Abstract
BACKGROUND
Recent studies have suggested differing toxicity patterns for patients with prostate cancer who receive treatment with 3-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), or proton beam therapy (PBT).
METHODS
The authors reviewed patient-reported outcomes data collected prospectively using validated instruments that assessed bowel and urinary quality of life (QOL) for patients with localized prostate cancer who received 3DCRT (n = 123), IMRT (n = 153) or PBT (n = 95). Clinically meaningful differences in mean QOL scores were defined as those exceeding half the standard deviation of the baseline mean value. Changes from baseline were compared within groups at the first post-treatment follow-up (2–3 months from the start of treatment) and at 12 months and 24 months.
RESULTS
At the first post-treatment follow-up, patients who received 3DCRT and IMRT, but not those who received PBT, reported a clinically meaningful decrement in bowel QOL. At 12 months and 24 months, all 3 cohorts reported clinically meaningful decrements in bowel QOL. Patients who received IMRT reported clinically meaningful decrements in the domains of urinary irritation/obstruction and incontinence at the first post-treatment follow-up. At 12 months, patients who received PBT, but not those who received IMRT or 3DCRT, reported a clinically meaningful decrement in the urinary irritation/ obstruction domain. At 24 months, none of the 3 cohorts reported clinically meaningful changes in urinary QOL.
CONCLUSIONS
Patients who received 3DCRT, IMRT, or PBT reported distinct patterns of treatment-related QOL. Although the timing of toxicity varied between the cohorts, patients reported similar modest QOL decrements in the bowel domain and minimal QOL decrements in the urinary domains at 24 months. Prospective randomized trials are needed to further examine these differences.
Keywords: 3-dimensional conformal radiotherapy, intensity-modulated radiotherapy, patient reported outcomes, prostate cancer, proton therapy, quality of life
INTRODUCTION
Three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), and proton beam therapy (PBT) are means of delivering high-dose radiation for localized prostate cancer with acceptable rates of acute and late toxicities.1,2 IMRT and PBT are technically advanced forms of conformal radiotherapy that may achieve radiation dose escalation to the prostate while minimizing toxicity to surrounding normal tissues, particularly the bladder and rectum. IMRT is now the predominant form of radiotherapy delivered for the treatment of prostate cancer in the United States.3–5 National attention has focused on IMRT and PBT because of increased costs and limited available evidence to demonstrate reduced toxicity.6–9
Recent reports using linked tumor registry and administrative claims data have suggested that PBT is associated with greater bowel toxicity than IMRT.7,9 Those authors appropriately echoed calls by the Institute of Medicine, National Cancer Institute, Centers for Medicare and Medicaid Services, and Agency for Healthcare Research and Quality for a randomized trial to compare the 2 modalities.6,10–12 The Massachusetts General Hospital and the University of Pennsylvania, in collaboration with the National Cancer Institute and other institutions, have recently opened such a trial. To further inform the current national debate, we report patient-reported quality-of-life (QOL) data for 3 contemporary cohorts that received treatment with 3DCRT, IMRT, or PBT mono-therapy, which have informed the design of this randomized trial.
MATERIALS AND METHODS
Patients and Treatments
We collected and examined the most current patient-reported QOL data from 3 prospective cohort studies involving patients with localized prostate cancer who received radiation therapy without androgen-suppression therapy. The PBT cohort comprised 95 men who received treatment at Massachusetts General Hospital between August 2004 and December 2008 and were surveyed at baseline and 3 months, 12 months, and 24 months from the start of treatment. The IMRT cohort comprised 153 men who received treatment at 9 university-affiliated hospitals comprising the Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment (PROST-QA) Consortium between March 2003 and March 2006 and were surveyed at baseline and 2 months, 6 months, 12 months, and 24 months after the start of treatment. The 3DCRT cohort comprised 123 men who received treatment at Harvard-affiliated hospitals between June 1994 and August 2000 and were surveyed at baseline and 3 months, 12 months, and 24 months after the start of treatment. Each study was reviewed and approved by the institutional review board for the participating site, and all participating patients provided informed written consent.
Measurement of Patient-Reported Quality of Life
Domain-specific QOL was assessed with the Prostate Cancer Symptom Indices (PCSI) scale for the PBT and 3DCRT cohorts and with the Expanded Prostate Cancer Index Composite (EPIC) instrument for the IMRT cohort. The PCSI and EPIC are similar instruments that measure prostate cancer treatment-related QOL.13,14 Both instruments contain domains that measure bowel/ rectal, urinary irritation/obstruction, and urinary incontinence QOL, which were included in the current analysis.
The primary endpoints of this study were the mean change in QOL scores from pretreatment to post-treatment in the acute (eg, 2–3 months after treatment initiation) and late (eg, 12–24 months post-treatment) time periods. Change scores within cohorts were calculated only for patients who reported data at baseline and at the specified time point for a given domain. The PCSI scale was inverted such that both instruments produced scores from 0 to 100, with lower scores indicating worse function.15–17
Statistical Analysis
Cohort characteristics were compared using the Fisher exact test or the Kruskal-Wallis test. Mean score changes from baseline within treatment cohorts were analyzed using a t test for paired data. To adjust for multiple pairwise comparisons, a 2-sided P value < .006 (eg, .05 for 9 comparisons) was considered significant, so that the overall Type 1 error was 0.05 for each QOL domain. For statistically significant mean score changes from baseline, clinically meaningful change was defined as a mean change score exceeding half the standard deviation of the baseline value.18 All calculations were performed using SAS 9.2 (SAS, Cary, NC).
RESULTS
Patient characteristics are listed in Table 1. Patients in the PBT cohort were younger than those in the IMRT or 3DCRT cohorts. A greater proportion of patients in the IMRT cohort were black. The 3DCRT cohort had higher baseline prostate-specific antigen values and included more patients with clinical T2 and T3 disease than the PBT or IMRT cohorts. Radiotherapy dose ranges were from 66.4 to 79.2 Gy for the 3DCRT cohort, from 75.6 to 79.2 Gy for the IMRT cohort, and from 74.0 to 82.0 Gy (relative biologic effectiveness) for the PBT cohort. Radiotherapy was delivered according to each center’s preferred practice at 1.8 to 2.0 Gy per day. Planning target volume margins were not explicitly mandated but were typically 10 mm for the 3DCRT cohort and 5 to 10 mm (with 5-mm to 7-mm rectal margins) for the IMRT cohort (personal communication with treating physicians at the participating institutions). Patients in the PBT cohort received treatment with 5-mm margins.15
TABLE 1.
Characteristics of Patients who Received 3-Dimensional Conformal Radiotherapy, Intensity-Modulated Radiotherapy, and Proton Beam Therapy
| Characteristic | No. of Patients (%)
|
Pa | ||
|---|---|---|---|---|
| PBT | IMRT | 3DCRT | ||
| Total no. of patients | 95 | 153 | 123 | |
| Enrollment period | 2004–2008 | 2003–2006 | 1994–2000 | |
| Age: Median [Range] y | 64 [49–78] | 69 [47–83] | 70 [54–82] | < .001 |
| Race | < .001 | |||
| White | 87 (93) | 121 (79) | 116 (94) | |
| Black | 6 (6) | 27 (18) | 2 (2) | |
| Other | 1 (1) | 2 (1) | 1 (1) | |
| Missing | 1 (1) | 3 (2) | 4 (3) | |
| Marriage status | .20 | |||
| Married or cohabiting | 72 (77) | 124 (81) | 105 (85) | |
| Other | 22 (23) | 29 (19) | 15 (12) | |
| Missing | 1 (1) | 0 | 3 (2) | |
| Education level | .14 | |||
| College graduate | 60 (64) | 77 (50) | 67 (54) | |
| Other | 34 (36) | 76 (50) | 53 (43) | |
| Missing | 1 (1) | 0 | 3 (2) | |
| Clinical tumor classification | 5.2 [2.3–15] | 5.8 [0.5–25.8] | 7.5 [0.9–77.4] | < .001 |
| T1 | 75 (80) | 123 (80) | 49 (40) | |
| T2 | 19 (20) | 30 (20) | 63 (51) | |
| T3 | 0 | 0 | 7 (6) | |
| Missing | 1 (1) | 0 | 4 (3) | |
| Gleason score | .53 | |||
| 4–6 | 63 (67) | 97 (63) | 66 (54) | |
| 7 | 30 (32) | 56 (37) | 38 (31) | |
| 8–10 | 1 (1) | 0 | 15 (12) | |
| Missing | 1 (1) | 0 | 4 (3) | |
Abbreviations: 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; PBT, proton beam therapy; PSA, prostate-specific antigen.
P values were calculated for comparisons across all 3 cohorts.
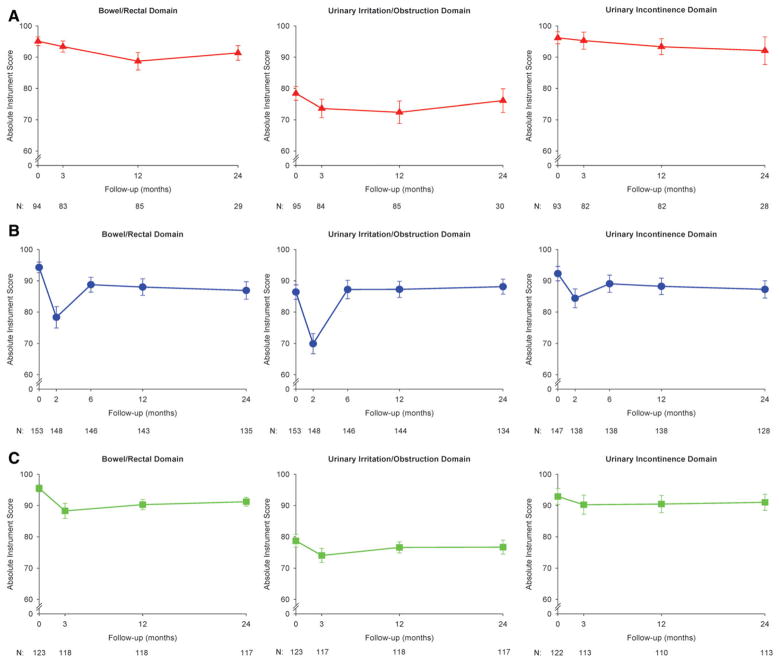
In the immediate post-treatment period (2 months from the start of treatment for the IMRT cohort and 3 months for the 3DCRT and PBT cohorts), patients in the IMRT and 3DCRT cohorts, but not in the PBT cohort, reported a clinically meaningful decrement in bowel/rectal QOL (Fig. 1, Table 2). At 12 months and 24 months, patients from all 3 cohorts reported clinically meaningful decrements in bowel QOL.
Figure 1.
Longitudinal patient-reported mean quality-of-life scores are illustrated for patients with prostate cancer who received (A) proton beam therapy (PBT), (B) intensity-modulated radiotherapy (IMRT), or (C) 3-dimensional conformal radiotherapy (3DCRT) in the bowel/rectal, urinary irritation/obstruction, and urinary incontinence domains. Error bars represent 95% confidence intervals. N indicates the number of patients reporting data at the given time point.
TABLE 2.
Comparison of Patient-Reported Quality of Life Scores Across the 3 Assessed Domains at Baseline, Immediately (2–3 Months) Post-Treatment, and 12 and 24 Months Post-Treatment
| Domain/ Cohort | Baseline
|
Immediately Post-Treatment
|
12 Months Post-Treatment
|
24 Months Post-Treatment
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean Score | SD | No. | Mean Score Change From Baseline | SD | Pa | Mean Change >0.5 SD | No. | Mean Score Change From Baseline | SD | Pa | Mean Change >0.5 SD | No. | Mean Score Change From Baseline | SD | Pa | Mean Change >0.5 SD | |
| Bowel/rectal | ||||||||||||||||||
| PBT | 94 | 95.1 | 6.7 | 83 | −1.7 | 8.3 | .062 | − | 85 | −6.4 | 13.2 | < .001 | + | 29 | −3.7 | 6.4 | .004 | + |
| IMRT | 153 | 94.3 | 10.9 | 148 | −16.0 | 21.4 | < .001 | + | 143 | −6.3 | 16.2 | < .001 | + | 135 | −7.4 | 16.6 | < .001 | + |
| 3DCRT | 123 | 95.5 | 6.2 | 118 | −7.2 | 13.4 | < .001 | + | 118 | −5.2 | 8.9 | < .001 | + | 117 | −4.3 | 7.8 | < .001 | + |
| Urinary irritation/obstruction | ||||||||||||||||||
| PBT | 95 | 78.4 | 11.0 | 84 | −4.8 | 13.8 | .002 | − | 85 | −6.0 | 16.9 | .002 | + | 30 | −2.3 | 10.5 | .241 | − |
| IMRT | 153 | 86.4 | 14.4 | 148 | −16.5 | 19.9 | < .001 | + | 144 | 0.9 | 15.8 | .513 | − | 134 | 1.7 | 14.2 | .164 | − |
| 3DCRT | 123 | 78.7 | 11.6 | 117 | −4.7 | 12.3 | < .001 | − | 118 | −2.1 | 9.8 | .021 | − | 117 | −2.0 | 12.4 | .080 | − |
| Urinary incontinence | ||||||||||||||||||
| PBT | 93 | 96.2 | 9.2 | 82 | −0.9 | 12.6 | .516 | − | 82 | −2.9 | 11.8 | .032 | − | 28 | −4.1 | 12.0 | .080 | − |
| IMRT | 147 | 92.3 | 13.9 | 138 | −7.9 | 18.0 | < .001 | + | 138 | −4.1 | 15.6 | .003 | − | 128 | −5.1 | 16.0 | .001 | − |
| 3DCRT | 122 | 92.9 | 13.7 | 113 | −2.6 | 16.7 | .097 | − | 110 | −2.4 | 14.7 | .089 | − | 113 | −1.9 | 14.1 | .161 | − |
Abbreviations: 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; PBT, proton beam therapy; PSA, prostate-specific antigen; SD, standard deviation.
P values refer to comparisons of baseline mean scores with the indicated time points.
In the immediate post-treatment period, patients in the IMRT cohort reported clinically meaningful decrements in QOL in the urinary irritation/obstruction and urinary incontinence domains that were not observed in the other 2 cohorts. At 12 months, only patients in the PBT cohort reported clinically meaningful score decrements in the urinary irritation/obstruction domain. At 24 months, clinically meaningful changes in urinary QOL were not observed in any of the cohorts.
DISCUSSION
We undertook this study to present the best available evidence examining prospective patient-reported outcomes before and after treatment with 3DCRT, IMRT, or PBT for localized prostate cancer. We observed that, in the acute setting after radiotherapy, 3DCRT and IMRT were associated with modest but clinically meaningful reductions in bowel and/or urinary QOL. This same pattern was not observed in patients who received PBT. At 24 months, patients who received all 3 radiation modalities reported modest but clinically meaningful changes in bowel QOL.
These data are consistent with and extend previously published patient-reported and physician-reported toxicity and QOL studies after external-beam radiotherapy for prostate cancer.15–17,19–24 Although it has been suggested that IMRT reduces bowel toxicity compared to 3DCRT, recent cohort studies comparing these 2 modalities demonstrated no clear differences in patient-reported outcomes.3,25,26
Late bowel toxicity has been correlated with the volume of rectum receiving specific doses of radiotherapy; however, acute bowel toxicity after radiotherapy is less studied.27,28 We observed that PBT was not associated with a clinically meaningful decrement in bowel QOL in the acute period after radiation. Assuming equal prescription doses and target margins, PBT reduces low-dose radiation exposure to the whole rectum and delivers high doses (similar to 3DCRT and IMRT) along the small strip of the anterior rectal wall immediately posterior to the prostate.29,30 IMRT in particular is associated with a low-dose radiation bath over a larger pelvic area; and its effects may be an important driver of acute bowel toxicity. This hypothesis is supported by data indicating that intermediate-dose rectal exposure or mean rectal dose is a better predictor of acute bowel toxicity than the absolute dose delivered to the prostate (and, thus, received by the nearby anterior rectal wall).31–33 Acute bowel toxicity also may predict for higher late toxicity, such as rectal bleeding.34 Furthermore, data also suggest that rectal volumes receiving intermediate doses of radiotherapy are the best predictors of late toxicity.35–37 Other researchers, however, have published data to suggest that the volume of rectum exposed to the highest doses of radiation (ie, ≥70 Gy) is the strongest predictor of late toxicity and that such toxicity in fact may be independent of the prescription dose.38–40 Given this lack of consensus, further prospective studies are needed to address this issue.
In the acute setting, IMRT also was associated with modest but clinically meaningful changes in the urinary irritation/obstruction and incontinence domains while similar decrements were not seen in the PBT cohort. A similar early but time-limited benefit for PBT was also noted in a recent comparison of Medicare claims data.41 One possible contributor is that IMRT may produce small radiation ‘‘hot spots,’’ which exceed the prescribed radiation dose by up to 15% within the prostate and/or prostatic urethra (which are not typically observed with 3DCRT or PBT).29 Although patients in the 3DCRT cohort did not report significant urinary QOL decrements, lower radiation doses may have mitigated symptoms in this group. Patients in the PBT cohort reported clinically meaningful urinary toxicity at 12 months, a pattern not observed in the other 2 cohorts. Differential treatment dose also may have contributed to this finding because many patients in the PBT cohort received doses of 82 gray equivalent (GyE), which has been shown to increase urinary toxicity compared with doses of 78 to 79 Gy.2 However, we observed no clinically meaningful score decrements in urinary QOL in any of our 3 cohorts at 24 months, suggesting that, although these symptoms may vary in their onset, typically, they are transient.
Our findings differ from those in 2 recent reports that examined bowel and urinary complications after radiotherapy using the linked Surveillance, Epidemiology, and End Results-Medicare database.7,9 Both studies identified radiotherapy complications based on billing claims for diagnoses and procedures and observed increased bowel toxicity in patients who received PBT compared with IMRT or 3DCRT. Medicare codes are a coarse measure of treatment complications, insensitive to patient symptoms, and subject to both confounding and misclassification bias.3,42 Furthermore, those studies did not collect or report information on radiation dose, target margins, or dose-volume histogram characteristics, which may have varied significantly between groups. In addition, Kim et al reported significantly lower gastrointestinal toxicity among patients who received treatment during the last 2 years of their analyzed PBT cohort, suggesting that a learning curve for this advanced technology may be present. Nonetheless, those studies presented important and complementary views of these modalities, highlighting the need for a randomized trial.7
Our study has several limitations. Although we present updated, prospectively collected data for each cohort, the nonrandomized design and significant differences between the baseline characteristics of the cohorts preclude a direct statistical comparison of QOL outcomes between groups. In addition, data for patients were collected using 2 different validated instruments. Although these instruments measure outcomes in a similar way across the analyzed domains, subtle differences in question wording may affect patient responses.17,23,43 To partially overcome this limitation, we defined a clinically meaningful score change as a score that exceeded half the standard deviation of the baseline value. This approach provides a more standardized measure across QOL instruments.18
The patients in our study were treated according to the standard practice at each center, including the selection of dose, target margins, and normal tissue constraints, as noted above. Given the similar relative biologic effectiveness of protons and photons, it is likely that any differences in toxicity between the techniques relate to differences in dose-volume factors, although underlying radiobiologic differences cannot be ruled out. However, because different target margins and doses were used in the 3 cohorts, it is difficult to disentangle the relative contribution of these factors from the potential benefits of a given treatment modality.
Our data also are limited by a 1-month difference in survey delivery between the cohorts measured by the PCSI and EPIC instruments. This difference may introduce bias into the interpretation of toxicity in the acute setting, because patients may report greater toxicity closer to treatment. Although this may be the case, at least 1 study has suggested that many of the effects on the bowel associated with pelvic radiotherapy are present with similar intensity 2 months after treatment relative to 2 weeks.44 Furthermore, because the patients in our study who received 3DCRT reported significant decrements in their bowel QOL score at 3 months (despite lower treatment doses), both 2 months and 3 months after the start of therapy appear to be reasonable times to assess such toxicity. Finally, substantially fewer patients in our PBT cohort returned their 24-month questionnaire relative to the other cohorts, which may have introduced sampling bias.
Patient experience of treatment-associated morbidity is complex and likely is influenced by numerous factors, including radiation dose, target margins, dose-volume histogram characteristics, data-collection methods, and perhaps treatment modality. Given the limitations in the available evidence and the potential promise but expense of PBT, a carefully designed randomized controlled trial that accounts for all of these issues presents an important opportunity to examine the comparative effectiveness of PBT before its widespread adoption and diffusion. Although, to our knowledge, there are no randomized data to suggest that 3DCRT is an inferior modality, currently, it is rarely used in the United States. Therefore, the recently launched trial will randomize men with localized prostate cancer to receive either PBT or IMRT and will follow them longitudinally to assess subsequent patient-reported bowel, urinary, and erectile function. Health state utilities and economic endpoints also will be measured. We have observed that nearly 60% of eligible patients state they are likely to enroll in such a trial.45 The results of this trial will provide patients, clinicians, payers, and policy makers with the most valid comparison of modern radiation-based technologies for the treatment of localized prostate cancer.
Acknowledgments
FUNDING SOURCES
This work was supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center, by National Institutes of Health (NIH) grants R01CA95662, 1RC1CA146596, and K07CA163616, and by internal funding from the University of Pennsylvania.
We thank the Prostate Cancer Outcomes and Satisfaction with Treatment Quality Assessment (PROST-QA) Consortium study group. The PROST-QA Consortium includes contributions in cohort design, patient accrual, and follow-up from the following investigators: Meredith Regan (Dana-Farber Cancer Institute, Boston, MA); Larry Hembroff (Michigan State University, East Lansing, MI); John T. Wei, Dan Hamstra, Rodney Dunn, Laurel Northouse, and David Wood (University of Michigan, Ann Arbor, MI); Eric A Klein and Jay Ciezki (Cleveland Clinic, Cleveland, OH); Jeff Michalski and Gerald Andriole (Washington University, St. Louis, MO); Mark Litwin and Chris Saigal (University of California-Los Angeles Medical Center, Los Angeles, CA); Thomas Greenfield, PhD (Berkeley, CA); Louis Pisters and Deborah Kuban (The University of Texas M. D. Anderson Cancer Center, Houston, TX); Howard Sandler (Cedars Sinai Medical Center, Los Angeles, CA); Jim Hu and Adam Kibel (Brigham and Women’s Hospital, Boston, MA); Douglas Dahl and Anthony Zietman (Massachusetts General Hospital, Boston, MA); and Irving Kaplan and Martin G. Sanda (Beth Israel Deaconess Medical Center, Boston, MA).
We acknowledge PROST-QA Data Coordinating Center Project Management by Jill Hardy, MS (Michigan State University, East Lansing, MI), Erin Najuch and Jonathan Chipman (Dana-Farber Cancer Institute, Boston, MA), and Catrina Crociani, MPH (Beth Israel Deaconess Medical Center, Boston, MA); grant administration by Beth Doiron, BA (Beth Israel Deaconess Medical Center, Boston, MA); and technical support from coordinators at each clinical site. We also acknowledge the help of Mark S. Cary, PhD and Kathleen Propert, ScD (University of Pennsylvania) as well as Meredith M. Regan, ScD (Dana-Farber Cancer Institute) for preliminary analyses in the initial stages of this project.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95–09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coen JJ, Bae K, Zietman AL, et al. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology phase II study 03–12. Int J Radiat Oncol Biol Phys. 2011;81:1005–1009. doi: 10.1016/j.ijrobp.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with non-metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinan MA, Robinson TJ, Zagar TM, et al. Changes in initial treatment for prostate cancer among Medicare beneficiaries, 1999–2007. Int J Radiat Oncol Biol Phys. 2012;82:e781–e786. doi: 10.1016/j.ijrobp.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efstathiou JA, Trofimov AV, Zietman AL. Life, liberty, and the pursuit of protons: an evidence-based review of the role of particle therapy in the treatment of prostate cancer. Cancer J. 2009;15:312–318. doi: 10.1097/PPO.0b013e3181b14ec0. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 9.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodge M, Pijls-Johannesma M, Stirk L, Munro AJ, De Ruysscher D, Jefferson T. A systematic literature review of the clinical and cost-effectiveness of hadron therapy in cancer. Radiother Oncol. 2007;83:110–122. doi: 10.1016/j.radonc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Moya del Pina B. [Accessed November 1, 2012];Proton therapy for cancer: a new technology brief. Available at: http://www.cancer.gov/aboutnci/ncicancerbulletin/archive/2009/090809/page8.
- 12.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 13.Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care. 2001;39:1118–1130. doi: 10.1097/00005650-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 15.Coen JJ, Paly JJ, Niemierko A, et al. Long-term quality of life outcome after proton beam monotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:e201–e209. doi: 10.1016/j.ijrobp.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 17.Talcott JA, Manola J, Clark JA, et al. Time course and predictors of symptoms after primary prostate cancer therapy. J Clin Oncol. 2003;21:3979–3986. doi: 10.1200/JCO.2003.01.199. [DOI] [PubMed] [Google Scholar]
- 18.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 19.Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer M, Suarez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe BS, Nichols RC, Henderson RH, et al. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer. 2012;118:4619–4626. doi: 10.1002/cncr.27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pederson AW, Fricano J, Correa D, Pelizzari CA, Liauw SL. Late toxicity after intensity-modulated radiation therapy for localized prostate cancer: an exploration of dose-volume histogram parameters to limit genitourinary and gastrointestinal toxicity. Int J Radiat Oncol Biol Phys. 2012;82:235–241. doi: 10.1016/j.ijrobp.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Pinkawa M, Piroth MD, Fischedick K, et al. Self-assessed bowel toxicity after external beam radiotherapy for prostate cancer—predictive factors on irritative symptoms, incontinence and rectal bleeding [serial online] Radiat Oncol. 2009;4:36. doi: 10.1186/1748-717X-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler HM, Liu PY, Dunn RL, Khan DC, Tropper SE, Sanda MG, et al. Reduction in patient-reported acute morbidity in prostate cancer patients treated with 81-Gy intensity-modulated radio-therapy using reduced planning target volume margins and electromagnetic tracking: assessing the impact of margin reduction study. Urology. 2010;75:1004–1008. doi: 10.1016/j.urology.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalski JM, Yan Y, Watkins-Bruner D, et al. Preliminary analysis of 3D-CRT vs IMRT on the high dose arm of the RTOG 0126 Prostate Cancer Trial: toxicity report. Int J Radiat Oncol Biol Phys. 2011;81:S1–S2. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkins Bruner D, Hunt D, et al. Preliminary analysis of 3D-CRT vs IMRT on the High Dose Arm of the RTOG 0126 Prostate Cancer Trial: patient reported outcomes. J Radiat Oncol Biol Phys. 2011;81:S44–S44. [Google Scholar]
- 27.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz M, Pierelli A, Fiorino C, et al. Helical tomotherapy and intensity modulated proton therapy in the treatment of early stage prostate cancer: a treatment planning comparison. Radiother Oncol. 2011;98:74–80. doi: 10.1016/j.radonc.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Dias RS, Giordani AJ, Souhami L, Segreto RA, Segreto HR. Rectal planning risk volume correlation with acute and late toxicity in 3-dimensional conformal radiation therapy for prostate cancer. Technol Cancer Res Treat. 2011;10:585–590. doi: 10.1177/153303461101000608. [DOI] [PubMed] [Google Scholar]
- 32.Valdagni R, Rancati T, Fiorino C, et al. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D-CRT. Int J Radiat Oncol Biol Phys. 2008;71:1065–1073. doi: 10.1016/j.ijrobp.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Vavassori V, Fiorino C, Rancati T, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007;67:1401–1410. doi: 10.1016/j.ijrobp.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Rancati T, Fiorino C, Fellin G, et al. Inclusion of clinical risk factors into NTCP modelling of late rectal toxicity after high dose radiotherapy for prostate cancer. Radiother Oncol. 2011;100:124–130. doi: 10.1016/j.radonc.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Skwarchuk MW, Jackson A, Zelefsky MJ, et al. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): multivariate analysis and dose-response. Int J Radiat Oncol Biol Phys. 2000;47:103–113. doi: 10.1016/s0360-3016(99)00560-x. [DOI] [PubMed] [Google Scholar]
- 36.Karlsdottir A, Muren LP, Wentzel-Larsen T, Dahl O. Late gastrointestinal morbidity after 3-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70:1478–1486. doi: 10.1016/j.ijrobp.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 37.Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685–698. doi: 10.1016/s0360-3016(00)01414-0. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen PL, Chen RC, Hoffman KE, et al. Rectal dose-volume histogram parameters are associated with long-term patient-reported gastrointestinal quality of life after conventional and high-dose radiation for prostate cancer: a subgroup analysis of a randomized trial. Int J Radiat Oncol Biol Phys. 2010;78:1081–1085. doi: 10.1016/j.ijrobp.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Tucker SL, Dong L, Michalski JM, et al. Do intermediate radiation doses contribute to late rectal toxicity? An analysis of data from Radiation Therapy Oncology Group protocol 94-06. Int J Radiat Oncol Biol Phys. 2012;84:390–395. doi: 10.1016/j.ijrobp.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorino C, Fellin G, Rancati T, Vavassori V, Bianchi C, Borca VC, et al. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys. 2008;70:1130–1137. doi: 10.1016/j.ijrobp.2007.07.2354. [DOI] [PubMed] [Google Scholar]
- 41.Yu JB, Soulos PR, Herrin J, Cramer LD, Potosky AL, Roberts KB, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 suppl):IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 43.Muanza TM, Albert PS, Smith S, et al. Comparing measures of acute bowel toxicity in patients with prostate cancer treated with external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1316–1321. doi: 10.1016/j.ijrobp.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 44.Hovdenak N, Karlsdottir A, Sorbye H, Dahl O. Profiles and time course of acute radiation toxicity symptoms during conformal radio-therapy for cancer of the prostate. Acta Oncol. 2003;42:741–748. doi: 10.1080/02841860310011302. [DOI] [PubMed] [Google Scholar]
- 45.Shah A, Efstathiou JA, Paly JJ, et al. Prospective preference assessment of patients’ willingness to participate in a randomized controlled trial of intensity-modulated radiotherapy versus proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:e19–e19. doi: 10.1016/j.ijrobp.2011.11.072. [DOI] [PubMed] [Google Scholar]