Sanchez-Guijo FM, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010 Dec 15;35(26):2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 16.Yoon SH, Miyazaki M, Hong SW, et al. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J Neurosurg Spine. 2008 May;8(5):450–457. doi: 10.3171/SPI/2008/8/5/450. [DOI] [PubMed] [Google Scholar]
- 17.Niinimaki J, Ruohonen J, Silfverhuth M, Lappalainen A, Kaapa E, Tervonen O. Quantitative magnetic resonance imaging of experimentally injured porcine intervertebral disc. Acta Radiol. 2007 Jul;48(6):643–649. doi: 10.1080/02841850701326933. [DOI] [PubMed] [Google Scholar]
- 18.Cinotti G, Della Rocca C, Romeo S, Vittur F, Toffanin R, Trasimeni G. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine (Phila Pa 1976) 2005 Jan 15;30(2):174–180. doi: 10.1097/01.brs.0000150530.48957.76. [DOI] [PubMed] [Google Scholar]
- 19.Sheyn D, Pelled G, Zilberman Y, et al. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells. 2008 Apr;26(4):1056–1064. doi: 10.1634/stemcells.2007-0858. [DOI] [PubMed] [Google Scholar]
- 20.Aslan H, Zilberman Y, Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006 Apr;12(4):877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 21.Kimelman-Bleich N, Pelled G, Sheyn D, et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009 Sep;30(27):4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010 Jul;5(7):1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SH, Yen HJ, Tsai CL. The response of articular chondrocytes to type II collagen-Au nanocomposites. Artif Organs. 2007 Dec;31(12):854–868. doi: 10.1111/j.1525-1594.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol J. 2003;4(2):92–96. doi: 10.1038/sj.thj.6200232. [DOI] [PubMed] [Google Scholar]
- 26.Omlor GW, Nerlich AG, Wilke HJ, et al. A new porcine in vivo animal model of disc degeneration: response of anulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine (Phila Pa 1976) 2009 Dec 1;34(25):2730–2739. doi: 10.1097/BRS.0b013e3181b723c9. [DOI] [PubMed] [Google Scholar]
- 27.Hegewald AA, Endres M, Abbushi A, et al. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J Neurosurg Spine. 2011 Feb;14(2):273–280. doi: 10.3171/2010.10.SPINE10223. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Moutsoglou D. Osteogenic and adipogenic differentiation potential of an immortalized fibroblast-like cell line derived from porcine peripheral blood. In Vitro Cell Dev Biol Anim. 2009 Dec;45(10):584–591. doi: 10.1007/s11626-009-9231-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006 Aug;15(Suppl 3):S303–S311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010 Aug;239(8):2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1(2):18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003 Aug;9(4):667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 33.McCann MR, Bacher CA, Seguin CA. Exploiting notochord cells for stem cell-based regeneration of the intervertebral disc. J Cell Commun Signal. 2011 Mar;5(1):39–43. doi: 10.1007/s12079-010-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aslan H, Zilberman Y, Kandel L, et al. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006 Jul;24(7):1728–1737. doi: 10.1634/stemcells.2005-0546. [DOI] [PubMed] [Google Scholar]
- 35.Wiesmann A, Buhring HJ, Mentrup C, Wiesmann HP. Decreased CD90 expression in human mesenchymal stem cells by applying mechanical stimulation. Head Face Med. 2006;2:8. doi: 10.1186/1746-160X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002 Apr;196(4):374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 37.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007 Sep;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 38.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006 Jun;5(3):213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Robins JC, Akeno N, Mukherjee A, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005 Sep;37(3):313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 40.Svanvik T, Henriksson HB, Karlsson C, Hagman M, Lindahl A, Brisby H. Human disk cells from degenerated disks and mesenchymal stem cells in co-culture result in increased matrix production. Cells Tissues Organs. 2010;191(1):2–11. doi: 10.1159/000223236. [DOI] [PubMed] [Google Scholar]
- 41.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002 Mar;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 42.Dorotka R, Bindreiter U, Vavken P, Nehrer S. Behavior of human articular chondrocytes derived from nonarthritic and osteoarthritic cartilage in a collagen matrix. Tissue Eng. 2005 May-Jun;11(5–6):877–886. doi: 10.1089/ten.2005.11.877. [DOI] [PubMed] [Google Scholar]
- 43.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Buhring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007 Dec;25(12):3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 45.Dudics V, Kunstar A, Kovacs J, et al. Chondrogenic potential of mesenchymal stem cells from patients with rheumatoid arthritis and osteoarthritis: measurements in a microculture system. Cells Tissues Organs. 2009;189(5):307–316. doi: 10.1159/000140679. [DOI] [PubMed] [Google Scholar]
- 46.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion 63-54. [DOI] [PubMed] [Google Scholar]
- 47.Gruber HE, Hanley EN., Jr Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord. 2000 Oct 23;1:1. doi: 10.1186/1471-2474-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine (Phila Pa 1976) 2003 Oct 15;28(20):2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 49.Le Maitre CL, Freemont AJ, Hoyland JA. Expression of cartilage-derived morphogenetic protein in human intervertebral discs and its effect on matrix synthesis in degenerate human nucleus pulposus cells. Arthritis Res Ther. 2009;11(5):R137. doi: 10.1186/ar2808. [DOI] [PMC free article] [PubMed] [Google Scholar]