Abstract
Purpose of review
Glycogen synthase kinase-3 (GSK3) is an enzyme that is gaining prominence as a critical signaling molecule in the epithelial cells of renal tubules. This review will focus on recent findings exploring the role of GSK3 in renal collecting ducts, especially its role in urine concentration involving vasopressin signaling.
Recent findings
Recent studies using inhibition or tissue-specific gene deletion of GSK3 revealed the mechanism by which GSK3 regulates aquaporin 2 water channels via adenylate cyclase or the prostaglandin-E2 pathway. In other studies, postnatal treatment with lithium, an inhibitor of GSK3, increased cell proliferation and led to microcyst formation in rat kidneys. These studies suggest that loss of GSK3 activity could interfere with renal water transport at two levels. In the short term, it could disrupt vasopressin signaling in collecting duct cells and in the long term it could alter the structure of the collecting ducts, making them less responsive to the hydro-osmotic effects of vasopressin.
Summary
Ongoing studies reveal the crucial role played by GSK3 in the regulation of vasopressin action in the renal collecting ducts and suggest a possible use of GSK3 inhibitors in disease conditions associated with disrupted vasopressin signaling.
Keywords: aquaporin 2, cyclooxygenase, glycogen synthase kinase-3, nephrogenic diabetes insipidus, prostaglandin, vasopressin
Introduction
Glycogen synthase kinase-3 (GSK3) is a serine/ threonine protein kinase that was discovered in 1980 by Sir Dr Phillip Cohen and named for its ability to phosphorylate and inhibit glycogen synthase, a key regulator of glycogen synthesis [1]. However, studies since then have identified GSK3 as a critical enzyme which coordinates multiple signaling pathways that regulate cellular processes including gene transcription, cytoskeletal organization, cell cycle progression, cell differentiation and normal epithelial function and survival [2,3]. Hence, GSK3 is currently considered a key target for drug discovery in Alzheimer's disease, cancer and diabetes [4▪,5▪,6]. GSK3 is highly conserved throughout evolution in eukaryotes [7] and exists in two isoforms, GSK3α and GSK3β, encoded by distinct genes located on separate chromosomes [8▪]. Since the α and β isoforms share 98% sequence homology in their kinase domains [9], no truly isoform-specific inhibitors have been developed yet [8▪,10]. GSK3 is active under resting/unstimulated conditions and is inhibited by rapid and reversible phosphorylation of serine 9 of GSK3β and serine 21 of GSK3α [11–13]. GSK3 is also regulated by protein–protein interaction and intracellular sequestration [14].
There is increasing evidence that GSK3 plays an important role in renal water transport. New evidence emerging from studies using tissue-specific gene knockout or inhibitors of GSK3 in rodent models and cell culture systems suggests that this kinase could play a crucial role in vasopressin-mediated urine concentration by the renal collecting ducts. This review will highlight recent studies that explore two key factors that regulate the collecting duct's response to vasopressin: firstly, the role of GSK3 in prostaglandin (PGE) and adenylate cyclase-mediated effects on aquaporin 2 (AQP2), and secondly, its role in maintenance of epithelial morphology of the renal collecting duct.
Glycogen Synthase Kinase-3 in The Kidneys
Glycogen synthase kinase-3 isoforms are ubiquitously expressed in the body. However, most studies have focused on GSK3β, since global knockout of GSK3β is lethal whereas GSK3α knockout mice survive [15,16]. Both GSK3α and GSK3β are expressed in the kidneys [17], and GSK3β expression can be detected in renal collecting ducts (principal cells as well as intercalated cells) in mice [18], rats and humans [19▪▪]. During postnatal development, GSK3β protein abundance decreases in the renal cortex with time, whereas in the medulla it remains unchanged [19▪▪]. No published reports exist regarding the expression of GSK3α in the kidneys. On the basis of discoveries on the functional role of renal GSK3β as a pro-apoptotic and antiproliferative signaling factor, studies have found that GSK3 inhibition could be protective in acute kidney injury [20–22]. Whereas inhibition of GSK3 could be beneficial in this regard, recent studies also suggest that GSK3 is important for the maintenance of water homeostasis by the kidneys.
Glycogen Synthase Kinase-3 in Water Transport: Role in Vasopressin Signaling
Regulation of water balance by the kidney is one of its fundamental homeostatic functions and is tightly controlled by vasopressin. In response to an antidiuretic stimulus, the pituitary gland secretes vasopressin, which binds to its type 2 receptor (V2R) in renal collecting ducts, activating adenylate cyclase and increasing intracellular cyclic adenosine monophosphate (cAMP) levels. cAMP activates protein kinase A or exchange protein directly activated by cAMP (EPAC), which in turn potentiates transcription [23–25], as well as trafficking to the apical plasma membrane [26] of the AQP2 water channels. Following an osmotic gradient, water enters the principal cells through AQP2 and exits through the AQP3 or AQP4 water channels on the basolateral membrane, resulting in the formation of concentrated urine.
Studies over the past 6 years have explored the role of GSK3 in the maintenance of water homeostasis by the kidneys [18,19▪▪,27,28▪▪,29,30]. Initial speculations on a possible role of GSK3 in renal water transport were based on the observation that lithium (Li+), which is commonly used for treatment of bipolar disorders, inhibits GSK3 in the clinical therapeutic range and can cause renal toxicity [31]. Li+ has been used therapeutically for more than 150 years and remains an important treatment for bipolar disorders [32,33]. Patients on long-term Li+ treatment often have an irreversible and clinically important reduction in maximal urinary concentrating ability, which may lead to nephrogenic diabetes insipidus (NDI), with detectable impairment in renal concentrating ability reported in up to 40% patients [34]. This important renal side effect of Li+ therapy was known long before the discovery of GSK3.
Lithium is a reversible inhibitor of GSK3 with an IC50 value of approximately 1mM [35], acting as a direct competitive inhibitor of Mg2+[36] or indirectly by increasing the inhibitory phosphorylation of GSK3α and GSK3β [13,37]. In the kidney, Li+ inhibits GSK3β, as demonstrated by a decrease in renal GSK3β kinase activity [18], as well as an increase in serine-9 phosphorylated GSK3β in mice and cultured renal cells [18,19▪▪,27,28▪▪,29,30,38]. Li+-induced reduction in urinary concentrating capacity and polyuria can be detected as early as 8 weeks in humans [39] and within 5–7 days in rodents [29,30,40,41]. It is accompanied by a drastic down-regulation of AQP2 expression along the entire collecting duct [42–44]. In LiCl-treated mice, the time course of GSK3 inhibition coincides with decreases in AQP2 expression [29], as well as increases in polyuria [29,30], suggesting that loss of GSK3 activity could be a crucial factor in the impaired urine-concentrating ability in these mice. Though Li+ is a highly specific inhibitor of GSK3, it is not selective and is known to activate multiple pathways including protein kinase B/phosphoinositide 3-kinase, protein phosphate 2A as well as mitogen-activated protein kinases [29,35]. Nevertheless, studies in cultured renal cortical collecting duct cells have demonstrated that LiCl or GSK3-selective small molecule inhibitors could reduce AQP2 expression induced by desmopressin (dDAVP), a synthetic analog of vasopressin [27]. Hence, many of the effects of Li+ treatment could overlap with those expected from GSK3 inhibition.
Further evidence for a direct role of GSK3β in urine concentration was provided by studies with renal collecting duct-specific GSK3β knockout mice [18]. These mice were not overtly polyuric under basal conditions. However, their ability to concentrate urine in response to water deprivation or dDAVP treatment was diminished, accompanied by significant reduction in AQP2 mRNA, protein levels as well as its trafficking to the apical membrane [18]. This suggests that GSK3β gene deletion reduces the collecting duct's response to the hydro-osmotic effects of vasopressin.
In humans, Li+-induced polyuria is not sensitive to vasopressin, whereas in rats, polyuria and down-regulation of AQP2 expression can be partially reversed by water deprivation, dDAVP treatment or by stopping LiCl treatment [44]. Evidence obtained from cell culture studies and rodent models suggests that GSK3 could affect vasopressin signaling, indirectly via the cyclooxygenase (COX) pathway, or directly at the level of adenylate cyclase activity.
Glycogen Synthase Kinase-3 and The Cyclooxygenase– Prostaglandin Pathway
Cyclooxygenases are rate-limiting enzymes in the production of PGEs, a group of fatty acid derivatives of which PGE1 and PGE2 are known to reduce vasopressin-stimulated water reabsorption in renal collecting ducts [45–47]. COX1, the constitutively expressed isoform, is particularly abundant in collecting ducts [48,49], whereas COX2, the inducible isoform, is restricted to the macula-densa and renal medullary interstitial cells (RMICs) [50,51].
COX2 is negatively regulated by GSK3. In cultured RMICs, treatment with GSK3-selective small molecule inhibitors like SB216763 or LiCl increased COX2 expression [38]. Similarly in LiCl-treated mice, inhibition of renal GSK3 is associated with an increase in COX2 and urinary PGE2, as well as an onset of NDI within 3–5 days of treatment [30]. Administration of a COX2 inhibitor to LICl-treated mice reduced PGE2 levels as well as NDI [30]. Any contribution of renal COX1 to the increased PGE2 in LiCl-treated mice could be ruled out since COX1 levels did not change in these mice and LiCl treatment caused polyuria in COX1 knockout mice. Furthermore, LiCl treatment for 5 days was seen to increase COX2 in vasopressin-deficient Brattleboro rats, suggesting that the increase in COX2 was independent of vasopressin [30]. These studies suggest that Li+ inhibits GSK3 leading to up-regulation of COX2 and PGE2 in RMICs. The increased PGE2 produced could act in a paracrine manner on the adjoining collecting duct cells, antagonizing vasopressin-mediated cAMP generation, thus leading to polyuria (Fig. 1).
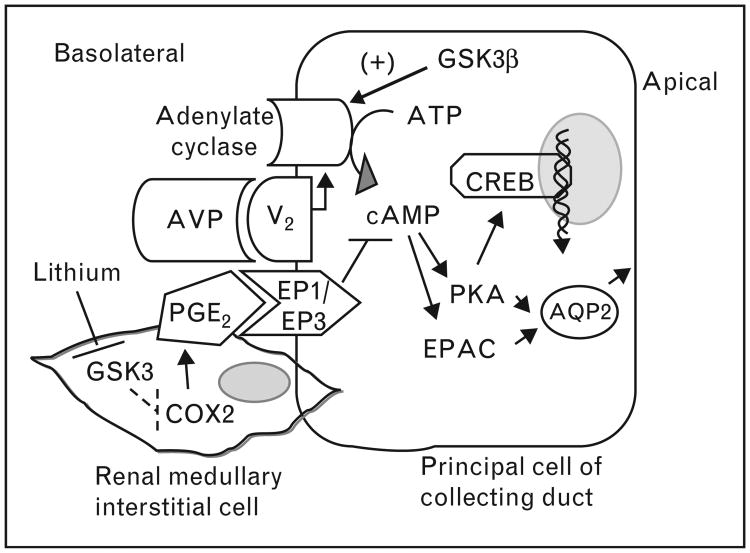
Figure 1.
GSK3-mediated regulation of vasopressin signaling in the collecting duct: inhibition of GSK3 by Li+ increases COX2 expression and PGE2 production in the renal medullary interstitial cells. PGE2 binds to EP1/EP3 receptors, antagonizing the AVP (vasopressin)-mediated cAMP generation. GSK3 also positively regulates adenylate cyclase activity, cAMP generation and AQP2 expression and trafficking in response to AVP. COX, cyclooxygenase; GSK3, glycogen synthase kinase-3; PGE, prostaglandin.
In a recent study, Kortenoeven et al. [28▪▪] investigated this hypothesis further using a mouse cortical collecting duct cell line (mpkCCD), in which LiCl treatment was demonstrated to inhibit GSK3, increase COX2 abundance and decrease AQP2 expression. Inhibition of COX2 alone or COX1 and COX2 in these cells increased AQP2 abundance. This study supports the observation in mice that GSK3 could regulate urine concentration via the COX pathway. Interestingly, Li+ did not significantly increase PGE2 production in mpkCCD cells or increase levels of the PGE receptors (EP1 and EP3) known to antagonize vasopressin action in collecting ducts [28▪▪]. However, addition of PGE2 to the medium reduced AQP2 abundance mainly by mediating AQP2 protein degradation [28▪▪]. The authors suggested that LiCl treatment reduces AQP2 expression by two mechanisms: by up-regulation of the COX2–PGE2 pathway, which leads to degradation of AQP2, which supports the hypothesis of Li+-induced up-regulation of COX2 in the RMICs [38] that naturally express this protein and the possible paracrine action of PGE2 on the collecting duct in the kidney [30], or by reducing AQP2 gene transcription in a PGE-independent fashion. This could be via inhibition of adenylate cyclase activity as described in the next section.
In a contrasting study, chronic treatment with LiCl for 4 weeks induced NDI in rats, but was accompanied by reduced COX1 and COX2 in the inner medulla [52]. Whereas GSK3 activity was not examined in these rats, these differences in COX expression could possibly be attributed to the different rodent models (rats vs. mice) and more importantly to the difference in length of LiCl treatment.
Glycogen Synthase Kinase-3 and Adenylate Cyclase Activity
Adenylate cyclase is a critical component of vasopressin signaling. Previous studies carried out in isolated collecting ducts or kidneys from LiCl-treated rats attributed the decrease in AQP2 expression to reduced vasopressin-dependent adenylate cyclase activity and cAMP generation [53–57]. However, in a contrasting study, Li+ did not affect vasopressin-induced cAMP generation in dDAVP-treated mpkCCD cells or Brattleboro rats, though Li+ reduced dDAVP-induced cAMP in normal rats [58].
A direct evidence for a role of GSK3β in the regulation of adenylate cyclase activity was provided by studies with renal collecting duct-specific GSK3β knockout mice [18]. The maximal urinary concentrating ability in these mice in response to water deprivation or dDAVP treatment was significantly compromised when compared to wild-type mice. Consistent with this, total AQP2 mRNA and protein levels, as well as its apical membrane localization, were reduced in the knockout mice. Furthermore, adenylate cyclase activity as well as intracellular cAMP levels in the renal papilla of the knockout mice was reduced in response to dDAVP or forskolin (a ligand of adenylate cyclase). This was the first demonstration that GSK3β regulates adenylate cyclase activity, and that its inactivation leads to reduced cAMP, AQP2 abundance and trafficking, and reduced responsiveness to the antidiuretic actions of vasopressin [18].
Glycogen Synthase Kinase-3 and Collecting Duct Morphology
Glycogen synthase kinase-3 isoforms play a pivotal role in cell cycle progression in embryonic stem cells and other cultured cell types, with GSK3 inhibition being pro-proliferation and anti-differentiation (reviewed by Force and Woodgett [3,59]). The relative importance of GSK3α and GSK3β in proliferation is not clear. Targeted global knockout of GSK3β in mice resulted in hyperproliferation of cardiomyocytes during embryonic development, partly due to the failure of the cardiomyocytes to adequately differentiate [16]. Mice with global knockout of GSK3α appeared to be normal in this respect [17].
Though a direct link between GSK3 and renal cell proliferation has not been established, studies based on LiCl treatment suggest that inhibition of GSK3 in the renal collecting duct could increase cell proliferation, possibly cause de-differentiation and hence reduce the principal cell's response to vasopressin. In a recent rat study, dams with litters were fed LiCl on postnatal days 7–28 [19▪▪]. The kidneys from LiCl-treated pups developed microcysts and showed an increased rate of tubular cell proliferation. Similar microcysts were observed in the cortical tubules of kidneys from long-term Li+-treated human patients. The authors attributed the high rate of collecting duct cell proliferation to GSK3β inhibition. Though a direct link between GSK3β inhibition and increased cell proliferation was not demonstrated, the presence of high levels of the inhibited, phosphorylated GSK3β in the epithelia lining the microcysts suggest that inhibition of GSK3β could have contributed to the increased rate of proliferation. Past studies have demonstrated that short-term (3–10 days) or chronic (4 weeks) treatment with LiCl in rats can lead to an increased rate of proliferation, as well as a change in the fraction of intercalated compared to principal cells of the collecting duct [41,42]. Proteomic analysis of renal collecting ducts from LiCl-treated rats demonstrated inhibition of GSK3β as well as increases in several pro-proliferative signaling factors and pathways [29]. It could be hypothesized that GSK3 activity is essential for collecting duct cells to be maintained in their differentiated form and that GSK3 inactivity could lead to proliferation and de-differentiation and hence less AQP2 expression.
Conclusion
Studies so far suggest that GSK3β could play an important role in urine concentration by renal collecting ducts. In contrast, the role of GSK3α in the kidney and especially in renal water transport has not been explored. It is difficult to examine the role of GSK3 in the renal collecting ducts using LiCl in animals, since the effects of Li+ are systemic rather than tissue-specific, and over time Li+ accumulates in the tubules leading to secondary and tertiary effects, which might involve multiple signaling pathways. Furthermore, prolonged treatment with Li+ is known to cause natriuresis and metabolic acidosis, though the connection with GSK3 is not clear in this respect. A better understanding of the role of GSK3 in the renal response to vasopressin could provide a scientific basis for the possible use of GSK3 inhibitors for the treatment of diseases associated with dysregulated vasopressin signaling like congestive heart failure, cirrhosis or polycystic kidney disease.
KEY POINTS
Glycogen synthase kinase-3 (GSK3) inhibition by LiCl increases cyclooxygenase 2 expression and reduces aquaporin 2 AQP2 expression.
GSK3 gene knockout in the collecting ducts disrupts the collecting duct's response to vasopressin by reducing adenylate cyclase activity, cAMP generation and reduced AQP2 expression and trafficking.
GSK3 inhibition by chronic LiCl treatment could lead to hyperproliferation of collecting duct epithelial cells.
Acknowledgments
Funding was received for this work by Rao from the National Institutes of Health (R01-DK083525).
Footnotes
Conflicts of interest: There are no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 564).
- 1.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- 2.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multitasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodgett JR, Force T. Unique and overlapping functions of GSK-3 isoforms in cellular differentiation, proliferation, and cardiovascular development. J Biol Chem. 2008;284:9643–9647. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Cai Z, Zhao Y, Zhao B. Roles of glycogen synthase kinase 3 in Alzheimer's disease. Curr Alzheimer Res. 2012 doi: 10.2174/156720512802455386. Epub ahead of print This review summarizes the recent findings on the role of GSK3 in neuronal degeneration and proposes it to be an attractive drug target for Alzheimer's and other diseases. [DOI] [PubMed] [Google Scholar]
- 5▪.Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol. 2011;51:607–613. doi: 10.1016/j.yjmcc.2010.11.020. This review presents a concise and up to date discussion on the role of GSK3α and GSK3β in cell proliferation and cell differentiation and suggests that loss of GSK3 activity could lead to dedifferentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacAulay K, Woodgett JR. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of type 2 diabetes. Expert Opin Ther Targets. 2008;12:1265–1274. doi: 10.1517/14728222.12.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- 8▪.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Frontiers Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. Excellent review with a historic perspective on research carried out on GSK3 with a biochemistry and cell biology perspective and a complete list of mouse models available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor a. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 12.De Sarno P, Li X, Jope RS. Regulation of akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 13.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/ threonine kinase akt-1 and suppresses glutamate-induced inhibition of akt-1 activity in neurons. Proc Natl Acad Sci U S A. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3: an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 15.Hoeflich KP, Luo J, Rubie EA, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 16.Kerkela R, Kockeritz L, Macaulay K, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacAulay K, Doble BW, Patel S, et al. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007;6:329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Rao R, Patel S, Hao C, et al. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol. 2010;21:428–437. doi: 10.1681/ASN.2009060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3beta-positive epithelium. Am J Physiol Renal Physiol. 2012;302:F455–F465. doi: 10.1152/ajprenal.00144.2011. In this rat study, rat litters exposed to LiCl on postnatal days 7–28 developed urine concentrating defect as adults and their kidneys developed microcysts. This study shows that GSK3β expression and activity is regulated in the postnatal kidney and inhibition of GSK3 could cause hyperproliferation of collecting duct cells. This study for the first time suggests the possibility that inhibition of GSK3 in the renal collecting duct cells could cause cyst formation. [DOI] [PubMed] [Google Scholar]
- 20.Dugo L, Collin M, Allen DA, et al. Gsk-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Havasi A, Gall J, et al. Gsk3{beta} promotes apoptosis after renal ischemic injury. J Am Soc Nephrol. 2010;21:284–294. doi: 10.1681/ASN.2009080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao HB, Shaw PC, Wong CC, Wan DC. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat. 2002;23:291–297. doi: 10.1016/s0891-0618(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Uchida S, Rai T, et al. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol. 1997;8:861–867. doi: 10.1681/ASN.V86861. [DOI] [PubMed] [Google Scholar]
- 24.Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta. 2006;1758:1100–1105. doi: 10.1016/j.bbamem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on cre and AP1 elements. Am J Physiol. 1997;272:F443–F450. doi: 10.1152/ajprenal.1997.272.4.F443. [DOI] [PubMed] [Google Scholar]
- 26.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 27.Kortenoeven ML, Li Y, Shaw S, et al. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76:44–53. doi: 10.1038/ki.2009.91. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Kortenoeven ML, Schweer H, Cox R, et al. Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol. 2012;302:C131–C140. doi: 10.1152/ajpcell.00197.2011. In this study, LiCl treatment was demonstrated to inhibit GSK3, increase COX2 abundance and decrease AQP2 expression in mpkCCD cells. This study shows a distinct difference between PGE2-mediated regulation of AQP2 by its protein degradation and Li+-mediated regulation by suppression of AQP2 protein expression and trafficking. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen J, Hoffert JD, Knepper MA, et al. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105:3634–3639. doi: 10.1073/pnas.0800001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao R, Zhang MZ, Zhao M, et al. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol. 2005;288:F642–F649. doi: 10.1152/ajprenal.00287.2004. [DOI] [PubMed] [Google Scholar]
- 31.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5:270–276. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 33.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol. 1999;10:666–674. doi: 10.1681/ASN.V103666. [DOI] [PubMed] [Google Scholar]
- 34.Stone KA. Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract. 1999;12:43–47. doi: 10.3122/15572625-12-1-43. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien WT, Klein PS. Validating GSK3 as an in vivo target of lithium action. Biochem Soc Trans. 2009;37:1133–1138. doi: 10.1042/BST0371133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Phiel CJ, Spece L, et al. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 38.Rao R, Hao CM, Breyer MD. Hypertonic stress activates glycogen synthase kinase 3beta-mediated apoptosis of renal medullary interstitial cells, suppressing an NFkappaB-driven cyclooxygenase-2-dependent survival pathway. J Biol Chem. 2004;279:3949–3955. doi: 10.1074/jbc.M309325200. [DOI] [PubMed] [Google Scholar]
- 39.Bendz H, Sjodin I, Aurell M. Renal function on and off lithium in patients treated with lithium for 15 years or more A controlled, prospective lithium-withdrawal study. Nephrol Dial Transplant. 1996;11:457–460. [PubMed] [Google Scholar]
- 40.Jia Z, Wang H, Yang T. Mice lacking mPGES-1 are resistant to lithium-induced polyuria. Am J Physiol Renal Physiol. 2009;297:F1689–F1696. doi: 10.1152/ajprenal.00117.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F39–F48. doi: 10.1152/ajprenal.00383.2005. [DOI] [PubMed] [Google Scholar]
- 42.Christensen BM, Marples D, Kim YH, et al. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol. 2004;286:C952–C964. doi: 10.1152/ajpcell.00266.2003. [DOI] [PubMed] [Google Scholar]
- 43.Kwon TH, Laursen UH, Marples D, et al. Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2000;279:F552–F564. doi: 10.1152/ajprenal.2000.279.3.F552. [DOI] [PubMed] [Google Scholar]
- 44.Marples D, Christensen S, Christensen EI, et al. Lithium-induced down-regulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest. 1995;95:1838–1845. doi: 10.1172/JCI117863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orloff J, Handler JS, Bergstrom S. Effect of prostaglandin (PGE-1) on the permeability response of toad bladder to vasopressin, theophylline and adenosine 3′,5′-monophosphate. Nature. 1965;205:397–398. doi: 10.1038/205397a0. [DOI] [PubMed] [Google Scholar]
- 46.Lipson L, Hynie S, Sharp G. Effect of prostaglandin E-1 on osmotic water flow and sodium transport in the toad bladder. Ann N Y Acad Sci. 1971;180:260–277. doi: 10.1111/j.1749-6632.1971.tb53196.x. [DOI] [PubMed] [Google Scholar]
- 47.Grantham JJ, Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3′,5′ -monophosphate, and theophylline. J Clin Invest. 1968;47:1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 49.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 50.Guan Y, Chang M, Cho W, et al. Cloning, expression, and regulation of rabbit cyclooxygenase-2 in renal medullary interstitial cells. Am J Physiol. 1997;273:F18–F26. doi: 10.1152/ajprenal.1997.273.1.F18. [DOI] [PubMed] [Google Scholar]
- 51.Harris RC, McKanna JA, Akai Y, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotnik P, Nielsen J, Kwon TH, et al. Altered expression of COX-1, COX-2, and mPGES in rats with nephrogenic and central diabetes insipidus. Am J Physiol Renal Physiol. 2005;288:F1053–F1068. doi: 10.1152/ajprenal.00114.2004. [DOI] [PubMed] [Google Scholar]
- 53.Christensen S, Kusano E, Yusufi AN, et al. Pathogenesis of nephrogenic diabetes insipidus due to chronic administration of lithium in rats. J Clin Invest. 1985;75:1869–1879. doi: 10.1172/JCI111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg H, Clayman P, Skorecki K. Mechanism of Li inhibition of vasopressin-sensitive adenylate cyclase in cultured renal epithelial cells. Am J Physiol. 1988;255:F995–F1002. doi: 10.1152/ajprenal.1988.255.5.F995. [DOI] [PubMed] [Google Scholar]
- 55.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol. 1996;270:C1695–C1702. doi: 10.1152/ajpcell.1996.270.6.C1695. [DOI] [PubMed] [Google Scholar]
- 56.Jackson BA, Edwards RM, Dousa TP. Lithium-induced polyuria: effect of lithium on adenylate cyclase and adenosine 3′,5′-monophosphate phospho-diesterase in medullary ascending limb of Henle's loop and in medullary collecting tubules. Endocrinology. 1980;107:1693–1698. doi: 10.1210/endo-107-6-1693. [DOI] [PubMed] [Google Scholar]
- 57.Yamaki M, Kusano E, Tetsuka T, et al. Cellular mechanism of lithium-induced nephrogenic diabetes insipidus in rats. Am J Physiol. 1991;261:F505–F511. doi: 10.1152/ajprenal.1991.261.3.F505. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Shaw S, Kamsteeg EJ, et al. Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity. J Am Soc Nephrol. 2006;17:1063–1072. doi: 10.1681/ASN.2005080884. [DOI] [PubMed] [Google Scholar]
- 59.Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009;284:9643–9647. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]