Abstract
mTOR is a highly conserved serine/threonine protein kinase that serves as a central regulator of cell growth, survival and autophagy. Deregulation of the PI3K/Akt/mTOR signaling pathway occurs commonly in cancer and numerous inhibitors targeting the ATP-binding site of these kinases are currently undergoing clinical evaluation. Here we report the characterization of Torin2, a second generation ATP-competitive inhibitor that is potent and selective for mTOR with a superior pharmacokinetic profile to previous inhibitors. Torin2 inhibited mTORC1-dependent T389 phosphorylation on S6K (RPS6KB1) with an EC50 of 250 pM with approximately 800-fold selectivity for cellular mTOR versus PI3K. Torin2 also exhibited potent biochemical and cellular activity against PIKK family kinases including ATM (EC50 28 nM), ATR (EC50 35 nM) and DNA-PK (EC50 118 nM) (PRKDC), the inhibition of which sensitized cells to Irradiation. Similar to the earlier generation compound Torin1 and in contrast to other reported mTOR inhibitors, Torin2 inhibited mTOR kinase and mTORC1 signaling activities in a sustained manner suggestive of a slow dissociation from the kinase. Cancer cell treatment with Torin2 for 24 hours resulted in a prolonged block in negative feedback and consequent T308 phosphorylation on Akt. These effects were associated with strong growth inhibition in vitro. Single agent treatment with Torin2 in vivo did not yield significant efficacy against KRAS-driven lung tumors, but the combination of Torin2 with MEK inhibitor AZD6244 yielded a significant growth inhibition. Taken together, our findings establish Torin2 as a strong candidate for clinical evaluation in a broad number of oncological settings where mTOR signaling has a pathogenic role.
Keywords: mTOR, ATM, ATR, lung cancer, kinase inhibitors
Introduction
The mammalian target of rapamycin (mTOR) is a highly conserved and widely expressed serine/threonine kinase that is a member of the phosphatidylinositol-3 kinase–like kinase (PIKK) family which includes the serine/threonine kinases ATR, ATM, DNA-PK, and SMG-1(1, 2).mTOR serves as a pivotal node in the PI3K/Akt/mTOR signaling pathway which senses growth factor and nutrient signals and controls fundamental cellular processes such cell growth, autophagy, translation and metabolism(3, 4). Hyper-activation of this pathway through loss of negative regulators such as PTEN, or mutational activation of receptor tyrosine kinases of PI3K is a frequent occurrence in cancer (5). mTOR exists in at least two multi-protein complexes which are named mTORC1 and mTORC2(6, 7). mTORC1 contains mTOR, mLST8 and raptor as core components and regulates cell growth, protein synthesis and autophagy through its downstream effectors, including S6K1, 4EBP1 and ATG13. mTORC2 consists of mTOR, mLST8, rictor, PRR5 and SIN1 as core components and regulates cell survival and actin organization through effectors such as Akt, SGK1 and PKCα. Through its inclusion in these two protein complexes mTOR functions both upstream of Akt through mTORC2-dependent phosphorylation of S473 located in the hydrophobic-motif (HM) and downstream of Akt in the context of mTORC1(4).
Rapamycin, a streptomyces-derived natural product that is an allosteric inhibitor of mTORC1, has been critical to understanding the functions of mTOR(8). Rapamycin acts by forming a complex with the small 12 kDa protein FKBP-12, and binding to the FRB domain of mTOR, acutely inhibiting mTORC1 activity(9). Rapamycin does not acutely inhibit the activity of mTORC2, although prolonged treatment can disrupt its activity by destabilizing complex formation(10). Prolonged suppression of mTORC1 also results in disruption of a negative feedback loop and consequently results in hyper-phosphorylation of Akt through activation of IRS1 and PI3K(11). Although rapamycin was long thought to completely disable mTORC1 activity, a new class of ATP competitive mTOR inhibitors, such as Torin1 and PP242, have revealed that many mTORC1 functions, such as phosphorylation of the 4EBP family of translational repressors, are resistant to rapamycin. (12–14). Moreover, this new class of inhibitors also potently targets mTORC2. Together, these features have generated hope that the new generation of ATP-competitive mTOR inhibitors will exhibit broader clinical efficacy relative to the rapalogs
Many members of this new class of ATP-competitive mTOR inhibitors have been developed using previously identified PI3K inhibitors as starting points. For example, LY294002, one of the original and most frequently used PI3K inhibitors, is structurally related to many dual PI3K/mTOR inhibitors, including SF1126, GSK1059615, BEZ235, XL765, PKI-587, PF-04691502, GSK2126458 and PKI-179, several of which have been advanced into clinical trials(15–22). mTOR inhibitors with little or no activity against PI3K, such as Torin1, PP242, WYE354, KU63794 have served as important research tools to advance the basic understanding of the mTOR signaling pathway and AZD8055, WYE-125132, INK-128, OSI-027 are currently undergoing clinical evaluation(23–28). Here we report detailed cellular and in vivo evaluation of Torin2, a compound recently developed to overcome the pharmacological limitations of Torin1. Chemical proteomic profiling followed by cellular pathway profiling demonstrates that Torin2, unlike Torin1, is also a potent inhibitor of ATR, ATM and DNA-PK(29–31). Torin2 displays dramatic anti-proliferative activity across a panel of cancer cell lines and elicited a combinatorial response with the MEK kinase inhibitor AZD6244 against genetically engineered mutant KRAS driven lung tumors.
Materials and Methods
Inhibitors
Torin1, Torin2 was prepared as previously described(13, 29). AZD8055, PP242 and Staurosporine were purchased from Haoyuan Chemexpress Co. (China, Shanghai). Acridine orange was purchased from Invitrogen.
ATP competition assay
Human mTORC1 complex was obtained as reported(12). In vitro mTORC1 activity was assayed using the Lanthascreen time-resolved FRET assay (Invitrogen). Briefly, mTORC1 was incubated with serially diluted inhibitors (3-fold, 10 points) for 30 min in 5 µL of kinase buffer (25 mM HEPES, pH 7.4, 8 mM MgCl2, 6 mM MnCl2, 4 mM DTT) in a 384-well low-volume white plate (Corning). The kinase reaction was initiated by the addition of an equal volume of the kinase buffer containing 0.6 µM GFP-labeled 4E-BP1 and 20 µM ATP. After incubation at room temperature for 90 min, the reaction was stopped by the addition of a 5 µL of solution containing 45 mM EDTA and 4.5 nM Tb-labeled antiphospho-4E-BP1 (T46) antibody. After 30 min, the FRET signal between Tb and GFP within the immune complex was read using an Envision plate reader (PerkinElmer). Each data point was duplicated and IC50 values were calculated using Prism4 software (GraphPad). For ATP competitiveness test, IC50 values were determined at a range of ATP concentrations in duplicate.
Immunoblot assays
ATR, ATM and DNA-PK cellular activity: HCT-116 Cells were seeded in 6-well plates (0.5×106 /well and grown overnight. After one hour of pretreatment with appropriate compounds at 37 °C, culture media was removed and saved. For ATR assay, the cells were treated with 50 mJ of UV radiation energy using strata linker (10 gray Ionizing radiation for ATM and DNA-PK assay). The culture media were added back to the cells and incubated at 37 °C. After one hour, cells were rinsed once with ice-cold PBS were lysed in ice-cold lysis buffer (40 mM HEPES [pH 7.4], 2 mM EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 1% Triton X-100, and one tablet of EDTA-free protease inhibitors per 25 ml). The soluble fractions of cell lysates were then isolated by centrifugation at 13,000 rpm for 10 min in a microcentrifuge. After the lysates from all the plates were collected the concentration of the protein was normalized by Bradford assay. 50 µL sample buffer was added to the normalized lysates and boiled for 5 min. Samples were subsequently analyzed by SDS-PAGE and immunoblotting. Results are shown in Fig. 1C, D and E.
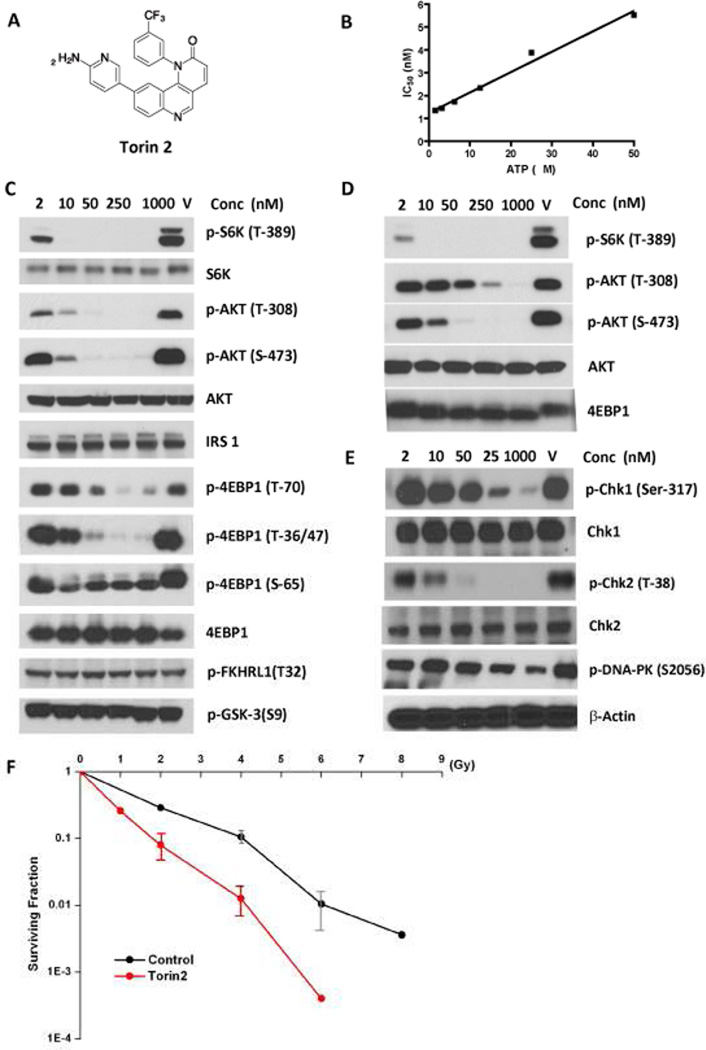
Figure 1.
Torin2 is a potent inhibitor of mTOR, ATR, ATM and DNA-PK. A, Chemical structure of Torin2. B, Torin2 is an ATP competitive inhibitor of mTOR. C, Torin2 is a potent mTOR inhibitor in HCT-116 cells. D, Torin2 selectively inhibits mTOR-regulated sites over PI3K-regulated sites in a PC3 AktS473D cell line. E, Torin2 inhibits ATR (UV radiation), ATM and DNA-PK (ionizing radiation) strongly. F. Torin2 sensitize IR treatment of human fibroblast cell line AG01522.
Biochemical and cellular mTOR kinase assays
1) in vitro assay
mTORC1 was incubated with inhibitors (0.5 ≠M, 1% DMSO) in 5 ≠L of reaction buffer (25 mM HEPES pH 7.4, 8 mM MgCl2 and 6 mM MnCl2) for 1 hour at room temperature. Then, drug-ATP competition was induced by the addition of 245 ≠L of the reaction buffer containing 500 ≠M ATP, 4 mM DTT, and 0.3 ≠M GFP-4EBP1 (Invitrogen). The reaction mixture was dispensed (10 ≠L, triplicate) into a low volume white plate (Corning) and the kinase reaction was stopped at various times with 5 ≠L of stop solution (Invitrogen). The stop solution (5 ≠L) containing 4 nM Tb-labeled p-4EBP1 (T46) antibody (Invitrogen) was added, then the FRET signal was read using Envision (PerkinElmer) after 30 min of incubation. Results are shown in Fig. 2A.
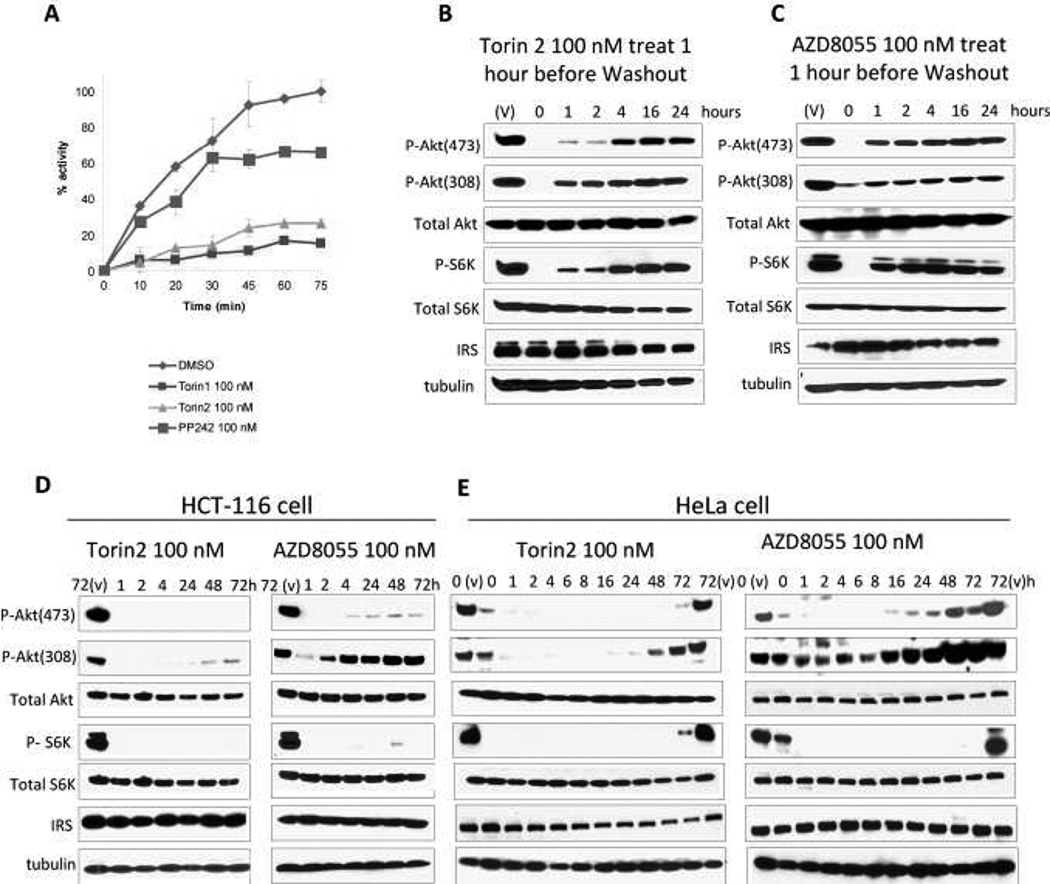
Figure 2.
Torin2 exhibits slow off-rate kinetics and suppresses feedback activation of PI3K. A, Enzymatic recovery assay of mTORC1 activity following incubation with Torin2, Torin1, and PP242. B, mTORC1/2 activity in cells following incubation and removal of Torin2. C, mTORC1/2 activity in cells following incubation and removal of AZD8055. C, HCT-116 cells were treated with 100 nM Torin2 or AZD8055 for the indicated times and analyzed for the indicated proteins by western blot. D, Hela cells were treated as in A.
2) Cellular assay
HCT116 cells were treated with 100 nM Torin2 or AZD8055 for 1 hour before they were thoroughly washed out by 3×PBS and 1xDMEM medium. Then cells were incubated in DMEM medium for indicated time before they are lysed and collected using M-PER (Pierce) according to the manufacturer’s instructions. Protein concentrations were measured and equal amount of proteins were loaded. Experiments were repeated three times and one set of results are shown in Fig. 2B.
3) Ionizing irradiation assay
Clonogenic cell survival in the human fibroblast AG01522 cell line was assessed by colony formation, using our standard protocols (Yang et al. 2005). Culture media was α-modified MEM (Sigma) with 20% fetal bovine serum (Hyclone), 100µg/ml streptomycin and 100U/ml penicillin. 100nM Torin 2 was added 30min before irradiation. The cells were irradiated using 250kVp X-rays (Siemens Stabilipan 2) and incubated for 24 hours then reseeded into 60mm petri dishes. Colonies were stained with methylene blue after 12 days of incubation in a 37°C incubator supplied with 5% CO2. Colonies containing at least 50 cells were scored under a bright field microscope. Plating efficiencies were calculated as colonies per number of cells plated and surviving fractions as ratios of plating efficiencies for irradiated and unirradiated cells. All experiments consisted of 3 independent repeats. Fig. 1F
Longer-term cellular signaling analysis
HCT116 and HeLa cells were treated with 100 nM of Torin2 or AZD8055 for indicated time before they are lysed and collected using M-PER (Pierce) according to the manufacturer’s instructions. Protein concentrations were measured and equal amount of proteins were loaded. Experiments were repeated three times and one set of results are shown in Fig 2. D. E.
Apoptosis assays
Different concentrations of AZD8055, Torin2 or staurosporine was added to five cancer cell lines for 16 hours before the cells were collected and analyzed by western blot using anti-PARP and anti-tubulin antibodies. Results are shown in Fig. 3B
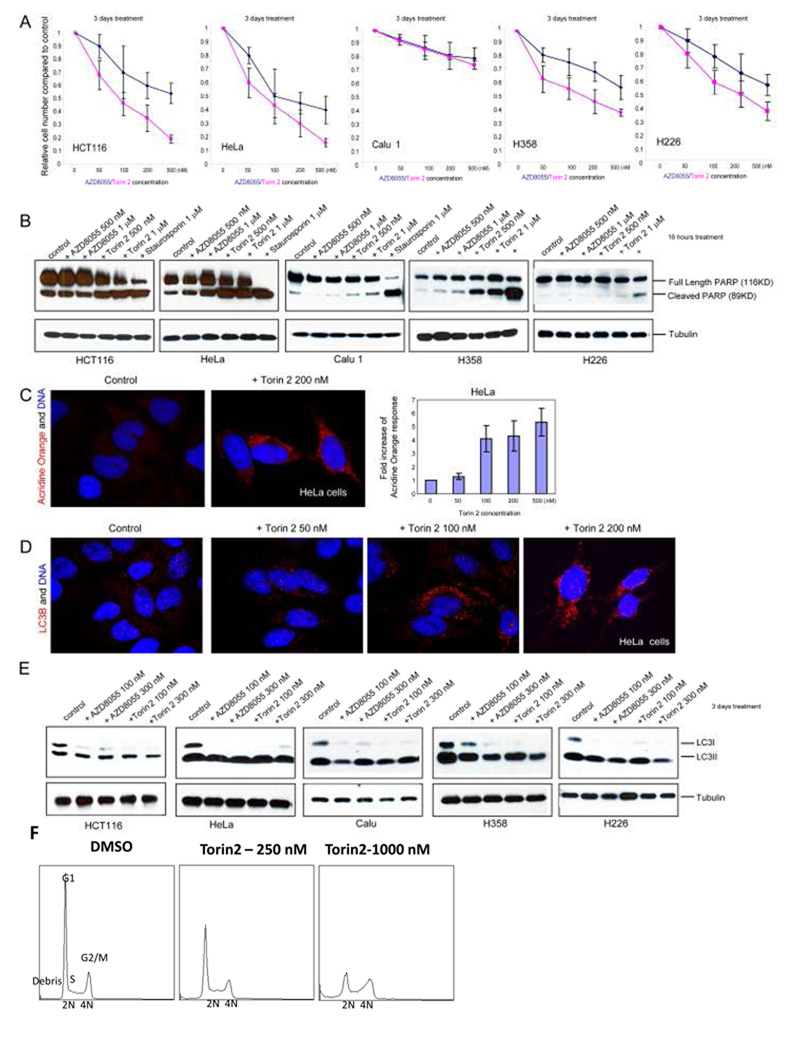
Figure 3.
Torin2 induces apoptosis and autophagy in vitro. A, B, Cells were treated with the indicated concentrations of AZD8055, Torin2 or staurosporin overnight and analyzed by western blot using antibodies specific for the indicated proteins. C, Hela cells were treated with different concentrations of Torin2 for 3 days and then stained for acridine orange and DAPI. D, Hela cells were treated with indicated concentrations of Torin2 for 3 days stained with antibody specific for LC3B and DAPI. E, Indicated cell lines were treated with increasing concentrations of AZD8055 or Torin 2 for 3 days before the cells and analyzed by western blot using anti-LC3B and anti-tubulin antibodies. F, Torin2 effects on cell cycles.
Autophagy assay
Hela cells were plated on coverslips, treated with different concentrations of Torin2 for 3 days before 1µg/ml Acridine orange was added for 15 min. Cells were washed in 3× PBS, fixed in PBS + 4% formaldehyde and stained with DAPI before they are mounted in Prolong Gold (invitrogen). Pictures were taken in Nikon Imaging Center of Harvard Medical School. Total fluorescence of acridine orange in each frame was quantified using MetaMorph and divided by the total number of cells within the frame. Numbers are then normalized to DMSO treated cells to show the acridine orange fold changes. Experiments were repeated three times. Mean values are shown for each condition and error bars represents standard deviations. For Fig. 3D, Hela cells were treated with different concentrations of Torin2 for 3 days before fixed and stained with anti-LC3B antibody and DAPI. Pictures were taken in Nikon Imaging Center of Harvard Medical School. For figure 3E, different concentrations of AZD8055 or Torin2 was added to five cancer cell lines for 3 days before the cells are collected in M-PER as introduced above and analyzed by western blot using anti-LC3B and anti-tubulin antibodies. Results are shown in Fig. 3 C, D and E.
Fluorescence-Activated Cell Sorting Analysis (FACS)
Hela S3 cells were treated with Torin1, Torin2, or DMSO control for 48 hrs. Cells were trypsinized, washed once in phosphate-buffered saline (PBS), and fixed overnight at −20 °C with 80% ethanol in PBS. Cells were washed three times with PBS. Finally, cells were resuspended in PBS containing 0.1% Triton X-100, 25 µg· ml-1 propidium iodide (Molecular Probes), and 0.2 mg·ml-1 RNase A (Sigma) and incubated for 45 minutes at 37 °C. Samples were then analyzed and results are shown in Fig. 3F.
Tumor cell growth assay
All lung cancer cell lines (H2122, H358, H1792, A549, H441, H1355, H460, H226, H1299, H292) were purchased from ATCC. Cells were cultured in RPMI1640 media with 10% FBS at 37°C with 5% CO2. 2000 cells of each well were seeded in 96-well plate (BD Falcon, #353936). After cells attachment, the drugs were added into the plate, and then incubated the cells for 72 hours. 10ul/well CCK-8 reagent (DOJINDO, #CK04-11) was added into 96 well plate and incubated cells for 2h. After incubation, the 450nm absorbance was measured by using 650nm as reference. All data was calculated by GraphPad Prism5 software to get IC50 of each drug.
All breast cancer cell lines (BT20, HS578T, MCF10A, MCF7, MDAMB231, SKBR3) were purchased from ATCC. Cells were plated in 96-well microscopy plates (Corning) and plated in recommended media at 3000 cells/well. After 24h cells were treated with different concentrations of inhibitor and incubated for 72h. Cells were fixed in 2% paraformaldehyde for 10 min at room temperature and washed with PBS-T (phosphate buffered saline, 0.1% Tween 20). Cells were washed once in PBS-T, once in PBS and incubated in 250 ng/ml Hoechst 33342 (Invitrogen) and 1:1000 Whole Cell Stain (blue; Thermo Scientific) solution for 15 min. Cells were then washed two times with PBS and imaged in an imageWoRx high-throughput microscope (Applied Precision). The images were analyzed using ImageRail and the average GI50 of six experiments was calculated using DataPflex (32, 33). Results are shown in Table 2.
Table 2.
Torin2 shows broad anti-proliferative effects. GI50 was determined based on a 72 h growth assay.
| Cell lines | GI50 (nM) | Tissue | Cell lines | GI50 (nM) | Tissue |
|---|---|---|---|---|---|
| H2122 | 13.2 | Lung Cancer | H1299 | 23.6 | Lung Cancer |
| H358 | 105.2 | Lung Cancer | H292 | 10.1 | Lung Cancer |
| H1792 | 19.8 | Lung Cancer | BT-20 | 219 | Breast cancer |
| A549 | 31.1 | Lung Cancer | HS578T | 62.4 | Breast cancer |
| H441 | 20.5 | Lung Cancer | MCF10A | 32.1 | Breast cancer |
| H1355 | 25.8 | Lung Cancer | MCF7 | 41.1 | Breast cancer |
| H460 | 45.7 | Lung Cancer | MDAMB231 | 108 | Breast cancer |
| H226 | 74.6 | Lung Cancer | SKBR3 | 123 | Breast cancer |
| HCT-116 | 60.7 | Colorectal cancer | Hela | 29.3 | Cervical cancer |
Combination study
Human non-small cell lung cancer cell lines H226, H358, and Calu-1 were grown in RPMI 1640/10% FBS/1% pen/strep/2mM L-Glutamine at 37°C in a humidified incubator with 5% CO2. To do combinational inhibition analysis, cells were plated into 96-well plates at 2000 cells per well in 100 µl medium containing 4-fold serial dilution of AZD starting from 10 µM to 0.002 µM and/or Torin2 starting from 0.2 µM to 0.04 µM. After three-day incubation, viable cells were counted using celltiter-glo (promega). All reactions were performed in triplicate. Results are shown in Fig. 4
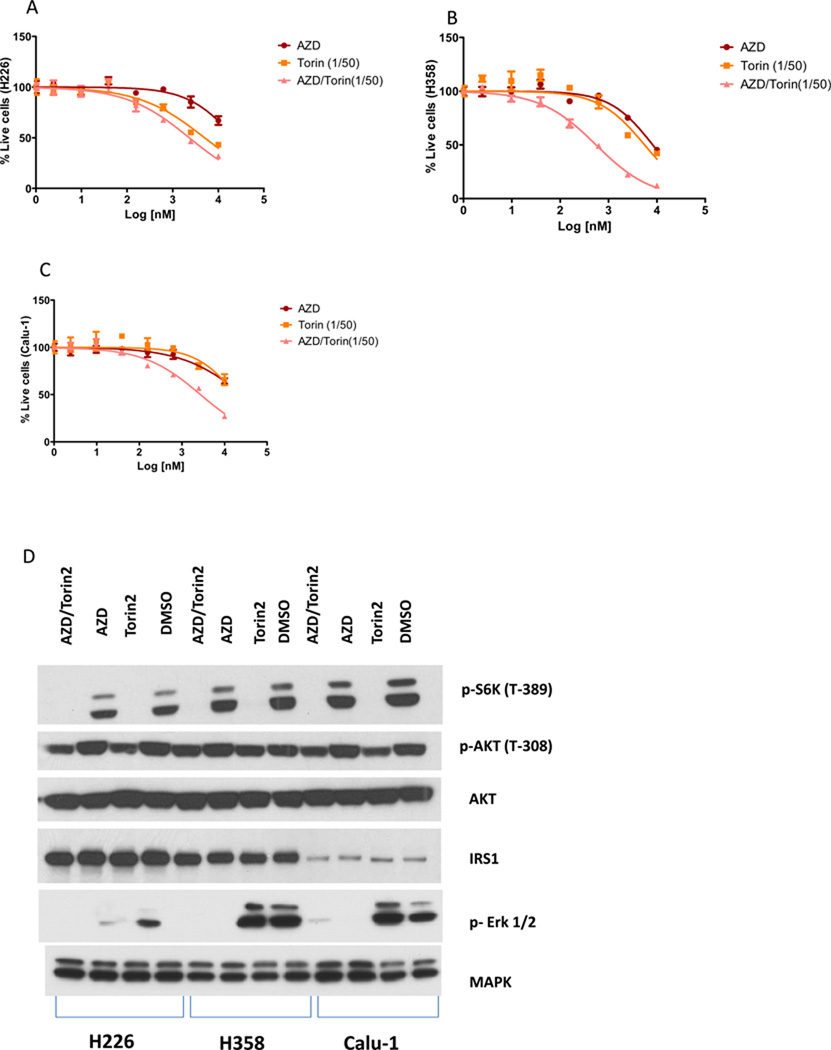
Figure 4.
Combined effect of Torin2 and AZD6244 in vitro. A. Proliferation of H226 cells treated with increasing concentrations of Torin2, AZD6244 or a ratio of Torin2:AZD6244 of 1:50; B, H358 cells were treated as in A; C. Calu-1 cells were treated as in A; E. The indicated cell lines were treated with vehicle, Torin2, AZD6244 or Torin2+AZD6244 for 1 h and then analyzed for the indicated proteins by western blot.
Mice cohort and drug treatment
Mice harbouring a conditional activating mutation Lox-Stop-Lox-Kras (G12D) allele (34)were housed in a pathogen- free environment at the Harvard School of Public Health and were handled in strict accordance with Good Animal Practice as defined by the Office of Laboratory Animal Welfare, and all animal work was done with Dana-Farber Cancer Institute IACUC approval. Mice were given Ad-Cre by nasal inhalation at 5–7 weeks of age to induce Kras G12D expression. After initial imaging, the mice bearing tumor were given Torin 2 (40mpg) or/and AZD6244 (25mpg) garage daily. Torin2 was suspended in saline; AZD6244 was reconstituted in 0.5% methylcellulose (Fluka) and 0.4% polysorbate (Tween 80; Fluka).
MRI and PET/CT study
The mice were imaged by Magnetic resonance imaging (MRI) biweekly to determine the reduction in tumor volume during the respective treatments as described previously(35). The tumor burden volume and quantification were reconstructed using 3D slicer software (http://www.slicer.org)(36). The early effects of single-agent (AZD or Torin2) or combined dual-agent treatments on tumor glucose utilizations were studied in vivo using 18F-fluoro-2-deoxy-glucose positron emission tomography (FDG-PET). Each selected Kras tumor-bearing mouse underwent both baseline and post-Rx FDG-PET imaging, the latter of which was performed after only two doses of respective single- or dual- agent treatments. The changes in tumor hypermetabolic activity, as quantified by the maximum standard uptake value (SUVmax) obtained from FDG-PET images, were compared for each treatment regimen. The operation of FDG-PET and quantification of SUVmax were described previously(37).
Immunohistochemical Analyses
Hematoxylin and eosin (H&E) staining of tumor sections was performed at the Department of Pathology at the Brigham and Women’s Hospital. Antibodies of pAKT (S473), pS6K (T389), p4EBP1 (T37/46), pERK 1/2 (T202/204) and pCHK1(S345) were purchased from Cell Signaling. Immunohistochemistry was performed on formal fixed paraffin embedded tumor sections using previously described methods (38).
Results
Torin2 is a potent and selective ATP-competitive mTOR inhibitor in vitro
Torin2 was discovered through a systematic medicinal chemistry effort to improve the pharmacological and solubility properties of Torin1, a previously reported highly potent and selective mTOR inhibitor(29) (Fig. 1A). Biochemical kinase assays with increasing ATP concentrations demonstrate that Torin2 inhibits mTOR in an ATP-competitive fashion (Fig.1B). To broadly survey the cellular targets of Torin2, we subjected the compound to profiling using the Kinativ® chemical proteomics methodology(30). In this assay the ability of Torin2 to protect kinases and other nucleotide-dependent enzymes from labeling with an electrophilic ATP-biotin compound in cellular lysates is measured using mass spectrometry. Kinativ profiling provides the most comprehensive coverage currently available for PIKK-family kinases, which bear the greatest homology to the ATP-binding site of mTOR. Comparison profiling of Torin2 and Torin1 revealed that Torin2 exhibits an apparent IC50 of less than 10 nM against many PIKK family members including mTOR, ATR, ATM, and DNA-PK as well as PI3Kα while Torin1 only strongly inhibits ATR, mTOR and DNA-PK(Table 1 and full list shown in supplemental Table 1)(39).
Table 1.
Biochemical and cellular characterization of Torin2 in comparison with Troin1 IC50 was determined using ActivX with Kinativ™ technology. EC50 was determined by western blot analysis in Figure 1 C, D, E.
| Kinase | Torin2 /Torin1 |
Torin2/Torin1 EC50((µM) |
Kinase | Torin2/Torin1 IC50((µM) |
Torin2/Torin1 EC50((µM) |
|---|---|---|---|---|---|
| IC50(µM) | |||||
| ATM | <0.01/0.64 | 0.028/>1 | PIK3CA | <0.01/0.26 | N/D |
| ATR | <0.01/<0.01 | 0.035/>1 | PIK3CB | 0.18/4.9 | N/D |
| DNAPK | <0.01/<0.052 | 0.118/>0.25 | PIK3CD | 0.018/1.6 | N/D |
| FRAP(mTOR) | <0.01/<0.01 | 0.0025/0.02 | PIP4K2C | 0.99/>10 | N/D |
| PI4KB | 0.13/>10 | N/D | PIP5K3 | 2.0/>10 | N/D |
| PIK3C3 | 0.014/0.75 | N/D | SMG1 | 0.27/4.4 | N/D |
To determine which of these targets are inhibited in a cellular context, we analyzed the phosphorylation status of downstream substrates following cellular treatment. As expected, T389 of S6K, a downstream target of TORC1, was potently inhibited with an EC50 of 250 pM and S473 of AKT, a downstream target of mTORC2, was potently inhibited with an EC50 of less than 10 nM (Fig.1C)(13). Torin2 potently inhibits T308 of Akt, a direct substrate of PDK1 and an indirect substrate of PI3Ks, with an EC50 of less than 10 nM. When the mTORC2 contribution to Akt phosphorylation is abrogated by introduction of a S473D mutant of Akt, the apparent EC50 against T308 is 200 nM. This demonstrates that Torin2 potently blocks the phosphorylation of Akt at both T308 and S473 sites and that the compound exhibits approximately 100-fold more potent functional inhibition of mTOR relative to PI3K activity (Fig. 1C, D and Supplemental Fig. 1). Torin2 inhibited the cellular activity of ATR with an EC50 of 35 nM as assessed by phosphorylation status of S317 of Chk1 following exposure of the cells to ultraviolet (UV) induced DNA damage (Fig. 1E). Torin2 inhibited the cellular activity of ATM with an EC50 of 28 nM as assessed by phosphorylation status of Chk2 following exposure of cells to 10 gray ionizing radiation (IR). Torin2 also inhibited DNA-PK with an EC50 of 118 nM as assessed by phosphorylation status of S2056 of DNA-PK following exposure of cells to 10 gray ionizing radiation (IR). In addition, torin2 shows a dose dependent sensitization of the IR treatment for human fibroblast cell line AG01522 which presumably due to the inhibition of the ATM or DNA-PK(Fig. 1F). The combined biochemical and cellular profiling results establish that Torin2 is a broadly active pan-PIKK family kinase inhibitor that most potently inhibits mTORC1 and mTORC2 at concentrations of less than 10 nM but that also inhibits ATR, ATM and DNA-PK at concentrations of between 20–100 nM and that can inhibit PI3K at concentrations above 200 nM. In contrast, Torin1 only exhibits moderate inhibition of DNA-PK (250 nM) but is inactive against other PIKK-family kinases.
Torin2 displays slow off-rate kinetics
Torin1 has previously been shown to display sustained inhibition of mTORC1 in biochemical and cellular assays, presumably as a result of slow dissociation from the complex(39). The ability of Torin2 to inhibit mTORC1 kinase activity in a time-dependent fashion demonstrated that Torin2 exhibited sustained inhibition of mTORC1, similar to what has been observed to Torin1 and in contrast to other ATP-competitive inhibitors such as PP242 (Fig. 2A). After removal of drug, the ability of mTORC1 to phosphorylate 4EBP1 was measured. Under these assay conditions, Torin1 demonstrated sustained inhibition of mTORC1 kinase activity for approximately 75 minutes in contrast to PP242, which showed full recovery of activity within this time. Treatment of cells with Torin2 for 1 hour followed by ‘wash-out’ (washing of cells and switching them into drug-free media) demonstrated that mTORC1 function - as measured by the phosphorylation status of S6KT389 - and mTORC2 function -as monitored by the phosphorylation status of AktS473 -required approximately 4 hours to recover while phosphorylation of both of these targets was regained within 1 hour following treatment with another ATP-competitive mTOR inhibitor, AZD8055(25)(Fig. 2B,C). Interestingly, recovery of AktT308 phosphorylation, which is downstream of PI3K, displayed similar kinetics for both compounds suggesting that Torin2 does not display prolonged inhibition of PI3K. The results suggest that Torin2 displays prolonged kinetics for inhibition of mTOR in biochemical and cellular contexts.
Torin2 limits negative feedback reactivation of Akt
Prolonged treatment of cells with rapamycin is well known to result in hyper-phosphorylation of Akt as a result of inhibition of the S6K/IRS-1/Akt negative feedback loop(11). To determine whether ATP-competitive mTOR inhibitors elicit a similar response, we examined the ability of Torin2 and AZD8055 to inhibit phosphorylation of AktT308, AktS473 and S6KT389 over a 72-hour time course in HCT116 and HeLa cells (Fig. 2D, E and Supplemental Fig. 2). Treatment with Torin2 at 100 nM was able to maintain strong suppression of both mTORC1 (S6KT389) and mTORC2 (AktS473) throughout the time course, however by 72 hours the phosphorylation of AktT308 was partially (HCT116) or completely recovered (HeLa). The data show that this feedback loop can reactivate Akt as assessed by T308 phosphorylation status despite potent inhibition of both mTORC1 and mTORC2. Treatment with 100 nM AZD8055 also suppressed both mTORC1 and mTORC2 activity however the phosphorylation of T308 of Akt recovered more quickly.
Torin2 inhibits proliferation of cancer cell, progression of the cell cycle and induces apoptosis and autophagy
Torin2 displays two to three-digit nanomolar GI50s for inhibition of proliferation of a diverse panel of cancer lines including lung, breast, colorectal and cervical Table 2. In a 72-hour proliferation assay, Torin2 exhibits greater anti-proliferative activity relative to AZD8055 in all 5 tested cancer cell lines (Fig. 3A). While the majority of tested cancer cell lines are potently inhibited, we did find one lung cancer cell line, Calu1, that was relatively more resistant to both Torin2 and AZD8055. Both Torin2 and AZD8055 could induce apoptosis as measured by PARP cleavage at concentrations of 0.5 or 1 µM in cells that exhibited strong inhibition of proliferation (HCT116, Hela), but much less in cells that were resistant (Calu 1 and H226), while only Torin2 induced strong apoptosis in H358. (Fig. 3B)
Both rapamycin and AZD8055 are potent inducers of autophagy (24), and so we also asked whether Torin2 would affect this process. The effect of Torin2 on autophagosome formation in Hela cells was first assessed by measuring the formation of punctate acidic vesicles in the cytoplasm using acridine orange as an indicator (Fig. 3C) A dose dependent increase in punctate acridine staining was observed after 72 h, consistent with induction of autophagy. A dose dependent increase of LC3-1/II autophagosome markers was also observed in 5 different cell lines including HCT-116, Hela, Calu-1, H358 and H226 (Fig. 3D, E). Cell cycle analysis using flow cytometry showed that Torin2 induced a dose-dependent decrease in G1 cells and an increase in S-phase, sub-G1 phase cells and cell death (Fig. 3F and supplemental table 2).
Torin2 and the MEK inhibitor AZD6244 synergistically suppress proliferation
Our cellular profiling results suggest that cell lines carrying mutations in KRAS (such as H226, H358 and Calu-1) were somewhat less sensitive to Torin2 than other lines. Given that these lines show strong activation of the Ras/Raf/Mek/Erk pathway, we investigated the potential combinatorial effect of Torin2 with the potent allosteric MEK inhibitor AZD6244(40). A 1:50 molar ratio of Torin2 to AZD6244 at concentrations that inhibited both mTORC1 and MEK activity resulted in the most differential growth inhibition relative to treatment with the single agents (Fig. 4 and supplemental table 3).
Anti-tumor efficacy of combined Torin2/AZD6244 treatment in a KRAS-driven model of lung cancer
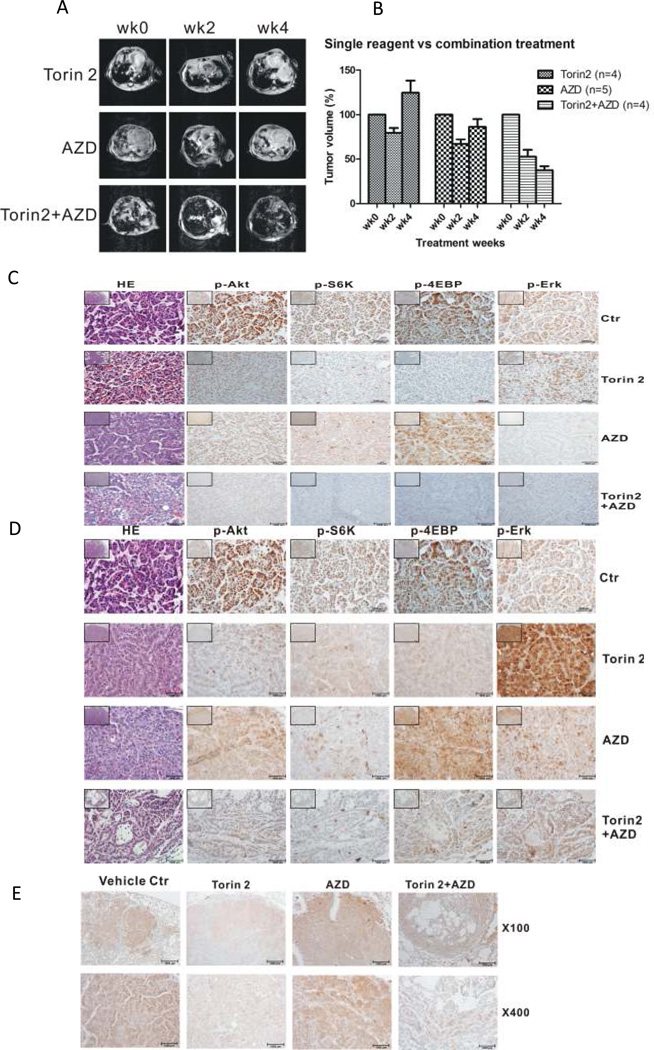
The potent anti-proliferative effect of the Torin2/AZD6244 combination in vitro suggested the possibility of similar efficacy in vivo. To determine whether Torin2 inhibits mTOR in vivo, we performed a 2-day pharmacodynamics assay following a single daily dosing of 40 mg/kg in a genetically engineered KRAS mutant driven lung cancer model (KRAS G12D model) and monitored pS6K(T389), pAkt(T308) and p4EBP1(T37/46) by immunohistochemistry. As expected, Torin2 strongly suppressed pS6K(T389) and p4EBP1(T37/46) and partly suppressed pAkt(T308). Treatment of mice with AZD6244 at 25 mg/kg resulted in a profound inhibition of pERK(40). Combined administration of Torin2 (40 mg/kg) and AZD6244 (25 mg/kg) demonstrated strong inhibition of all pharmacodynamics markers. Having established the ability to inhibit the intended targets, we evaluated tumor size by MRI and pharmacodynamic markers after 4 weeks of treatment. Treatment with neither Torin2 (40 mg/kg, q.d.) nor AZD6244 (25 mg/kg, q.d.) alone resulted in a significant inhibition of tumor volume as determined by MRI. In contrast, the combination of two drugs showed significant reduction in tumor growth (p<0.0001) (Fig. 5A, B). Moreover, PET-CT demonstrated a clear metabolic rate reduction in tumors treated with both compounds, but not those treated with either compound alone (Supplemental Fig. 3,4). Examination of pharmacodynamic markers following 4-week treatment revealed some recovery of mTOR and MEK activity. In the Torin2 treatment group, pS6K(T389) and p4EBP1(T37/46) levels showed some recovery (Fig. 5C, D), while pERK1/2 levels was significantly increased. In the AZD6244 treatment group, the pERK1/2 levels had slightly recovered. In addition, The pCHK1(S345) of Torin2 group was slightly decreased indicating a inhibition of ATR kinase.
Figure 5.
Single and combined effect of Torin2 and AZD6244 on tumor growth in vivo. A, MRI images of tumor size after treatment with vehicle, Torin2, AZD6244, or Torin2 + AZD6244 for the indicated times. B, Measurements of tumor volume for animals treated as in A. C, Sections of tumors from animals treated with the indicated compounds for 2 days, analyzed by immunohistochemistry for the indicated proteins D, Sections of tumors from animals treated with the indicated compounds for 4 weeks, analyzed by immunohistochemistry for the indicated proteins E, Sections from D were analyzed for levels of pChk1 by immunohistochemistry.
Discussion
We have described Torin2 as a potent orally bioavailable mTOR kinase inhibitor with significant selectivity over other protein kinases. In cells, Torin2 demonstrated over 800 fold selectivity against PI3K, approximately 100 fold selectivity against ATR and ATM, and 500 fold selectivity against DNA-PK. Like other mTOR active site inhibitors, Torin2 causes rapid dephosphorylation of rapamycin-sensitive mTORC1 substrates, such as S6K (T389), rapamycin-insensitive mTORC1 substrates, such as 4EBP1(T37/46), and mTORC2 substrates such as Akt(S473). Consistent with these effects, Torin2 is a potent inducer of phenotypes associated with mTOR inhibition, such as autophagy and, at higher concentrations, apoptosis.
Similarly to other mTOR inhibitors, including rapamycin, Torin2 also exhibited a bimodal effect on Akt activity. Acute inhibition of Akt S473 phosphorylation reduces recruitment to the plasma membrane where PDK1 phosphorylates T308, a site in the activation loop that directly regulates Akt kinase activity. However, prolonged inhibition of mTORC1 de-represses a feedback loop that ultimately leads to PI3K hyperactivation(11). In this context, Akt is recruited to the plasma membrane despite dephosphorylation at S473, and Akt phosphorylation at T308 and kinase activity is reactivated to near normal levels. These effects are likely to be directly mediated through dual inhibition of mTORC1 and mTORC2 as the highly selective ATP-competitive mTOR inhibitor AZD8055 has been reported to exhibit the same bimodal effect on Akt activity(41). Nonetheless, the absence of phosphorylation at S473 is thought to limit levels of Akt activity below a threshold required for tumorigenesis(42). Thus, mTOR active site inhibitors may permit Akt activity within physiological bounds while preventing the level of hyperactivation often observed with rapamycin and related rapalogs.
Torin2 retains the slow off-rate kinetics that we previously observed with Torin1, but not with other mTOR inhibitors. This long residence is potentially desirable because it results it results in sustained pharmacodynamics which appear to compensate for the relatively short half-lives of Torin1 and Torin2 in vivo(13,29). The recovery rate for Torin2 is, however, faster than for Torin1. Torin1 suppressed S6K T389 phosphorylation for up to 16 h after removal of the drug, while Torin2 maintained suppression for only 4 h (39). The difference was somewhat surprising given that Torin2 is a structural analogue of Torin1, and molecular modeling indicates that both compounds utilize the same binding mode(13, 29). Moreover, both Torin1 and Torin2 show similar EC50s for mTOR substrates in cells, indicating that the difference in recovery times cannot be explained simply by different affinities for the binding pocket. One possible explanation is that Torin1 induces a conformational change in the kinase that is energetically more difficult to recover from.
There currently exists significant interest in the use of mTOR inhibitors as anti-cancer therapeutics, as evidenced by efforts from Wyeth, OSI and Intellikine etc. Like other mTOR inhibitors, Torin2 had broad efficacy against a panel of cancer cell lines, although we were unable to associate any particular genetic alteration with resistance or sensitivity. Indeed, we identified only one cell line (Calu-1) that was markedly resistant. In general, cell lines harboring mutations in KRAS were more resistant to the effect of mTOR inhibition on proliferation, consistent with previous reports(43). However, cell lines with KRAS mutations exhibit a broad range of responses to mTOR inhibitors, and therefore a single lesion is unlikely to determine sensitivity. Analysis of a more comprehensive panel of tumor cell lines may better clarify the spectrum of alterations that determine sensitivity to the effects of mTOR inhibition.
We also asked whether Torin2 might synergize with other anti-cancer treatments. Unlike Torin1, Torin2 at slightly higher concentrations also targets the PIKK kinases ATM, ATR, and DNA-PK, which is similar with PI3K/mTOR dual inhibitor BEZ235(44). Each of these kinases plays important roles in the response to DNA damage. BEZ235 has been shown to sensitize the chemotherapy treatment such as cisplatin due to the inhibition of ATR, and here we demonstrated that torin2 can sensitization of the IR treatment in human fibroblast cells. In addition, we detected no synergy between Torin2 and these treatments (data not shown). One explanation may be that the cell cycle arrest caused by mTOR inhibition protects cells from DNA damaging agents, which tend to act during S-phase. However, tumor cell lines that maintain proliferation when treated with Torin2 alone, such as Calu-1, may be more susceptible to combined treatment with DNA-damaging agents and deserve further investigation.
Previous work has indicated that molecules targeting the Ras/MAPK pathway might also synergize with mTOR inhibition(45). Various combinations of inhibitors have been reported to achieve better efficacy in a range of cancer models(45, 46) and some of these regimes are currently in clinical trials for treatment of solid tumors such as PF04691502(PI3K/mTOR)/PD-0325901(MEK) from Pfizer and GSK2126458(PI3K/mTOR)/GSK1120212(MEK)(47) from GSK. Here, we tested the efficacy of Torin2 either alone or in combination with the MEK inhibitor AZD6244 against a mouse model of lung cancer driven by expression of KRASG12D mutant allele. Despite efficacy against cell lines harboring similar mutations in culture, neither Torin2 nor AZD6244 alone showed apparent anti-tumor activity in this model as single agents. Short-term treatment with either compound reduced target phosphorylation, such as pS6K(T389) for Torin2 and pErk1/2 for AZD6244 as determined by immunohistochemistry. However, a longer 4-week treatment resulted in partial re-activation of the targeted pathways. In tumors treated with Torin2, immunohistochemistry revealed increased levels of both pAkt(T308) and pS6K(T389) relative to short-term treatment. Likewise, tumors treated with AZD6244 exhibited partially recovered levels of pERK. Both observations of pathway reactivation are consistent with previous reports and further the paradoxical but increasingly common theme that prolonged inhibition of individual signaling pathways leads to compensatory activation through secondary mechanisms.
In contrast to their single agent efficacy, 4-week treatment of tumors with a combination of Torin2 and AZD6244 significantly reduced tumor volume. Moreover, this regimen prevented the reactivation of each pathway as observed when each inhibitor was used alone. Why this occurs is unclear, as the currently understood mechanisms for mTOR re-activation do not involve the Ras/MAPK pathway, and vice-versa. Thus, MEK and mTOR may play complementary and unappreciated roles in the reactivation of the other’s signaling pathways. Nonetheless, the maintenance of pathway suppression is likely an important contributor to the anti-tumor efficacy of dual-inhibitor regimen. The dual mTOR/PI3K inhibitor BEZ235 also synergized with AZD6244 in the same lung cancer model(45). As Torin2 does not significantly impair PI3K activity, our results indicate that the mTOR-specific activity of BEZ235 is key to its efficacy in this model.
We have described Torin2 as a potent pan-PIKK kinase inhibitor with significant activity and selectivity against mTOR, ATM, ATR and DNA-PK and both in vitro and in vivo anti-tumor efficacy. As described previously for the dual mTOR/PI3K inhibitor BEZ235, Torin2 potently synergized with the MEK inhibitor AZD6244 in a KRAS-driven model of lung cancer. The efficacy of Torin2 in this particular model is likely due mTOR inhibition. However, the capacity of Torin2 to target other PIKK kinases may prove useful in other contexts where mTOR inhibition alone is ineffective, potentially in combination DNA-damaging therapies.
Supplementary Material
Acknowledgment
We thank ActivX Biosciences for providing KiNativ™ profiling services and Dana Farber cancer institute animal facility for providing animal study platform.
Grant support
Qingsong Liu and Mario Niepel are supported by NIH grant HG006097; Chunxiao Xu, Yan Liu, Peng Gao, Tanya Tupper and Kwok-Kin Wong are supported by NIH RO1 CA122794, CA140594 and NIH Lung SPORE P50CA090578; Carson C. Thoreen is supported by Postdoctoral fellowship from the American Cancer Society and Seong A. Kang are supported by NIH RO1 AI47389 and CA103866; Hidemasa Kawamura, and Kathryn D. Held are supported by NIH C06 CA059267; Stephen J. Elledge and David M. Sabatini are Howard Hughes Medical Institute investigators.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT. PI 3-kinase related kinases: 'big' players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3:883–887. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 5.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 12.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benz o[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53:7146–7155. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casagrande F, Bacqueville D, Pillaire MJ, Malecaze F, Manenti S, Breton-Douillon M, et al. G1 phase arrest by the phosphatidylinositol 3-kinase inhibitor LY 294002 is correlated to up-regulation of p27Kip1 and inhibition of G1 CDKs in choroidal melanoma cells. FEBS Lett. 1998;422:385–390. doi: 10.1016/s0014-5793(98)00043-x. [DOI] [PubMed] [Google Scholar]
- 16.Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008;68:206–215. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 17.Knight SD, Nicholas AD, Burgess JL, Chaudhari AM, Darcy MG, Donatelli GA, et al. Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 20.Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011;17:3193–3203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 21.Yuan J, Mehta PP, Yin MJ, Sun S, Zou A, Chen J, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol Cancer Ther. 2011;10:2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- 22.Venkatesan AM, Chen Z, dos Santos O, Dehnhardt C, Santos ED, Ayral-Kaloustian S, et al. PKI-179: an orally efficacious dual phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor. Bioorg Med Chem Lett. 2010;20:5869–5873. doi: 10.1016/j.bmcl.2010.07.104. [DOI] [PubMed] [Google Scholar]
- 23.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, et al. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 26.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, et al. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Garcia C, Ibrahim YH, Serra V, Calvo MT, Guzman M, Grueso J, et al. Dual mTORC1/2 and HER2 Blockade Results in Antitumor Activity in Preclinical Models of Breast Cancer Resistant to Anti-HER2 Therapy. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 28.Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10:1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, et al. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem. 2011;54:1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patricelli MP, Nomanbhoy TK, Wu J, Brown H, Zhou D, Zhang J, et al. In situ kinase profiling reveals functionally relevant properties of native kinases. Chem Biol. 2011;18:699–710. doi: 10.1016/j.chembiol.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patricelli MP, Szardenings AK, Liyanage M, Nomanbhoy TK, Wu M, Weissig H, et al. Functional interrogation of the kinome using nucleotide acyl phosphates. Biochemistry. 2007;46:350–358. doi: 10.1021/bi062142x. [DOI] [PubMed] [Google Scholar]
- 32.Millard BL, Niepel M, Menden MP, Muhlich JL, Sorger PK. Adaptive informatics for multifactorial and high-content biological data. Nature methods. 2011;8:487–493. doi: 10.1038/nmeth.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendriks BS, Espelin CW. DataPflex: a MATLAB-based tool for the manipulation and visualization of multidimensional datasets. Bioinformatics. 2010;26:432–433. doi: 10.1093/bioinformatics/btp667. [DOI] [PubMed] [Google Scholar]
- 34.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, Oxnard GR, Moskowitz CS, Kris MG, Pao W, Guo P, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res. 2010;16:4647–4653. doi: 10.1158/1078-0432.CCR-10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Kirubakaran S, Hur W, Niepel M, Westover K, Thoreen CC, et al. Kinome-wide Selectivity Profiling of ATP-competitive Mammalian Target of Rapamycin (mTOR) Inhibitors and Characterization of Their Binding Kinetics. J Biol Chem. 2012;287:9742–9752. doi: 10.1074/jbc.M111.304485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 41.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat Struct Mol Biol. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Greger JG, Eastman SD, Zhang V, Bleam MR, Hughes AM, Smitheman KN, et al. Combinations of BRAF, MEK, PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by NRAS or MEK Mutations. Mol Cancer Ther. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.