Abstract
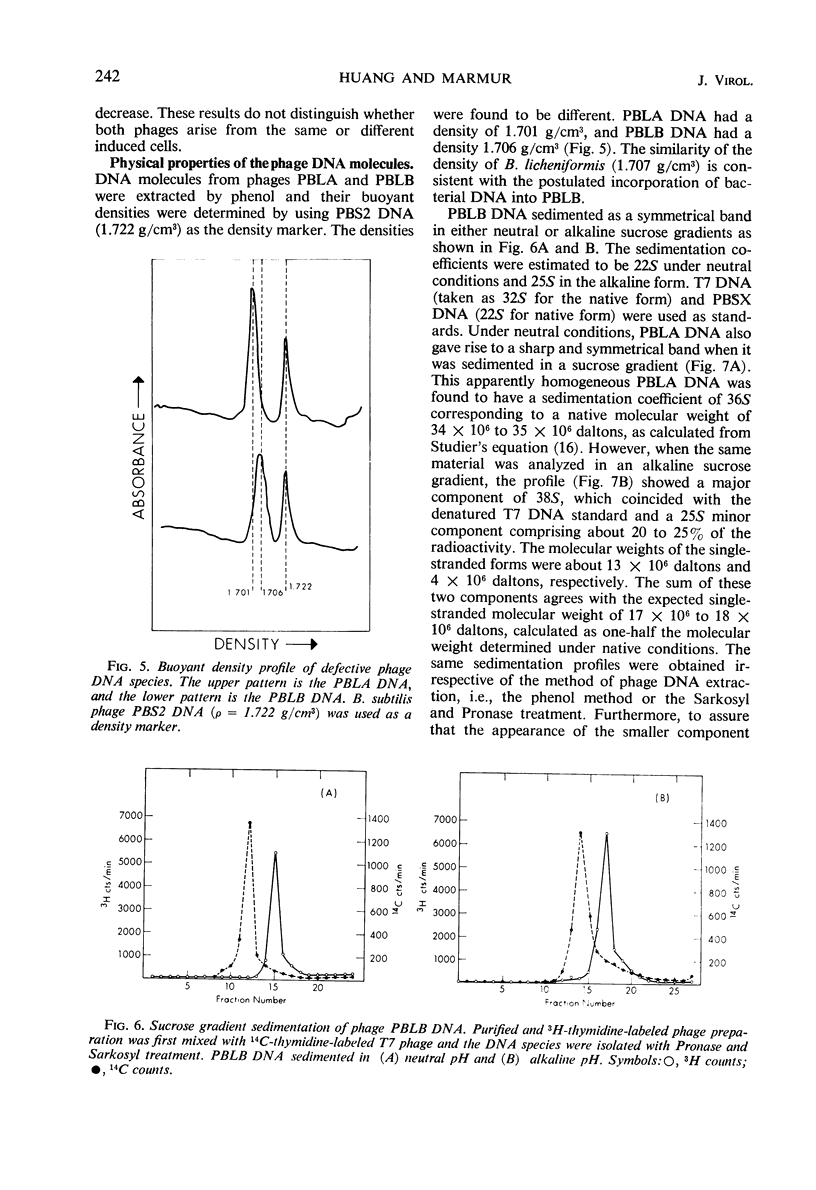
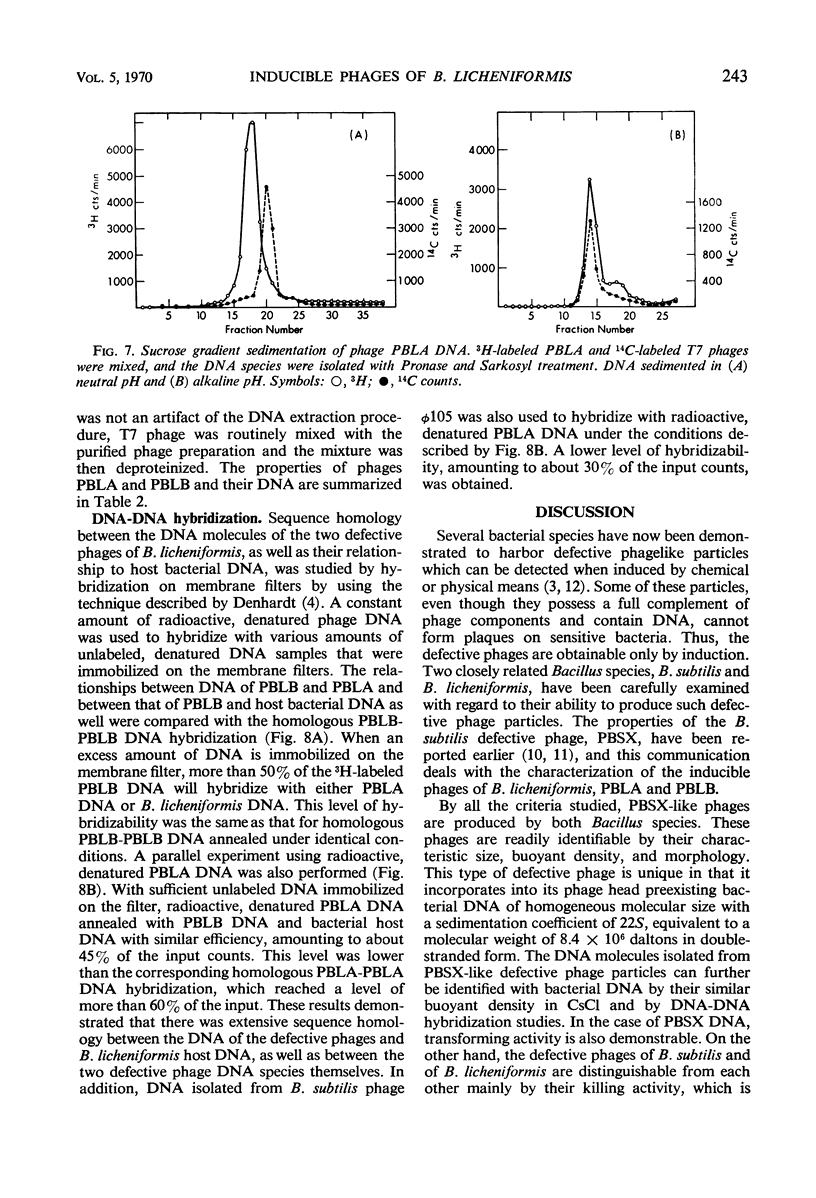
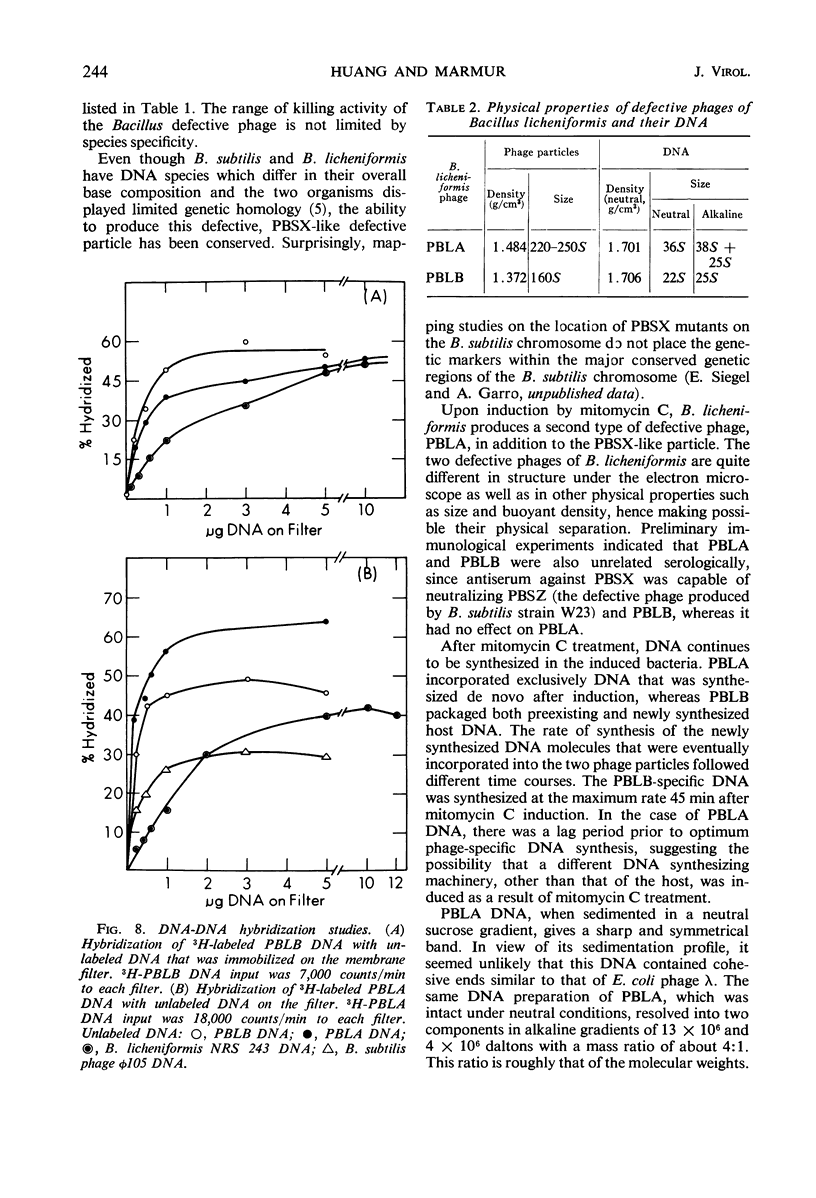
Two morphologically distinct and physically separable defective phages have been found in Bacillus licheniformis NRS 243 after induction by mitomycin C. One of them (PBLB) is similar to the defective phage PBSX of B. subtilis, which has a density of 1.373 g/cm3 in CsCl and a sedimentation coefficient of 160S. PBLB incorporates into its head mainly bacterial deoxyribonucleic acid (DNA) which has a sedimentation coefficient of 22S and a buoyant density in CsCl of 1.706 g/cm3. The other phage (PBLA) has a morphology similar to the temperate phage φ105 of B. subtilis; the head diameter is about 66 nm, and it possesses a long and noncontractile tail. PBLA has a density of 1.484 g/cm3 in CsCl and the phage-specific DNA, which is exclusively synthesized after induction by mitomycin C, has a density of 1.701 g/cm3. PBLA DNA is double-stranded and has a sedimentation coefficient of 36S, corresponding to a molecular weight of 34 × 106 to 35 × 106 daltons. The phage DNA has one interruption per single strand, giving single-stranded segments with molecular weights of 13 × 106 and 4 × 106 daltons. Common sequences between the two phage DNA species and with their host DNA have been demonstrated by DNA-DNA hybridization studies. Both phage particles kill sensitive bacteria. However, all attempts thus far to find an indicator strain to support plaque formation have been unsuccessful.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boice L., Eiserling F. A., Romig W. R. Structure of bacillus subtilis phage SPO2 and its DNA: similarity of Bacillus subtilis phages SPO2, phi 1O5 and SPP1. Biochem Biophys Res Commun. 1969 Feb 21;34(4):398–403. doi: 10.1016/0006-291x(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- LEONARD C. G., MATTHEIS M. J. DIFFERENT TRANSFORMING CHARACTERISTICS OF COLONIAL VARIANTS FROM AUXOTROPHIC MUTANTS OF BACILLUS LICHENIFORMIS. J Bacteriol. 1965 Aug;90:558–559. doi: 10.1128/jb.90.2.558-559.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. Elective production of thymine-less mutants. Nature. 1960 Oct 22;188:340–341. doi: 10.1038/188340a0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Marmur J. Conversion of Bacillus subtilis DNA to phage DNA following mitomycin C induction. J Mol Biol. 1968 Jun 28;34(3):429–437. doi: 10.1016/0022-2836(68)90170-8. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Altenbern R. A. Detection of bacteriophages from two strains of Clostridium tetani. J Virol. 1967 Oct;1(5):1085–1086. doi: 10.1128/jvi.1.5.1085-1086.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Farmer J. L., Rothman F. Thymidylate synthesis and aminopterin resistance in Bacillus subtilis. J Bacteriol. 1966 Jul;92(1):186–196. doi: 10.1128/jb.92.1.186-196.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Tieze G. A., Wendelaar Bonga S., Stouthamer A. H. Purification and genetic determination of bacteriocin production in Enterobacter cloacae. J Bacteriol. 1968 Feb;95(2):631–640. doi: 10.1128/jb.95.2.631-640.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]