Abstract
Background
Current guidelines recommend mammography every 1 or 2 years starting at age 40 or 50 years, regardless of individual risk for breast cancer.
Objective
To estimate the cost-effectiveness of mammography by age, breast density, history of breast biopsy, family history of breast cancer, and screening interval.
Design
Markov microsimulation model.
Data Sources
Surveillance, Epidemiology, and End Results program, Breast Cancer Surveillance Consortium, and the medical literature.
Target Population
U.S. women aged 40 to 49, 50 to 59, 60 to 69, and 70 to 79 years with initial mammography at age 40 years and breast density of Breast Imaging Reporting and Data System (BI-RADS) categories 1 to 4.
Time Horizon
Lifetime.
Perspective
National health payer.
Intervention
Mammography annually, biennially, or every 3 to 4 years or no mammography.
Outcome Measures
Costs per quality-adjusted life-year (QALY) gained and number of women screened over 10 years to prevent 1 death from breast cancer.
Results of Base-Case Analysis
Biennial mammography cost less than $100 000 per QALY gained for women aged 40 to 79 years with BI-RADS category 3 or 4 breast density or aged 50 to 69 years with category 2 density; women aged 60 to 79 years with category 1 density and either a family history of breast cancer or a previous breast biopsy; and all women aged 40 to 79 years with both a family history of breast cancer and a previous breast biopsy, regardless of breast density. Biennial mammography cost less than $50 000 per QALY gained for women aged 40 to 49 years with category 3 or 4 breast density and either a previous breast biopsy or a family history of breast cancer. Annual mammography was not cost-effective for any group, regardless of age or breast density.
Results of Sensitivity Analysis
Mammography is expensive if the disutility of false-positive mammography results and the costs of detecting nonprogressive and nonlethal invasive cancer are considered.
Limitation
Results are not applicable to carriers of BRCA1 or BRCA2 mutations.
Conclusion
Mammography screening should be personalized on the basis of a woman’s age, breast density, history of breast biopsy, family history of breast cancer, and beliefs about the potential benefit and harms of screening.
Primary Funding Source
Eli Lilly, Da Costa Family Foundation for Research in Breast Cancer Prevention of the California Pacific Medical Center, and Breast Cancer Surveillance Consortium.
Using screening mammography to detect early-stage invasive breast cancer reduces breast cancer mortality by 15% to 25% (1– 6) and is cost-effective for women at average risk for breast cancer (7–13). However, the frequency with which women should receive mammography is controversial. Some guidelines recommend mammography every 1 to 2 years for all women aged 40 years or older (14, 15). The U.S. Preventive Services Task Force (USPSTF) recently issued guidelines recommending that mammography be done biennially for women aged 50 to 74 years, but not routinely for women younger than 50 years (16).
These guidelines do not consider the influence of common risk factors for breast cancer other than age. Breast cancer risk is strongly associated with breast density (17– 19), with low breast density (Breast Imaging Reporting and Data System [BI-RADS] category 1) associated with less-than-average risk and high breast density (categories 3 and 4) with higher-than-average risk (20). Family history of breast cancer and a previous breast biopsy are also risk factors for breast cancer (20). The health benefits and cost utility of screening mammography may be strongly influenced by a woman’s risk for breast cancer, which can be estimated from her age, breast density on an initial mammogram (20, 21), history of breast biopsy, and family history of breast cancer (20). Our objective was to examine the health benefits and cost utility of mammography performed every 3 to 4 years, biennially, or annually in women with different profiles of breast cancer risk.
Methods
Perspective and Threshold
Our analysis was based on data from women in the United States and assumed the perspective of a national health payer. Two cost-effectiveness thresholds were considered: $100 000 or less and $50 000 or less per quality-adjusted life-year (QALY) gained.
Model Structure
We constructed a Markov cost–utility model to compare the lifetime costs and health benefits of having mammography annually, biennially, or every 3 to 4 years or not having mammography. Each strategy included 6 health states: healthy (no breast cancer); ductal carcinoma in situ (DCIS); localized, regional, or distant invasive breast cancer; and death. All women started in the healthy state and could stay healthy, die, or transition to DCIS or one of the invasive breast cancer states. Those with DCIS could transition to an invasive breast cancer state or die of causes other than breast cancer. Those with invasive breast cancer could die of breast cancer or other causes. No transitions from localized to regional or distant or from regional to distant breast cancer were included; stage distribution at the time of diagnosis would capture the effects of these transitions up to the point of diagnosis, and our data tracked mortality and costs over years since diagnosis according to stage at the time of diagnosis.
Breast Cancer Incidence Rates
We estimated the incidence rates of invasive breast cancer and DCIS by age (Supplement, available at www.annals.org) by using 1975 to 2005 data from the Surveillance, Epidemiology, and End Results (SEER) database (22). Data from Tice and colleagues’ study (20) were used to adjust these rates for breast density (Figure 1), history of breast cancer in a first-degree relative, and history of breast biopsy (Supplement).
Figure 1. Markov model of possible state transitions.
The dotted-and-dashed lines indicate transitions from the healthy state; the dashed lines indicate transitions from the DCIS state; and the solid lines indicate transitions from the invasive breast cancer states. DCIS = ductal carcinoma in situ.
Ductal carcinoma in situ is usually discovered by screening mammography (23). The incidence of DCIS was assumed to be 4-fold higher among women who had mammography than among those who did not (23, 24). We also assumed that women with DCIS who had no mammography would have a 3.4-fold greater risk for subsequent invasive breast cancer than healthy women, and those with DCIS who had mammography would have a 1.9-fold greater risk (25, 26).
Stages of Incident Invasive Breast Cancer
We assumed that invasive breast cancer is more likely to be diagnosed at an advanced stage in women who have no or less frequent screening mammography. The proportions of women in each stage of invasive breast cancer who have screening mammography were estimated by using data (number of years between the date invasive breast cancer was detected and the most recent previous mammogram) from the Breast Cancer Surveillance Consortium (BCSC) from 1996 through 2006 (27). Women who last had mammography 0.5 to 1.5 years (6000 women), 1.5 to 2.5 years (2846 women), or 2.5 to 5.5 years (1433 women mean, 3.5 years) before the date on which their cancer was detected were assigned to annual mammography group, biennial mammography group, and mammography every 3 to 4 years group, respectively (Table 1). The stage distributions at each mammography frequency thus included both women whose invasive breast cancer was detected by mammography (true-positive results) and those whose breast cancer was diagnosed by other means (false-negative results). The proportions of women with local, regional, and distant breast cancer did not significantly differ between the annual and biennial mammography groups (Supplement).
Table 1.
Base-Case Values for Each Breast Cancer Stage
| Characteristic | Breast Cancer Stage |
|||
|---|---|---|---|---|
| Ductal Carcinoma In Situ | Local Invasive | Regional Invasive | Distant Invasive | |
| Breast cancer cost, $* | ||||
| First year | 8893 | 11 710 | 22 139 | 34 192 |
| After first year | 777 | 554 | 3209 | 10 061 |
| Last year of life† | 31 694 | 37 516 | 52 620 | |
| Proportion of healthy-state QALYs lost | ||||
| First year | 0.096 | 0.154 | 0.247 | 0.247 |
| After first year | 0.000 | 0.020 | 0.095 | 0.168 |
| Proportions in each stage‡ | ||||
| Aged 40–49 y | ||||
| No mammography$ | – | 0.515 | 0.431 | 0.079 |
| Mammography every 3–4 years$ | – | 0.643 | 0.327 | 0.030 |
| Low breast density | – | 0.676 | 0.296 | 0.028 |
| High breast density | – | 0.617 | 0.353 | 0.031 |
| Mammography every 2 years | ||||
| Low breast density | – | 0.713 | 0.269 | 0.018 |
| High breast density | – | 0.657 | 0.323 | 0.020 |
| Aged 50–59 y | ||||
| No mammography$ | – | 0.483 | 0.443 | 0.074 |
| Mammography every 3–4 years$ | – | 0.660 | 0.323 | 0.017 |
| Low breast density¶ | – | 0.703 | 0.276 | 0.021 |
| High breast density** | – | 0.647 | 0.330 | 0.023 |
| Mammography every 2 years | ||||
| Low breast density¶ | – | 0.737 | 0.249 | 0.014 |
| High breast density** | – | 0.685 | 0.299 | 0.015 |
| Aged 60–69 y | ||||
| No mammography$ | – | 0.496 | 0.407 | 0.097 |
| Mammography every 3–4 years$ | – | 0.695 | 0.273 | 0.032 |
| Low breast density¶ | – | 0.740 | 0.234 | 0.026 |
| High breast density** | – | 0.689 | 0.283 | 0.028 |
| Mammography every 2 years | ||||
| Low breast density¶ | – | 0.771 | 0.213 | 0.016 |
| High breast density** | – | 0.724 | 0.258 | 0.018 |
| Aged 70–79 y | ||||
| No mammography$ | – | 0.533 | 0.378 | 0.080 |
| Mammography every 3–4 years$ | – | 0.764 | 0.219 | 0.017 |
| Low breast density¶ | – | 0.775 | 0.209 | 0.015 |
| High breast density** | – | 0.731 | 0.252 | 0.017 |
| Mammography every 2 years | ||||
| Low breast density¶ | – | 0.803 | 0.187 | 0.010 |
| High breast density** | – | 0.762 | 0.227 | 0.011 |
BI-RADS = Breast Imaging Reporting and Data System; QALY = quality-adjusted life-year.
In 2008 U.S. dollars.
Applied only to women who are dying of breast cancer and not other causes.
Stage distributions for annual mammography (not shown) did not significantly differ from those for biennial mammography. Distributions by age and screening frequency were calculated from Breast Cancer Surveillance Consortium data (Supplement, available at www.annals.org).
Stage distributions stratified by age only.
Proportion does not fall between the estimates for high and low breast density because of a very slight estimation error when the stage distributions stratified by both age and breast density were estimated from generalized ordinal logit regressions. Sensitivity analyses showed that this error did not significantly influence the costs per QALY gained (data not shown).
BI-RADS category 1 or 2 breast density.
BI-RADS category 3 or 4 breast density.
The BCSC data include only women who have had mammography. We used SEER program data from 1975 through 1979 (28), when screening mammography was infrequently used, to estimate the proportions of women not receiving mammography who had localized, regional, and distant breast cancer at the time of diagnosis, stratified by age alone (29). These proportions were not stratified by breast density because those data are not available for these women (Table 1).
Proportion of Mammography That Yields False-Positive Results
The proportions of false-positive mammography results were estimated by using data from the BCSC (20, 30) for each subgroup defined by age and breast density (range, 3.1% [age 70 to 79 years, BI-RADS category 1] to 9.9% [age 40 to 49 years, category 3 or 4]) (Supplement).
Mortality Due to Breast Cancer or Other Causes
Breast cancer mortality by age at diagnosis, stage at diagnosis, and years since diagnosis was calculated from the SEER data (28). We assumed no excess breast cancer mortality more than 20 years beyond the year of diagnosis. Overall mortality by age was calculated by using vital statistics data for 2003 for all U.S. women (31).
Direct Medical Costs of Mammography and Breast Cancer
We calculated the annual cost of film mammography by dividing $108, the median Medicare reimbursement in 2008 (32), by the interval between mammography screenings. The direct costs of DCIS and invasive breast cancer (Table 1) were calculated by using data from Taplin and colleagues (33) and Yabroff and colleagues (34), respectively, as well as Medicare reimbursement rates, and updated to 2008 U.S. dollars by using the Consumer Price Index for medical services (35) for the initial year after diagnosis, the final year of life, and the years in between according to stage at diagnosis. False-positive mammography results were assumed to generate additional procedures costing $396 in 2008 U.S. dollars (36).
Loss of Quality of Life Due to Breast Cancer
Quality-of-life values for the healthy state were estimated from the general female population of Sweden, according to age (37). We calculated the loss in quality of life during the first and subsequent years after diagnosis of DCIS or invasive local, regional, or distant breast cancer by using EuroQol-5D values for Swedish women with breast cancer at different stages (Table 1) (38).
Base Case and Secondary Analyses
We assumed no family history of breast cancer and no previous breast biopsy for the base-case analysis and a family history of breast cancer or previous breast biopsy for the secondary analyses. Our analyses compared all 3 mammography frequencies and no mammography in models with breast cancer stage distributions stratified by age only and also compared the 3 mammography frequencies in models with distributions stratified by age and breast density.
Sensitivity Analyses
We performed univariate sensitivity analyses that compared screening mammography every 3 to 4 years with no mammography for women aged 40 and 49 years who had BI-RADS category 3 breast density, and we varied DCIS incidence, breast cancer incidence, mortality, costs, and disutility over wide ranges. Additional univariate sensitivity analyses varied the cost of film screening mammography from $78 to $138 and assumed a smaller or larger stage shift from advanced to local disease (proportion of local breast cancer ± 0.05 compared with the base case) for mammography every 3 to 4 years. Sensitivity analyses were done that assumed a 1-time QALY loss of 0.013 years (4.7 days) after a false-positive mammography result (39) and an overdetection rate (percentage of detected cases of invasive breast cancer that are nonprogressive lesions that pose no threat to the life or health of the person) of 10% (40– 42). The Supplement provides details of these distributions.
Probabilistic sensitivity analyses were used to estimate the degree of uncertainty of the estimates of cost-effectiveness. We ran simulations that allowed all of the assumptions to vary at random across reasonable ranges (Table 2) except for the cost of mammography, which was fixed at the base-case value of $108.
Table 2.
Univariate Sensitivity Analyses for Mammography Every 3 to 4 Years Versus No Mammography*
| Variable | Value Range | Cost per QALY Gained $ |
|
|---|---|---|---|
| Low Value | High Value | ||
| Breast cancer costs | 50% to 150% of base-case values | 74 765 | 65 856 |
| Incidence of invasive breast cancer | 70% to 130% of base-case values | 101 478 | 50 534 |
| Invasive breast cancer mortality | 70% to 130% of base-case values | 86 857 | 58 467 |
| Incidence of ductal carcinoma in situ | 50% to 150% of base-case values | 43 840 | 93 571 |
| Mammography cost | $78 to $138 | 55 774 | 91 807 |
| Disutility of invasive breast cancer | 50% to 150% of base-case values | 77 792 | 56 150 |
| Proportion of false-positive mammography results | 1% to 10% | 54 015 | 98 566 |
| Disutility of false-positive mammography results | 0 to 0.013 QALY | 72 184 | 118 798 |
| Overdetection of invasive breast cancer | 0% to 10% | 72 184 | 108 432 |
| Stage proportion change† | Local, 0.078 to 0.178; regional, −0.054 to −0.154 | 104 720 | 54 048 |
QALY = quality-adjusted life-year.
In patients aged 50–59 y with Breast Imaging Reporting and Data System category 1 breast density.
Proportions of local and regional breast cancer were changed by 0.05 in opposite directions, resulting in lower or higher stage shifts.
Calculations
The base-case, secondary, and univariate sensitivity models were run as Monte Carlo simulations with 1 000 000 trials each. Both costs and health benefits were discounted at an annual rate of 3%. The costs per QALY gained for each strategy represented the incremental lifetime costs divided by the incremental lifetime QALYs, compared with the next less expensive alternative. Probabilistic sensitivity analyses were run with 500 simulations and 50 000 trials per simulation. Simulations that cost $100 000 or less per QALY were deemed to be cost-effective.
For each scenario, the number of women who would need to be screened over 10 years to prevent 1 death from breast cancer by using a more frequent screening strategy was calculated as the inverse of the difference in deaths from breast cancer between the less frequent and more frequent screening strategies.
Validation of the Model
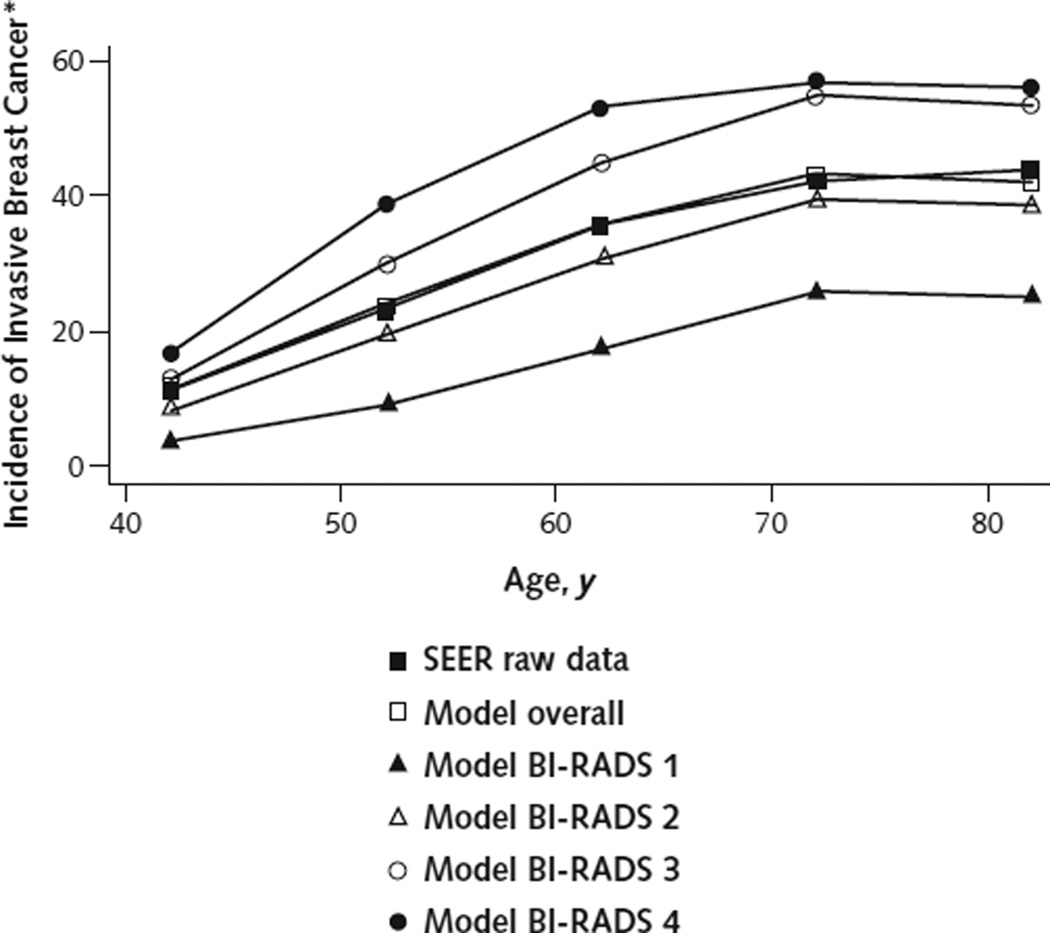
Age-adjusted breast cancer incidence in the BCSC data set and our model-predicted incidence of breast cancer both match that of the SEER database very closely (Figure 2). In addition, our model-predicted cumulative incidence ratios for invasive breast cancer in women with BI-RADS category 1, 3, or 4 breast density, compared with those with category 2 breast density, were all within 2% of the estimates published by Tice and colleagues (Supplement).
Figure 2. Incidence of invasive breast cancer as a function of age and breast density in U.S. women.
BI-RADS = Breast Imaging Reporting and Data System; SEER = Surveillance, Epidemiology, and End Results.
* Per 10 000 women per year.
Our model predicted a cumulative lifetime incidence of 12.35% and mortality rate of 2.99% for invasive breast cancer, starting at age 40 years. These values are close to the estimated lifetime incidence and mortality for invasive breast cancer estimated by SEER (11.92% and 2.89%, respectively) for cancer-free women aged 40 years (22). Our model estimated that biennial mammography for women aged 40 to 69 years and 40 to 79 years reduced breast cancer mortality by 15% and 23%, respectively, compared with no mammography. A systematic review by the USPSTF (6) estimated that biennial mammography reduced breast cancer mortality by 16% and 25%, respectively, for women in the same age ranges.
Role of the Funding Source
Our study was funded by an unrestricted grant from Eli Lilly and by the Da Costa Family Foundation for Research in Breast Cancer Prevention of the California Pacific Medical Center. Data collection for this work was supported by grants from the BCSC. The collection of cancer incidence data used in this study was supported by several state public health departments and cancer registries throughout the United States. The funding sources had no role in the design, data collection or analysis, or interpretation of the study or in the decision to submit the manuscript for publication.
Results
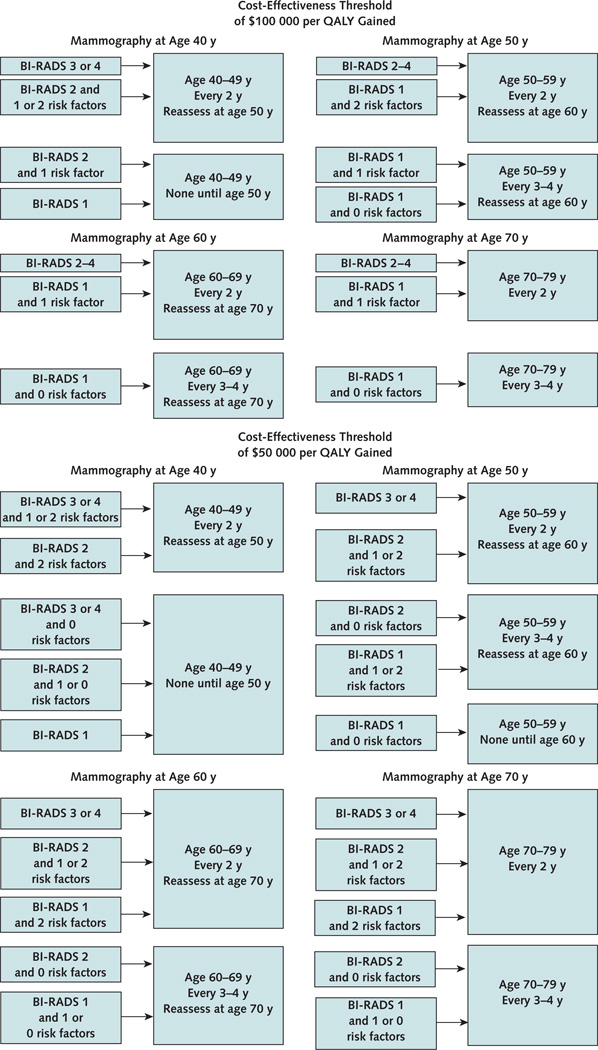
The most cost-effective frequency of mammography depended on a woman’s age, breast density, family history of breast cancer, and history of breast biopsy (Table 3 and 4). Assuming a cost-effectiveness threshold of $100 000 per QALY gained, biennial mammography is cost-effective for women aged 40 to 49 years who have BI-RADS category 3 or 4 breast density or both a previous breast biopsy and a family history of breast cancer (Figure 3). Biennial mammography is also cost-effective for women aged 50 to 59 years who have category 2, 3, or 4 breast density and for those with category 1 breast density and both a previous breast biopsy and a family history of breast cancer; for all other women in this age range with category 1 breast density, mammography every 3 to 4 years is cost-effective. Biennial mammography is cost-effective for all women aged 60 to 69 years except those with category 1 breast density and no additional risk factors; for that small subset, mammography every 3 to 4 years is cost-effective. Among women aged 70 to 79 years, biennial mammography is cost-effective for those with category 3 or 4 breast density and those with either a previous breast biopsy or a family history of breast cancer. For women with category 1 or 2 breast density and no additional risk factors, mammography every 3 to 4 years is cost-effective. The costs per QALY gained for annual compared with biennial mammography were more than $340 000 for all ages and categories of breast density (data not shown).
Table 3.
Outcomes of Mammography Every 2 Years, Mammography Every 3 to 4 Years, and No Mammography in Women With No Previous Breast Biospy or Family History of Breast Cancer
| Age and BI-RADS Category |
Patients % |
10-Year Incidence of Invasive Breast Cancer% |
10-Year Incidence of False-Positive Results%* |
Mammography Frequency Comparison |
Number Needed to Screen† |
Cost per QALY Gained$ |
|---|---|---|---|---|---|---|
| 40–49 y | ||||||
| 1 | 4.4 | 0.43 | 17.2 | 3–4 y vs. none | 8475 | 228 427 |
| 2 y vs. 3–4 y | 27 778 | 362 699 | ||||
| 2 | 35.3 | 0.89 | 33.3 | 3–4 y vs. none | 4870 | 120 113 |
| 2 y vs. 3–4 y | 12 195 | 140 048 | ||||
| 3 | 46.8 | 1.38 | 38.9 | 3–4 y vs. none | 4386 | 90 646 |
| 2 y vs. 3–4 y | 7813 | 87 769† | ||||
| 4 | 13.5 | 1.79 | 38.8 | 3–4 y vs. none | 2703 | 83 899 |
| 2 y vs. none | 6579 | 74 482† | ||||
| 50–59 y | ||||||
| 1 | 7.8 | 0.85 | 15.9 | 3–4 y vs. none | 3077 | 72 184 |
| 2 y vs. 3–4 y | 11 364 | 208 748 | ||||
| 2 | 46.6 | 1.79 | 30.0 | 3–4 y vs. none | 1582 | 36 212 |
| 2 y vs. 3–4 y | 7576 | 89 189 | ||||
| 3 | 39.2 | 2.78 | 36.8 | 3–4 y vs. none | 1196 | 22 878 |
| 2 y vs. 3–4 y | 4202 | 46 629 | ||||
| 4 | 6.4 | 3.63 | 35.9 | 3–4 y vs. none | 1010 | 17 131 |
| 2 y vs. 3–4 y | 2564 | 23 962 | ||||
| 60–69 y | ||||||
| 1 | 9.4 | 1.47 | 14.2 | 3–4 y vs. none | 1109 | 30 976 |
| 2 y vs. 3–4 y | 5556 | 129 117 | ||||
| 2 | 49.5 | 2.58 | 26.5 | 3–4 y vs. none | 646 | 16 724 |
| 2 y vs. 3–4 y | 4425 | 63 707 | ||||
| 3 | 35.9 | 3.80 | 33.0 | 3–4 y vs. none | 482 | 12 163 |
| 2 y vs. 3–4 y | 2732 | 30 948 | ||||
| 4 | 5.2 | 4.42 | 33.6 | 3–4 y vs. none | 396 | 8385 |
| 2 y vs. 3–4 y | 2041 | 21 425 | ||||
| 70–79 y | ||||||
| 1 | 11.9 | 1.94 | 12.4 | 3–4 y vs. none | 704 | 18 223 |
| 2 y vs. 3–4 y | 7143 | 150 568 | ||||
| 2 | 53.9 | 2.95 | 23.0 | 3–4 y vs. none 2 y vs. 3–4 y | 491 4065 | 13 574 96 004 |
| 3 | 30.8 | 4.08 | 24.7 | 3–4 y vs. none | 339 | 5214 |
| 2 y vs. 3–4 y | 2959 | 50 982 | ||||
| 4 | 3.4 | 4.13 | 22.7 | 3–4 y vs. none 2 y vs. 3–4 y |
337 2841 |
5400 40 540 |
BI-RADS = Breast Imaging Reporting and Data System; QALY = quality-adjusted life-year.
Estimates shown reflect empirical data from the Breast Cancer Surveillance Consortium for mammography every 2 y. Cumulative incidence of false-positive mammography results are assumed to be the same regardless of whether mammography is performed every 3 to 4 y or every 2 y.
Number of women needed to screen over 10 y to prevent 1 death from breast cancer.
Cost per QALY gained is for mammography every 2 y vs. no mammography because mammography every 2 y is weakly dominant over mammography every 3 to 4 y.
Table 4.
Effects of Previous Breast Biopsy or Family History of Breast Cancer on Health Benefits and Cost-Effectiveness of Mammography Every 2 Years Compared With Every 3 to 4 Years
| Age and Mammography Frequency Comparison |
BI-RADS Category |
10-Year Incidence of False-Positive Results%* | Added Risk Factors† |
10-Year Incidence of Breast Cancer% |
Number Needed to Screen† |
Cost per QAL Gained$ |
|---|---|---|---|---|---|---|
| 40–49 y | ||||||
| 3–4 y vs. none | 1 | 17.2 | Family history | 0.64 | 8197 | 136 601 |
| Previous biopsy | 0.72 | 9259 | 140 588 | |||
| Both | 1.11 | 6757 | 105 264 | |||
| 2 | 33.4 | Family history | 1.38 | 6250 | 96 505 | |
| Previous biopsy | 1.48 | 6535 | 91 496 | |||
| Both | 2.22 | 4717 | 68 761 | |||
| 2 y vs. 3–4 y | 2 | 33.4 | Family history | 1.39 | 6667 | 79 793 |
| Previous biopsy | 1.46 | 6173 | 61 343 | |||
| Both | 2.25 | 4348 | 37 865 | |||
| 3 | 38.9 | Family history | 2.12 | 4132 | 38 319 | |
| Previous biopsy | 2.27 | 4237 | 38 946 | |||
| Both | 3.54 | 2994 | 18 748 | |||
| 4 | 38.8 | Family history | 2.76 | 3311 | 23 779 | |
| Previous biopsy | 2.92 | 3401 | 27 757 | |||
| Both | 4.54 | 1961 | 9114 | |||
| 50–59 y | ||||||
| 3–4 y vs. none | 1 | 15.9 | Family history | 1.36 | 2398 | 41 884 |
| Previous biopsy | 1.45 | 2083 | 40 612 | |||
| Both | 2.21 | 1727 | 25 060 | |||
| 2 y vs. 3–4 y | 1 | 15.9 | Family history | 1.35 | 8197 | 121 244 |
| Previous biopsy | 1.43 | 8333 | 104 974 | |||
| Both | 2.24 | 5495 | 57 956 | |||
| 2 | 30.0 | Family history | 2.82 | 4348 | 41 881 | |
| Previous biopsy | 2.93 | 3571 | 38 920 | |||
| Both | 4.55 | 2703 | 18 672 | |||
| 60–69 y | ||||||
| 2 y vs. 3–4 y | 1 | 14.2 | Family history | 2.34 | 4274 | 66 217 |
| Previous biopsy | 2.47 | 3968 | 60 985 | |||
| Both | 3.74 | 3185 | 40 068 | |||
| 2 | 26.5 | Family history | 4.01 | 2618 | 31 189 | |
| Previous biopsy | 4.23 | 2427 | 28 903 | |||
| Both | 6.55 | 1563 | 10 884 | |||
| 70–79 y | ||||||
| 2 y vs. 3–4 y | 1 | 12.4 | Family history | 2.97 | 3937 | 84 079 |
| Previous biopsy | 3.21 | 4348 | 78 684 | |||
| Both | 4.89 | 2747 | 39 896 | |||
| 2 | 23.0 | Family history | 4.51 | 2994 | 47 508 | |
| Previous biopsy | 4.79 | 2463 | 40 630 | |||
| Both | 7.37 | 1634 | 21 365 | |||
BI-RADS = Breast Imaging Reporting and Data System; QALY = quality-adjusted life-year.
Estimates shown reflect empirical data from the Breast Cancer Surveillance Consortium for mammography every 2 y. Cumulative incidence of false-positive mammography results is assumed to be the same regardless of whether mammography is performed every 3 to 4 y or every 2 y.
Family history of breast cancer in a first-degree relative (12.5% of women in the Breast Cancer Surveillance Consortium database) or previous breast biopsy (20.3%).
Number of women needed to screen over 10 y to prevent 1 death from breast cancer.
Figure 3. Cost-effective mammography screening strategies for women aged 40 to 79 years, by age and breast density.
Strategies assume a willingness-to-pay threshold of $100 000 (top) or $50 000 (bottom) per QALY gained. BI-RADS = Breast Imaging Reporting and Data System; QALY = quality-adjusted life-year.
Assuming a cost-effectiveness threshold of $50 000 per QALY gained (Figure 3), biennial mammography was cost-effective for women aged 40 to 49 years with BI-RADS category 3 or 4 breast density and either a previous breast biopsy or a family history of breast cancer, aged 50 to 79 years with category 3 or 4 breast density, or aged 50 to 79 years with category 2 breast density and either a previous breast biopsy or a family history of breast cancer. Mammography every 3 to 4 years was cost-effective for women aged 50 to 79 years with category 2 breast density and additional risk factors, aged 50 to 59 years with category 1 breast density and both a previous breast biopsy and a family history of breast cancer, or aged 60 to 79 years with either a previous breast biopsy or a family history of breast cancer.
A similar pattern was found when we considered only the benefits of mammography and not its costs. As age or breast density increased, many fewer women needed to be screened to prevent 1 death from breast cancer (Table 3). For example, when mammography was performed every 3 to 4 years for 10 years, preventing 1 death from breast cancer required screening 337 women aged 70 to 79 years with BI-RADS category 4 breast density, compared with 4870 women aged 40 to 49 years with category 2 breast density (Table 3). When mammography was performed biennially instead of every 3 to 4 years for 10 years, preventing 1 breast cancer death required screening 2041 women aged 60 to 69 years with category 4 breast density, compared with 12 195 women aged 40 to 49 with category 2 breast density (Table 3). Of note, mammography detected far more false-positive lesions than true cases of invasive breast cancer.
Assuming a 1-time disutility of 0.013 QALY for a false-positive mammography result and an overdetection rate of 10% for invasive breast cancer significantly increased the costs per QALY gained for mammography (Table 2). Our results were also sensitive to the magnitude of excess DCIS detection with mammography compared with no mammography, the shift from advanced to local disease with more frequent mammography, breast cancer incidence, and the assumed proportion of false-positive mammograms (Table 2). Among women aged 40 to 49 years with no additional risk factors for breast cancer, probabilistic sensitivity analyses showed that the probability of mammography every 3 to 4 years being cost-effective compared with no mammography was less than 1% and 5.4%, respectively, for those with BI-RADS category 1 or 2 breast density. The Supplement presents additional probabilistic sensitivity analyses.
Discussion
Our analyses suggest that recommendations about the frequency of mammography should be personalized on the basis of a woman’s age, breast density, history of breast biopsy, and family history of breast cancer, as well as the effect of mammography on her quality of life. This differs from mammography guidelines that recommend mammography every 1 or 2 years starting at age 40 or 50 years regardless of other risk factors (14, 15, 43). To our knowledge, ours is the first cost-effectiveness study of mammography to consider the effects of breast density, family history of breast cancer, and previous breast biopsy on the cost-effectiveness of mammography and to directly compare the cost-effectiveness of different frequencies of mammography with each other.
The USPSTF concluded that the benefits of mammography outweigh the harms of biennial screening for women aged 50 to 74 years; our results for screening women aged 50 to 79 years who have BI-RADS category 2, 3, or 4 breast density (>90% of all women in this age range) are consistent with this guideline. In contrast, our analysis found that mammography every 3 to 4 years is cost-effective for women aged 50 to 79 years who have BI-RADS category 1 breast density, no previous breast biopsy, and no family history of breast cancer, but that biennial mammography may not be. Our results indicate that annual mammography is not cost-effective, which matches the conclusion of the USPSTF.
The USPSTF recommended basing the intensity of screening mammography for women aged 40 to 49 years on factors that may be unique to each person. Our analyses suggest that women should have initial screening mammography at age 40 years. Assuming a cost-effectiveness threshold of $100 000 per QALY gained, biennial mammography is cost-effective for women aged 40 to 49 years who have BI-RADS category 3 or 4 breast density or both a family history of breast cancer and a previous breast biopsy. At a threshold of $50 000 per QALY gained, mammography can be offered to women aged 40 to 49 years with BI-RADS category 3 or 4 breast density and either a previous breast biopsy or family history of breast cancer.
For women aged 40 to 49 years who have BI-RADS category 1 or 2 breast density and no other risk factors, mammography may reasonably be resumed at age 50 years, with the frequency of subsequent screening determined in part by the woman’s breast density (Figure 3, top). Assuming a cost-effectiveness threshold of $50 000 per QALY gained, less frequent mammography may be appropriate, especially for women with category 1 or 2 breast density (Figure 3, bottom). If breast density or other risk factors change with increasing age, the strategy can be altered accordingly. We believe that BI-RADS category should be included in mammography reports to assist primary care providers in recommending the best screening strategy to their patients.
Our analyses have limitations. First, our results are sensitive to the rates of DCIS detection and overdetection of invasive breast cancer with mammography, and they do not apply to women who carry the BRCA1 or BRCA2 mutation, for whom more frequent mammography and screening with magnetic resonance imaging may be indicated. Second, we could not determine the cost-effectiveness of mammography every 3 years compared with intervals of 4 years or longer, because relatively fewer women in the BCSC had mammography less often than every 2 years. Third, we used qualitative BI-RADS classifications, which have modest interrater reproducibility (44, 45), to assess breast density. However, our data came from the large BCSC, which includes hundreds of radiologists, so our results probably represent current practice for assessing BI-RADS breast density categories.
Fourth, we could estimate stage distributions by age but not breast density in the absence of mammography results. Stratifying the stage distributions by both age and breast density resulted in a lower estimated cost per QALY gained for women with BI-RADS category 3 or 4 breast density and a higher cost for women with category 1 or 2 breast density, compared with age alone, for mammography every 2 versus every 3 to 4 years. If this pattern is also true of mammography every 3 to 4 years compared with no mammography, we may have underestimated the costs per QALY gained for mammography every 3 to 4 years versus no mammography for those with category 1 or 2 breast density and overestimated the costs for those with category 3 or 4 breast density. However, the costs per QALY gained in scenarios in which mammography every 3 to 4 years seems to be the preferred strategy are so far below $100 000 that accounting for these biases would be unlikely to alter our conclusions. Fifth, early detection with screening mammography and improved treatment have decreased breast cancer mortality rates (3). If mortality reductions are greater for local than for more advanced breast cancer, then costs per QALY gained for mammography may be mildly overestimated, whereas if the mortality reductions are greater for advanced than for local breast cancer, the costs per QALY gained may be mildly underestimated.
Finally, our results are based on the use of film rather than digital mammography. Digital mammography is more cost-effective than film mammography for women with BI-RADS category 3 or 4 breast density and for women younger than 50 years, but not for women aged 50 years or older with category 1 or 2 breast density (36). Thus, we believe our results are applicable to women older than 50 years and those younger than 50 years with category 3 or 4 breast density. More data are needed regarding the accuracy of digital compared with film mammography in women aged 40 to 49 years with category 1 or 2 breast density.
These uncertainties underscore the need for better methods of stratifying breast cancer risk and additional data to support their cost-effectiveness. Until such methods are available, we believe that considering breast density, previous biopsy, and family history of breast cancer when deciding on a mammography screening strategy is appropriate, on the basis of our analyses.
We took the perspective of a national payer for health services. From this perspective, our cost-effectiveness analyses might inform person who are developing guidelines on screening mammography. In contrast, from the perspective of an individual woman, the decision about how frequently to have screening mammography may emphasize trade-offs among the potential benefits (indicated by the number of women who need to be screened to prevent 1 death), potential anxiety from false-positive results, and relief from worry that a normal result sometimes affords. The emotional effect of mammography is difficult to quantify or weigh in cost-effectiveness analyses. Therefore, the decision about the best frequency of mammography will depend on understanding and weighing its benefits, costs, and limitations. Our analysis contributes to these decisions by showing that the potential benefits depend on a profile of risk factors that women and physicians should consider.
We conclude that the frequency of screening mammography should be personalized on the basis of a woman’s breast density, age, family history of breast cancer, and history of breast biopsy. Women may choose to have mammography at age 40 years, and those with average or low breast density and no other breast cancer risk factors may choose to repeat screening at age 50 years (including reassessment of breast density) and start periodic screening at that point. In contrast to current guidelines, women aged 50 to 79 years who have low breast density and no other breast cancer risk factors (and who therefore are at lower risk for breast cancer) may consider having mammography less frequently than every 2 years. Biennial mammography is cost-effective for women aged 40 to 49 years who have relatively high breast density or additional risk factors for breast cancer. Other factors, such as the potential emotional effect of mammography, may also be considered when deciding when to start and how often to have screening mammography.
Supplementary Material
Context
The optimal timing and frequency of screening mammography are controversial.
Contribution
This analysis found that the cost-effectiveness of screening mammography depended on a woman’s age, breast density, family history, and history of breast biopsy. Mammography every 2 years was cost-effective for women aged 40 to 49 years with relatively high breast density or additional risk factors for breast cancer. Mammography every 3 to 4 years was cost-effective for women aged 50 to 79 years with low breast density and no other risk factors.
Implication
Decisions about when and how often to have screening mammography could be personalized on the basis of risk factors.
—The Editors
Acknowledgment
The authors thank the BCSC investigators, participating mammography facilities, and radiologists for the data they provided for this study.
Grant Support: By an unrestricted grant from Eli Lilly and by the Da Costa Family Foundation for Research in Breast Cancer Prevention of the California Pacific Medical Center. Data collection for this work was supported by grants U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, and U01CA70040 from the National Cancer Institute BCSC. The collection of cancer incidence data used in this study was supported by several state public health departments and cancer registries throughout the United States; a full description of these sources can be found at http://breastscreening.cancer.gov/work/acknowledgement.html.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M10-2871.
Reproducible Research Statement: Study protocol, statistical code, and data set: Procedures for requesting these data for research purposes are provided at http://breastscreening.cancer.gov/.
Author Contributions: Conception and design: J.T. Schousboe, K. Kerlikowske, S.R. Cummings.
Analysis and interpretation of the data: J.T. Schousboe, K. Kerlikowske, A. Loh, S.R. Cummings.
Drafting of the article: J.T. Schousboe, K. Kerlikowske, A. Loh, S.R. Cummings.
Critical revision of the article for important intellectual content: J.T. Schousboe, K. Kerlikowske, A. Loh.
Final approval of the article: J.T. Schousboe, K. Kerlikowske, A. Loh, S.R. Cummings.
Provision of study materials or patients: J.T. Schousboe, K. Kerlikowske, A. Loh.
Statistical expertise: J.T. Schousboe, K. Kerlikowske.
Obtaining of funding: K. Kerlikowske.
Administrative, technical, or logistic support: J.T. Schousboe, K. Kerlikowske.
Collection and assembly of data: J.T. Schousboe, K. Kerlikowske, A. Loh.
References
- 1.Lee CH. Screening mammography: proven benefit, continued controversy. Radiol Clin North Am. 2002;40:395–407. doi: 10.1016/s0033-8389(01)00015-x. [PMID: 12117183] [DOI] [PubMed] [Google Scholar]
- 2.Sirovich BE, Sox HC., Jr. Breast cancer screening. Surg Clin North Am. 1999;79:961–990. doi: 10.1016/s0039-6109(05)70056-6. [PMID: 10572546] [DOI] [PubMed] [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [PMID: 16251534] [DOI] [PubMed] [Google Scholar]
- 4.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. U.S. Preventive Services Task Force. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. W237-42. [PMID: 19920273] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2006:CD001877. doi: 10.1002/14651858.CD001877.pub2. [PMID: 17054145] [DOI] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [PMID: 19920274] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddy DM. Screening for breast cancer. Ann Intern Med. 1989;111:389–399. doi: 10.7326/0003-4819-111-5-389. [PMID: 2504094] [DOI] [PubMed] [Google Scholar]
- 8.Garuz R, Forcén T, Cabasés J, Antoñanzas F, Trinxet C, Rovira J, et al. Economic evaluation of a mammography-based breast cancer screening programme in Spain. Eur J Public Health. 1997;7:68–76. [Google Scholar]
- 9.Hall J, Gerard K, Salkeld G, Richardson J. A cost utility analysis of mammography screening in Australia. Soc Sci Med. 1992;34:993–1004. doi: 10.1016/0277-9536(92)90130-i. [PMID: 1631612] [DOI] [PubMed] [Google Scholar]
- 10.Szeto KL, Devlin NJ. The cost-effectiveness of mammography screening: evidence from a microsimulation model for New Zealand. Health Policy. 1996;38:101–115. doi: 10.1016/0168-8510(96)00843-3. [PMID: 10160378] [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Kåresen R, Hervik A, Thoresen SO. Mammography screening in Norway: results from the first screening round in four counties and cost-effectiveness of a modeled nationwide screening. Cancer Causes Control. 2001;12:39–45. doi: 10.1023/a:1008999403069. [PMID: 11227924] [DOI] [PubMed] [Google Scholar]
- 12.Arveux P, Wait S, Schaffer P. Building a model to determine the cost-effectiveness of breast cancer screening in France. Eur J Cancer Care (Engl) 2003;12:143–153. doi: 10.1046/j.1365-2354.2003.00373.x. [PMID: 12787012] [DOI] [PubMed] [Google Scholar]
- 13.Kattlove H, Liberati A, Keeler E, Brook RH. Benefits and costs of screening and treatment for early breast cancer. Development of a basic benefit package. JAMA. 1995;273:142–148. [PMID: 7799495] [PubMed] [Google Scholar]
- 14.American Cancer Society. American Cancer Society Guidelines for the Early Detection of Cancer. Vol. 2009. Atlanta: American Cancer Soc; 2008. [Google Scholar]
- 15.Von Eschenbach AC. NCI remains committed to current mammography guidelines [Editorial] Oncologist. 2002;7:170–171. doi: 10.1634/theoncologist.7-3-170. [PMID: 12065784] [DOI] [PubMed] [Google Scholar]
- 16.U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [PMID: 19920272] [DOI] [PubMed] [Google Scholar]
- 17.Olsen AH, Bihrmann K, Jensen MB, Vejborg I, Lynge E. Breast density and outcome of mammography screening: a cohort study. Br J Cancer. 2009;100:1205–1208. doi: 10.1038/sj.bjc.6604989. [PMID: 19293800] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [PMID: 17229950] [DOI] [PubMed] [Google Scholar]
- 19.Chiu SY, Duffy S, Yen AM, Tabár L, Smith RA, Chen HH. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1219–1228. doi: 10.1158/1055-9965.EPI-09-1028. [PMID: 20406961] [DOI] [PubMed] [Google Scholar]
- 20.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [PMID: 18316752] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R, et al. National Institutes of Health Breast Cancer Surveillance Consortium. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99:386–395. doi: 10.1093/jnci/djk066. [PMID: 17341730] [DOI] [PubMed] [Google Scholar]
- 22.Ries LA, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. Based on the November 2007 submission. Bethesda, MD: National Cancer Institute; 2008. SEER Cancer Statistics Review, 1975–2005. Accessed at http://seer.cancer.gov/csr/1975_2005/ on 4 May 2011. [Google Scholar]
- 23.Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [PMID: 16319971] [DOI] [PubMed] [Google Scholar]
- 24.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [PMID: 12381707] [DOI] [PubMed] [Google Scholar]
- 25.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [PMID: 10761960] [DOI] [PubMed] [Google Scholar]
- 26.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [PMID: 14625260] [DOI] [PubMed] [Google Scholar]
- 27.Breast Cancer Surveillance Consortium. NIH Publication No. 04–5490. Bethesda, MD: National Cancer Institute; 2004. Evaluating Screening Performance in Practice. Accessed at http://breastscreening.cancer.gov/espp.pdf on 4 May 2011. [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results (SEER) Program. Released April 2008, based on the November 2007 submission. Bethesda, MD: National Cancer Institute; 2007. Research Data (1973–2005). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. [Google Scholar]
- 29.Plevritis SK, Salzman P, Sigal BM, Glynn PW. A natural history model of stage progression applied to breast cancer. Stat Med. 2007;26:581–595. doi: 10.1002/sim.2550. [PMID: 16598706] [DOI] [PubMed] [Google Scholar]
- 30.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [PMID: 12558355] [DOI] [PubMed] [Google Scholar]
- 31.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PMID: 16681183] [PubMed] [Google Scholar]
- 32.Centers for Medicare & Medicaid Services. 2009 Physician Fee Schedule. Baltimore: Centers for Medicare & Medicaid Services; 2008. [Google Scholar]
- 33.Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [PMID: 7861461] [DOI] [PubMed] [Google Scholar]
- 34.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [PMID: 18445825] [DOI] [PubMed] [Google Scholar]
- 35.Bureau of Labor Statistics. Consumer Price Index Detailed Reports. vol. 2009. Washington, DC: U.S. Department of Labor; 2009. [Google Scholar]
- 36.Tosteson AN, Stout NK, Fryback DG, Acharyya S, Herman BA, Hannah LG, et al. DMIST Investigators. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148:1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. [PMID: 18166758] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burström K, Johannesson M, Diderichsen F. A comparison of individual and social time trade-off values for health states in the general population. Health Policy. 2006;76:359–370. doi: 10.1016/j.healthpol.2005.06.011. [PMID: 16214258] [DOI] [PubMed] [Google Scholar]
- 38.Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–1081. doi: 10.1007/s11136-007-9202-8. [PMID: 17468943] [DOI] [PubMed] [Google Scholar]
- 39.Brett J, Bankhead C, Henderson B, Watson E, Austoker J. The psychological impact of mammographic screening. A systematic review. Psychooncology. 2005;14:917–938. doi: 10.1002/pon.904. [PMID: 15786514] [DOI] [PubMed] [Google Scholar]
- 40.Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ. 2009;339:b2587. doi: 10.1136/bmj.b2587. [PMID: 19589821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahl PH, Maehlen J, Welch HG. The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med. 2008;168:2311–2316. doi: 10.1001/archinte.168.21.2311. [PMID: 19029493] [DOI] [PubMed] [Google Scholar]
- 42.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study. BMJ. 2006;332:689–692. doi: 10.1136/bmj.38764.572569.7C. [PMID: 16517548] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137:344–346. doi: 10.7326/0003-4819-137-5_part_1-200209030-00011. [PMID: 12204019] [DOI] [PubMed] [Google Scholar]
- 44.Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast. 2005;14:269–275. doi: 10.1016/j.breast.2004.12.004. [PMID: 16085233] [DOI] [PubMed] [Google Scholar]
- 45.Kerlikowske K, Grady D, Barclay J, Frankel SD, Ominsky SH, Sickles EA, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst. 1998;90:1801–1809. doi: 10.1093/jnci/90.23.1801. [PMID: 9839520] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.