Abstract
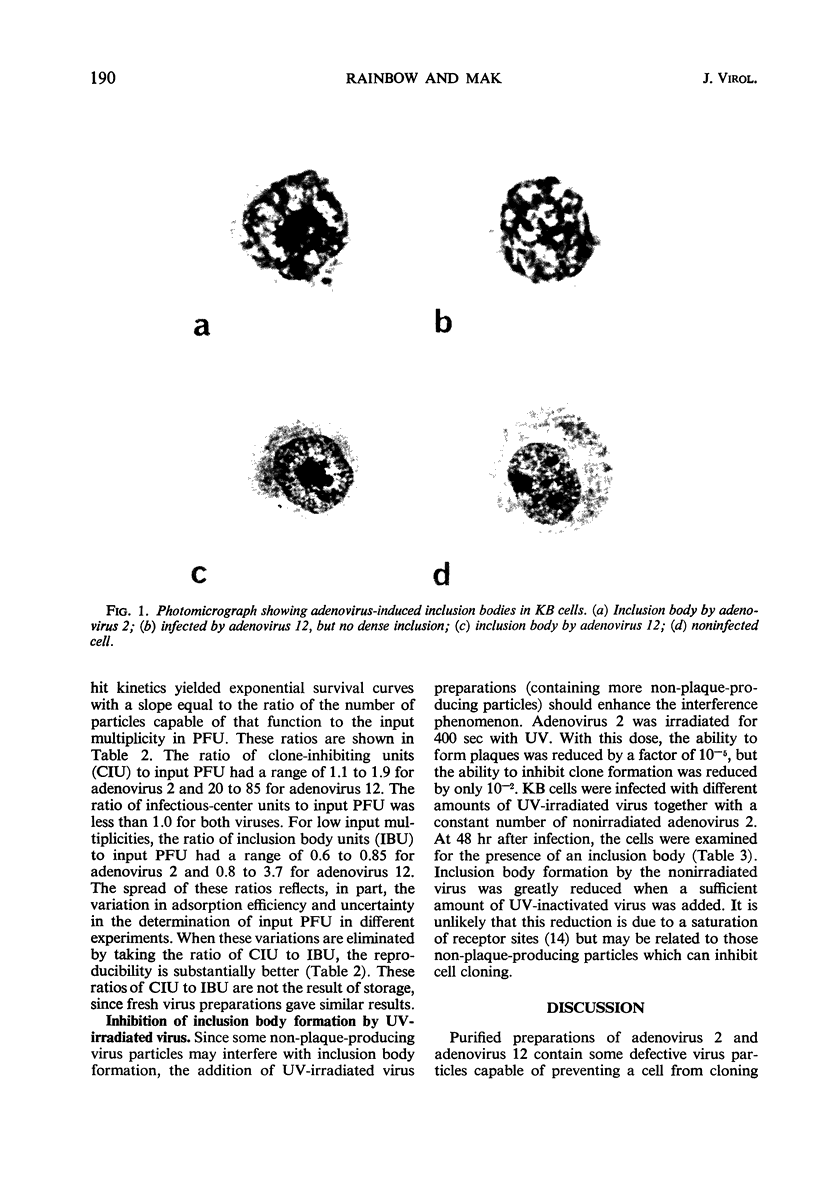
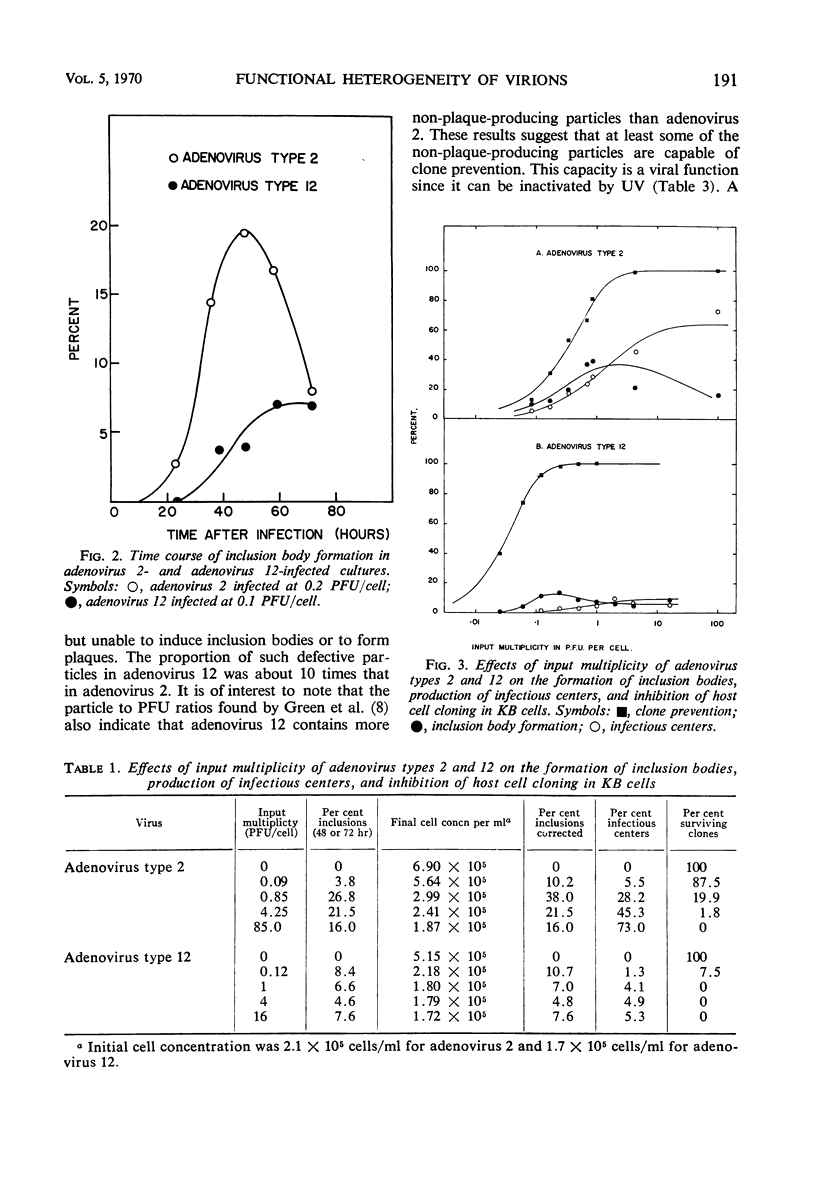
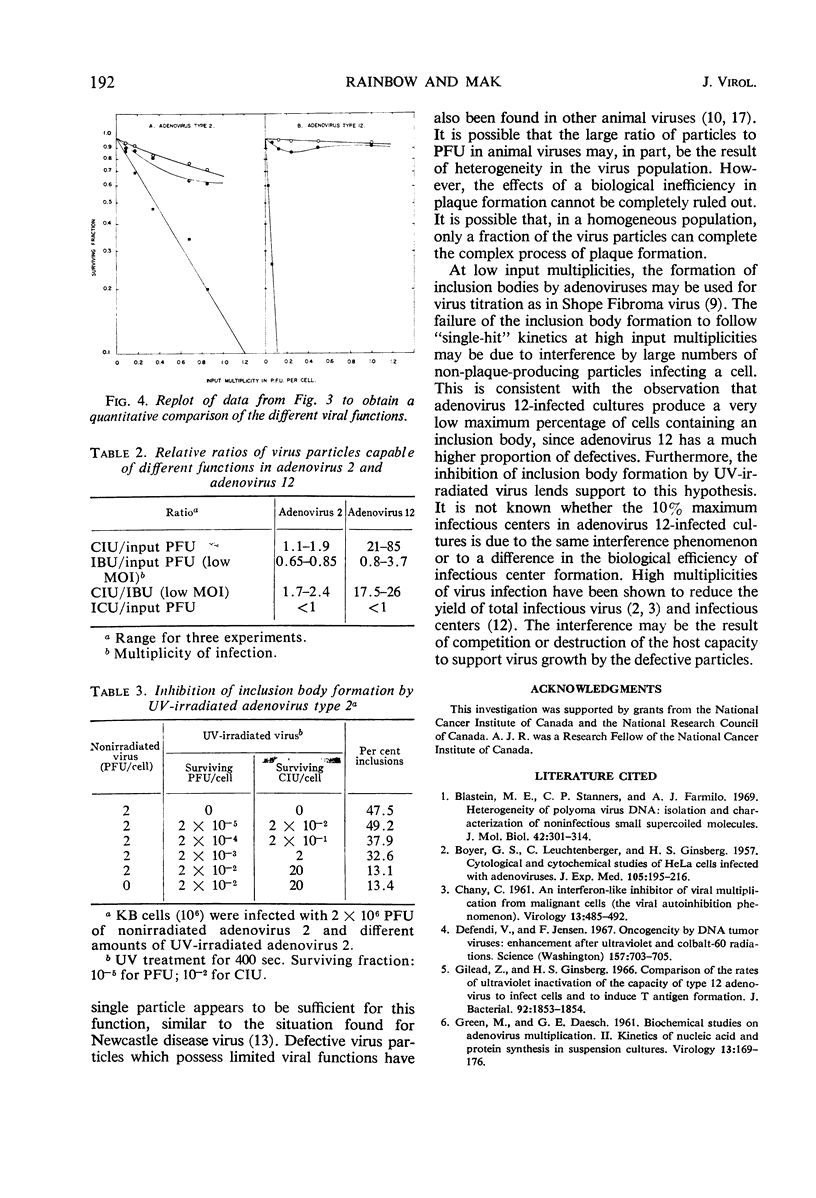
Purified preparations of adenovirus types 2 and 12 were used to infect KB cells at different input multiplicities. The resulting infected cultures were scored for inclusion body formation, production of infectious centers, and cloning efficiency. Both preparations were found to contain some defective particles capable of preventing a cell from cloning but unable to induce inclusion bodies or form plaques. The proportion of such defective particles in adenovirus 12 was about 10 times that in adenovirus 2. At high input multiplicities, the percentage of cells displaying an inclusion body was less than that predicted by the Poisson distribution and reached a maximum of 40 to 60% for adenovirus 2 and 12 to 15% for adenovirus 12. This reduction may be due to interference by large numbers of non-plaque-producing particles infecting each cell. The per cent of cells forming infectious centers was substantially greater for adenovirus 2 than for adenovirus 12 when compared at the same input plaque-forming units, reaching a maximum of 35 to 73% for adenovirus 2 and 5 to 10% for adenovirus 12. The low value for adenovirus 12 may be a result of the same interference phenomenon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER G. S., LEUCHTENBERGER C., GINSBERG H. S. Cytological and cytochemical studies of HeLa cells infected with adeno-viruses. J Exp Med. 1957 Mar 1;105(3):195–216. doi: 10.1084/jem.105.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstein M. E., Stanners C. P., Farmilo A. J. Heterogeneity of polyoma virus DNA: isolation and characterization of non-infectious small supercoiled molecules. J Mol Biol. 1969 Jun 14;42(2):301–313. doi: 10.1016/0022-2836(69)90045-x. [DOI] [PubMed] [Google Scholar]
- CHANY C. An interferon-like inhibitor of viral multiplication from malignant cells (the viral autoinhibition phenomenon). Virology. 1961 Apr;13:485–492. doi: 10.1016/0042-6822(61)90279-3. [DOI] [PubMed] [Google Scholar]
- Defendi V., Jensen F. Oncogenicity by DNA tumor viruses: enhancement after ultraviolet and cobalt-60 radiations. Science. 1967 Aug 11;157(3789):703–705. doi: 10.1126/science.157.3789.703. [DOI] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Comparison of the rates of ultraviolet inactivation of the capacity of type 12 adenovirus to infect cells and to induce T antigen formation. J Bacteriol. 1966 Dec;92(6):1853–1854. doi: 10.1128/jb.92.6.1853-1854.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Latarjet R., Cramer R., Montagnier L. Inactivation, by UV-, x-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967 Sep;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Changes in metabolic and enzymatic activities of monkey kidney cells after infection with adenovirus 2. Virology. 1966 Apr;28(4):679–692. doi: 10.1016/0042-6822(66)90252-2. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I., PUCK T. T. Host-cell interaction of animal viruses. I. Titration of cell-killing by viruses. Virology. 1958 Oct;6(2):405–423. doi: 10.1016/0042-6822(58)90091-6. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T., Marcus P. I. A RAPID METHOD FOR VIABLE CELL TITRATION AND CLONE PRODUCTION WITH HELA CELLS IN TISSUE CULTURE: THE USE OF X-IRRADIATED CELLS TO SUPPLY CONDITIONING FACTORS. Proc Natl Acad Sci U S A. 1955 Jul 15;41(7):432–437. doi: 10.1073/pnas.41.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich H. F., Avila L., Yohn D. S. Viruses and mammalian chromosomes. IX. The capacity of UV-impaired adenovirus type 18 to induce chromosome abberations. Exp Cell Res. 1968 Oct;53(1):44–54. doi: 10.1016/0014-4827(68)90350-9. [DOI] [PubMed] [Google Scholar]
- WILDY P., WATSON D. H. Electron microscopic studies on the architecture of animal viruses. Cold Spring Harb Symp Quant Biol. 1962;27:25–47. doi: 10.1101/sqb.1962.027.001.006. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Zur Hausen H. Association of adenovirus type 12 deoxyribonucleic acid with host cell chromosomes. J Virol. 1968 Mar;2(3):218–223. doi: 10.1128/jvi.2.3.218-223.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]




