Abstract
Interleukin (IL)-27 is a unique cytokine that has a dual role in immune responses. It was originally described to promote Th1 differentiation but also suppresses inflammation by inhibiting these and other inflammatory T cell subsets. Inhibition of inflammatory activity in macrophages has also been reported. These reports have largely focused on cytokine profiles or signaling mechanisms. To date, there have been no reports of how IL-27 may directly influence cellular mechanisms that operate to control microbial growth. Formation of a phagolysosome that acquires antimicrobial properties is an essential step for destruction of pathogens or pathogen-derived materials that are internalized by macrophages. Here we report that IL-27 has a profound influence on this critical innate immunity pathway. Treatment of human macrophages with IL-27 interferes with the acidification of phagosomes by reducing protein levels of V-ATPase and impairs control of bacterial pathogens.
Keywords: IL-27, Latex beads, Phagosome, Phagolysosome, V-ATPase
1. Introduction
Interleukin (IL)-27 is a member of the IL-6 sub-family of type I cytokines. IL-27 is produced by antigen presenting cells in response to a variety of activation stimuli, notably microbial-derived products [1,2]. IL-27 is known to promote TH1 differentiation [3]. However, IL-27 inhibits differentiation of TH-17 cells and IL-17 production [4]. Murine and human macrophage inflammatory signaling and cytokine production is diminished in response to IL-27 [4]. Mice deficient in the IL-27 receptor are unable to control inflammation during chronic infection [5,6]. Thus, while IL-27 has the ability to both enhance and suppress inflammation, the latter seems to dominate.
In the endosomal/lysosomal trafficking pathway, the phagosome fuses with endosomes that mature to lysosomes to mediate the clearance of microbial pathogens in macrophages [7]. IFN-γ promotes lysosomal fusion with endosomes and acidification [8]. Activated macrophages acidify phagosomes or endosomes by vacuolar H+-ATPases (V-ATPase) [9]. These multisubunit protein complexes transport protons across membranes by hydrolyzing ATP to acidify endosomal compartments [10]. Here, we are showing for the first time that IL-27 decreases lysosomal acidification in human macrophages by influencing the expression of V-ATPase.
2. Materials and methods
2.1. Cell culture
Human buffy coats were purchased from the New York Blood Center (New York, NY). Eligible donors were 16 years of age or older, at least 110 lb, and in good physical health. The donor samples were anonymous and deidentifed. Monocytes were isolated by successive density gradient centrifugation as described previously [2]. Monocytes were differentiated to macrophages in DMEM supplemented with 2 mM glutamine, 25 mM HEPES, 20% FBS, and 10% human serum AB and incubated at 37 °C with 5% CO2 for 7 days. The cells were washed with PBS and plated onto new culture dishes in DMEM supplemented with 1% human serum, 2 mM glutamine, and 25 mM HEPES.
2.2. Bacterial infection and gentamycin protection assay
Pseudomonas aeruginosa 1244 and Staphylococcus aureus RN6390 were kindly provided by Dr. Joseph Horzempa (West Liberty University) and Dr. Mark Hart (University of North Texas Health Science Center), respectively. The bacteria were grown overnight in Tryptic Soy broth at 37 °C and washed in PBS. The bacteria were adjusted to 1 × 108 CFU/ml using a spectrophotometer (OD590 = 0.140: P. aeruginosa, OD600 = 0.4: S. aureus). Macrophages were pretreated with IL-27 (30 ng/ml), bafilomycin (100 nM), or left untreated and then incubated with bacteria at an MOI of 10 for 4 h at 37 °C with 5% CO2. At this time gentamycin (100 µg/ml) was included in the cultures. For the 24 h time point, gentamycin was maintained in the culture for an additional 20 h. At each end point, culture supernatants were removed and macrophages permeabilized with 1% saponin to release bacteria. Serial dilutions were plated on Tryptic Soy agar and incubated overnight at 37 °C.
2.3. Fluorescent bead uptake in macrophages
Human macrophages were cultivated in 24-well plates (2 × 105/well). Yellow-green fluorescent- labeled 2 µm latex beads (~10 beads/macrophage; Sigma–Aldrich) were supplied to macrophages treated with or without IL-27 (eBioscience) or bafilomycin (Sigma). The bead conjugated fluorophore is stable at acidic pH.
2.4. Analysis of lysosomal acidification and V-ATPASE immunolabeling
At the indicated time point, macrophages were treated with Lysotracker DND-99 Red (Life Technologies) for 45 min and then fixed with PBS that contained 4% paraformaldehyde (PFA). The slides were examined using a Zeiss Meta 510 laser scanning confocal microscope. A total of ten fields containing 10–20 macrophages per field were examined in each experiment. The mean fluorescent intensity (MFI) for each macrophage was calculated using Image J software. For immunostaining, mouse anti-V1-ATPase H antibody (Santa Cruz Biotechnology) was visualized by anti-mouse-Alexafluor 588-conjugated secondary antibody. The percent V-ATPase expression was analyzed by MFI similar to as described above with approximately five macrophages per field. Protein lysates for immunoblots were prepared in parallel with imaging experiments by standard techniques. The same primary antibody was revealed on nitrocellulose by anti-mouse HRP.
2.5. Statistical analysis
A Student’s t test was used to determine statistical significance in the 95% confidence interval (P < 0.05).
3. Results
3.1. Latex bead compartment acidification was decreased by IL-27
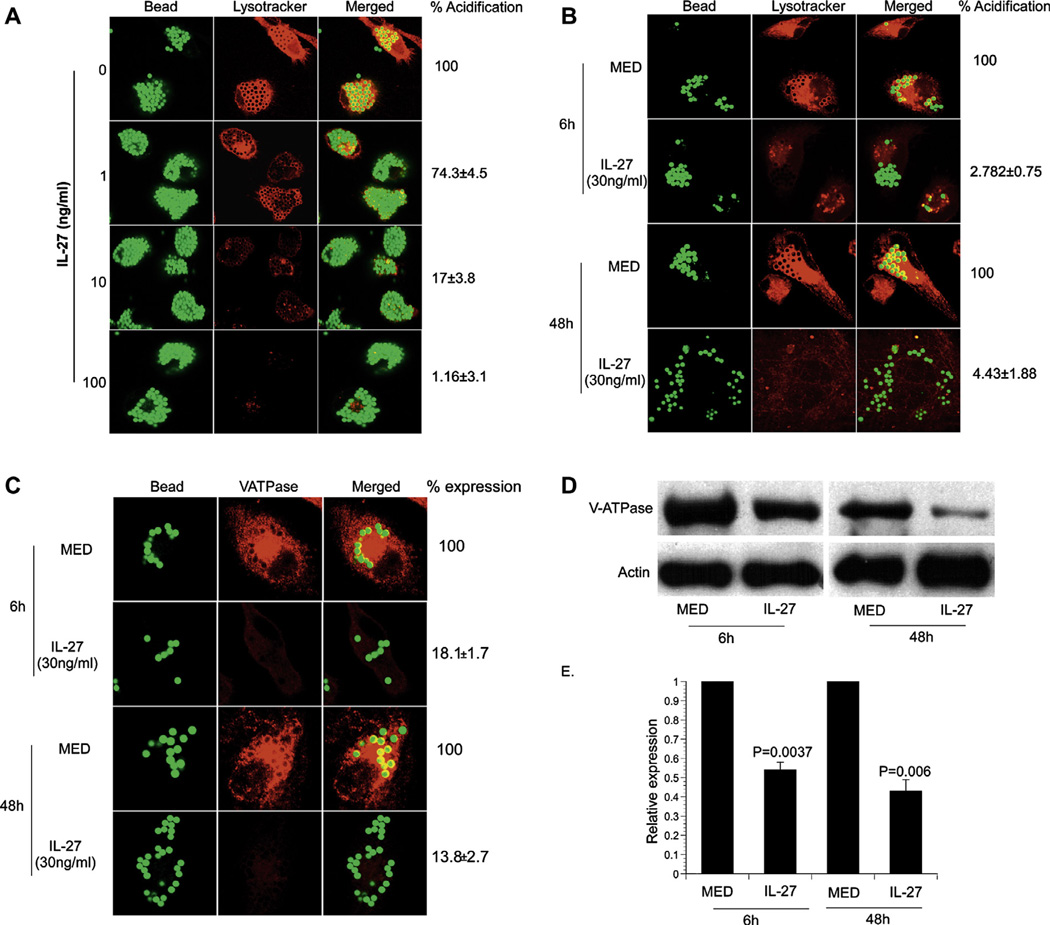
IL-27 exhibits anti-inflammatory activity toward macrophages [2,4,5]. Therefore, we hypothesized that IL-27 may negatively regulate trafficking to lysosomes. To investigate this possibility, we first evaluated lysosomal acidification in the latex bead compartment (LBC) of human macrophages. The macrophages were treated with IL-27 (0–100 ng/ml) for 6 h prior to supplying latex beads. At 48 h, macrophages were stained with Lysotracker (200 nM). After fixation, cells were analyzed by confocal microscopy. In the absence of IL-27, Lysotracker (red) was densely localized around beads (green) indicating the acidification of LBC phagosomes (Fig. 1A). In contrast, acidification dose-dependently decreased when macrophages were treated with IL-27 (Fig. 1A). Next, we evaluated the kinetics of the block in acidification. Macrophages were treated with IL-27 (30 ng/ml) for 6 h prior to internalization of beads for 6–48 h. Nearly all LBCs were acidified (red) by 6 h in the absence of IL-27 and this was maintained through 48 h (Fig. 1B). IL-27 (30 ng/ml) blocked acidification of lysosomes by 97% at 6 h and through 48 h post-treatment (Fig. 1B). Collectively, these results show that IL-27 negatively regulates the phagosomal/lysosomal trafficking pathway in a rapid and dose-dependent manner by blocking acidification.
Fig. 1.
IL-27 decreases the acidification of latex bead compartments by reducing expression of V-ATPase. Macrophages were either untreated (0 or MED) or treated with IL-27 as indicated. (A, B, and C) Cells were subjected to yellow-green fluorescent labeled latex beads for an additional 6 or 48 h as indicated. (A and B) Acidified lysosomes (red) were stained by Lysotracker (200 nM) following latex bead uptake. The images shown are from an individual experiment representative of three. The MFI obtained from untreated macrophages was set to 100%. All other conditions were expressed relative to this value. The values presented here are combined results from two (A) or three independent experiments (B). (C) Macrophages were stained with anti-V-ATPase H antibody as described. Labeled proteins were detected with anti-mouse IgG conjugated with Alexa-568 (red). Representative images from four experiments are shown. Percent expression of the protein was calculated as is described in Section 2. (D) Cell lysates were prepared at indicated times for immunoblot analysis. An image representative of three experiments is shown, and (E) The ratio of V-ATPase/actin band intensity was expressed relative to medium alone for three combined experiments. A Student’s t test was used to compare ratios from the IL-27-treated group with the control (MED) at each time point in the 95% confidence interval.
3.2. Expression of V-ATPase is inhibited by IL-27
Intracellular V-ATPases are composed of five different membrane-bound subunits that interact with six cytosolic subunits to acidify phagosomes in macrophages [9,10]. Localization of V-ATPases at the phagosome allows for a decrease in pH from 6.5 to 5.0 and is critical to the maturation of the LBC phagosome [11]. This acidic environment inhibits the growth of microorganisms and further enhances the recruitment and activity of hydrolytic enzymes. To determine the mechanism of decrease in lysosomal acidification, we examined localization of V-ATPases. Macrophages were treated with IL-27 (30 ng/ml) for 6 h prior to internalization of beads for 6 or 48 h. In the absence of IL-27, V-ATPase was strongly recruited to the LBC phagosome (Fig. 1C). Conversely, signal intensity of V-ATPase was dramatically decreased by 6 h in the presence of IL-27 (30 ng/ml) (Fig. 1C). This was further substantiated by immunoblot analysis that showed V-ATPase levels were significantly decreased by IL-27 (Fig. 1 panels D and E). Overall, this data demonstrates that IL-27 decreases the level of V-ATPase expression resulting in the loss of lysosomal acidification.
3.3. IL-27 promotes bacterial survival in human macrophages
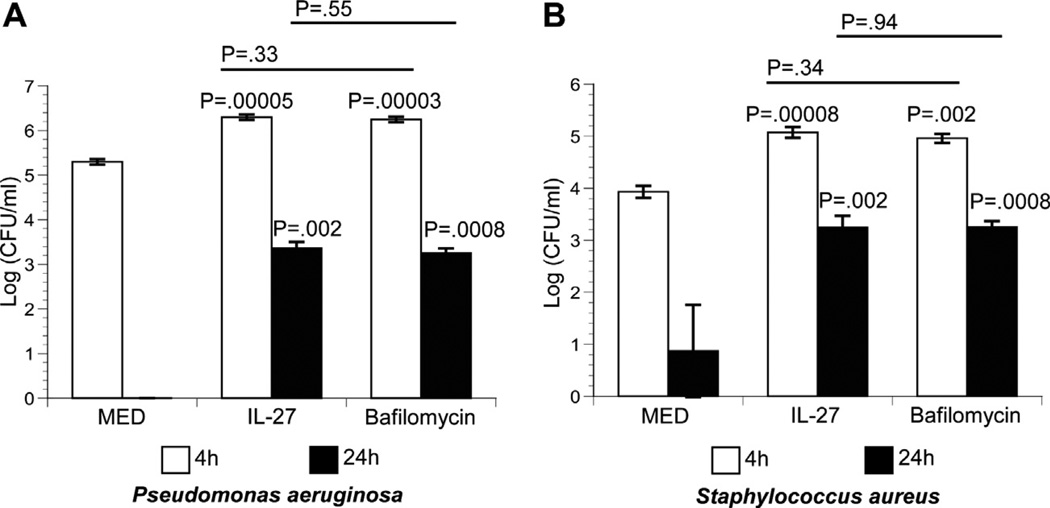
Acidification of the phagosome is critical for macrophage-mediated control of bacteria. The P. aeruginosa and S. aureus bacterial load is decreased by 4 h following infection of mouse macrophages [12,13]. Since IL-27 decreases the acidification of phagosomes, we hypothesized that IL-27 may reverse control of these bacteria in infected human macrophages. To test this idea, human macrophages were treated either with IL-27 or bafilomycin as a control prior to infection by P. aeruginosa or S. aureus. Bafilomycin inhibited lysosomal acidification in latex bead-treated macrophages (data not shown). Treatment with IL-27 increased viable bacteria compared with medium alone at both time points; the increase was more pronounced at 24 h (Fig. 2A and B). Treatment with bafilomycin resulted in a similar increase in viable bacteria demonstrating the specificity of the effects of IL-27 on phagosomal acidification and the consequence to bacterial control.
Fig. 2.
IL-27 promotes the survival of Pseudomonas aeruginosa and Staphylococcus aureus. Macrophages were either untreated (MED) or treated with IL-27 (30 ng/ml) or bafilomycin (100 nM) for 6 h. Macrophages were then infected with P. aeruginosa (A) or S. aureus. Data is represented as the mean CFU/ml recovered from infected macrophages ±SE at 4 h and 24 h. Results representative of three independent experiments are shown. P values above bars are relative to medium alone. P values above solid line are relative to bafilomycin-treated samples.
4. Discussion
Pro and anti-inflammatory activity of IL-27 toward T cells and macrophages has been reported. In addition to previously mentioned influences on T cells [4], IL-27 enhanced TLR responsiveness in murine and human monocytes [4,14]. However, in murine and human macrophages, IL-27 suppressed the production of MTB-induced inflammatory cytokines [2,5]. One of the hallmarks of macrophage activation by pro-inflammatory signals is activation of the phagosomal/lysosomal trafficking pathway so that activated macrophages can effectively destroy phagocytosed foreign materials. Anti-inflammatory cytokines have been implicated in interference with this pathway. Increased localization of mycobacteria with lysosomes has been observed in IL-10-deficient murine macrophages [15]. Similarly, IL-10 decreased localization of endocytosed horseradish peroxidase particles to lysosomes in human macrophages [8]. Here we have described a novel inhibitory mechanism of action for IL-27 on human macrophage lysosomal acidification.
In the presence of IL-27, the LBC phagosome was significantly less acidified in a dose-dependent manner. The kinetics of this response were rapid, occurring within 6 h (Fig. 1). Deacidification was due to reduced expression of V-ATPases (Fig. 1). Even though IL-27 inhibits the expression of V-ATPases, it is also possible that existing lysosomes derived from early endosomes (EE) are unable to fuse with LBC phagosomes; LBC phagosomes transiently interact with early endosomes, late endosomes, and lysosomes [11]. We also observed that there are some acidified lysosomes available in the presence of IL-27. However, those lysosomes were not recruited to the LBC phagosomes (Fig. 1). Therefore, IL-27 may also inhibit the fusion between phagosomes and lysosomes. Future research will further investigate this possibility and define the effect of IL-27 on trafficking to lysosomes in pathogen-specific models.
Since IL-27 specifically inhibits the expression of V-ATPases and lysosomal acidification, it could create an intracellular environment that favors bacterial growth. P. aeruginosa and S. aureus are known to be killed by macrophages when phagocytosed [12,13]. Viability of P. aeruginosa and S. aureus was higher in the presence of IL-27 (Fig. 2). The number of viable bacteria recovered from IL-27-treated macrophages was similar to that recovered from bafilomycin-treated macrophages. This further demonstrates the specific control of bacteria mediated by the influence of IL-27 on lysosomal trafficking. Massive clearance of bacteria was demonstrated at 24 h. This is likely due to the increased recruitment of VATPase that is observed in untreated macrophages through 24 h of fluorescent bead internalization. There may also be a contribution of other anti-bacterial mechanisms such as reactive oxygen in addition to the phagolysosomal pathway. Overall, the data presented in this manuscript provide novel mechanisms that enhance our understanding of the anti-inflammatory properties of IL-27.
Acknowledgement
This work was supported by NIH Grant HL093300.
References
- 1.Beadling C, Slifka MK. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch Immunol Ther Exp. 2006;54:15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- 2.Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27 heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 5.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2006;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 6.Pearl JE, Khader SA, Solache A, Gilbermartin L, Ghilardi N, DeSauvage F, et al. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 7.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 8.Montaner LJ, Da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, et al. Type 1 and Type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J Immunol. 1999;162:4606–4613. [PubMed] [Google Scholar]
- 9.Hackam DJ, Rotstein OD, Zhang WJ, Demaurex N, Woodside M, Tsai O, et al. Regulation of phagosomal acidification. J Biol Chem. 1997;47:29810–29820. doi: 10.1074/jbc.272.47.29810. [DOI] [PubMed] [Google Scholar]
- 10.Jefferies KC, Cipriano DJ, Forgac M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys. 2008;476:33–42. doi: 10.1016/j.abb.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci USA. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Descamps D, LeGars M, Bally V, Barbier D, Maschalidi S, et al. Toll-like receptor5, IL-1 secretion, and asparagine endopeptidase are critical factors for alveolar macrophages phagocytosis and bacterial killing. Proc Natl Acad Sci USA. 2012;109:1619–1624. doi: 10.1073/pnas.1108464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddie IPWK, Sokolovska A, Charriere GM, Boyer L, Dejardin S, et al. Phagocytosis and phagosome acidification are required for pathogen processing and MyD 88-dependent response to Staphylococcus aureus. J Immunol. 2010;184:7071–7081. doi: 10.4049/jimmunol.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalliolias GD, Ivashikiv LB. IL-27 activates human monocytes via STAT-1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLR and p38. J Immunol. 2008;180:6325–6333. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 15.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]