Abstract
In 2011, the prevalence of prescription drug abuse exceeded that of any other illicit drug except marijuana. Consequently, efforts to curtail abuse of new medications should begin during the drug development process, where abuse liability can be identified and addressed before a candidate medication has widespread use. The first step in this process is scheduling with the Drug Enforcement Agency so that legal access is appropriately restricted, dependent upon levels of abuse risk and medical benefit. To facilitate scheduling, the Food and Drug Administration (FDA) has published guidance for industry that describes assessment of abuse liability. The purpose of this paper is to review methods that may be used to satisfy the FDA’s regulatory requirements for animal behavioral and dependence pharmacology. Methods include psychomotor activity, self-administration (an animal model of the rewarding effects of a drug), drug discrimination (an animal model of the subjective effects of a drug), and evaluation of tolerance and dependence. Data from tests conducted at RTI with known drugs of abuse illustrate typical results, and demonstrate that RTI is capable of performing these tests. While using preclinical data to predict abuse liability is an imperfect process, it has substantial predictive validity. The ultimate goal is to increase consumer safety through appropriate scheduling of new medications.
Introduction
Data from public health surveillance systems indicate that over the past decade, prescriptions for neuropsychiatric medications (e.g., opioids, stimulants, sedatives) have increased dramatically (Fortuna, Robbins, Caiola, Joynt, & Halterman, 2010; Manchikanti et al., 2012; Moloney, Konrad, & Zimmer, 2011). While nonmedical use of prescription drugs has remained fairly steady since 2008, prevalence in 2011 still exceeded that of any illicit drug except marijuana (Substance Abuse and Mental Health Services Administration, 2012). Further, the rates of adverse events associated with nonmedical use of prescription drugs have significantly increased. For example, poisoning recently overtook motor vehicle accidents as the leading cause of accidental death in 2008 (Warner et al., 2011). The majority of these poisoning deaths (89 percent) were caused by drugs, with 77 percent ruled as unintentional overdoses (Warner, Chen, Makuc, Anderson, & Miniño, 2011). Unlike many types of illicit drugs used primarily for euphoria and not for improving health, prescription drugs are legal and carry both euphoric and health-promoting properties. Given the range of outcomes associated with use of prescription medications, it is not surprising that a large number of terms have been developed to refer to their illicit consumption, varying along the spectrum from benign (e.g., use/misuse) to harmful (addiction/dependence). One specific term, “abuse,” has become a rather generic term, referring to any consumption practice along the spectrum of harmful use. In a draft guidance document issued by the Food and Drug Administration (FDA), “[a]buse potential refers to a drug that is used in nonmedical situations, repeatedly or even sporadically, for the positive psychoactive effects it produces” (FDA, 2010).
Assessment of Abuse Potential: 8-Factor Analysis
One of the first steps in prevention of prescription drug abuse is effective scheduling, or categorizing, of candidate medication prior to approval by the FDA. The Controlled Substances Act, or CSA (21 U.S.C. 811(b), 811(c)) describes eight factors that are used in determination of appropriate scheduling of a new molecular entity: (1) its actual or relative potential for abuse (also called its abuse liability); (2) scientific evidence of the drug’s pharmacological effects; (3) the state of current scientific knowledge regarding the drug or other substance; (4) its history and current pattern of abuse; (5) the scope, duration, and significance of abuse; (6) what, if any, risk there is to the public health; (7) its psychic or physiological dependence liability; (8) whether the substance is an immediate precursor of a substance already controlled. The FDA should ensure that abuse liability is examined for new candidate medications that target changes in neurochemistry, for currently prescribed medications that show unexpected abuse potential, and when changes are made to the formulation or route of administration.
In collaboration with the FDA, the Drug Enforcement Agency (DEA) uses this information to schedule a drug into one of five classifications (schedule I–V), with increasing abuse potential and decreasing legal access at lower schedule numbers. For example, most schedule I drugs have high abuse potential and no accepted medical use in the US. (e.g., heroin, lysergic acid diethylamide [LSD]). According to federal law, possession of a schedule I drug is illegal without an appropriate license (e.g., for research purposes). In contrast, schedule V drugs have relatively low abuse potential. Many of them can be purchased without a prescription, and others are readily available through prescription (e.g., many anticonvulsants). Notably, this classification system applies only to drugs that are covered by the CSA. Drugs without abuse potential (e.g., those that do not cross the blood-brain barrier, and many over-the-counter drugs such as aspirin) are approved by the FDA but are not reviewed by the DEA and are unscheduled.
For a candidate medication that an 8-factor analysis has indicated may have abuse liability, the manufacturer must include an abuse potential section containing detailed information on the drug’s chemistry, pharmacology, and effects in humans in the New Drug Application (NDA) that it submits to the FDA. The NDA also must have a subsection on animal behavioral and dependence pharmacology. The purpose of this occasional paper is to describe four specific methods that are most commonly used to satisfy the animal behavioral and dependence pharmacology requirement (FDA, 2010), including psychomotor activity, self-administration, drug discrimination, and assessment of tolerance and dependence. The following section provides an overview of each procedure, accompanied by presentation of actual data for known drugs of abuse to illustrate results similar to those that might be obtained with drugs having potential abuse liability.
Behavioral and Dependence Pharmacology
Psychomotor Activity Tests
Psychomotor activity tests examine effects of the candidate medication on locomotor behavior. Because this procedure does not require prior training of the animal or prolonged drug exposure, it is amenable to high throughput studies (Morgan, Dupree, Bibbey, & Sizemore, 2012). This test does not specifically screen for abuse liability per se, but rather provides information on pharmacological similarity to known drugs of abuse as well as possible toxic effects of the drug. Additionally, activity tests can facilitate later assessment in more specific abuse liability tests by determining relevant experimental parameters such as the behaviorally active dose range, the latency for drug effects to begin, and the total duration of a drug’s effects (Morgan et al., 2012).
Activity tests are generally conducted in open field chambers made of clear Plexiglas surrounded by arrays of infrared photocell beams that detect movement (Figure 1). The activity tests monitor and record ambulatory movements (movement of the entire body to a new position), fine movements (small movements of a particular body part such as stereotypy or grooming), and distance traveled. More sophisticated activity tests calculate time spent in particular areas of the open field, rearing, and jumping (Kaliva, Duffy, DuMarus, & Skinner, 1988).
Figure 1.

Standard locomotor chamber used for measuring psychomotor behavior
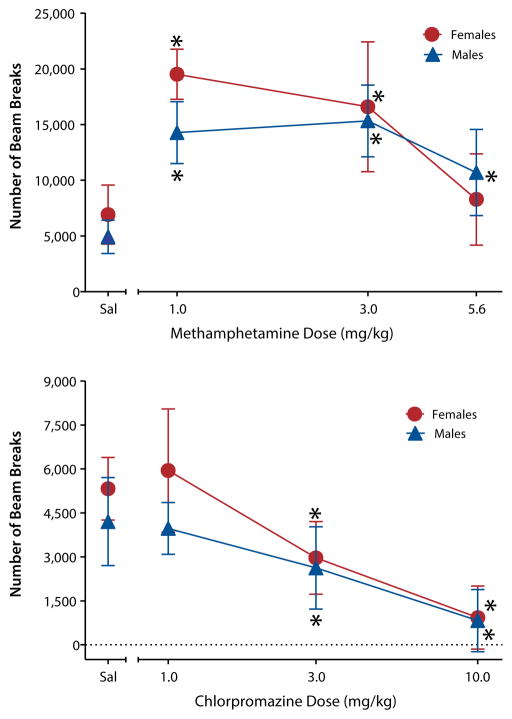
Since the direction of effect on locomotor activity varies across different classes of abused drugs, one requirement of a locomotor activity system that is used for screening candidate medications is that it must be able to measure increases and decreases in motor activity. For example, a defining behavioral characteristic of psychomotor stimulants is their propensity to increase locomotion in rodents, an effect that is strongly associated with their high addiction potential (Calabrese, 2008; Wise & Bozarth, 1987). Amphetamine and other psychomotor stimulants dose-dependently increase locomotion and, at higher doses, may induce stereotypy, or repetitive movements (see methamphetamine results in Figure 2; Balster & Chait, 1978; French & Witkin, 1993). In contrast, barbiturates and other CNS depressants dose-dependently decrease locomotion (Darnell, McCloskey, & Commissaris, 1986; Hayakawa et al., 2008; Savić et al., 2009; Vinkers et al., 2009). An important point to note, however, is that drug-induced changes in locomotor activity do not necessarily indicate abuse potential (e.g., see results with chlorpromazine in Figure 2), but must be considered in conjunction with results from more specific functional tests such as drug discrimination and self-administration.
Figure 2. Male and female Sprague-Dawley rats exhibited increased locomotor activity compared to drug vehicle (saline) when injected with the psychomotor stimulant methamphetamine (top panel), as evidenced by an increase in the number of beam breaks.
Rats exhibited decreased activity when injected with the prototypic antipsychotic chlorpromazine, as evidenced by fewer and fewer beam breaks as the dose of chlorpromazine increased (bottom panel). These data indicate that the procedure has sufficient sensitivity to detect both increases and decreases in motor activity. Note the different Y-axis scales. Sal stands for saline vehicle. Asterisks indicate significant differences from vehicle (p < 0.05).
Self-Administration
Self-administration is an animal model of the rewarding effects of a drug. Drugs that are self-administered by animals are very likely to be abused by humans, providing the model with high face and predictive validity (O’Connor, Chapman, Butler, & Mead, 2011; Panlilio & Goldberg, 2007). For this procedure, researchers surgically implant an animal with an intravenous jugular catheter. Experimental sessions occur in an operant chamber in which the external port of the catheter is attached to tubing that exits the chamber and is connected to an infusion pump (Figure 3). The researchers train the animals to make a response (e.g., lever press, nose poke) to receive an intravenous infusion of drug. Drug infusions are available following a fixed number of responses (fixed ratio), usually between 1 and 10 responses per infusion (abbreviated FR1–FR10; Weeks, 1962). Alternatively, the researchers may use a schedule in which each subsequent infusion requires progressively more responses than the prior infusion (known as a progressive ratio schedule; Richardson & Roberts, 1996). Progressive ratio schedules allow for a more precise determination of the efficacy of the drug as a reinforcer and allow comparisons of efficacy across drugs.
Figure 3.

Standard operant chamber used for drug self-administration
For screening candidate medications, researchers most commonly train animals to self-administer a known drug of abuse such as cocaine, which produces stable responding in rodents and nonhuman primates (Griffiths, Lamb, Sannerud, Ator, & Brady, 1991; Johanson & Balster, 1978). Because self-administration behavior can differ across drug classes, however, the training drug might be selected from the same pharmacological class as the candidate drug if the class is known to be abused (O’Connor et al., 2011). Once the animal has acquired drug self-administration, researchers substitute the candidate medication for the training drug. The researchers assess various doses of the candidate medication, along with its vehicle, and compare responding for infusion of the candidate medication to that for infusion of the training drug (e.g., compare results between stimulants and chlorpromazine in Figure 4). Maintenance of responding above vehicle level indicates that a drug is reinforcing, whereas maintenance of responding below vehicle level indicates that a drug may be aversive (Numan, 1981; Sinden & Le Magnen, 1982).
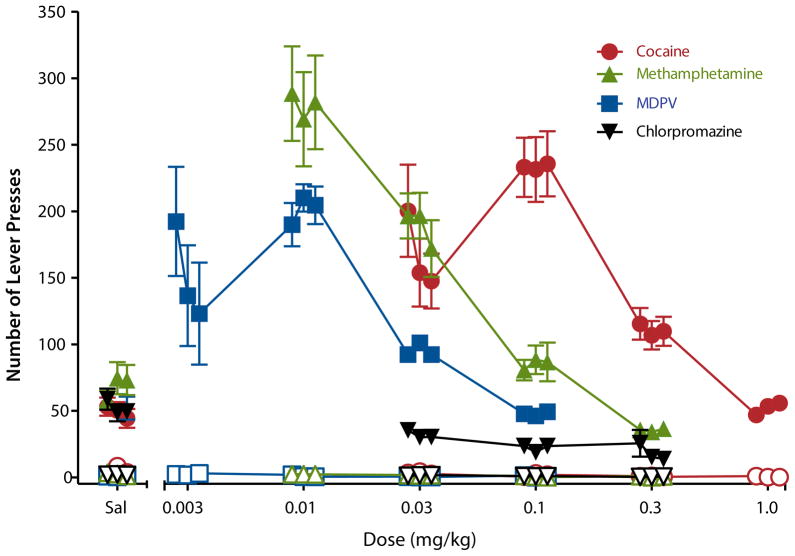
Figure 4. Male Sprague-Dawley rats were trained to press a lever to self-administer i.v. infusions of cocaine (five lever presses were required for each infusion).
The figure shows the average number of lever presses as a function of dose of drug across 3 days of access to each dose on the active (filled symbols) and inactive (open symbols) levers. “Sal” stands for saline vehicle. Rats pressed the active lever to receive infusions of cocaine and methamphetamine at much higher rates than for infusions of saline, indicating that these drugs are reinforcing. Responding for higher doses of cocaine and methamphetamine dropped to saline levels, whereas lower doses of cocaine and methamphetamine also resulted in lesser responding. This inverted U-shaped dose-effect function is typical of self-administration of many known drugs of abuse and may represent the incapacitating effect of higher doses and the lack of reinforcing effect from lower doses. MDPV produced effects that were similar to those of cocaine but at lower doses, suggesting that MDPV was also reinforcing to the rats and was more potent than cocaine. MDPV, a synthetic cathinone that has been commonly identified in products labeled “bath salts,” was recently designated by the DEA as a schedule I drug due to its abuse in humans. In contrast, the antipsychotic chlorpromazine was not self-administered and, in fact, shows signs that it may be aversive, in that it produced lower than baseline rates of infusions.
The self-administration procedure is useful for testing candidate stimulants, opioids, barbiturates, and benzodiazepines, since animals readily self-administer these drug classes (Davis, Smith, & Smith, 1987; Van Ree, Slangen, & de Wied, 1978; Weeks & Collins, 1987). A few classes of drugs that are abused by humans but are not generally self-administered by rodents are psychoactive components of the marijuana plant and hallucinogens (French, Lopez, Peper, Kamenka, & Roberts, 1995; Van Ree et al., 1978; Weeks & Collins, 1987). Hence, some classes of abused drugs may appear to be false negatives in the self-administration procedure. Consequently, for the most accurate prediction, results obtained from self-administration studies should be examined as one part of an overall abuse potential assessment package that includes other behavioral tests as well as data collected as part of the 8-factor analysis.
Drug Discrimination
Drug discrimination is a pharmacologically selective behavioral model of the interoceptive effects of a drug, or the internal sensations produced by a drug (Barrett, Caul, & Smith, 2005; Solinas, Panlilio, Justinova, Yasar, & Goldberg, 2006). Like self-administration, this model has high predictive validity, with a strong correlation between drugs that animals identify as sharing discriminative stimulus properties (i.e., producing similar internal sensations) and those that humans characterize as having similar subjective effects (Appel et al., 1991; Mori, Yoshizawa, Shibasaki, & Suzuki, 2012). The apparatus for this procedure usually is a standard two-lever operant chamber in which an animal is trained to press the levers in order to receive a food pellet (Figure 5). After the animal learns the lever press response, discrimination training begins.
Figure 5.

Standard operant chamber used for drug discrimination
During discrimination training, the animal is administered a training drug (e.g., delta-9-tetrahydrocannabinol [THC]) or its vehicle before being placed in the chamber. On days when the animal receives the training drug, lever presses on only one lever will result in food delivery, whereas on days when it receives vehicle, lever presses on only the other lever will result in food delivery (Leberer & Fowler, 1977; York & Winter, 1975). Over time, the animal learns that the interoceptive effects associated with the training drug serve as a signal that the drug-associated lever will be active, and the absence of those effects indicates that the other (vehicle-associated) lever will be active.
Once the animal is performing the discrimination accurately, researchers can substitute a candidate medication to evaluate the extent to which it shares discriminative stimulus effects with the training drug (i.e., whether it elicits responding primarily on the drug-associated lever). If the training drug is a drug of abuse, similar discriminative stimulus effects, or internal sensations, suggest that the candidate medication would produce similar subjective effects and might also be abused in humans (Holtzman, 1985). On the other hand, lever presses predominantly on the vehicle lever suggests that the drug does not share interoceptive effects with the training drug.
Failure to substitute for an abused training drug does not rule out abuse liability, nor does it suggest that the candidate medication would not have subjective effects. Drug discrimination is very specific to the pharmacological action of a drug (Glennon, 1991). In many cases, drugs that share discriminative stimulus effects produce these effects through similar actions in the brain. Hence, the candidate medication may still be a drug of abuse, but may have a different mechanism of action than the training drug, and therefore may not substitute for the training drug (Appel et al., 1991). For example, morphine does not substitute for THC in rats trained to discriminate THC from vehicle (Figure 6), even though both drugs are abused by humans. In addition, centrally active drugs that are not abused may serve as discriminative stimuli (e.g., rodents have been trained to discriminate the antipsychotic clozapine; Goudie, Smith, Taylor, Taylor, & Tricklebank, 1998; Porter, Prus, Vann, & Varvel, 2005).
Figure 6. Two groups of male Sprague-Dawley rats were trained to discriminate 3 mg/kg THC from vehicle.
For both panels, V stands for vehicle and T stands for the training dose (3 mg/kg THC). The data points above these labels show data from when animals were trained to discriminate THC from vehicle. In control tests with vehicle and 3 mg/kg THC (left side of top panel), rats in both groups responded almost exclusively on the appropriate lever. When other doses of THC were substituted for the 3 mg/kg training dose, dose-dependent substitution occurred, with rats responding most on the drug lever at doses at or above the training dose (top panel, unfilled black triangles and filled inverted black triangles).
In group 1, dose-dependent substitution also occurred upon substitution of JWH-018 (top panel, filled red squares), a synthetic cannabinoid that has been abused by humans in products marketed under names such as “Spice.” Lower doses of JWH-018 substituted for THC, suggesting that JWH-018 is more potent than THC. Consistent with the high predictive validity of this model, humans who have ingested these products report a marijuana-like intoxication.
In contrast, rats that were injected with morphine (group 2) did not respond on the THC-associated lever (top panel, filled blue circles), suggesting that morphine failed to substitute. As predicted by the model, morphine does not possess marijuana-like subjective effects in humans, although it does share subjective effects with other mu opioid agonists such as heroin.
Together, these results demonstrate the pharmacological selectivity and predictive validity of the drug discrimination procedure. Overall response rates calculated as responses per second, a measure of nonspecific, direct effects of the drugs (e.g., effects on motor behavior), are shown in the bottom panel.
For this reason, the selection of a training drug is crucial, and whenever possible, it is best to use a training drug from the same drug class as the candidate medication (Comer, France, & Woods, 1991). Typically, researchers determine an entire dose-effect curve for each drug examined, including the training drug (e.g., see results in Figure 6). Drug discrimination is a unique method for predicting abuse liability of candidate medications that focuses on subjective effects of the drug and provides insight into the mechanism of action (Meert, 1991).
Tolerance and Dependence
Drug abuse commonly implies repeated administration of the drug. To incorporate this aspect into abuse liability testing, researchers assess tolerance and dependence. Tolerance is defined as a decreased magnitude of effect following repeated dosing with the drug, resulting in the need for larger doses to produce the initial effects (Stewart & Badiani, 1993). Drug dependence may also occur after a period of chronic administration and may be physiological or psychological in nature, leading to physical withdrawal symptoms or drug craving, respectively (Ator & Griffiths, 2003; Dawe & Gray, 1995). Most abuse liability assessments focus on physiological withdrawal (Ator & Griffiths, 2003).
Researchers generally assess tolerance and dependence by administering the drug repeatedly, taking physiological and behavioral measurements before, during, and following cessation of drug administration. Often researchers administer drugs for 2 to 4 weeks and they may administer them multiple times per day, or continuously, depending upon the drug’s duration of action (Lukas & Griffiths, 1982; Weerts, Ator, Grech, & Griffiths, 1998). The measurements they choose for assessing tolerance depend upon the specific drug class, whereas they typically evaluate dependence/withdrawal through direct observation of the animal’s overt behavior following termination of drug administration or administration of an antagonist (known as precipitated withdrawal; Hughes et al., 1994).
Although describing the myriad of methods and measurements used to examine tolerance and dependence is beyond the scope of this paper (see Ator & Griffiths, 2003, and Maldono, 2002 for a review), two points are relevant here. First, because chronic administration of the candidate medication often occurs during the course of the overall toxicological evaluation of the drug, measuring neurobehavioral effects is prudent if the drug crosses the blood-brain barrier and may conceivably be abused. Second, evaluation of tolerance and dependence does not provide information about abuse potential per se, but rather serves as an adjunct that is useful in determining the consequences should the drug be abused (West & Gossop, 1994). For example, the withdrawal symptoms (labeled “discontinuation syndrome”) associated with abrupt termination of a period of chronic use of certain antidepressants (e.g., venlafaxine, paroxetine) might be conceived of as a form of dependence (Zajecka, Tracy, & Mitchell, 1997), yet antidepressants are not typically used recreationally and are not considered to be drugs of abuse. Hence, although evaluation of tolerance and dependence is most appropriately considered in the context of safety toxicology, its implications should be discussed in light of the candidate medication’s overall abuse liability profile as determined by 8-factor analysis.
Discussion and Conclusions
Four major procedures are most commonly used in the preclinical assessment of abuse liability: pharmacological equivalence, drug discrimination, self-administration, and physical dependence assessment (FDA, 2010). When used alone, each technique has disadvantages; together, however, they create a strong package with high predictive validity for compounds that are likely to be abused by humans. Designer drugs are a case in point. Although these chemicals were originally developed for biomedical research or as potential therapeutics (Chen, Ensor, Russell, & Bohner, 1959), published information on their synthesis was later diverted and used to manufacture compounds that are consumed for the purpose of inducing desired emotional states (e.g., euphoria; Carroll, Lewin, Mascarella, Seltzman, & Reddy, 2012; Wiley, Marusich, Huffman, Balster, & Thomas, 2011).
Familiar examples of designer drugs are phencyclidine (PCP), “China White” (fentanyl analog), and 3,4-methylenedioxy-N-methamphetamine (MDMA, or ecstasy). More recently, synthetic cathinones (e.g., methylenedioxypyrovalerone [MDPV]) have been marketed in products labeled as “bath salts” or “plant food” and synthetic cannabinoids (e.g., JWH-018) have been marketed in products labeled as “herbal incense” or “spice.” In the studies presented here, the abuse liability of MDPV is predicted by its self-administration in rats trained to self-administer i.v. cocaine, whereas the propensity of JWH-018 to produce marijuana-like intoxication is predicted by its THC-like effects in drug discrimination. In summary, tests within the abuse liability assessment battery are responsive to known drugs of abuse (e.g., methamphetamine, cocaine, THC) and to designer drugs in two different classes (MDPV and JWH-018).
Because abuse liability tends to be associated with increases in compulsive use of a drug, it may be financially beneficial for manufacturers of designer drugs to produce drugs with high abuse liability. In contrast, high potential for abuse is a liability for therapeutic drug development, and responsible pharmaceutical companies work to minimize it (e.g., through changes in formulation). In cases where medications under development are similar to known substances of abuse in structure, binding affinity, or other biochemical or behavioral properties, prediction of psychotropic effects is of particular importance, and scientists often perform preclinical evaluation of abuse liability, as described herein, before these novel drugs are widely marketed. Indeed, within the increasingly stringent regulatory climate governing pharmaceutical development, evidence of any central nervous system activity indicating stimulant, depressant, hallucinogenic, or mood-elevating properties has become a threshold criterion for assessment of abuse liability of a candidate medication (Schoedel & Sellers, 2008). Prediction of whether a drug is likely to be abused is critical for appropriate scheduling with the DEA, as well as for development of accompanying educational material aimed at the consumer and for organization of post-marketing surveillance strategies. The ultimate goal of this endeavor is to increase consumer safety by decreasing the probability of misuse and abuse of prescription drugs.
Acknowledgments
The self-administration and locomotor activity research reported herein was supported by RTI International internal research and development funds. The drug discrimination research was supported by National Institute on Drug Abuse (NIDA) grants DA-031988 and DA-03672 to Jenny Wiley. THC and JWH-018 were supplied by the NIDA Drug Supply Program. Synthesis of MDPV was supported by NIDA grant DA-12970 to Bruce Blough of RTI.
Contributor Information
Julie A. Marusich, Pharmacology group at RTI International.
Timothy W. Lefever, RTI’s Pharmacology group.
Scott P. Novak, RTI’s Behavioral Health Epidemiology program.
Bruce E. Blough, RTI’s Organic and Medicinal Chemistry group.
Jenny L. Wiley, RTI’s Pharmacology group.
References
- Appel JB, Baker LE, Barrett RL, Broadbent J, Michael EM, Riddle EE, Van Groll BJ. Use of drug discrimination in drug abuse research. NIDA Research Monograph. 1991;116:369–397. doi: 10.1037/e496182006-023. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug and Alcohol Dependence. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Balster RL, Chait LD. The effects of phencyclidine on amphetamine stereotypy in rats. European Journal of Pharmacology. 1978;48:445–450. doi: 10.1016/0014-2999(78)90173-5. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Caul WF, Smith R. Withdrawal, tolerance, and sensitization to dopamine mediated interoceptive cues in rats trained on a three-lever drug-discrimination task. Pharmacology, Biochemistry, and Behavior. 2005;81:1–8. doi: 10.1016/j.pbb.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Addiction and dose response: The psychomotor stimulant theory of addiction reveals that hormetic dose responses are dominant. Critical Reviews in Toxicology. 2008;387:599–617. doi: 10.1080/10408440802026315. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Annals of the New York Academy of Sciences. 2012;1248:18–38. doi: 10.1111/j.1749-6632.2011.06199.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Ensor CR, Russell D, Bohner B. The pharmacology of 1-(1-phenylcyclohexyl)piperidine-HCl. The Journal of Pharmacology and Experimental Therapeutics. 1959;127:241–250. [PubMed] [Google Scholar]
- Comer SD, France CP, Woods JH. Training dose: Influences in opioid drug discrimination. NIDA Research Monographs. 1991;116:145–161. doi: 10.1037/e496182006-010. [DOI] [PubMed] [Google Scholar]
- Darnell RJ, McCloskey TC, Commissaris RL. Convulsant versus typical barbiturates: effects on locomotor activity. Pharmacology, Biochemistry, and Behavior. 1986;24:727–731. doi: 10.1016/0091-3057(86)90581-2. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith TE, Smith SG. Intravenous and intragastric self-administration of chlordiazepoxide in the rat. Alcohol and Drug Research. 1987;7:511–516. [PubMed] [Google Scholar]
- Dawe S, Gray JA. Craving and drug reward: A comparison of methadone and clonidine in detoxifying opiate addicts. Drug and Alcohol Dependence. 1995;39:207–212. doi: 10.1016/0376-8716(95)01159-8. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). Draft guidance for industry: Assessment of abuse potential of drugs. 2010 Retrieved from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf.
- Fortuna RJ, Robbins BW, Caiola E, Joynt M, Halterman JS. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics. 2010;126:1108–1116. doi: 10.1542/peds.2010-0791. [DOI] [PubMed] [Google Scholar]
- French ED, Lopez M, Peper S, Kamenka JM, Roberts DC. A comparison of the reinforcing efficacy of PCP, the PCP derivatives TCP and BTCP, and cocaine using a progressive ratio schedule in the rat. Behavioural Pharmacology. 1995;6:223–228. [PubMed] [Google Scholar]
- French D, Witkin JM. Effects of the dopamine release inhibitor, CGS 10746B, on the locomotor stimulant and discriminative stimulus effects of cocaine and methamphetamine. Pharmacology, Biochemistry, and Behavior. 1993;46:989–993. doi: 10.1016/0091-3057(93)90233-j. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Introduction. NIDA Research Monograph. 1991;116:1–3. [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD. Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: Tests with subtype selective receptor ligands. Behavioural Pharmacology. 1998;9:699–710. doi: 10.1097/00008877-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. Self-injection of barbiturates, benzodiazepines, and other sedative anxiolytics in baboons. Psychopharmacology. 1991;103:154–161. doi: 10.1007/BF02244196. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, Egawa T, Kitamura Y, Uchida N, Nishimura R, Egashira N, Iwasaki K, Fujiwara M. Cannabidiol potentiates pharmacological effects of delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Research. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Drug discrimination studies. Drug and Alcohol Dependence. 1985;14:263–282. doi: 10.1016/0376-8716(85)90061-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: Similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bulletin on Narcotics. 1978;30:43–54. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMarus LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. The Journal of Pharmacology and Experimental Therapeutics. 1988;245:485–492. [PubMed] [Google Scholar]
- Leberer MR, Fowler SC. Drug discrimination and generalization in pigeons. Pharmacology, Biochemistry, and Behavior. 1977;7:483–486. doi: 10.1016/0091-3057(77)90219-2. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR. Precipitated withdrawal by a benzodiazepine receptor antagonist (Ro 15-1788) after 7 days of diazepam. Science. 1982;217:1161–1163. doi: 10.1126/science.6287579. [DOI] [PubMed] [Google Scholar]
- Maldono R. Study of cannabinoid dependence in animals. Pharmacology & Therapeutics. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Helm S, II, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–ES38. [PubMed] [Google Scholar]
- Meert TF. Application of drug discrimination with drugs of abuse to develop new therapeutic agents. NIDA Research Monograph. 1991;116:307–323. doi: 10.1037/e496182006-019. [DOI] [PubMed] [Google Scholar]
- Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: a public health concern. American Journal of Public Health. 2011;101:1429–1433. doi: 10.2105/AJPH.2010.300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Dupree JP, Bibbey AD, Sizemore GM. Assessing locomotor-stimulating effects of cocaine in rodents. In: Kobeissy FH, editor. Psychiatric disorders: Methods and protocols. Methods in Molecular Biology. Vol. 829. New York: Humana Press; 2012. pp. 321–327. [DOI] [PubMed] [Google Scholar]
- Mori T, Yoshizawa K, Shibasaki M, Suzuki T. Discriminative stimulus effects of hallucinogenic drugs: a possible relation to reinforcing and aversive effects. Journal of Pharmacological Sciences. 2012;120:70–76. doi: 10.1254/jphs.12r08cp. [DOI] [PubMed] [Google Scholar]
- Numan R. Multiple exposures to ethanol facilitate intravenous self-administration of ethanol by rats. Pharmacology, Biochemistry, and Behavior. 1981;15:101–108. doi: 10.1016/0091-3057(81)90346-4. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neuroscience and Biobehavioral Reviews. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JH, Prus AJ, Vann RE, Varvel SA. Discriminative stimulus properties of the atypical antipsychotic clozapine and the typical antipsychotic chlorpromazine in a three-choice drug discrimination procedure in rats. Psychopharmacology. 2005;178:67–77. doi: 10.1007/s00213-004-1985-5. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr, Joksimović S, Van Linn M, Cook JM. The differential role of alpha1-and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. The International Journal of Neuropsychopharmacology. 2009;12:1179–1193. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Sellers EM. Assessing abuse liability during drug development: Changing standards and expectations. Clinical Pharmacology and Therapeutics. 2008;83:622–626. doi: 10.1038/sj.clpt.6100492. [DOI] [PubMed] [Google Scholar]
- Sinden JD, Le Magnen J. Parameters of low-dose ethanol intravenous self-administration in the rat. Pharmacology, Biochemistry, and Behavior. 1982;16:181–183. doi: 10.1016/0091-3057(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nature Protocols. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behavioural Pharmacology. 1993;4:289–312. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. The Journal of Pharmacology and Experimental Therapeutics. 1978;204(3):547–557. [PubMed] [Google Scholar]
- Vinkers CH, Klanker M, Groenink L, Korte SM, Cook JM, Van Linn ML, Hopkins SC, Olivier B. Dissociating anxiolytic and sedative effects of GABAAergic drugs using temperature and locomotor responses to acute stress. Psychopharmacology. 2009;204:299–311. doi: 10.1007/s00213-009-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. Drug poisoning deaths in the United States, 1980–2008. Hyattsville, MD: Centers for Disease Control and Prevention; 2011. National Center for Health Statistics, Data Brief 81. [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weeks JR, Collins RJ. Screening for drug reinforcement using intravenous self-administration in the rat. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 35–43. [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. The Journal of Pharmacology and Experimental Therapeutics. 1998;285:41–53. [PubMed] [Google Scholar]
- West R, Gossop M. Overview: A comparison of withdrawal symptoms from different drug classes. Addiction. 1994;89:1483–1489. doi: 10.1111/j.1360-0443.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF. Hijacking of basic research: The case of synthetic cannabinoids. Research Triangle Park, NC: RTI Press; 2011. RTI Press publication No. OP-0007-1111. Retrieved from http://www.rti.org/rtipress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- York JL, Winter JC. Long-term effects of barbital on spontaneous activity of rats trained to use the drug as a discriminative stimulus. Psychopharmacologia. 1975;42:47–50. doi: 10.1007/BF00428824. [DOI] [PubMed] [Google Scholar]
- Zajecka J, Tracy KA, Mitchell S. Discontinuation symptoms after treatment with serotonin reuptake inhibitors: A literature review. The Journal of Clinical Psychiatry. 1997;58:291–297. doi: 10.4088/jcp.v58n0702. [DOI] [PubMed] [Google Scholar]