Abstract
Males outperform females on some spatial tasks, and this may be partially due to the effects of sex steroids on spatial strategy preferences. Previous work with rodents indicates that low estradiol levels bias females toward a striatum-dependent response strategy, whereas high estradiol levels bias them toward a hippocampus-dependent place strategy. We tested whether testosterone influenced the strategy preferences in male rats. All subjects were castrated and assigned to one of three daily injection doses of testosterone (0.125, 0.250, or 0.500 mg/rat) or a control group that received daily injections of the drug vehicle. Three different maze protocols were used to determine rats’ strategy preferences. A low dose of testosterone (0.125 mg) biased males toward a motor-response strategy on a T-maze task. In a water maze task in which the platform itself could be used intermittently as a visual cue, a low testosterone dose (0.125 mg) caused a significant increase in the use of a cued-response strategy relative to control males. Results from this second experiment also indicated that males receiving a high dose of testosterone (0.500 mg) were biased toward a place strategy. A third experiment indicated that testosterone dose did not have a strong influence on the ability of rats to use a nearby visual cue (floating ball) in the water maze. For this experiment, all groups seemed to use a combination of place and cued-response strategies. Overall, the results indicate that the effects of testosterone on spatial strategy preference are dose dependent and task dependent.
Keywords: Testosterone, Androgen, Spatial memory, Spatial strategy, Place strategy, Response strategy, Water maze, Radial arm maze, Rats
Introduction
It is now well established that men outperform women on certain spatial tasks (Voyer et al., 1995). These include mental rotation of objects (Kaufman, 2007; Parsons et al., 2004), judgment of line angles (Cherney et al., 2008), route learning (Holding and Holding, 1988; Postma et al., 2004), and maze navigation (Astur et al., 1998; Moffat et al., 1998). Similarly, male rats have better spatial learning and memory abilities than females based on performance in the Morris water maze (Harris et al., 2008; Jonasson et al., 2004; Markowska, 1999; Roof and Stein, 1999) and the radial-arm maze (Gibbs and Johnson, 2008; Luine and Rodriguez, 1994; Seymoure et al., 1996).
One underlying cause of this sex difference seems to be that the two sexes use different strategies to solve spatial tasks. A place strategy involves using knowledge about the positions of environmental cues relative to a goal and relative to one’s own position to locate the goal (Packard and McGaugh, 1996; Schmidt et al., 2009). A response strategy involves using stimulus–response relationships to locate a goal (Packard and McGaugh, 1996; Schmidt et al., 2009). We will use the term motor-response strategy to refer to the use of proprioceptive stimuli (e.g., turn to the left or turn to the right in the T-maze) and the term cued-response strategy to refer to the use of stimuli proximal to the goal (e.g., visible goal platform in the water maze). Many studies with rodents involving localized brain lesions have demonstrated that the use of a place strategy relies on the hippocampus, whereas the use of response strategies (both motor-response and cued-response) relies on the dorsal striatum (Devan et al., 1996; McDonald and White, 1993; Packard and McGaugh, 1996; Pearce et al., 1998). Because both the motor-response strategy and the cued-response strategy rely on a functional striatum (McDonald and White, 1994; Packard and McGaugh, 1996), we consider these different types of response strategy to be functionally similar. When solving spatial tasks, men preferentially use a place strategy involving Euclidian cues, whereas women preferentially use a response strategy involving local landmarks (Cherney et al., 2008; Dabbs et al., 1998; Lawton, 1994; Silverman and Choi, 2006). This sex difference in preferred strategy seems to be because men are better able than women to employ a place strategy (Goyette et al., 2012; Sandstrom et al., 1998).
Experiments with rats generally support the findings of human studies. In the Morris water maze, female rats show a strong bias for using local landmarks to find the escape platform (Jonasson et al., 2004; Kanit et al., 1998, 2000), whereas males seem to rely more heavily on extra-maze cues than do females (Sava and Markus, 2005). Males outperformed females in the water maze when a place strategy was needed to solve the task, but no sex difference was observed when response strategies (local cues or a turn bias) could be used to solve the task (Blokland et al., 2006). Additionally, in a task that required rats to switch between place and response strategies, females performed more errors than males when they had to switch from a response strategy to a place strategy (Schmidt et al., 2009).
Interestingly, Kanit et al. (2000) found that unlike adult rats, juveniles did not show a sex difference in spatial strategy preference. This suggests that the sex difference in spatial strategy preference may be due to the activational effects of sex steroids. The effects of estrogens on spatial strategies have been extensively tested using female rats (Korol, 2004). The estrus cycle influences strategy preference, with females in proestrus (high estradiol) showing a bias toward a place strategy and females in estrus (low estradiol) showing a bias toward a response strategy (Korol et al., 2004; McElroy and Korol, 2005; Pleil and Williams, 2010; Sava and Markus, 2005). Similarly, high or low doses of estradiol given to ovariectomized females cause a bias toward a place strategy or a response strategy, respectively (Quinlan et al., 2008). Females given estradiol replacement performed better than ovariectomized females without estradiol on tasks requiring a place strategy, whereas for tasks requiring a response strategy females without estradiol performed better than females given estradiol (Davis et al., 2005; Korol and Kolo, 2002). One study found that ovariectomized females relied more heavily on local landmarks (stimulus–response strategy) in the water maze than did females with relatively low circulating levels of estradiol (Daniel and Lee, 2004). Finally, intra-hippocampal infusions of estradiol improved place learning in females rats (Zurkovsky et al., 2007), whereas intra-striatal infusions of estradiol impaired motor-response learning (Zurkovsky et al., 2007, 2011). Overall, these results indicate that increasing a female’s estradiol levels improves her ability to use a place strategy but impairs her ability to use a response strategy.
Estradiol’s strong role in regulating spatial strategies suggests that testosterone may also have activational effects on spatial strategies. In support of this idea, androgen receptors have been found in both the hippocampus and striatum of male rats (Feng et al., 2010; Kerr et al., 1995; Li et al., 1997; Xiao and Jordan, 2002). Although recent literature reviews have concluded that elevated circulating testosterone levels do not consistently improve spatial ability in men (Puts et al., 2010; Ulubaev et al., 2009), some human studies have shown that testosterone improves mental rotation ability (Christiansen and Knussmann, 1987; Hooven et al., 2004; Silverman et al., 1999), route-learning (Choi and Silverman, 2002), and performance on a block design task (Thilers et al., 2006). Among male rats, the effects of testosterone have been shown to differ between working and reference memory. Working memory is a form of short-term memory that involves storage of information from a particular task only for as long as it is useful to complete that task, and reference memory refers to the long-term storage of memories that are used from one task to the next (Olton and Papas, 1979). Numerous studies have shown that castrating male rats impairs their spatial working memory (Daniel et al., 2003; Gibbs and Johnson, 2008; Hasegawa and Mochizuki, 2009; Kritzer et al., 2001; Spritzer et al., 2008), and some studies have shown that testosterone replacement restores spatial working memory (Bimonte-Nelson et al., 2003; Kritzer et al., 2001; Spritzer et al., 2011b). In contrast, most studies to date indicate that testosterone has no effect on spatial reference memory (Hodosy et al., 2010; Naghdi et al., 2005; Sandstrom et al., 2006; Spritzer et al., 2008), but a few have demonstrated testosterone-induced improvements in reference memory (Khalil et al., 2005; Spritzer et al., 2011b).
Only two studies to date have specifically addressed the effects of testosterone on spatial strategies used by male rats (Gibbs, 2005; Hawley et al., 2012). Gibbs (2005) observed no differences in strategy preference among castrated, intact, and testosterone-implanted male rats and none of the groups showed a significant bias toward a particular strategy. Hawley et al. (2012) found that intact males had a significant bias toward a place strategy, and castration had no significant effect on this bias. In combination, these two studies suggest that testosterone does not have activational effects that influence spatial strategy preference. However, some evidence indicates that the effects of testosterone on spatial memory are dose dependent (Naghdi et al., 2001; Spritzer et al., 2011b), and the effects of testosterone may also be task dependent (Spritzer et al., 2008, 2011b). For the current study, we tested three different physiological doses of testosterone and used three different maze tasks to assess learning strategy.
The overarching goal of the current study was to clarify the activational effects of testosterone on the spatial strategy preference of male rats. Our three experiments assessed bias for three slightly different types of response strategies, and estradiol has been previously shown to influence female strategy preferences for all three tasks.Experiment 1 employed a dual-solution T-maze in which the motor-response strategy involved learning to turn either to the right or left (proprioceptive cues). For this task, high estradiol and low estradiol levels bias females toward place and motor-response strategies, respectively (Korol et al., 2004; Quinlan et al., 2008). Experiments 2 and 3 both involved versions of the Morris water maze that could be solved using either a place strategy or a cued-response strategy. The distinction between the two experiments is that for Experiment 2 the goal platform itself could be used as a cue, whereas for Experiment 3 a floating ball near the platform was the only proximal cue. Females show a strong bias toward a cued-response strategy in the visible-platform version of the water maze, and this bias does not emerge until after puberty (Kanit et al., 2000). For the floating-ball task, ovariectomy caused a strong bias toward a cued-response strategy relative to estrogen-replaced females (Daniel and Lee, 2004). Given the previous results for females on these tasks and the fact that testosterone can be aromatized to estradiol in the male brain (Shibuya et al., 2003), we predicted that castration would bias males toward a response strategy and that testosterone replacement would bias them toward a place strategy. As an alternative hypothesis, the limited data for male rats suggested that we might instead observe no effects of testosterone on spatial strategy preferences (Gibbs, 2005; Hawley et al., 2012).
Methods
Subjects
Adult male Long-Evans rats (approximately 60 days old) were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). For all experiments, rats were individually housed in opaque polypropylene cages (21 × 42 × 21 cm) with Tek-Fresh Bedding (Harlan Laboratories, Indianapolis, IN, USA). Animals had free access to water and a soy-protein-free rodent diet (Harlan Teklad Diet 2020X), except during food restriction for rats tested on the T-maze. The housing and testing rooms were temperature controlled (21 ± 1 °C) with a 12:12 h light/dark cycle (lights on at 0700 h). All animal procedures were approved by the Middlebury College Animal Care and Use Committee and were carried out in accordance with ethical guidelines set by the National Institutes of Health.
All subjects were bilaterally castrated 7–8 days after they arrived in the animal facility. Surgeries were performed with aseptic technique under isoflurane anesthesia (3.5–4.0% in oxygen during induction, 2.0–2.5% in oxygen during maintenance). The analgesic Ketofen (5 mg/kg body mass, s.c.) was administered just before surgery. Immediately after surgery, topical antibiotic (vetryopolycin) and analgesic (2.5% lidocaine, 2.5% prilocaine) were applied to the incision area. Each testis was excised through a small incision at the posterior end of the scrotum and ligated with chromic gut suture material (Ethicon, Somerville, NJ, USA). The muscular sheath was closed with chromic gut sutures, and the skin layer was closed with ethilon sutures (Ethicon). For Experiments 2 and 3, the rats were castrated over two days with half the rats castrated each day (Table 1). Rats were checked daily for one week after surgery to ensure full recovery, and during the last four days of recovery, animals were handled an additional 4–5 min/day to become acclimated to the researchers. Seven days after surgery, the average mass of the subjects was 345.3 ± 3.1 g.
Table 1.
Timelines for the Experiments 1–3 with the days shown during which each procedure was conducted.
| Exp. | GDX | Handle | Maze habituation |
Testosterone injections |
Acquisition trialsa |
Probe trialsa |
Blood draw |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6–9 | 2–15 | 9–17 | 16 | 16 | 17 |
| 2 | 1–2 | 6–9 | NA | 10–27 | 17–25 | 26 | 27 |
| 3 | 1–2 | 6–9 | NA | 10–28 | 17–26 | 27 | 28 |
NA (not applicable) refers to procedures that were not used for a particular experiment. GDX (gonadectomy) refers to the days when castration surgeries were performed.
Acquisition and probe trials were conducted in the T maze for Experiment 1 and the Morris water maze for Experiments 2 and 3.
For each experiment, subjects were divided into 4 groups (n = 11–12/group). The Control group received daily s.c. injections of 0.1 ml sesame oil. The three treatment groups received s.c. injections of specific doses of testosterone propionate (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.1 ml of sesame oil: 0.125 mg, 0.250 mg, or 0.500 mg per rat per day. For a typical male rat, serum testosterone levels range from 1 to 4 ng/ml (Kalra and Kalra, 1977; Lee et al., 1975; Mock et al., 1978), with some studies showing testosterone peaks as high as 6–15 ng/ml (Bartke et al., 1973; Kamel and Frankel, 1978). Our injection doses were chosen because they produce serum testosterone levels that are within this physiological range (Spritzer et al., 2011b). Injections started 8–9 days after castrations and were carried out between 0900 and 1000 h each day. For each experiment, seven days of injections of the respective doses were given prior to starting acquisition trials (Table 1). We previously found that one week of preliminary testosterone injections was necessary to observe treatment effects in a reference memory version of the Morris water maze (Spritzer et al., 2011b).
Apparatus
For Experiment 1, testing was conducted in a polycarbonate plus maze elevated 91 cm above the floor. The maze was dark gray and consisted of four arms (40.5 × 12.7 cm) with 12.5 cm high walls. The arms were arranged at 90° around a 14 × 14 cm center. To convert the plus maze into a T-maze, entrance into the arm opposite the start arm was prevented with an opaque plastic door. During testing, 45 mg dustless reward pellets (Bio-Serv, Frenchtown, New Jersey) were placed in small food troughs 1 cm from the end of each arm, which prevented rats from seeing the pellets until they reached the end of an arm. Testing was conducted in a small room with standard fluorescent overhead lighting, and the maze remained in the same location for the duration of behavioral testing. Each side of the room was visually distinct from the others to create clear extra-maze cues. Large, high-contrast posters were mounted on two of the walls, one wall had no obvious cues, and the remaining side of the room was where the researcher and cart with rats were positioned.
For Experiments 2 and 3, the Morris water maze used was a circular white steel pool (180 cm diameter; 60 cm high). The pool was filled to approximately 40 cm with water at equilibrium with room temperature (20 ± 2 °C), which was made opaque with non-toxic white powdered paint (Sargent Art, Hazelton, PA, USA). The pool was divided virtually into four quadrants with four equidistant release points around the edge. The Plexiglas goal platform (10 × 10 cm square) was positioned 35 cm from the pool edge and was positioned in the center of one of the quadrants, which varied by experiment and day of testing. Except when raised above the water periodically for Experiment 2, the platform was hidden 2.5 cm below the water for all trials. Each trial was recorded by a digital USB camera mounted on the ceiling directly above the maze and interfaced with a laptop computer running a video tracking program (ANY-maze™ Video Tracking System, 4.62a beta; Stoelting Co., Wood Dale, IL, USA). Distal cues around the maze remained constant throughout testing, including two large, high-contrast posters mounted on two walls and a large white curtain used to obscure observers on one side of the room. Immediately after placing each rat in the maze, the observer went behind the curtain to minimize the use of the observer as a visual cue.
Experiment 1
Experiment 1 tested the effects of testosterone on spatial strategy preference using a “dual-solution” T-maze task designed to distinguish between rats using a place strategy or a motor-response strategy (Packard and McGaugh, 1996). Eight days after castration surgeries, all animals were placed on food restriction and were maintained at 85–90% of free-feeding body mass until the end of the experiment. Free-feeding reference rats were used to determine expected daily growth rates, and the target mass for each rat was adjusted accordingly. All animals received ten reward pellets in their home cages daily during the habituation phase of testing.
The testing protocol involved four days of maze habituation followed by a single day of acquisition and probe trials. These procedures were carried out in the afternoon (1300–1600 h) each day starting 4–6 h after testosterone injections. The maze was in the same T configuration relative to the room for all habituation and acquisition trials, with the arm opposite the start arm blocked. Habituation was divided into two phases, each lasting two days. For the first phase, two reward pellets were placed in the troughs at the end of each of the two goal arms. Each rat was released into the start arm for a single trial each day and was allowed to explore until it had eaten the pellets or 10 min had elapsed. For the second phase of habituation, only one reward pellet was placed in the troughs at the end of each of the two goal arms. Rats were individually placed in the start arm and allowed to explore until both pellets were eaten or 5 min had elapsed. This entire procedure was repeated until each rat had a total of 8 trials. Between trials rats were placed in a holding cage for 15 s while the maze was rotated randomly 90° to the left or right to reduce the use of intra-maze cues. For all trials, the start and goal arms remained in the same position relative to the room.
Acquisition trials were conducted the day after habituation. Each animal was assigned to receive a reward pellet in either the left or right goal arm, counter-balanced such that half the rats were rewarded in the left arm and half in the right. Each rat was placed in the start arm and allowed to explore the maze until it made a clear choice by having its hind legs cross into an arm. Rats that chose correctly were allowed to eat the food reward in the arm before being returned to the holding cage for a 15 s inter-trial interval, while rats that chose incorrectly were allowed to investigate the empty food bowl before being taken out of the arm and placed into the holding cage for the 15 s inter-trial interval. This process was repeated until each rat made the correct choice in 9 out of 10 consecutive trials. Rats that could not perform this criterion within a maximum of 80 trials were not given a probe trial. Testing was continued until 11 rats from each group (Control and testosterone treatments) had met criterion. In all, eight rats failed to reach criterion.
For the probe trial, the arm opposite the normal start arm (i.e., the probe arm) was unblocked and the original start arm was blocked, creating a T-maze oriented in the opposite direction with respect to the room cues. Immediately after a rat reached criterion, it was given a probe trial in which it was released into the probe arm. If the animal made the same directional turn for which it had been rewarded during acquisition trials, then it was scored as using a motor-response strategy. If the animal turned in the opposite direction and chose the arm that had been rewarded during acquisition trials, then it was scored as using a place strategy.
Experiment 2
Experiment 2 tested the effects of testosterone on spatial strategy preference using a dual-solution version of the Morris water maze that distinguished between rats using a place strategy or a cued-response strategy (Bizon et al., 2003; Kanit et al., 2000). The local cue was the platform itself, which was periodically raised 3 cm above the water using bricks, and the edge of the platform was lined with black tape so that it was easily visible to the swimming rats. The testing protocol involved 9 consecutive days of acquisition trials followed by one day of probe trials. All trials were conducted 4–6 h after testosterone injections (1300–1700 h). The order in which rats were tested was the same each day and was counter-balanced with respect to treatment group.
The platform remained in the center of the same quadrant (southeast) for all acquisition trials. In three three-day sequences, the platform was alternately visible above the water for two days (days 1–2, 4–5, and 7–8) and hidden below the water on the third day (days 3, 6, and 9). Rats were subjected to blocks of four consecutive acquisition trials each day, in which they were released from each of the four release sites around the pool in a random order. Rats were always released into the maze facing the wall of the pool and they were allowed to remain on the platform for 15 s whenever they found it. Rats that failed to find the platform in 90 s were placed on the platform and allowed to remain there for 15 s. Following each trial, rats were towel dried and placed in a holding cage for a 45 s inter-trial interval. Poor performance on days when the platform was hidden indicated that a rat was relying on a cued-response strategy to locate the platform.
On the day immediately after the ninth day of acquisition trials, each rat received a single probe trial to determine their preferred spatial strategy. For probe trials, the platform was made visible above the water and it was moved to the quadrant opposite of where it had been during the acquisition trials (i.e., moved from southeast quadrant to northwest quadrant). Rats were released from one of the two release sites, chosen randomly, that were equidistant from the new target quadrant and at either edge of the old target quadrant. Rats that swam within 5 cm of the previous platform location were classified as using a place strategy, whereas all other rats were classified as using a cued-response strategy (Fig. 1). This classification method did not result in any ambiguous trials. Specifically, no rats first swam near the new platform and then swam to the previous location. Additionally, all rats classified as using a place strategy showed a clear diversion in their course toward the old platform location, often making a looping pattern near the old platform location (Fig. 1).
Fig. 1.
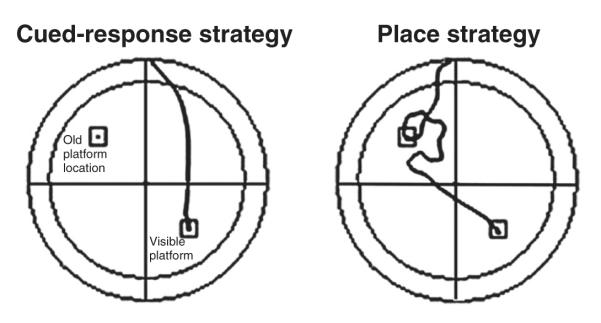
Representative swim paths of two rats that used a cued-response strategy and a place strategy during the probe trial of Experiment 2. For the probe trials, the platform was positioned in the lower right quadrant, which was opposite of where it had been during the 9 days of acquisition trials (i.e. upper left). Rats using a cued-response strategy swam directly to the visible platform, whereas rats using a place strategy swam first to the old platform location before swimming to the visible platform. The inner circle demarcates the region within 20 cm of the edge of the pool, which was used to score thigmotaxis in both Experiment 2 and Experiment 3.
Experiment 3
Experiment 3 tested the effects of testosterone on spatial strategy preference using a version of the Morris water maze that was similar to that used for Experiment 2 except that rather than the platform itself being used as a cue for response learning, a nearby local cue was employed (Daniel and Lee, 2004; Pearce et al., 1998). The local cue was a floating Styrofoam ball (5 cm diameter) tethered to an anchor, providing a constant cue that could not support the mass of the rats. The top of the ball was white, so as not to interfere with video tracking, but black tape was wrapped around the middle of the ball to ensure that it was visible to the swimming rats. The testing protocol involved 10 consecutive days of acquisition trials followed by one day of probe trials. All trials were conducted 4–6 h after testosterone injections (1300–1700 h). The order in which rats were tested was the same each day and was counter-balanced with respect to treatment group.
For acquisition trials, the location of the goal platform remained constant within each day of testing, but the platform was moved to the center of a new quadrant at the start of each day. The quadrant of the pool where the platform was placed each day was determined in a quasi-random manner such that over four-day blocks the platform was placed in each quadrant only once, while the sequence of locations within each block was randomized. The floating ball was also moved at the start of each day so that it was consistently located 20 cm to the south of the platform. Thus, the floating ball provided a cue that was always near the platform. All other testing procedures were identical to those described previously for the acquisition trials in Experiment 2. Good performance on the first trial of each day of acquisition trials was indicative of reliance on the local cue (cued-response strategy), whereas improved performance over the four trials within a day of testing indicated that a rat was relying on a place strategy to locate the platform.
On the day immediately after the tenth day of acquisition trials, each rat received four probe trials to determine the extent to which it used the local cue to locate the goal platform. At the start of probe trials, the platform was moved to a quadrant adjacent to where it had been during the tenth day of acquisition trials, and the local cue (floating ball) was removed from the pool. All other procedures used during the probe trials were identical to those used for acquisition trials. Good performance during probe trials indicated the use of a place strategy.
Testosterone assays
Blood samples were collected from all rats one day after behavioral testing was completed to assay circulating testosterone levels. As during behavioral testing, testosterone or oil (Control group) injections were given in the morning (0900–1000 h) and blood samples were collected in the afternoon (1300–1700 h) to correspond with the timing of behavioral testing. Rats were euthanized by either CO2 inhalation (Experiments 1 and 3) or a lethal i.p. injection sodium pentobarbital (Experiment 2) and blood was collected via heart puncture into microcentrifuge tubes on ice. Blood was refrigerated overnight at 4 °C before being centrifuged (15 min, 9000 g), and the serum was extracted and stored at −20 °C in microcentrifuge tubes until assaying.
Total serum testosterone was assayed using coated-tube radio-imunnoassay (RIA) kits with all samples run in duplicate. The kits (Siemens Healthcare Diagnostics, Los Angeles, CA, USA) had a lower limit of detection of 0.04 ng/ml, and the testosterone antibody had limited cross-reactivity with dihydrotestosterone (3.3%), other androgens (<1.0%), and very low cross-reactivity with progesterone, estrogens, or glucocorticoids (<0.1%). Based on sample duplications, the intra-assay coefficient of variation for all experiments combined was 9.0 ± 0.6%. We did not run any samples in more than one assay, but the manufacturer reported inter-assay coefficient of variation was 6.0–12.0%.
Statistical analysis
For all experiments, comparisons of means among groups that did not involve a within subjects factor were made using univariate analysis of variance (ANOVA) with treatment (Control, 0.125 mg T, 0.250 mg T, or 0.500 mg T) as a fixed factor. For all behavioral data, we were specifically interested in how the treatment groups differed from the Control group; therefore, the results of pair-wise comparisons between the Control group and each of the three treatment groups (2-tailed version of Dunnett’s test) are reported for each ANOVA even when the main effects were not significant. For analysis of serum testosterone levels, Fisher’s LSD was used to make pairwise comparisons among the three treatment groups. IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL, USA) was used for all analyses, and P < 0.05 was considered statistically significant. All averages are reported as mean ± SEM.
For Experiments 1 and 2, goodness-of-fit tests (χ2) were used to test for significant biases in the proportion of rats using place vs. response strategies with expected values of 50% for each strategy. Goodness-of-fit tests were also used to compare the proportions of rats from each treatment group that used either strategy to the proportion observed in the Control group.
For Experiments 2 and 3, path lengths to reach the platform were analyzed as an index of learning and memory during acquisition trials in the water maze. Escape latencies during acquisition trials were also analyzed for these experiments, but the observed differences between groups were identical to those obtained from analyses of path length, and therefore the escape latency data are not reported. For Experiments 2 and 3, percentage of time spent in a particular quadrant of the water maze was compared to chance levels (25% of time) using one-sample t-tests. For Experiment 2, path lengths during acquisition trials were first analyzed using a repeated measure ANOVA with testing day as the within-subjects factor and testosterone treatment as a between-subjects factor. For the days when the platform was hidden (3, 6, and 9), univariate ANOVAs and Dunnett’s tests were used to compare the mean path lengths of the Control group to that of each of the treatment groups. For Experiment 3, path lengths during acquisition trials were analyzed using repeated measures ANOVA, with day and trial as within-subjects factors and treatment as a between-subjects factor. Similarly, path lengths during the final day of acquisition trials in Experiment 3 were compared to path lengths during the probe trials using repeated measures ANOVA. For both Experiments 2 and 3, percentage of time spent thigmotactic (within 20 cm of the pool edge) was analyzed using repeated measures ANOVA. High levels of thigmotaxis are considered to be indicative of a stronger stress response (Beiko et al., 2004; Perrot-Sinal et al., 1996) and possibly an inability to initiate spatial learning in the water maze (Devan et al., 1999).
Results
Experiment 1: spatial strategies in a T-maze
A total of 52 rats were tested in the dual-solution T-maze, and 8 rats failed to reach criterion (9 out of 10 consecutive trials correct) within 80 trials. One rat in the Control group did not move out of the start arm within 2 min for 20 consecutive trails and was dropped from all further analyses. Three of the 7 remaining rats that failed to meet criterion were from the Control group, but the differences between groups in the proportion of rats that failed to reach criterion were not significant (P > 0.50). Of the rats that met criterion (n = 11/group), there was no significant difference between groups in the number of trials needed to reach criterion (P = 0.78). When the rats that failed to meet criterion were included in this analysis and assigned a value of 80 trials to meet criterion, there were still no significant differences in number of trials to criterion between the groups (P = 0.52). Thus, testosterone treatment had no detectable effect on the ability of rats to learn the T-maze task. The amount of time spent in the maze per trial during the acquisition phase did not differ between the groups when the rats that failed to meet criterion were included (P = 0.13) or excluded (P = 0.12), indicating that testosterone had no effect on the motivation of rats to perform in the maze. Additionally, the number of trials needed to reach criterion during the acquisition phase did not differ between rats exhibiting the two different strategies during the probe trials (place strategy: 35.93 ± 3.73 trials; response strategy: 32.41 ± 2.97 trials; P = 0.48), suggesting that neither strategy was better than the other in solving the maze.
Probe trials indicated that testosterone had a significant dose-dependent effect on which strategy rats used to solve the T-maze. Specifically, rats in the 0.125 mg T group showed a significant bias toward a motor-response strategy (Fig. 2; χ2 = 4.46, d.f. = 1, P = 0.035) compared to chance levels (i.e., 50% place strategy and 50% motor-response strategy), and none of the other groups showed a significant bias for either strategy (all P > 0.13). The Control group performed near chance levels, with six of the eleven rats (55%) showing a motor-response strategy. The strategy preference for the 0.125 mg T group was nearly significantly different from that observed in the Control group (Fig. 2; χ2 = 3.30, d.f. = 1, P = 0.069), and neither of the other two treatment groups showed a strategy preference that differed from the Control group (both P > 0.20).
Fig. 2.
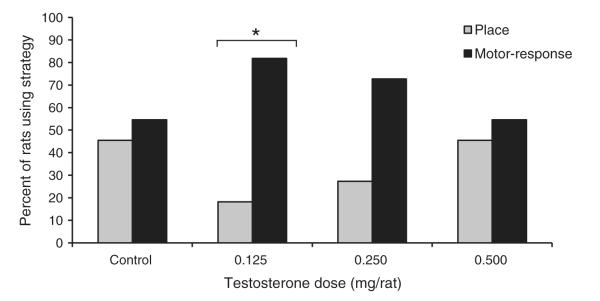
Percent of rats in each group that exhibited a place strategy or a motor-response strategy during probe trials in the T-maze for Experiment 1. All rats were castrated, and Control rats received daily injections of sesame oil and rats in the three treatment groups received daily injections of testosterone propionate. The 0.125 mg T group showed a significant bias for a motor-response strategy (*P < 0.05), but none of the other groups showed a significant bias for either strategy.
Experiment 2: spatial strategies in water maze with direct visual cue
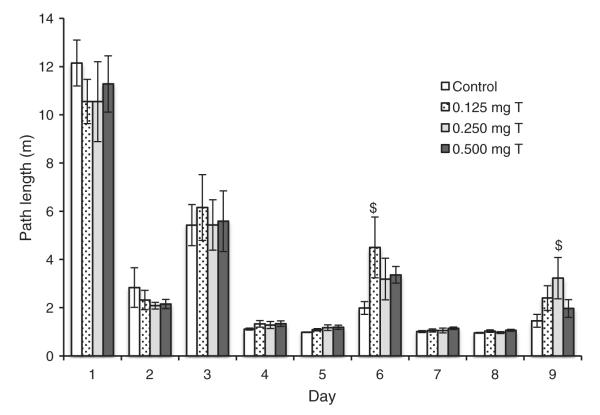
There were significant differences in path length to the platform across the nine days of acquisition trials (F8,352 = 107.3, P < 0.0005), with a rapid decrease in path lengths from day 1 to day 2 and increased path lengths on days 3, 6, and 9 when the platform was hidden (Fig. 3). Univariate ANOVAs indicated no significant differences in path lengths among groups overall on the three hidden-platform days (all P > 0.15). However, Dunnett’s tests indicated that on day 6, the 0.125 mg T group showed nearly significantly longer path lengths than the Control group (P = 0.077) and on day 9, the 0.250 mg T group showed nearly significantly longer path lengths than the Control group (P = 0.071). Dunnett’s tests indicated no other significant differences between the treatment groups and Control group on any of the hidden-platform days (all P > 0.48). These results suggest that the 0.125 mg T and 0.250 mg T groups may have relied more heavily on the platform as a cue (i.e., a cued-response strategy) than did the Control group.
Fig. 3.
Path lengths (mean ± SEM) to reach the water maze platform during Experiment 2 for castrated male rats injected daily with either sesame oil (Control) or specific doses of testosterone propionate. The platform was visible on days 1–2, 4–5, and 7–8 and hidden below the water on days 3, 6, and 9. Path lengths differed significantly among the days (P < 0.0005). Dunnett’s test showed that on Day 6 and 9, the 0.125 mg T and 0.250 mg T groups, respectively, had nearly significantly longer path lengths than did the Control group ($P < 0.10).
Percentage of time spent thigmotactic (within 20 cm of edge) during the acquisition trials showed a significant decrease over the testing days (F8,352 = 192.1, P < 0.0005), but the main effect of treatment (P = 0.83) and day × treatment interaction (P = 0.57) were not significant. This suggests that testosterone injections did not influence stress levels in the water maze.
The results from the probe trials provided evidence that testosterone injections had a significant effect on spatial strategy preference. Specifically, the 0.500 mg T group showed a significant bias toward a place strategy compared to chance levels (Fig. 4; χ2 = 5.33, d.f. = 1, P = 0.021). None of the other groups showed a significant bias for either strategy, although the Control group showed a marginally significant bias for a place strategy (χ2 = 3.00, d.f. = 1, P = 0.083). The 0.125 mg T group was significantly more likely to use a cued-response strategy than was the Control group (Fig. 4; χ2 = 11.11, d.f. = 1, P = 0.001), whereas neither of the other two treatment groups showed a strategy preference that differed from the Control group (both P > 0.50).
Fig. 4.
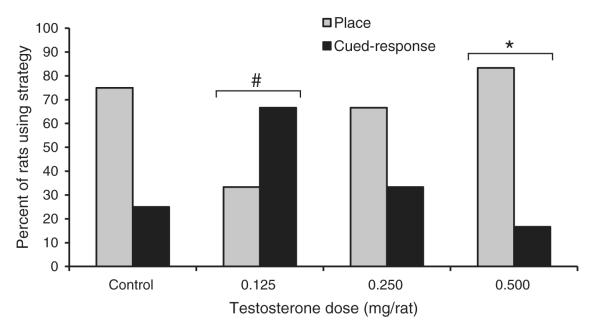
Percent of rats in each group that exhibited a place strategy or a cued-response strategy during probe trials of Experiment 2, when the platform was moved to a new quadrant of the water maze and made visible above the water. All rats were castrated, and Control rats received daily injections of sesame oil and rats in the three treatment groups received daily injections of testosterone propionate. The 0.500 mg T group showed a significant bias for a place strategy (*P < 0.05), but none of the other groups showed a significant bias for either strategy. The 0.125 mg T group was significantly more biased toward a cued-response strategy than was the Control group (#P < 0.05).
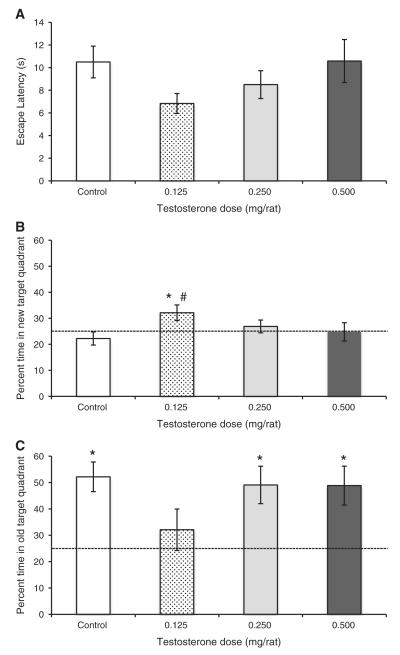
The time that it took rats to reach the new platform location did not differ among the groups during the probe trials (Fig. 5A; P = 0.19). Dunnett’s test indicated that none of the treatment groups differed from the Control groups (all P > 0.17), but it is noteworthy that the 0.125 mg T group found the platform quicker than the Control group and there was a large effect size for this difference (Cohen’s d = 0.906). Additionally, the 0.125 mg T group was the only group that spent significantly more than 25% of its time (chance levels) during probe trials in the new target quadrant (Fig. 5B; t11 = 2.37, P = 0.037). In contrast, all of the other groups spent significantly more than 25% of their time swimming in the old target quadrant where the platform had been during acquisition trials (Fig. 5C; all t11 > 2.30 and P < 0.01). The 0.125 mg T group showed no bias toward swimming in the old target quadrant (P = 0.39). Further analyses showed that there was no difference among groups in the percentage of time spent in the new target quadrant (P = 0.12), but Dunnett’s test indicated that the 0.125 mg T group spent a greater percentage of time in the new target quadrant than did the Control group (P = 0.040; Cohen’s d = 0.847), whereas the other two treatment groups did not differ from the Control group (both P > 0.54). The overall difference among groups in percentage of time spent in the old target quadrant was not significant (P = 0.19), and Dunnett’s test indicated no significant differences between the Control group and any of the treatment groups (all P > 0.12), but there was a large effect size for the difference between the 0.125 mg T group and the Control group (Cohen’s d = 1.026).
Fig. 5.
Performance of castrated male rats injected daily with either sesame oil (Control) or specific doses of testosterone propionate during probe trials of Experiment 2. Each rat was given a single probe trial in which the platform was moved from to the quadrant opposite of where it had been during acquisition trials and made visible above the water. (A) There were no significant differences among the groups in the time that it took rats to reach the new platform location (escape latency). (B) Only the 0.125 mg T group spent significantly more than a chance percentage of time (dashed line at 25%) in the new target quadrant (*P < 0.05), and the 0.125 mg T group was the only treatment group to spend a larger percentage of time in the new target quadrant than the Control group (#P < 0.05). (C) In contrast, all of the other groups showed a significant bias for the old target quadrant (*P < 0.05). All values are reported as mean ± SEM.
Experiment 3: spatial strategies in water maze with nearby visual cue
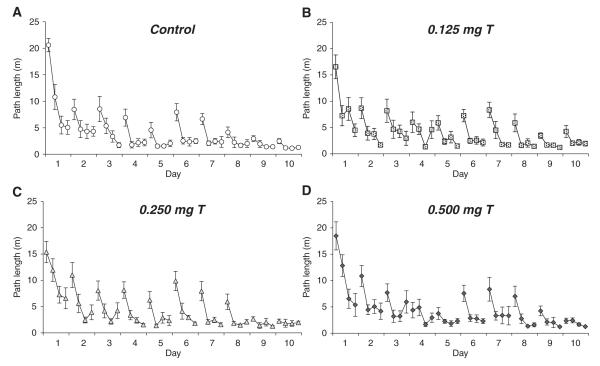
For path length to the platform, there was a significant day × trial interaction (F27,1188 = 5.89, P < 0.0005) as well as significant main effects of day (F9,396 = 68.05, P < 0.0005) and trial (F3,132 = 125.6, P < 0.0005). Rats showed improved performance over the 10 days of testing and improved performance over the 4 trials within each day (Fig. 6). Treatment had no significant main effect on path length (P = 0.61), and treatment had no significant interaction effects with trial or day (all P > 0.80). These results suggest that all groups were equally efficient at using the floating cue to locate the hidden platform.
Fig. 6.
Path lengths (mean ± SEM) to reach the water maze platform during Experiment 3 for castrated male rats injected daily with either sesame oil (A) or specific doses of testosterone propionate (B–D). The hidden platform was moved to a new target quadrant at the start of each day of four trials, and a floating ball was consistently positioned 20 cm to the south of the platform. All groups showed a significant decrease in path lengths over the 10 days of acquisition trials (P < 0.0005) and a significant decrease over the four trials within each day (P < 0.0005). However, testosterone treatment had no significant effects on performance.
Percentage of time spent thigmotactic (within 20 cm of edge) showed a pattern similar to path lengths, with a significant day × trial interaction (F27,1080 = 11.79, P < 0.0005) and significant main effects of day (F9,360 = 71.759, P < 0.0005) and trial (F9,120 = 71.759, P < 0.0005). Treatment had no significant main effect or interaction effects for percentage of time thigmotactic (all P > 0.13). This suggests that testosterone injections did not influence stress levels in the water maze.
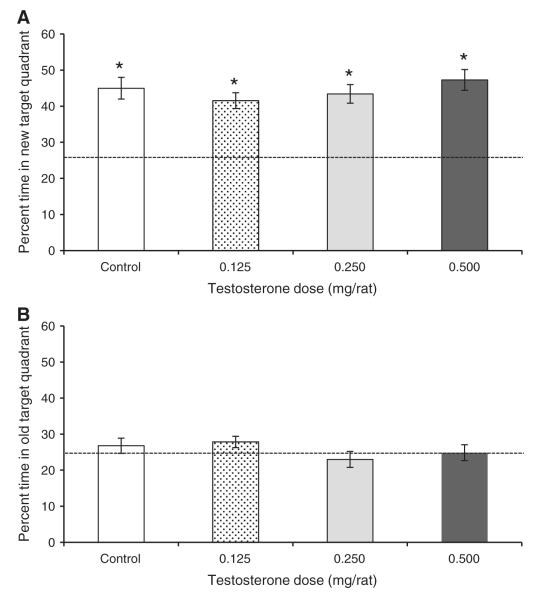
Considering only the first trial of each acquisition day, all groups spent significantly more than 25% of their time (chance levels) swimming in the current target quadrant, which was cued with the floating ball (Fig. 7A; all t11 > 6.60 and P < 0.0005). In contrast, none of the groups spent greater than chance levels swimming in the old target quadrant where the platform had been on the preceding day (Fig. 7B; all P > 0.10). Additionally, neither the percentage of time spent in the old target quadrant (P = 0.36) nor the percentage of time spent in the new target quadrant (P = 0.49) differed among the groups, and Dunnett’s tests indicated that the Control group did not differ from any of the treatment groups in percentage of time spent in the old or new target quadrants (all P > 0.43). Thus, all groups seemed to be using the local cue to navigate to the new platform location at the start of each day of acquisition trials.
Fig. 7.
Percentage of time spent in either the current target quadrant of the water maze (A) or the target quadrant from the proceeding day (B) by castrated male rats injected daily with either sesame oil (Control) or specific doses of testosterone propionate. These results are for only the first trial of each day of acquisition trials. For Experiment 3, the platform was moved to a new target quadrant at the start of each day of four trials, and a floating ball was consistently positioned 20 cm to the south of the platform. All groups spent significantly more than a chance percentage of time (dashed line at 25%) in the new target quadrant (*P < 0.0005), but none of the groups spent more than a chance percentage of time in the old target quadrant. There were no significant differences between the groups for either of these percentages.
Subjects had longer path lengths during the probe trials, when the floating cue was removed, compared to the final day (day 10) of acquisition trials, but performance improved over the four probe trials until path lengths were similar to the acquisition data by the fourth probe trial. The day × trial interaction for path length was significant (F3,129 = 6.76, P < 0.0005), and there were significant main effects of trial (F3,129 = 17.26, P < 0.0005) and day (F1,43 = 29.23, P < 0.0005). Treatment had no significant main effect on path length (P = 0.16), and treatment had no significant interaction effects with trial or day (all P > 0.19). In summary, these results suggest that all groups relied to some degree on using the floating ball to locate the platform.
Serum testosterone
For all three experiments, all of the Control rats had serum testosterone concentrations below the detection limit for the assay (0.04 ng/ml). For all three experiments, serum testosterone levels differed significantly among the three treatment groups (Table 2; Experiment 1: F2,30 = 25.57, P < 0.0005; Experiment 2: F2,33 = 87.37, P < 0.0005; Experiment 3: F2,33 = 43.23, P < 0.0005). For Experiment 1, the 0.125 mg T and 0.250 mg T groups had significantly less circulating testosterone than the 0.500 mg T group (both P < 0.0005) but these two groups did not differ significantly from each other (P = 0.11). For Experiments 2 and 3, post-hoc tests indicated that all three groups had serum testosterone levels that differed significantly from each other (all P < 0.020).
Table 2.
Testosterone concentrations (mean ± SEM) in serum collected 4–6 h after testosterone or oil (Control group) injections on the day after the last day of memory testing for each experiment.
| Experiment | Treatment | N | Serum testosterone (ng/ml) |
|---|---|---|---|
| 1 | Control | 11 | 0.00 ± 0.00* |
| 0.125 mg T | 11 | 1.51 ± 0.26a | |
| 0.250 mg T | 11 | 2.93 ± 0.20a | |
| 0.500 mg T | 11 | 7.32 ± 0.97b | |
| 2 | Control | 12 | 0.00 ± 0.00* |
| 0.125 mg T | 12 | 2.35 ± 0.20a | |
| 0.250 mg T | 12 | 4.10 ± 0.29b | |
| 0.500 mg T | 12 | 8.46 ± 1.62c | |
| 3 | Control | 12 | 0.00 ± 0.00* |
| 0.125 mg T | 12 | 1.24 ± 0.09a | |
| 0.250 mg T | 12 | 2.43 ± 0.17b | |
| 0.500 mg T | 12 | 5.51 ± 0.55c |
Letters indicate treatment groups that differed from each other within an experiment (P < 0.05).
All samples fromControl ratswere belowthe detection limit of the assay (0.04 ng/ml) and were assigned a value of 0 ng/ml.
Discussion
Overall, the results of our three experiments indicate some effects of testosterone on spatial strategy preferences in male rats, but the effects were dose-dependent and task-dependent. For both Experiment 1 and Experiment 2, the low dose of testosterone (0.125 mg/rat) was shown to increase the use of a response strategy. Specifically, the 0.125 mg T group showed a significant bias toward a motor-response strategy in the dual-solution T-maze (Experiment 1). In the dual-solution water maze (Experiment 2), the 0.125 mg T group was significantly more likely to employ a cued-response strategy than was the Control group, and this was the only group to show a significant bias toward swimming in the new cued quadrant during the probe trials. The high dose of testosterone (0.500 mg/rat) caused a significant bias toward a place strategy, but only in Experiment 2.
Testosterone injections had no effect on the ability of the rats to learn the three tasks used in our study. This is in contrast to numerous past studies indicating that testosterone improves spatial working memory (Bimonte-Nelson et al., 2003; Daniel et al., 2003; Gibbs and Johnson, 2008; Hasegawa and Mochizuki, 2009; Kritzer et al., 2001; Spritzer et al., 2008, 2011b). However, the tasks used in our experiments were designed primarily to test for strategy preferences rather than to test for the effects of testosterone on spatial memory. One previous study involving a T-maze protocol comparable to Experiment 1 also demonstrated that testosterone treatment had no effect on spatial learning (Gibbs, 2005). Hawley et al. (2012) found a small but significant tendency for castrated male rats to perform more errors than intact males in a dual-solution water T-maze task. One explanation for this contradiction is that Hawley et al. (2012) used an aversively motivated task, suggesting that motivation or stress levels may interact with testosterone level to influence spatial learning and memory. Past studies testing the effects of testosterone on performance in the Morris water maze have shown improvement of (Khalil et al., 2005; Spritzer et al., 2011b), impairment of (Goudsmit et al., 1990; Naghdi et al., 2001), or no effect of testosterone on performance (Hodosy et al., 2010; Isgor and Sengelaub, 1998; Naghdi et al., 2005; Sandstrom et al., 2006; Spritzer et al., 2008). We previously found that a wide range of physiological doses of testosterone, including those used in the current study, did not cause clear differences among treatment groups in their ability to learn reference memory or working memory versions of the Morris water maze (Spritzer et al., 2011b). Thus, in spite of the fact that testosterone dose may bias male rats toward a particular spatial strategy in the water maze, this does not seem to directly impact their ability to learn the task. However, the effects of castration on spatial memory have been most clearly demonstrated using appetitively motivated working memory tasks, and it will be useful to test how testosterone dose impacts performance on such tasks.
Dose-dependent effects
We observed the clearest effects of testosterone on spatial strategy with the 0.125 mg/rat dose, which increased the use of a response strategy in both Experiments 1 and 2. Careful examination of Figs. 2 and 4 suggests that giving castrated male rats a low dose of testosterone (0.125 mg/rat) caused a shift in spatial strategy toward a response strategy, but as testosterone levels were raised (0.250 and 0.500 mg/rat) rats showed increased use of a place strategy and showed strategy preferences similar to those observed in the Control groups. Past studies have demonstrated that the serum testosterone levels for a male rat typically range from 1 to 4 ng/ml with occasional daily peaks as high as 6–15 ng/ml (Bartke et al., 1973; Kalra and Kalra, 1977; Mock et al., 1978). This indicates that our low dose (0.125 mg/rat) resulted in testosterone levels that were typical for an adult male rat and our high dose (0.500 mg/rat) resulted in high but not supra-physiological levels of circulating testosterone. Therefore, our results suggest that an individual male rat might show shifts in preferred spatial strategy that vary within a day or between days. Individual variation in testosterone levels may explain why past studies with intact male rats have shown no bias in spatial strategy for either the dual-solution T-maze (McIntyre et al., 2003) or the dual-solution water maze (Bizon et al., 2003; Devan and White, 1999).
Experiment 1 was similar to an experiment conducted by Gibbs (2005), and both studies indicated that castrated rats without hormone replacement do not show a strong strategy preference. Experiment 1 also showed that a low dose of testosterone (0.125 mg/rat) biased males toward a motor-response strategy, but Gibbs (2005) found no effects on testosterone replacement on strategy preference in the T-maze. However, the maze protocol used by Gibbs (2005) differed from ours in that it involved “delayed matching-to-position” and trials were conducted in blocks of 8 per day for up to 30 days. This protocol primarily tested working memory, but unlike Experiment 1 it also had a reference memory component because rats had to remember the task from day to day. Previous work suggests that testosterone enhances working memory but not reference memory (Gibbs and Johnson, 2008; Sandstrom et al., 2006; Spritzer et al., 2008, 2011b); therefore, the reference memory component of the Gibbs’ (2005) task may have obscured any effects of testosterone on strategy choice. Another explanation is that the testosterone dose used by Gibbs (2005) was too high to bias rats toward a response strategy. Gibbs (2005) used silastic implants that resulted in serum testosterone concentrations near 14 ng/ml, which corresponds with our high dose (0.500 mg/rat), and we observed no significant strategy bias in the 0.500 mg T group for Experiment 1. Thus, the results of both studies support the conclusion that castrated rats without any testosterone replacement or with a high dose of testosterone display no strategy bias in an appetitively motivated task. However, the bias toward a response strategy in Experiment 1 seems to be unique to our low dose of testosterone (0.125 mg T).
Hawley et al. (2012) used a dual-solution water T-maze in which rats had to learn which direction to swim in order to reach the goal platform. They found that both castrated and intact males showed a strong bias toward a place strategy. We observed no significant strategy bias among the Control rats in any of our experiments. This contradiction may be due to differences in protocol, as we did not assess a motor-response strategy in the water maze. Because Hawley et al. (2012) did not report circulating testosterone levels, it is difficult to compare their intact males to any of our groups. If their rats had relatively high testosterone levels, then the results would correspond with our finding for Experiment 2 indicating that high circulating testosterone levels bias males toward a place strategy. Based on their results, Hawley et al. (2012) concluded that learning strategy was not influenced by testosterone, but our results suggest that a low dose of testosterone causes a bias toward a response strategy that is distinct from both castrated males and males with relatively high circulating testosterone levels.
To some degree, our findings that low doses of testosterone favored the use of a response strategy (Experiment 1) and that high doses favored the use of a place strategy (Experiment 2) parallel the findings for the effects of estradiol on the spatial strategy preferences of female rats. Multiple studies have shown that the stage in the estrus cycle influences a female’s strategy preferences, with peaks in estradiol biasing females toward a place strategy and relatively low estradiol levels biasing females toward a response strategy (Korol et al., 2004; McElroy and Korol, 2005; Pleil and Williams, 2010; Sava and Markus, 2005). Ovariectomized females show worse performance on tasks that require the use of a place strategy and better performance on tasks that require the use of a motor-response strategy compared to ovariectomized females given relatively high doses of estradiol (Davis et al., 2005; Korol and Kolo, 2002). In contrast, we found no bias for a particular strategy among castrated Control males for any of our experiments. These results suggest that following gonadectomy, males have no strong strategy preference, whereas females prefer a response strategy. Our results for Experiment 2 suggest that giving castrated male rats a low dose of testosterone (0.125 mg/rat) caused a shift in spatial strategy toward a cued-response strategy. In contrast, giving ovariectomized female rats a low dose of estradiol seems to cause no change in strategy preference (i.e., response bias remains), and it is not until estradiol levels are elevated that there is a shift in strategy toward a place bias (Quinlan et al., 2008). Thus, although both male and female rats show dose-dependent responses to testosterone and estradiol, respectively, the effects for the two sexes are distinctly different.
Task-dependent effects
Both our results and the results of past studies show that the effects of testosterone on spatial strategy are task dependent. This is especially apparent among rats with high levels of testosterone, with these males showing no bias on appetitively motivated tasks (Experiment 1; (Gibbs, 2005)) and a bias toward a place strategy on aversively motivated tasks (Experiment 2; (Hawley et al., 2012)). This suggests that high testosterone levels may allow rats to flexibly use the strategy that is most appropriate for a particular task. The T-maze (Experiment 1) is a relatively simple task that can be quickly learned using a simple turn bias without reference to spatial cues. In contrast, the Morris water maze (Experiment 2) is arguably more spatially complex in that the rat’s movements are less restricted than in the T-maze. This increased spatial complexity may encourage rats to attend to distal spatial cues and employ a place strategy. In support of this idea, we previously found that rats receiving a high dose of testosterone (0.500 mg/rat) showed more behavioral flexibility in the water maze than did males receiving a low dose (0.250 mg/rat) (Spritzer et al., 2011b).
Unlike Experiments 1 and 2, Experiment 3 indicated no clear distinctions in strategy preference among the groups. The finding that all groups showed some impairment on the first trial of each day of acquisition trials relative to the final trial on the previous day suggests that all groups were using a place strategy to some degree, and this is supported by previous experiments using this protocol (Daniel and Lee, 2004; Pearce et al., 1998). However, even on the first trial of each acquisition day, all groups showed a bias to swim in the current target quadrant rather than the target quadrant from the previous day, suggesting that all groups were also making some use of the floating ball as a proximal cue. Our inability to distinguish the strategy preferences of the groups may have been because optimal performance on this particular task required the use of a combination of place and cued-response strategies. One strategy could have been to navigate directly to the floating ball and then make a random search in the vicinity of the ball, but this would be less effective than using the floating ball in combination with distal cues around the room to triangulate to the exact position of the goal each day.Pearce et al. (1998) found that moving the goal to the opposite side of the floating ball resulted in reduced performance among male rats, indicating that the subjects were indeed using a combination of proximal and distal cues to locate the goal. Thus, this task may not be well suited for distinguishing between place and response learning strategies. All groups showed improved performance during the acquisition trials in Experiment 3, which suggest that all groups were capable of using a combination of proximal and distal cues to locate a goal. In contrast, the results of Experiment 2 indicated that the 0.125 mg T dose caused increased (relative to Control) bias toward using the goal platform itself as a navigational cue. Perhaps as cues become more distal from the goal, the influence of testosterone on strategy preference is reduced.
Daniel and Lee (2004) used methods identical to those used in Experiment 3 and found that removal of the floating ball impaired performance of ovariectomized females but not ovariectomized females given estradiol replacement. In contrast, Experiment 3 demonstrated no clear effects of testosterone on male performance in this task. Sava and Markus (2005) found that moving a proximal cue (large white panel) along the edge of a water maze caused females with low estrogen levels to navigate toward the cue rather than using a place strategy, while both females with high estrogen levels and males showed no bias to navigate toward the proximal cue. Another study showed that females are more likely than males to use the experimenter as a proximal cue during navigation in the water maze (Roof and Stein, 1999). In combination, these studies suggest that the effects of testosterone on the use of a nearby visual cue by males are less dramatic than are the effects of estradiol on cue use by females.
Physiological mechanisms
The effects of testosterone on the brain are primarily through its two major metabolites, estradiol and dihydrotestosterone (DHT), which are strong agonists of estrogen and androgen receptors, respectively (Edinger and Frye, 2004; Stoffel-Wagner, 2003). The effects of testosterone on spatial strategy preference may involve either an androgen-dependent or estrogen-dependent pathway. Some studies have tested the effects of DHT and estradiol on spatial ability in male rats. For example, male rats with dysfunctional androgen receptors throughout the brain exhibit impaired performance on the reference memory version of the Morris water maze (Jones and Watson, 2005). Systemic implants of estradiol improved spatial working memory in castrated male rats (Gibbs, 2005; Luine and Rodriguez, 1994), and intrahippocampal injections of estradiol enhanced the performance of intact male rats on a reference memory version of the Morris water maze (Packard et al., 1996). Bimonte-Nelson et al. (2003) found that DHT implants caused a small improvement in spatial working memory among aged male rats, but larger memory improvements were observed in response to testosterone implants. Both estradiol and DHT implants were shown to improve memory on an object location task relative to castrated control rats (McConnell et al., 2012). It remains to be tested as to whether or not these memory-enhancing effects of DHT and estradiol correspond with changes in spatial strategy.
The hippocampus and striatum are critically involved in employing place and response strategies, respectively (Devan et al., 1996; McDonald and White, 1993; Packard and McGaugh, 1996; Pearce et al., 1998). Given that androgen and estrogen receptors are expressed in both the hippocampus and striatum of male rats (Feng et al., 2010; Kerr et al., 1995; Li et al., 1997; Xiao and Jordan, 2002), testosterone or its metabolites may influence spatial memory by having different effects on these two regions. Injections of estradiol into the hippocampus of ovariectomized females improved place learning while having no effect on response learning (Zurkovsky et al., 2006, 2007), and injections of estradiol into the dorsal striatum of ovariectomized females had no effect on place learning while impairing response learning (Zurkovsky et al., 2007). Thus, estradiol seems to have independent effects on the hippocampus and striatum for females, and it will be interesting to determine whether testosterone also has different effects on these brain regions. Some studies have shown that DHT enhances neuroplasticity in the hippocampus of male rats whereas estradiol does not (Leranth et al., 2003; MacLusky et al., 2006; Spritzer et al., 2011a), suggesting that androgens could have effects on hippocampus-dependent memory that differ from estradiol’s effects on the hippocampus of females.
Unlike the rats receiving the low doses of testosterone (0.125 mg), the castrated Control rats did not show a bias toward a motor-response strategy in Experiment 1. Although no testosterone was detectable in the serum of Control rats, small amounts of androgens are produced both in the adrenal glands and in the brain itself (Shibuya et al., 2003). Thus, there seems to be a threshold concentration of testosterone that must be reached in order to bias males toward a response strategy, and the results of Experiment 2 suggest that even higher levels of testosterone cause the bias to shift toward a place strategy. Perhaps testosterone levels that are below the physiological norm fail to influence functioning of either the hippocampus or the striatum, and the striatum is more sensitive than the hippocampus to low, but physiological, levels of testosterone. Supporting this idea, immunoreactivity for androgen receptors was shown to be higher in the striatum than the hippocampus of male rats (Feng et al., 2010). There is also some evidence that the effects of testosterone on neuroplasticity are dose dependent. For example, relatively high doses of testosterone (0.500–1.00 mg/rat) were needed to enhance neurogenesis within the hippocampus of male rats (Spritzer and Galea, 2007; Spritzer et al., 2011a).
Chang and Gold (2003) demonstrated that intact male rats performing on a T-maze task identical to ours (Experiment 1) showed a transition from a place bias to a response bias over the course of 100 acquisition trials. Specifically, after 20 acquisition trials, rats showed a bias toward a place strategy, whereas after 40 trials most rats were biased toward a response strategy, and by 100 acquisition trials all rats showed a bias toward a response strategy (Chang and Gold, 2003). The rats in Experiment 1 took 34 trials on average to reach criterion, suggesting that one would not expect a strong bias for either strategy among the rats, and this was indeed the case for most of the groups. Perhaps the strong bias toward a motor-response strategy among the 0.125 mg T group is indicative of a more rapid transition from initial use of a place strategy to a motor-response strategy. Furthermore,Chang and Gold (2003) found that this transition corresponded with an initial rise in acetylcholine within the hippocampus, followed by a slow rise in acetylcholine within the striatum, so perhaps testosterone is influencing this transition.
A third memory system that may have influenced our results is the basolateral amygdala, which is critical for learning associations between stimuli and an affective response (White and McDonald, 2002). Evidence suggests that activation of the amygdala via stress can modify a rat’s ability to learn spatial tasks requiring place or response strategies. Some studies have shown that stress specifically impairs performance on tasks requiring a place strategy while actually improving performance on response tasks (Kim et al., 2001; Sadowski et al., 2009). Injections of an anxiogenic drug into the amygdala biased rats toward a motor-response strategy (Elliott and Packard, 2008; Packard and Wingard, 2004), and male rats with lower levels of anxiety were more likely to use a place strategy in the Morris water maze (Hawley et al., 2011). Therefore, testosterone may bias males toward a particular spatial strategy by acting directly on the amygdala and/or by blunting the hormonal stress response (Viau, 2002). However, a negative correlation between testosterone levels and anxiety level (Edinger and Frye, 2004) does not seem to fully explain our results. We used relatively cold water in our water maze experiments, suggesting that an acute stress response was likely induced in Experiment 2. Although we observed a transition from a cued-response strategy to a place strategy with increasing testosterone dose in that experiment, the Control group did not show a bias toward a response strategy that would be predicted based exclusively on a castration-induced increase in anxiety.
Conclusion
The results of this study suggest that circulating testosterone levels influence the strategy preferences of male rats. A low dose of testosterone (0.125 mg/rat) caused a bias toward a motor-response strategy in the dual-solution T-maze, and the same dose increased the use of a cued-response strategy in the dual-solution water maze. A high testosterone dose (0.500 mg/rat) caused a bias toward a place strategy in the water maze but not the T-maze. The dose-dependent effects of testosterone may be due to differential effects on the hippocampus and striatum, similar to the effects of estradiol on the brains of female rats (Zurkovsky et al., 2007). However, unlike past findings with females, we observed no clear strategy bias in gonadectomized males, suggesting a sex difference in the impact of sex steroids on strategy preference. Further work is needed to determine whether the testosterone-induced strategy biases that we observed among male rats actually influence their performance on spatial tasks.
Acknowledgments
We thank Meagan Coneeny, Molly Curtis, Kevin Grafmiller, Tyler Prince, Leanne Shulman, Julia Stern, and JaeHee Jane Yoon for assistance with data collection. We thank Vicki Major and the animal care staff for their assistance, and we thank Mark Stefani and Tom Root for their important contributions to this work. This project was funded by Middlebury College and the Vermont Genetics Network (INBRE grant number 2P20RR016462) from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this project are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water maze: a large and reliable sex difference. Behav. Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance. Behav. Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Han J, Hudon C, Gallagher M. Effects of hippocampal cholinergic deafferentation on learning strategy selection in a visible platform version of the water maze. Hippocampus. 2003;13:676–684. doi: 10.1002/hipo.10113. [DOI] [PubMed] [Google Scholar]
- Blokland A, Rutten K, Prickaerts J. Analysis of spatial orientation strategies of male and female Wistar rats in a Morris water escape task. Behav. Brain Res. 2006;171:216–224. doi: 10.1016/j.bbr.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J. Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney ID, Brabec CM, Runco DV. Mapping out spatial ability: sex differences in way-finding navigation. Percept. Mot. Skills. 2008;107:747–760. doi: 10.2466/pms.107.3.747-760. [DOI] [PubMed] [Google Scholar]
- Choi J, Silverman I. The relationship between testosterone and route-learning strategies in humans. Brain Cogn. 2002;50:116–120. doi: 10.1016/s0278-2626(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jr., Chang E, Strong RA, Milun R. Spatial Ability, navigation strategy, and geographic knowledge among men and women. Evol. Hum. Behav. 1998;19:89–98. [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol. Learn. Mem. 2004;82:142–149. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl.) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol. Learn. Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J. Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol. Learn. Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate–putamen lesion on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav. Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive enhancing effects may be due in part to actions of its 5a-reduced metabolites in the hippocampus. Behav. Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol. Learn. Mem. 2008;90:616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Feng Y, Weijdegård B, Wang T, Egecioglu E, Fernandez-Rodriguez J, Huhtaniemi I, Stener-Victorin E, Billig H, Shao R. Spatiotemporal expression of androgen receptors in the female rat brain during the oestrous cycle and the impact of exogenous androgen administration: a comparison with gonadally intact males. Mol. Cell. Endocrinol. 2010;321:161–174. doi: 10.1016/j.mce.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm. Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit E, Vandepoll NE, Swaab DF. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in your and middle-aged animals. Behav. Neural Biol. 1990;53:6–20. doi: 10.1016/0163-1047(90)90729-p. [DOI] [PubMed] [Google Scholar]
- Goyette SR, McCoy JG, Kennedy A, Sullivan M. Sex differences on the judgment of line orientation task: a function of landmark presence and hormonal status. Physiol. Behav. 2012;105:1045–1051. doi: 10.1016/j.physbeh.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Harris AP, D’Eath RB, Healy SD. Sex differences, or not, in spatial cognition in albino rats: acute stress is the key. Anim. Behav. 2008;76:1579–1589. [Google Scholar]
- Hasegawa N, Mochizuki M. Improved effect of pycnogenol on impaired spatial memory function in partial androgen deficiency rat model. Phytother. Res. 2009;23:840–843. doi: 10.1002/ptr.2702. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Dohanich GP. The relationships between trait anxiety, place recognition memory, and learning strategy. Behav. Brain Res. 2011;216:525–530. doi: 10.1016/j.bbr.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol. Behav. 2012;105:1014–1020. doi: 10.1016/j.physbeh.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Hodosy J, Pales J, Ostatnikova D, Celec P. The effects of exogenous testosterone on spatial memory in rats. Cent. Eur. J. Biol. 2010;5:466–471. [Google Scholar]
- Holding CS, Holding DH. Acquisition of route network knowledge by males and females. J. Gen. Psychol. 1988;116:29–41. [Google Scholar]
- Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42:782–790. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 cell morphology in rats. Horm. Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Jonasson Z, Cahill JFX, Tobey RE, Baxter MG. Sexually dimorphic effects of hippocampal cholinergic deafferentation in rats. Eur. J. Neurosci. 2004;20:3041–3053. doi: 10.1111/j.1460-9568.2004.03739.x. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kalra PS, Kalra SP. Circadian periodicities of serum androgens, progesterone, gonadotropins and Luteinizing hromone-releasing hormone in male rats: the effects of hypothalamic deafferentation, castradion, and adrenalectomy. Endocrinology. 1977;101:1821–1827. doi: 10.1210/endo-101-6-1821. [DOI] [PubMed] [Google Scholar]
- Kamel F, Frankel AI. Hormone release during mating in the male rat: time course, relation to sexual behavior, and interaction with handling procedures. Endocrinology. 1978;103:2172–2179. doi: 10.1210/endo-103-6-2172. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taşkiran K, Furedy JJ, Kulai B, McDonald R, Pöğün S. Nicotine interacts with sex in affecting rat choice between “look-out” and “navigational” cognitive styles in the Morris water maze place learning task. Brain Res. Bull. 1998;46:441–445. doi: 10.1016/s0361-9230(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgoren S, Fured JJ, Pogun S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res. Bull. 2000;52:243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution of hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Khalil R, King MA, Soliman MRI. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han J, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J. Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol. Learn. Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LI. Estrogen-induced changes in place and response learning in young adult female rats. Behav. Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Border KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm. Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirilis T. Gonadectomy impairs T-maze acquisition in adult male rats. Horm. Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lawton CA. Gender differences in way-finding strategies: relationship to spatial ability and spatial anxiety. Sex Roles. 1994;30:765–779. [Google Scholar]
- Lee VWK, DeKrestser DM, Hudson B, Wang C. Variations in serum FSH, LH and testosterone levels in male rats from birth to sexual maturity. J. Reprod. Fertil. 1975;42:121–126. doi: 10.1530/jrf.0.0420121. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-β-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav. Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrus cycle. J. Neurosci. 1999;15:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SEA, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm. Behav. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McElroy MW, Korol DL. Intrahippocampal muscimol shifts learning strategy in gonadally intact young adult female rats. Learn. Mem. 2005;12:150–158. doi: 10.1101/lm.86205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol. Learn. Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Mock EJ, Norton HW, Frankel AI. Daily rhythmicity of serum testosterone concentration in the male laboratory rat. Endocrinology. 1978;103:1111–1121. doi: 10.1210/endo-103-4-1111. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” maze: sex differences and correlation with psychometric measure of spatial ability in humans. Evol. Hum. Behav. 1998;19:73–87. [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Mohaddess G, Khamnei S, Arjomand M. No significant difference between intact and testosterone depleted or administrated male rats in spatial learning and memory. IJPR. 2005;1:29–32. [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of the hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol. Learn. Mem. 2004;82:243–252. doi: 10.1016/j.nlm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interactions with cholinergic systems. Behav. Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, Rizzo AA. Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia. 2004;42:555–562. doi: 10.1016/j.neuropsychologia.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Roberts ADL, Good M. Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature. 1998;396:75–77. doi: 10.1038/23941. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav. Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Williams CL. The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm. Behav. 2010;57:360–367. doi: 10.1016/j.yhbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma A, Jager G, Kessels RPC, Kippeschaar HPF, van Honk J. Sex differences for selective forms of spatial memory. Brain Cogn. 2004;54:24–34. doi: 10.1016/s0278-2626(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Puts DA, Cardenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Horm. Behav. 2010;58:282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm. Behav. 2008;53:185–191. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav. Brain Res. 2009;205:19–25. doi: 10.1016/j.bbr.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman JA, Huettel S. Males and females use different distal cues in a virtual environment navigation task. Cogn. Brain Res. 1998;6:351–360. doi: 10.1016/s0926-6410(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sava S, Markus EJ. Intramaze cue utilization in the water maze: effects of sex and estrous cycle in rats. Horm. Behav. 2005;48:23–33. doi: 10.1016/j.yhbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Jacobson TK, Markus E. Hippocampal and striatal dependent navigation: sex differences are limited to acquisition. Horm. Behav. 2009;56:199–205. doi: 10.1016/j.yhbeh.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymoure P, Dou H, Juraska JM. Sex differences in radial maze performance: influence of rearing environment and room cues. Psychobiology. 1996;24:33–37. [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori T, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim. Biophys. Acta Gen. Subj. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Silverman I, Choi J. Non-Euclidean navigational strategies of women: compensatory response or evolved dimorphism? Evol. Psychol. 2006;4:75–84. [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Develop. Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LAM. Castration differentially affects spatial working and reference memory in male rats. Arch. Sex. Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011a;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm. Behav. 2011b;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteriod biosynthesis in the human brain and its clinical implications. Ann. N. Y. Acad. Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Thilers PP, MacDonald SWS, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ulubaev A, Lee DM, Purandare N, Pendleton N, Wu FCW. Activational effects of sex hormone on cognition in men. Clin. Endocrinol. 2009;71:607–623. doi: 10.1111/j.1365-2265.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic–pituitary–gonadal and – adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol. Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm. Behav. 2002;42:327–336. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol. Learn. Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm. Behav. 2011;60:470–477. doi: 10.1016/j.yhbeh.2011.07.014. [DOI] [PubMed] [Google Scholar]