Abstract
Mild traumatic brain injury (mTBI) leads to long-term cognitive and emotional difficulties and behavioral disturbances, but the diagnosis and treatment of mTBI have historically been hampered by a lack of evidence-based correlates of these clinical manifestations. Unlike moderate and severe TBI, mTBI does not show significant tissue lesions or cavities in the cortex. Moreover, neuroimaging by magnetic resonance imaging or computed tomography is usually negative, suggesting that the damage is beyond the resolution of current structure-based scanning technologies. Therefore, we investigated the morphologies of spared neurons in the mouse cortex after mTBI in a controlled cortical impact injury model. Our results indicate that, although mTBI caused only a mild extent of cell death, it led to extensive dendrite degeneration and synapse reduction in the cortex in this model. This study sheds light on the neuropathologic consequences of mTBI in humans and suggests that neurodegeneration may be a novel target for developing diagnostic methods and therapeutic approaches for mTBI.
Keywords: Dendrite, mTBI, Neural degeneration, Spine, Synapse
INTRODUCTION
There are 1.5 million civilian traumatic brain injuries (TBIs) annually in the United States. Traumatic brain injury is conventionally graded on a severity continuum ranging from mild to severe. Because injuries at the more severe end of this continuum are associated with significant morbidity and mortality, considerable attention has been devoted to understanding and managing the most severe injuries (1). On the other hand, both clinical definitions and results of surveys indicate that mild TBI (mTBI) accounts for nearly 75% of all treated cases (2–5). Many more mild injuries undoubtedly go unreported or are otherwise unaccounted for (5, 6). Furthermore, it is estimated that more than 300,000 US veterans of the wars in Iraq and Afghanistan have sustained mTBI from improvised explosive device blast waves (20% of 1.6 million) (7, 8). Therefore, mTBI is a major public health problem.
Unlike victims of moderate and severe TBI (9–14), individuals who experience mTBI may show cognitive impairment without identifiable tissue lesions or cavities in the cerebral cortex (15). Neuroimaging by magnetic resonance imaging (MRI) or computed tomography (CT) is usually negative (16–20); therefore, mTBI has been called an “invisible wound.” Owing in part to poor understanding of the neuropathology leading to neurologic dysfunction after mTBI, there are no effective treatments. In particular, the absence of gross alterations makes the diagnosis, therapy evaluation, and outcome prediction after mTBI difficult. Here, we induced mTBI using a rodent controlled cortical impact (CCI) model and assessed neuropathologic changes in detail, including cortex spared volume, cell death, dendrite degeneration, dendritic spine degeneration, and synaptic loss after mTBI. The results support the concept that mTBI produces dramatic dendrite and synapse degeneration despite minimal cell death in the neocortex.
MATERIALS AND METHODS
Male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were housed in groups with a 12-hour/12-hour light-dark cycle and free access to food and water ad libitum. They were used in experiments at 8 weeks of age. All procedures were performed under protocols approved by the Animal Care and Use Committee at Indiana University.
Controlled Cortical Impact TBI
The CCI injury model replicates focal abnormalities seen in many human TBI cases (21–23), and the mouse CCI model has been used to investigate posttraumatic cell death (24, 25). Here, 72 mice were subjected to mild CCI injury or sham treatment, as previously described (26, 27), with the following exceptions: the amount of deformation was set at 0.2 mm and the piston velocity was controlled at 2.8 m/s. These modifications result in a mild level of injury using an electromagnetic model (28).
Tissue Processing for Fluoro-Jade B Staining and Immunostaining
At 3 days after TBI surgery, the animals were deeply anesthetized with an overdose of sodium pentobarbital and then perfused transcardially with cold 0.9% saline, followed by a fixative containing 4% paraformaldehyde (Sigma, St Louis, MO) in phosphate-buffered saline (PBS). The brains were removed and postfixed in paraformaldehyde overnight, followed by cryoprotection with 30% sucrose for 48 hours. Thirty-micrometer-thick serial coronal sections were cut using a cryostat (Leica CM 1950; Leica, Wetzlar, Germany) and stored at − 20°C. The sections were then processed for Fluoro-Jade B (FJB) staining and immunohistochemical analysis as described (26, 27, 29–32). Briefly, to perform FJB staining (12 mice), sections were first hydrated in distilled water. The sections were then incubated in a solution of 0.06% potassium permanganate (Sigma) for 10 minutes on a rotating stage. After rinsing in distilled water for 2 minutes, the sections were incubated in a 0.0004% solution of FJB and 0.0004% 4′,6-diamidino-2-phenylindole (DAPI) (both from Sigma) for 20 minutes. Sections were then rinsed in distilled water for 2 minutes and air-dried. The dry slides were cleared in xylene for 2 minutes and mounted with DPX (Sigma). For immunostaining (30 mice), sections were rinsed in PBS 3 times and incubated in a blocking solution (0.1% Triton X-100, 1% bovine serum albumin, 5% normal goat serum in PBS) for 1 hour at room temperature (RT), followed by an overnight incubation with primary antibody (1:1,000 NeuN and 1:1,000 synaptophysin; Sigma) at 4°C. Sections were then washed with PBS 3 times and incubated with the secondary antibody at RT for 2 hours. After treatment with DAPI for 2 minutes, the sections were washed with PBS 3 times and mounted with FluoroGel mounting medium (GeneTex, Irvine, CA). Secondary antibodies from Jackson Immunoresearch (West Grove, PA) were all applied 1:1,000 dilutions.
Golgi Staining
In total, 20 mice were used for Golgi staining (10 each for TBI and sham groups). We used the FD Rapid Golgi Stain kit (FD NeuroTechnologies, Ellicott City, MD) to perform Golgi staining following the vendor’s protocol. Briefly, the freshly dissected brains were immersed in impregnation solution (made by mixing equal volumes of solutions A and B) and stored at RT for 2 weeks in the dark. The brains were then transferred into solution C and kept for 48 hours at 4°C in the dark. They were then sliced using a horizontal sliding slicer (VT1000S; Leica) at a thickness of 150 µm and stained using standard staining procedures.
Dendrite Analysis
Pyramidal neurons in layers II/III of the cortex in the epicenter were analyzed as previously described (33). Briefly, the criteria used to select neurons for reconstruction were as follows: the neurons should be fully impregnated and located in layers II/III with triangular soma and apical dendrites extending toward the pial surface without truncated branches (34–36). For each selected neuron, all branches of the dendritic tree were reconstructed at 40 × magnification using a motorized microscope (Olympus BX60; Olympus, Tokyo, Japan) with Neurolucida software (Microbrightfield, Williston, VT). A 3-dimensional analysis of the reconstructed neurons was performed using NeuroExplorer software (Microbrightfield). Ten neurons exhibiting all of the criteria were randomly chosen from the 3 epicenter sections of each animal in the different experimental groups, and neurons from the same animal were averaged. Several aspects of dendritic morphology were examined. To assess overall changes, the total length of the basal and apical trees and the number of basal dendrites and apical dendritic branches were compared across groups. To assess the complexity of the dendritic tree, Sholl analysis was performed (37, 38). The numbers of intersections of dendrites were calculated with concentric spheres positioned at radial intervals of 10 µm.
Spine Quantification
The spine density of the dendrite branch was measured by considering only apical branches that were either parallel or at acute angles to the coronal surface of the section and did not overlap with other branches (5 mice/group). Because mTBI-induced changes in the apical dendritic branches varied depending on the distance from the soma, segments were randomly selected in the proximal and distal parts of the tree, where maximal effects were observed. To minimize bias, only spines that emerged perpendicular to the dendritic shaft were counted. To assess mTBI-induced changes in spine morphology, spines in the selected segments were classified as mushroom, stubby, or filopodia, and the proportions of spines in each category were compared between the groups. High-magnification images were captured using a camera (DXC-390; Sony Corporation, Tokyo, Japan) attached to the BX60 Olympus upright microscope under a 100× oil objective.
Cell Number Quantification
The optical fractionator method (39–41) was used to quantify total (DAPI-positive) and NeuN-positive cells in the epicenter in layers II/III of the cortex. Briefly, using a Bioquant stereology workstation (BioQuant Image Analysis Corp, Nashville, TN), the step size across sections was set as 100 × 100 µm . The dissector height was set as 20 µm, and a 5-µm zone at the uppermost part of the section was excluded from the analysis at every step as the upper guard zone. The upper guard zone is 5 µm, the dissector is set 20 µm, and the lower guard zone is, on average, 3.3 µm. The cells in a counting frame of 50 × 50 µm2 were counted under a 40 × objective, which allowed accurate recognition. Each DAPI- or NeuN-positive cell was counted when it came into focus as the plane of focus was moved up and down through the height of the dissector (20 µm) following the unbiased counting rules for the optical dissector (40). The total numbers of DAPI- or NeuN-positive cells in the epicenter were determined and normalized to the sham control.
Synapse Assessment
Synaptic densities were analyzed using ImageJ (National Institutes of Health, Bethesda, MD). The images were compared to the threshold to outline immunopositive puncta, and the numbers of the puncta were determined using the “analyze particle” module of the program. These numbers were then normalized to the control to determine the synaptic density.
Statistical Analysis
Collected data were expressed as mean ± SD, and differences were analyzed using Student t test with the significant level set at p < 0.05.
RESULTS
mTBI Results in Mild Cell Death in the Injury Area of the Neocortex
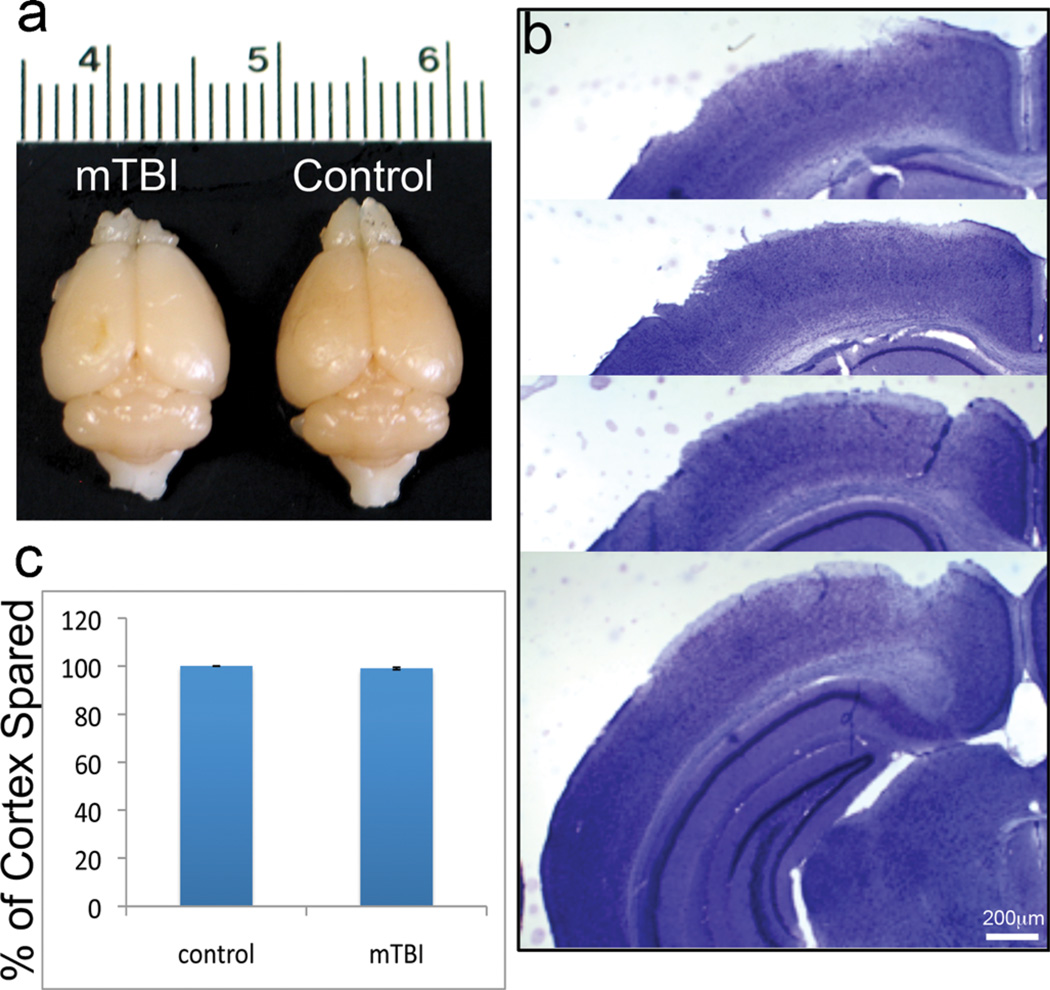
To assess the neuropathologic changes after mTBI, we first analyzed mouse brains 3 days after TBI, that is, the peak of neuronal degeneration that appears after TBI (unpublished data). The brains showed very limited tissue disruption without cavitation (Fig. 1A). Staining with crystal violet showed that cortical tissue was generally intact without dramatic lesions (Fig. 1B). The volume of the spared cortex in mice with TBI was 98.3% ± 0.52% of the cortex volume of sham controls (Fig. 1C); this reduction was not statistically significant (p > 0.05). These data indicate that the cortex does not show major tissue lesions after mTBI.
FIGURE 1.
Mild traumatic brain injury (mTBI) in mice. (A) Gross examination of the brains 3 days post-TBI showed very limited tissue lesions without cavitation in the mTBI mouse brain (left) compared to the sham control (right). (B) Crystal violet staining shows that the tissue in the injured cortex is nearly intact without major disruption. (C) Quantitative measurement of the volume of the damaged brain region revealed that a reduction in the volume of the spared cortex was less than 5% and not significantly different versus the contralateral hemisphere (n = 10, p > 0.05).
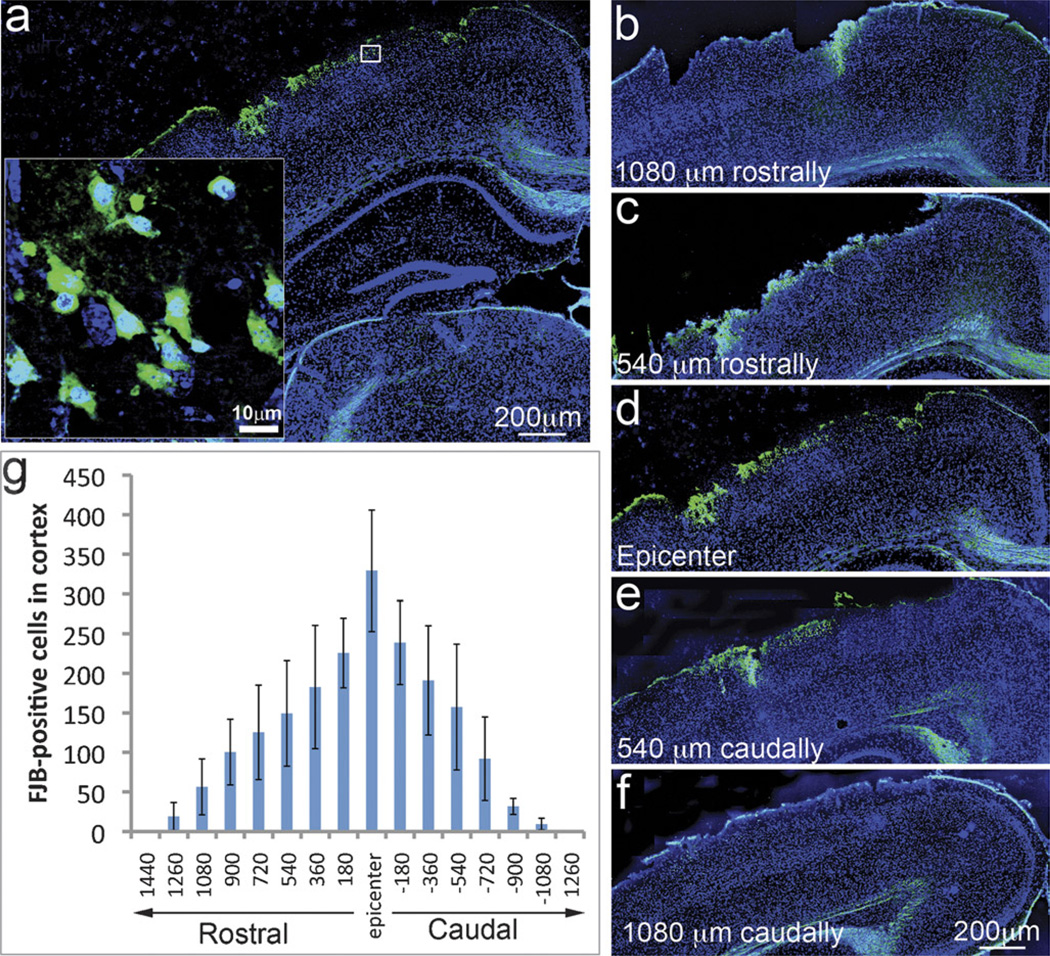
Fluoro-Jade B staining is a widely used method to detect dying cells in the cortex (29, 30). Animals with a mild level of CCI injury exhibited FJB-positive neurons (green) in the epicenter on the lesioned side of the neocortex (Fig. 2A) but not in the corresponding region on the contralateral side (data not shown). The somas of the degenerating neurons were highly stained by FJB (Fig. 2A, inset). The degenerating neurons were constrained within approximately 1 mm around the epicenter (Figs. 2, B–F). The greatest number of FJB-positive cells was observed in the epicenter (330 ± 77/section), and the number decreased to 150 ± 36/section at 540 µm and reached almost zero at a distance of 1260 µm rostrally and caudally from the epicenter (Fig. 2G).
FIGURE 2.
Cell death in the neocortex of mice with mild traumatic brain injury (mTBI). (A) Fluoro-Jade B (FJB)–positive cells are green in the epicenter on the ipsilateral side of the neocortex. (B–F) Distribution pattern of dying cells around the epicenter. (G) Quantitative data show that the highest number of FJB-positive cells was observed in the epicenter and gradually decreased rostrally and caudally away from it (n = 12).
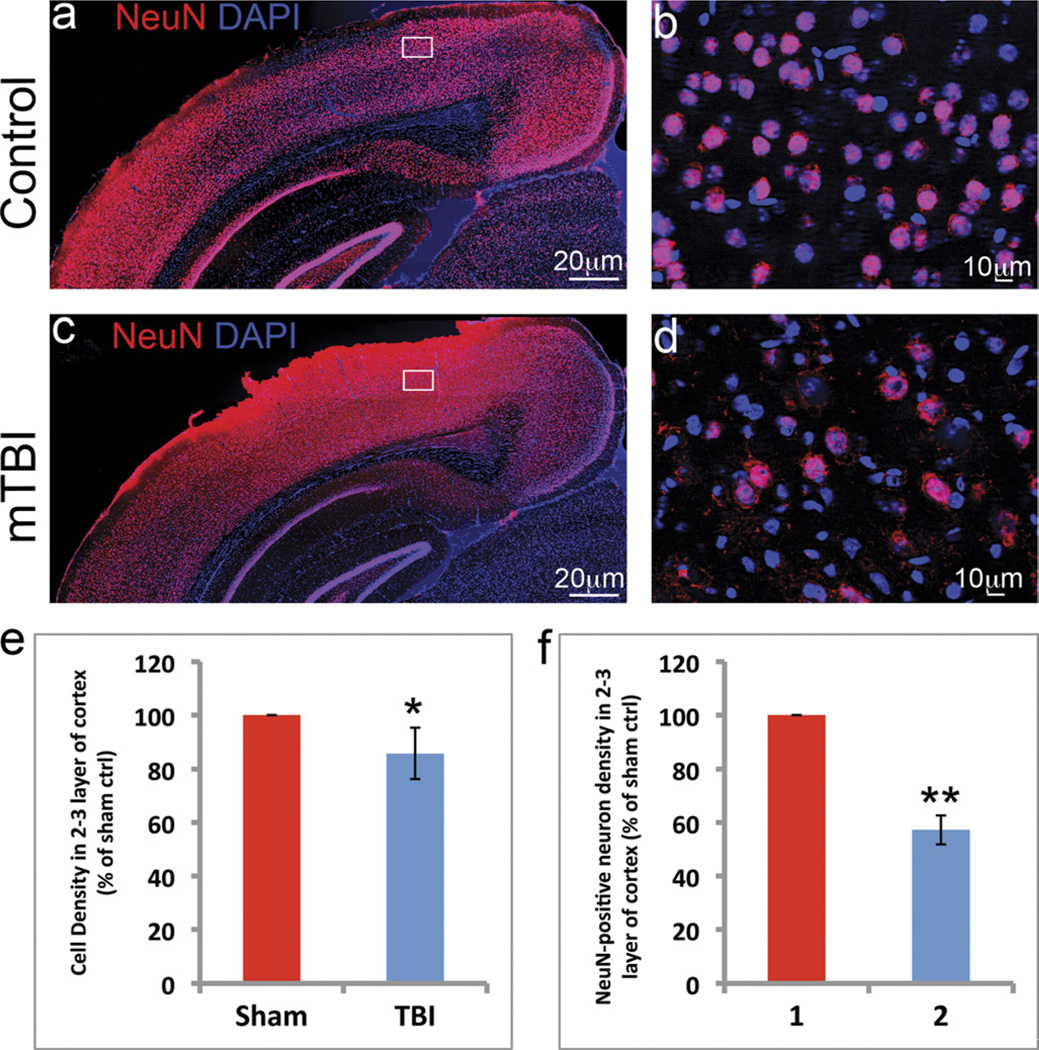
The total cell and neuron density in the injury area was measured using DAPI staining and immunostaining with an antibody to NeuN, a mature neuron marker. Compared with the sham control, the total cell density based on DAPI staining was slightly reduced to 85.8% ± 9.5% (*, p < 0.05) in the epicenter. Because DAPI stains the nuclei of both neurons and glia, NeuN staining was used to assess neuron loss in the injury area. These experiments revealed highly stained NeuN in the nuclei of neurons in the cortex of control (Figs. 3A, B) and mTBI-injured brains (Figs. 3C, D). At low resolution, NeuN staining resulted in a slightly higher background in the mTBI-injured brain (Fig. 3C) compared with the sham control (Fig. 3A). At higher magnification, NeuN-stained neurons were evident in both the mTBI-injured (Fig. 3D) and the sham control (Fig. 3B). We then assessed the density of the mature neurons in layers II/III at the epicenter of the mTBI-injured cortex and the corresponding layers in the control cortex. The density of NeuN-positive neurons in the epicenter was reduced to 57.2% ± 5.4% of the density in the controls (**, p < 0.005). These data suggest that, although mTBI did not cause gross neuroanatomic changes in the brain, it did result in some neuron death in the epicenter.
FIGURE 3.
Cells lost in the neocortex of mice with mild traumatic brain injury (mTBI). (A–D) DAPI (blue) and NeuN (red) staining revealed the nuclei of cells and mature neurons at the injury site in the cortex (A and B sham control, C and D mTBI). (E) DAPI staining followed by counting of the stained cells showed that the total cell density in the epicenter was slightly reduced to 85.8% ± 9.5% compared to the sham control (n = 10; *, p < 0.05). (F) The density of NeuN-positive neurons was dramatically reduced to 57.2% ± 5.4% (n = 10; **, p < 0.005).
mTBI Causes Extensive Neural Degeneration in the Neocortex
To determine whether mTBI causes degeneration in the spared neurons, we assessed the morphologies of the spared neurons by Golgi staining, a reliable and sensitive method for demonstrating morphologic details of individual neurons, especially dendritic spines. Golgi staining visualized individual neurons including their processes and spines at very high resolution in the control brains (Figs. 4A, C1–D3) and damaged brain after mTBI (Figs. 4B, E1–F3). The density of the Golgi-stained neurons in the outlined area of the injury brain was significantly decreased compared with the corresponding position of the control brains, consistent with the finding that mTBI induced cell death (Fig. 2) and decreased cell density in the injury area (Fig. 3). Higher-resolution images of the epicenter (Figs. 4, E1–E3) and 1 mm lateral to the epicenter (Figs. 4, F1–F3) also demonstrated that the dendrites of the spared neurons in the injured cortex were swollen with beadings, a hallmark of dendritic injury, compared to neurons in the corresponding regions of control mice (Figs. 4, C1–C3 and D1–D3). The cortex volume showed dendritic degeneration (Fig. 4B) and was surprisingly large. When degeneration was taken into account, the spared cortex decreased to 82.5% ± 4.3% (*, p < 0.05; Fig. 4G). The volume of neuronal degeneration was 10.3 times larger than the tissue lesion volume in the injured cortex after mTBI (Fig. 4H). These data indicate very extensive injury in the dendrites of the spared neurons in the injury area, that is, the actual injury was much greater than previously assessed using the percentage of spared tissue as an indicator.
FIGURE 4.
Mild traumatic brain injury (mTBI) causes extensive neural degeneration in the neocortex. (A–F) Golgi staining reveals individual neurons, their processes, and spines in a sham control (A, C1–C3, and D1–D3) and in a mouse with mTBI (B, E1–E3, and F1–F3). The density of the Golgi-stained neurons in the injury area on the ipsilateral side was decreased, and the dendrites and spine of the spared neurons in the same area were dramatically injured. (G) Quantitative data show that when degeneration is taken into account the spared cortex decreased dramatically to 82.5% compared with the sham control (n = 10; **, p < 0.005). (H) The volume of the neuronal degeneration region (marked by the red line in B) was 10.3 times higher than the tissue lesion volume in the injured cortex after mTBI.
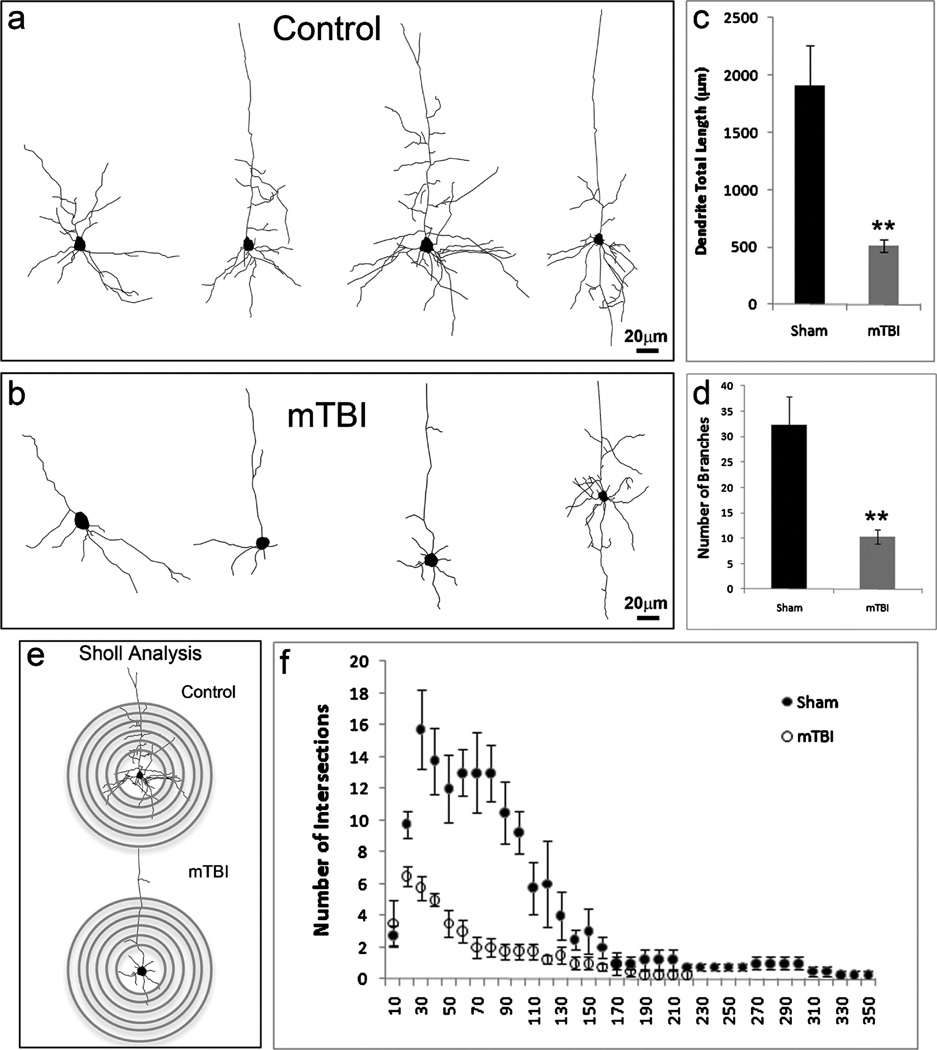
mTBI Causes Dendrite Degeneration
To characterize the morphologic characteristics of neurons in the cortex after mTBI, we reconstructed dendritic morphologies of individual neurons (n = 50) of layers II/III in the epicenter or in the corresponding positions in mTBI and control mice (Figs. 5A, B). The results showed that the total length of the apical dendrites and the number of dendrite branches of pyramidal cells were reduced in mTBI (Figs. 5C, D). To characterize the dendritic branching characteristics of individual neurons in the injury area and the corresponding control regions, we performed Sholl analysis, a quantitative method for morphometric neuronal studies (Figs. 5E. F). This analysis revealed that mTBI brains showed reduced dendritic material compared with the sham controls. This decreased complexity was particularly evident at a distance between 40 and 100 µm from the soma. The difference between neurons from mTBI and sham animals peaked at 70 µm from the soma; at this distance, the total number of crossings was 6 times lower for mTBI mice (2 ± 0.7 vs 13 ± 2.5 crossings in mTBI and sham mice, respectively; 50 cells in each group, p < 0.01; Fig. 5F). These results indicate a dramatic dendritic degeneration in the neurons in the injury area after mTBI.
FIGURE 5.
Mild traumatic brain injury (mTBI) causes dendrite degeneration. (A, B) Neurolucida reconstruction of Golgi-stained layers II/III neurons in the cortex of sham (A) and mTBI mice (B). (C, D) The total length (C) and the number of branches (D) of the dendrites in cells from sham and mTBI mice. The total length of the dendrites in spared neurons decreased from 1905 ± 357 to 508 ± 55 µm; branches are dramatically decreased from 32 ± 5 to 10 ± 1 in the cortex after mTBI (n = 5; *, p < 0.05). (E) Sholl analysis–derived distribution of layers II/III neuron dendritic complexity based on the distance from the cell body. Mean number of intersections of dendrite branches with consecutive 10-µm-spaced concentric spheres. (F) There is a dramatic reduction of dendritic complexity in the spared neurons in mTBI mice versus sham controls (n = 5; *, p < 0.05).
mTBI Leads to Spine Degeneration
To assess dendritic spines of spared neurons in the injury area, we imaged the dendritic spines in the cortexes of control (Fig. 6A) and mTBI (Fig. 6B) mice and determined their density and characteristics. The density of the dendritic spines was significantly reduced from 14.48 ± 0.89 per 10 µm to 9.09 ± 0.85 per 10 µm in the spared neurons in the injured cortex (Fig. 6F). Dendritic spine protrusions were categorized into 3 types based on their shapes and lengths as follows: mushroom-shaped spines, in which the diameter of the head is much larger than the diameter of the neck, as is usually seen in mature synapses (Fig. 6C) (42); stubby-shaped spines, which are short and wide and are considered to represent either a transitional growth stage between an early to mature spine or the stage during retraction of the mature spine for elimination (Fig. 6D); and filopodia-shaped spines, which are highly mobile, long, and thin with a fine tip and are seen at the early stage of spine formation (Fig. 6E) (43–46). The densities of mushroom-shaped (8.06 ± 0.41 vs 3.36 ± 0.39 per 10 µm) and filopodia-shaped spines (3.31 ± 0.44 vs 1.66 ± 0.26 per 10 µm) were decreased in mTBI versus controls, whereas the density of stubby-shaped spines increased slightly (Fig. 6G).
FIGURE 6.
Mild traumatic brain injury (mTBI) leads to dendritic spine degeneration. (A, B) High-resolution image of a single spine in sham control (A) and mTBI mice (B). (C–E) Mushroom-shaped (C), stubby-shaped (D), and filopodia-shaped (E) spines are shown. (E) Quantitative data show that the density of the dendritic spines is significantly reduced in spared neurons in the cortex of TBI mice (n = 5; **, p < 0.005). (G) The reduction is particularly apparent in mushroom-shaped and filopodia-shaped spines (n = 5; **, p < 0.005).
mTBI Decreases the Number of Synapses in the Injury Area
Because a reduction in the number of dendritic branches and degeneration of the dendritic spines would decrease the number of synapses, we determined whether mTBI-induced degeneration in dendrites and dendritic spines led to a decrease in synapses by quantifying immunostaining with an antisynaptophysin antibody (47). Synaptophysin immunostaining was observed in a punctate distribution, consistent with localization of presynaptic boutons. Cell nuclei showed negligible synaptophysin immunoreactivity (Fig. 7). The densities of synaptophysin-positive puncta in layers II/III in the epicenter of the TBI-injured mice and at the same anatomic position in the brains of control mice were counted. In the TBI-injured brains, the density of the synaptophysin-positive puncta was reduced to 65.5% ± 8.1% versus the sham control cortex, indicating that mTBI decreased the number of synapses.
FIGURE 7.
Mild traumatic brain injury (mTBI) decreases the number of synapses in the injury area. (A–D) Synaptophysin staining (red) reveals presynaptic puncta in layers II/III of the cortex in control (A, B) and mTBI mice (C, D). The synapse density decreased to 65.5% ± 8.1% of the control cortex in the cortex of injured mice (n = 10; *, p < 0.05).
DISCUSSION
Although patients with mTBI show a wide range of neurologic symptoms, including headache, blurred vision, dizziness, sleep problems, subjective memory problems, and other cognitive difficulties (48–57), neuroimaging using CT or MRI does not usually identify major structural lesions (16–20). Neurological dysfunction in experimental models of TBI has been attributed to rapid cell death (12, 58–60). However, unlike moderate and severe TBI, the model of mTBI we used does not induce significant tissue lesions or cavitation in the cortex, and cell death was limited to the epicenter. Previous work in the rat also showed prolonged memory impairment in the absence of hippocampal cell death after TBI (61).
Although mTBI caused only mild cell death, it resulted in extensive dendritic degeneration and synapse reduction. The cell body and the dendrites are the major domains of neurons that receive input, and dendrites play a critically important role in providing a massive receptive area on the neuronal surface for spine formation. An important specialization of the dendritic arbor of certain neurons is the presence of large numbers of spines with as many as 30,000 to 40,000 spines on large pyramidal neurons. Maintaining such complex dendrite arbors is a prerequisite for neuronal connections and, to a large degree, neuron functions. Using Golgi staining, we found that dendrites were degenerated in the ipsilateral cortex after mTBI; the abnormalities included swelling with beading, a hallmark of dendritic injury, decreased numbers of branches, and shorter branch lengths. Dendrite degeneration around the epicenter was very extensive. Indeed, the volume of neuronal degeneration in the cortex was 10.3 times higher than the tissue lesion volume after mTBI.
Dendritic spine morphology was also significantly affected in the injured neurons in the ipsilateral cortex. In particular, mushroom-shaped spines were dramatically reduced. Because these spines form synapses, reductions in their density suggest a reduction in the number of the synapses. This was confirmed with synaptophysin immunohis-tochemistry. Moreover, the injury was much more extensive than assessments based on the percentage of spared tissue. Because CNS function relies critically on the establishment and maintenance of synaptic connections between the neurons (62), extensive degeneration of dendrites and dendritic spines and loss of synapses would likely greatly impair neurologic function.
There is increased attention directed at finding objective physiological correlates for persistent cognitive and neuropsychiatric symptoms through experimental neuroimaging techniques. Because the diameter of synapses is less than 1 µm, the formation and elimination of synapses are beyond the resolution of conventional CT and MRI. On the basis of our results, functional imaging techniques may be more promising than structural imaging techniques for the immediate diagnosis and management of mTBI. Indeed, several recent studies using newer imaging techniques such as functional MRI, positron emission tomography, and single photon emission CT showed functional impairment in the cortex after mTBI (16, 63–66). Thus, these functional techniques may be more sensitive for detecting functional impairments related to synapse degeneration.
In summary, we showed that although mTBI led to limited tissue lesion and cell death, it caused widespread and significant synapse degeneration in the cortical neurons that were spared. This suggests dramatic neuronal degeneration after mTBI. The widespread synapse loss disrupts neural circuitry after mTBI would likely contribute to neurologic dysfunction. Furthermore, the spared neurons showed dramatic synapse degeneration without death after mTBI, suggesting that synapse degeneration is a program separate from neuron death and should be treated with different therapeutic approaches than those used to prevent cell death. The essential and defining characteristics of the injury will indicate possible novel targets for developing therapeutic approaches; facilitate techniques for diagnosis, therapy evaluation, and outcome prediction; and may drive an evidence-based approach to clinical management.
Acknowledgments
This work was supported by grants from the Indiana Spinal Cord & Brain Injury Research Grants (SCBI 200-12), the Ralph W. and Grace M. Showalter Research Award, and Indiana University Biological Research Grant to J. Chen.
REFERENCES
- 1.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: Differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Minino AM, Anderson RN, Fingerhut LA, et al. Deaths: Injuries, 2002. Natl Vital Stat Rep. 2006;54:1–124. [PubMed] [Google Scholar]
- 4.Cassidy JD, Carroll L, Cote P, et al. Mild traumatic brain injury after traffic collisions: A population-based inception cohort study. J Rehabil Med. 2004:15–21. doi: 10.1080/16501960410023688. [DOI] [PubMed] [Google Scholar]
- 5.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 6.Williamson IJ, Goodman D. Converging evidence for the under-reporting of concussions in youth ice hockey. Br J Sports Med. 2006;40:128–132. doi: 10.1136/bjsm.2005.021832. discussion 128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoge CW, Goldberg HM, Castro CA. Care of war veterans with mild traumatic brain injury-flawed perspectives. N Engl J Med. 2009;360:1588–1591. doi: 10.1056/NEJMp0810606. [DOI] [PubMed] [Google Scholar]
- 8.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto ST, Longhi L, Saatman KE, et al. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Bio-behav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Saatman KE, Feeko KJ, Pape RL, et al. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 11.McAllister TW, Flashman LA, McDonald BC, et al. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 12.Hall ED, Sullivan PG, Gibson TR, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: More than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- 13.Singh IN, Sullivan PG, Deng Y, et al. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 14.Milman A, Rosenberg A, Weizman R, et al. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- 15.Petchprapai N, Winkelman C. Mild traumatic brain injury: Determinants and subsequent quality of life. A review of the literature. J Neurosci Nurs. 2007;39:260–272. [PubMed] [Google Scholar]
- 16.Belanger HG, Vanderploeg RD, Curtiss G, et al. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Iverson GL. Clinical and methodological challenges with assessing mild traumatic brain injury in the military. J Head Trauma Rehabil. 2010;25:313–319. doi: 10.1097/HTR.0b013e3181d6f9bd. [DOI] [PubMed] [Google Scholar]
- 18.Iverson GL, Brooks BL, Ashton VL, et al. Interview versus questionnaire symptom reporting in people with the postconcussion syndrome. J Head Trauma Rehabil. 2010;25:23–30. doi: 10.1097/HTR.0b013e3181b4b6ab. [DOI] [PubMed] [Google Scholar]
- 19.Iverson GL, Langlois JA, McCrea MA, et al. Challenges associated with post-deployment screening for mild traumatic brain injury in military personnel. Clin Neuropsychol. 2009;23:1299–1314. doi: 10.1080/13854040903153902. [DOI] [PubMed] [Google Scholar]
- 20.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J Emerg Nurs. 2009;35:5–40. doi: 10.1016/j.jen.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Lighthall JW. Controlled cortical impact: A new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 22.Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith DH, Soares HD, Pierce JS, et al. A model of parasagittal controlled cortical impact in the mouse: Cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 24.Ariza M, Serra-Grabulosa JM, Junque C, et al. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Pullela R, Raber J, Pfankuch T, et al. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Chen J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J Neurotrauma. 2009;26:1325–1335. doi: 10.1089/neu.2008.0744. [DOI] [PubMed] [Google Scholar]
- 27.Gao X, Deng-Bryant Y, Cho W, et al. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brody DL, Mac Donald C, Kessens CC, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: A novel fluo-rochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 30.Schmued LC, Hopkins KJ. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Enikolopov G, Chen J. Direct isolation of neural stem cells in the adult hippocampus after traumatic brain injury. Journal of neurotrauma. 2008;25:985–995. doi: 10.1089/neu.2008.0460. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Enikolopov G, Chen J. Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp Neurol. 2009;219:516–523. doi: 10.1016/j.expneurol.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerqueira JJ, Taipa R, Uylings HB, et al. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- 34.Uylings HB, van Pelt J, Parnavelas JG, et al. Geometrical and topological characteristics in the dendritic development of cortical pyramidal and non-pyramidal neurons. Prog Brain Res. 1994;102:109–123. doi: 10.1016/s0079-6123(08)60535-x. [DOI] [PubMed] [Google Scholar]
- 35.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Uylings HB, Ruiz-Marcos A, van Pelt J. The metric analysis of three-dimensional dendritic tree patterns: A methodological review. J Neurosci Methods. 1986;18:127–151. doi: 10.1016/0165-0270(86)90116-0. [DOI] [PubMed] [Google Scholar]
- 37.Uylings HB, van Pelt J. Measures for quantifying dendritic arborizations. Network. 2002;13:397–414. [PubMed] [Google Scholar]
- 38.Sholl DA. The measurable parameters of the cerebral cortex and their significance in its organization. Prog Neurobiol. 1956:324–333. [PubMed] [Google Scholar]
- 39.Gundersen HJ, Bendtsen TF, Korbo L, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis [review] APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 40.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 41.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: A case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: Evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunaevsky A, Tashiro A, Majewska A, et al. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J Comp Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- 46.Fischer M, Kaech S, Knutti D, et al. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 47.Calhoun ME, Jucker M, Martin LJ, et al. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J Neurocytol. 1996;25:821–828. doi: 10.1007/BF02284844. [DOI] [PubMed] [Google Scholar]
- 48.Cline DM, Whitley TW. Observation of head trauma patients at home: A prospective study of compliance in the rural south. Ann Emerg Med. 1988;17:127–131. doi: 10.1016/s0196-0644(88)80297-x. [DOI] [PubMed] [Google Scholar]
- 49.Bazarian JJ, Wong T, Harris M, et al. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999;13:173–189. doi: 10.1080/026990599121692. [DOI] [PubMed] [Google Scholar]
- 50.Garraway M, Macleod D. Epidemiology of rugby football injuries. Lancet. 1995;345:1485–1487. doi: 10.1016/s0140-6736(95)91040-9. [DOI] [PubMed] [Google Scholar]
- 51.Lidvall HF, Linderoth B. Letter: Recovery after minor head injury. Lancet. 1974;2:1150–1151. doi: 10.1016/s0140-6736(74)90923-4. [DOI] [PubMed] [Google Scholar]
- 52.Lidvall HF, Linderoth B, Norlin B. Causes of the post-concussional syndrome. Acta Neurol Scand Suppl. 1974;56:3–144. [PubMed] [Google Scholar]
- 53.McCrea M, Iverson GL, McAllister TW, et al. An integrated review of recovery after mild traumatic brain injury (MTBI): Implications for clinical management. Clin Neuropsychol. 2009;23:1368–1390. doi: 10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- 54.Lowdon IM, Briggs M, Cockin J. Post-concussional symptoms following minor head injury. Injury. 1989;20:193–194. doi: 10.1016/0020-1383(89)90109-5. [DOI] [PubMed] [Google Scholar]
- 55.Macciocchi SN, Barth JT, Alves W, et al. Neuropsychological functioning and recovery after mild head injury in collegiate athletes. Neurosurgery. 1996;39:510–514. [PubMed] [Google Scholar]
- 56.Paniak C, Phillips K, Toller-Lobe G, et al. Sensitivity of three recent questionnaires to mild traumatic brain injury-related effects. J Head Trauma Rehabil. 1999;14:211–219. doi: 10.1097/00001199-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Paniak C, Reynolds S, Phillips K, et al. Patient complaints within 1 month of mild traumatic brain injury: A controlled study. Arch Clin Neuropsychol. 2002;17:319–334. [PubMed] [Google Scholar]
- 58.Adelson PD, Dixon CE, Kochanek PM. Long-term dysfunction following diffuse traumatic brain injury in the immature rat. J Neurotrauma. 2000;17:273–282. doi: 10.1089/neu.2000.17.273. [DOI] [PubMed] [Google Scholar]
- 59.Ciallella JR, Yan HQ, Ma X, et al. Chronic effects of traumatic brain injury on hippocampal vesicular acetylcholine transporter and M2 muscarinic receptor protein in rats. Exp Neurol. 1998;152:11–19. doi: 10.1006/exnr.1998.6831. [DOI] [PubMed] [Google Scholar]
- 60.Dixon CE, Liu SJ, Jenkins LW, et al. Time course of increased vulnerability of cholinergic neurotransmission following traumatic brain injury in the rat. Behav Brain Res. 1995;70:125–131. doi: 10.1016/0166-4328(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 61.Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 62.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Bruns JJ, Jr, Jagoda AS. Mild traumatic brain injury. Mt Sinai J Med. 2009;76:129–137. doi: 10.1002/msj.20101. [DOI] [PubMed] [Google Scholar]
- 64.Le TH, Gean AD. Neuroimaging of traumatic brain injury. Mt Sinai J Med. 2009;76:145–162. doi: 10.1002/msj.20102. [DOI] [PubMed] [Google Scholar]
- 65.Slobounov SM, Zhang K, Pennell D, et al. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: A functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McAllister TW, Sparling MB, Flashman LA, et al. Neuroimaging findings in mild traumatic brain injury. J Clin Exp Neuropsychol. 2001;23:775–791. doi: 10.1076/jcen.23.6.775.1026. [DOI] [PubMed] [Google Scholar]