Abstract
Reinnervation is needed to rescue muscle when motoneurons die in disease or injury. Embryonic ventral spinal cord cells transplanted into peripheral nerve reinnervate muscle and reduce atrophy but low motoneuron survival may limit motor unit formation. We tested whether transplantation of a purified population of embryonic motoneurons into peripheral nerve (mean ± SE: 146,458 ± 4011 motoneurons) resulted in more motor units and reinnervation than transplantation of a mixed population of ventral spinal cord cells (72,075 ± 12,329 motoneurons). Ten weeks after either kind of transplant, similar numbers of neurons expressed choline acetyl transferase and/or Islet-1. Motoneuron numbers always exceeded the reinnervated motor unit count. Most motor end plates were simple plaques. Reinnervation significantly reduced muscle fiber atrophy. These data show that the number of transplanted motoneurons or motoneuron survival do not limit muscle reinnervation. Incomplete differentiation of motoneurons in nerve and lack of muscle activity may result in immature neuromuscular junctions that limit reinnervation and function.
Keywords: Axon regeneration, Motoneuron transplantation, Motor unit, Muscle denervation, Muscle reinnervation, Neuromuscular junction
INTRODUCTION
Motoneuron death at the epicenter of human spinal cord trauma results in complete muscle denervation in 15% of cases (1). This death leaves no other endogenous sources of motoneurons to reinnervate the muscles. Motoneurons have to be replaced if the affected muscles are to be rescued from the severe atrophy that occurs with chronic denervation (2).
Replacement of neurons into the spinal cord has yielded equivocal functional benefits (3). An alternative approach, transplantation of rat embryonic day 14–15 (E14-15) ventral spinal cord cells into a peripheral nerve, re-establishes functional neuromuscular junctions (4). Axons sprout extensively and multiple axons terminate at any given motor end plate (5). However, muscle reinnervation is incomplete because only a few motor units are re-established 10 weeks after neuron transplantation. The paucity of motor units limits the number of muscle fibers that can be activated by electrical stimulation to generate functional behaviors (6).
Neuron survival is also low in peripheral nerves (7), a dilemma common to other neural transplantation models (8). Most neurons die within days of transplantation, in part because of an impoverished neurotrophic environment (9, 10). Purified rat embryonic motoneurons require a combination of trophic factors and elevated cAMP to survive and to support axon growth (11, 12). Indeed, adding the combination of glial cell line-derived neurotrophic factor, hepatocyte growth factor, and insulin-like growth factor-1 to the transplant media improved motoneuron survival in peripheral nerve and muscle reinnervation (13, 14).
In this study, we tested the concept that low motoneuron numbers may limit motor unit formation by transplanting different numbers of motoneurons into peripheral nerve. Differentiated motoneurons only constitute 5% to 10% of the total E14-15 ventral spinal cord cell population used for transplantation (15), but can be isolated to 70% to 90% purity by discontinuous gradient centrifugation because they are larger than most other cells at E14-15 (16, 17). Here, our aim was to test if transplantation of a purified population of motoneurons into peripheral nerve (mean ± SE: 146,458 ± 4011 motoneurons) resulted in more motor units and greater muscle reinnervation than transplantation of the mixed population of E14-15 ventral spinal cord cells (72,075 ± 12,329 motoneurons).
MATERIALS AND METHODS
Experiments involved the use of female Fischer 344 rats (Charles River, Wilmington, MA). All procedures were approved by the animal care and use committee of the University of Miami and adhered to the National Institutes of Health Guidelines for Care and Use of Animals.
Embryonic Ventral Spinal Cord Cell Isolation
E14-15 embryos were placed in ice-cold Ca2+-Mg2+-free Hank’s solution supplemented with 25 mM glucose, 25 mM HEPES buffer, and 100 U/ml penicillin-100 μg/ml streptomycin (Invitrogen, Carlsbad, CA) (5). For cell dissociation, sections of the ventral cord were enzymatically digested in Hank’s solution containing trypsin at 37°C for 45 minutes. Fetal bovine serum was added to 10 %, and the cells centrifuged at 300 g for 5 minutes. The cells were resuspended in Liebovitz’s L15 medium (Invitrogen) containing B27 supplement, 25 mM glucose, 25 mM HEPES buffer, glutaMax-1, insulin-transferrin-selenium supplement, N-2 (Invitrogen). Trituration using fire-polished Pasteur pipettes was used to disperse the cells. Growth factors were also added to the medium because they improve long-term survival of rat motoneurons in vitro and in vivo. These included brain-derived neurotrophic factor (10 ng/ml), ciliary neurotrophic factor (10 ng/ml), glial-derived neurotrophic factor (10 ng/ml), hepatocyte growth factor (10 ng/ml), insulin-like growth factor (100 ng/ml), and forskolin (10 μM) (11, 14). Transplants of these cells were termed “ventral preparations” (Ventral).
For purification of the motoneurons, the Ventral spinal cord cells were layered over 5 ml of optiprep (Axis Shield, Oslo, Norway) (16). After centrifugation at 470 g for 20 minutes at 4°C, the motoneurons were recovered from the band between the medium and the optiprep. The cells were washed and then resuspended in L15 medium that included the factors described above. Transplants of these cells were termed “purified preparations” (Pure).
Two additional cell preparations were used to estimate the number of motoneurons present in Ventral and purified transplants. Dissociated cells from Ventral spinal cord and Pure preparations were each cultured for 16 to 18 hours on poly-DL-ornithine hydrobromide (Sigma, St. Louis, MO P8638) and laminin-coated (Invitrogen 23017-015) acid-washed glass cover slips, as described for rat embryonic motoneurons (11, 15). Cells were placed into complete Leibovitz’s L-15 medium with 5% horse serum and growth factors. After incubation, the cells were fixed with warm 4% paraformaldehyde in PBS. The presence of neurons and motoneurons was assessed using immunohistochemistry. Cells with a neuronal phenotype were detected with a rabbit polyclonal to β-tubulin III (Covance, Princeton, NJ, PRB-435P). Motoneurons were detected with the motoneuron specific marker islet-1/2 (monoclonal 39.4D5; 11; Developmental Hybridoma Bank (DSHB), University of Iowa, Iowa City, IA) because islet-1/2 expression is high in embryonic motoneurons, whereas choline acetyl transferase (ChAT) expression is low. Fluorescently conjugated secondary antibodies were used to reveal the binding of primary antibodies (1:500, Invitrogen). For each preparation, the number of β-tubulin III-positive and islet-1/2-positive cells was counted in 15 fields of cells from at least 5 different cover slips. Prior to transplantation, the average composition of the 2 cellular preparations was different. In the Pure preparations, 79% ± 3% (mean ± SE) of the cells were positive for β-tubulin III and 73% ± 2% of cells were positive for islet 1/2. The corresponding data for the Ventral preparations were 63% ± 2% and 7% ± 1%, respectively. With 200,000 and 1 million cells transplanted in Pure and Ventral preparations, respectively, we estimate that an average of 146,458 ± 4011 and 72,075 ± 12,329 motoneurons were transplanted in the respective preparations. Thus, almost twice as many motoneurons were transplanted into the peripheral nerve for Pure vs. Ventral preparations.
Muscle Denervation and Cell Transplantation
Animals were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). The left sciatic nerve of adult female Fischer 344 rats (mean body weight ± SE: 169 ± 2 g) was transected at mid thigh level to paralyze and denervate many hind limb muscles (4); this situation mimics the muscle consequences of human spinal cord injury when there is extensive motoneuron death near the lesion epicenter and no possibility of muscle reinnervation from spinal motoneurons (1). The proximal nerve stump was sutured to hip muscles to prevent muscle reinnervation from peripheral axons.
One week later, embryonic ventral spinal cord cells or medium were injected into the distal tibial nerve stump 10 to 15 mm proximal to its entry into the medial gastrocnemius (MG) muscle. Transplants included either: i) 200,000 cells isolated using the density gradient, with factors in the medium (Pure; n = 15 rats). Of these cells, an average (± SE) of 146,458 ± 4,011 were estimated to be motoneurons; or ii) 1 million ventral spinal cord cells with factors in the medium (Ventral; n = 13 rats). Of these cells, 72,075 ± 12,329 were estimated to be motoneurons, resulting in transplantation of approximately half of the motoneurons present in purified preparations. A third group had medium injected into the tibial nerve without cells (n = 8 rats). Hind limb muscles in these animals were denervated long-term because there were no neurons or myelinated axons in the tibial nerve 10 weeks after injection of medium. A fourth group of animals (n = 8 rats) consisted of unoperated controls from which tissue was taken for analysis.
Physiological Recordings
Electrophysiological recordings were made from MG muscles of anesthetized rats (sodium pentobarbital, 40 mg/kg i.p.) 10 weeks after transplantation (4, 6). The left sciatic nerve was exposed. The proximal sciatic nerve stump remained sutured to the hip muscle, so this approach was effective at preventing peripheral axons from reinnervating MG muscle. The transplant and MG muscle were separated from surrounding tissue leaving the nerve and blood supply intact. For recordings, the animal lay prone on a pad that maintained body temperature at 37°C. Muscle temperature was maintained between 35–37° C with a heating lamp. The left knee and ankle were clamped. The transplant was laid over a pair of silver electrodes for electrical stimulation. The MG tendon was connected to a transducer (Model FT03, Astro-Med. Inc., West Warwick, RI) with suture to record isometric force at optimal muscle length. A pair of silver electrodes was positioned on the surface of the MG to record electromyographic activity (EMG). To determine the number of reinnervated motor units in each MG muscle, the number of EMG and force steps was counted in response to transplant stimulation (single pulses, 10, 20 or 50 μs duration, 1–30V using either 0.1 V or 1 V steps). Motor unit forces were determined by digital subtraction of successive force increments. The force and EMG signals were filtered (DC-100 Hz, 30–1000 Hz, respectively) and sampled online (400 Hz, 3200 Hz) using SC/Zoom (Department of Integrative Medical Biology, Physiology section, Umeå University, Sweden). Reinnervation of lateral gastrocnemius (LG) and plantaris muscles was only assessed by documenting whether visible contractions of these muscles occurred in response to electrical stimulation of the transplant.
Analysis of Neuron Survival
After the physiological recordings (10 weeks after transplantation), animals were killed using Euthasol (0.5 ml/kg). The transplant was removed, oriented on Whatman filter paper and fixed overnight in Zamboni’s fixative (2 % paraformaldehyde, 15 % picric acid in 0.1 M phosphate buffer, pH 7.3) at 4°C. The tissue was cryoprotected in graded sucrose solutions, frozen (−80° C), sectioned (25 μm) and mounted onto Superfrost microscope slides.
The following antibodies were used to detect neurons: (neuron-specific nuclear protein [NeuN], diluted 1:200, Millipore, Billerica, MA), motoneurons (goat antibody that recognizes ChAT], diluted 1:110, Millipore), a monoclonal antibody that recognizes the Islet-1 homeobox protein, diluted 1:50, DSHB 39.4D5; the Hb9 homeobox protein, diluted 1:100 (DSHB, 81.5C10), and mature neurons (monoclonal SMI-32, diluted 1:1000, Invitrogen), an antibody that demarcates somatic size and dendritic arborization by recognizing nonphosphorylated neurofilaments of medium and high molecular weight (18). The dilution of the antibodies was optimized by detection of proteins in E14-15 and/or adult spinal cord sections.
For analysis of neuron and motoneuron survival, sections were thawed at room temperature (RT) for 1 hour, rinsed with 1.5 T buffer saline (0.05 M TRIS pH 7.6 and 1.5% sodium chloride), and permeabilized with 100% methanol at −20° C. The sections were quenched for autofluorescence with sodium borohydride in 1.5 T buffer for 30 minutes, incubated overnight in blocking buffer consisting of 1.5 T buffer supplemented with 1% bovine serum albumin, 0.1% dry skim milk and 1% normal donkey serum then incubated with primary antibody for 24 to 48 hours at 4°C (7). The slides were then washed 3 times in PBS, each for 5 minutes and incubated with donkey anti-goat Alexa 488 conjugate (diluted 1:300, Invitrogen) and donkey anti-mouse Alexa 596 for 2 hours at RT. All secondary antibodies were diluted in 1.5 T buffer supplemented with 0.05% Nonidet-40. After 3 washes in buffer, the slides were covered with ProLong Gold antifade reagent (Invitrogen). Control sections were prepared in which primary antibodies were omitted during the overnight incubation. To ensure equal treatment of all experimental groups, sections from each group were always processed together.
Images were acquired with an Olympus BX60 microscope equipped with UPLAN fluorescent objectives and a Prior Optiscan automatic X,Y,Z-stage using a Sensicam QE camera (Cooke Corporation, Auburn Hills, MI) and Image Pro-Plus software (Media Cybernetics, Inc., Bethesda, MD). As in previous studies (7), the disector/fractionator method of stereology was used to estimate the total number of neurons and motoneurons in each transplant (19, 20). Only neurons with discernible nuclei were counted. The median (range) number of NeuN-positive neurons counted in a known volume of the Pure and Ventral transplants was 1449 (653–2559) and 1132 (301–2584), respectively. The corresponding data for ChAT-positive neurons was 153 (89–328) and 104 (76–214), counts that have proven to yield a relatively accurate estimate of the actual number of cells in tissues (20).
Myelinated Axon Counts
Nerves to MG, LG and the tibial nerve distal to these branches were fixed (4% paraformaldehyde in phosphate buffer, then 4% glutaraldehyde in phosphate buffer), embedded in Epon araldite, sectioned (1 μm thick) and stained with toluidine blue to show myelinated axons. The total number of myelinated axons within these nerves was measured using Metaporph software (Molecular Devices, Sunnyvale, CA).
Assessment of Neuromuscular Junctions
The MG and LG muscles were frozen longitudinally onto Tissue-Tek O.C.T. and cork using isopentane cooled by dry ice, then stored at −80° C. To analyze neuromuscular junctions, longitudinal sections (60 μm thick) were collected on glass slides and dried at RT in a drop of 3% disodium ethylenediaminetetraacetic acid (EDTA) to prevent muscle contraction. The sections were fixed in Zamboni’s fixative and 5% sucrose for 30 minutes at 4° C, washed several times in 1.5 T buffer, dipped into 0.1 M glycine in 1.5 T plus 0.05 % Igepal CO-630 (Sigma) for 30 minutes at RT, washed in 1.5 T, dipped into 100% methanol (−20° C) for 20 minutes, then washed (7). The tissue was blocked with 1% dry milk, 20 mM lysine and Igepal in 1.5 T overnight. To allow visualization of the axons and nerve terminals, the tissue was incubated in rabbit anti-neurofilament M (diluted 1: 220 in 1.5 T buffer; Millipore), 4% normal donkey serum, 0.3% milk, 0.1% rat serum and 0.05% Igepal. To localize motor end plates, postsynaptic acetylcholine receptors were stained with Alexa 594-labelled α-bungarotoxin (diluted 1: 300; Invitrogen). The SV-2 mouse monoclonal antibody was used to identify pre-synaptic acetylcholine vesicles (DSHB) (21). The sections were incubated for 2 hours with a secondary antibody (Alexa Fluor 488, diluted 1:250, or Alexa Fluor 594; Invitrogen). The sections were washed 4 times, dried and cover slipped with Prolong Antifade mounting medium.
Sections with en face views of innervated end plates (an axon entered the end plate) were identified. ImageJ software (version 1.32; http://rsb.info.nih.gov/ij) was used to convert these end plates to grayscale. Thresholding was used to identify only the α-bungarotoxin-stained areas, which were measured to provide the total end plate area.
Muscle Area Measurements
Cross sections (10 μm) from the mid belly of the MG muscle were stained with hematoxylin and eosin to analyze muscle morphology and measure muscle fiber areas. In each muscle, the area of 500 muscle fibers was measured from at least 100 different locations using MetaMorph software. Because muscle reinnervation from embryonic neurons in peripheral nerve is usually incomplete, the number and area of reinnervated fibers was estimated from the subset of large fibers (i.e. fibers with areas larger than 95% of the fibers in denervated muscles). This separation into reinnervated and denervated muscle fibers by size was verified in a previous study by repeatedly stimulating the transplant and comparing the areas of glycogen-depleted (reinnervated) fibers with the areas of fibers in completely denervated muscles (22).
Statistics
Medians, 25th and 75th percentiles are given. ANOVA on ranks was used to assess across group differences in the number of NeuN-positive, ChAT-positive and/or Islet-1-positive neurons, ChAT vs. Islet-1-positive neuron diameters, axon numbers in the tibial, MG and LG nerves, total end plate area, muscle fiber areas, percentage of reinnervated muscle fibers, and motor unit counts. Relative motoneuron survival was calculated by dividing the number of motoneurons in each transplant by the mean number of motoneurons transplanted in Pure or Ventral preparations. ANOVA was used to compare differences in motoneuron survival for Pure and Ventral preparations. Chi square analysis was used to compare whether the types of reinnervated and normally innervated motor end plates differed. ANOVA on ranks were used to assess within group differences in motoneuron, axon, and motor unit counts, percentage reinnervation and reinnervated fiber area with all data expressed relative to uninjured control values. Spearmann rank correlations were used to examine the significance of correlations between the percentage of reinnervated muscle fibers, and the numbers of myelinated axons and reinnervated motor units. Statistical significance was set at p < 0.05 (Systat Software, San Jose, CA).
Results
Neurons Expressed Motoneuron Markers Weeks after Transplantation into Peripheral Nerve
Many NeuN-positive neurons were present 10 weeks after transplantation of ventral spinal cord cells into peripheral nerve (Fig. 1A, F). These neurons had diverse phenotypes. Some neurons expressed motoneuron markers, indicated by positive staining for ChAT (Fig. 1A), Islet-1 (Fig. 1B), or both ChAT and Islet-1 (Fig. 1C). No neurons stained positively for the 81.5C10 monoclonal against the motoneuron distinct homeodomain protein HB9 (not shown). Some large neurons immunostained for SMI-32 (Fig. 1D), a monoclonal antibody that recognizes a non-phosphorylated epitope on the medium- and high-molecular weight subunits of neurofilament proteins; however, these SMI-32-positive neurons lacked a cholinergic phenotype, a feature that may be important for muscle reinnervation.
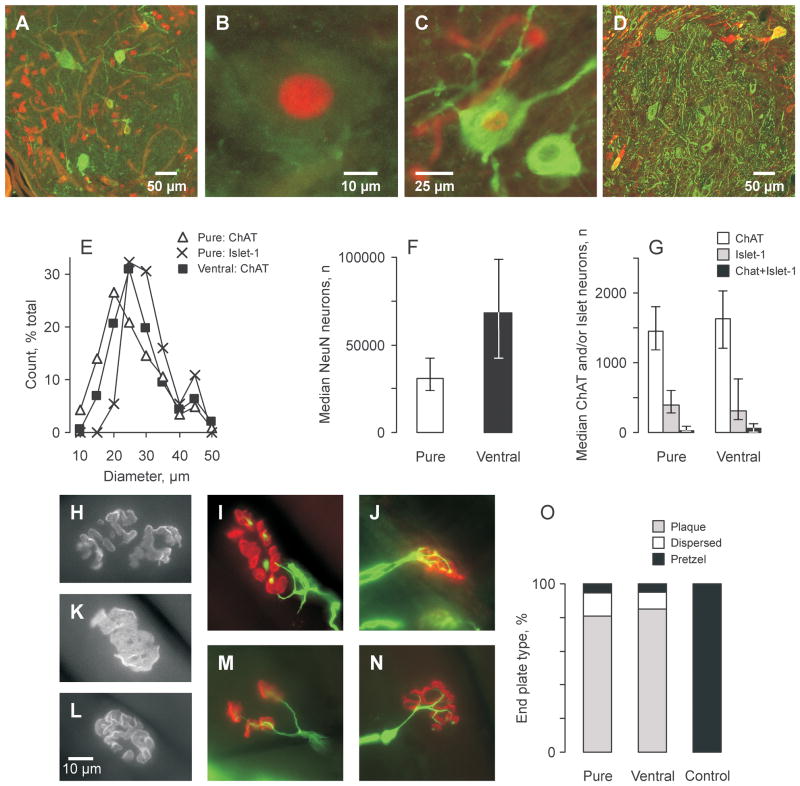
Figure 1.
Motoneurons survive and form neuromuscular junctions. (A–D) Transverse sections of transplants purified for motoneurons after 10 weeks in adult rat tibial nerve stained for NeuN (red) and choline acetyl transferase (ChAT) (green) (A), Islet-1 immunoreactive neurons (ChAT negative) with ChAT-positive axons (B), both ChAT-positive (green) and Islet-1-positive (red) neurons (C), and SM132-positive (green) and ChAT-positive neurons (red) (D). (E–G) Diameter distributions for ChAT-positive neurons across treatments and Islet-1-positive neurons (ChAT-negative) in transplants purified for motoneurons (E). Median (25th and 75th percentile) number of neurons positive for NeuN (F), ChAT alone, Islet-1 alone or both markers across groups (G). Pure = preparations purified for motoneurons; Ventral = ventral preparations. (H–N) End plates identified with α-bungarotoxin binding to acetylcholine receptors (red; neurofilaments, green) in reinnervated muscles (H–M), and 1 control muscle (N). Most end plates were plaques (H, K), or fragmented (I, M). Only a few end plates were pretzels (L). Synaptic vesicles at nerve terminals (red) (J). (O) Proportion of end plate types across groups compared to control muscles.
Neurons in the transplants varied in size. ChAT-positive neuron diameters ranged from 6–49 μm and were similar across groups (Fig. 1E). The distribution for Islet-1-positive neurons in purified preparations was shifted towards larger diameters (range: 16–45 μm). The median diameter for Islet-1-positive neurons (28 μm) was significantly larger than that for ChAT-positive neurons (21 μm). Thus, only a fraction of the neurons that expressed motoneuron markers at 10 weeks in this study were as large as those reported for mature rat motoneurons (23, 24), and our previous results for transplanted neurons that were retrogradely labeled from reinnervated muscle (13).
Transplantation of More Motoneurons Did Not Increase Motoneuron Survival
Most neurons that expressed markers for motoneurons stained for ChAT alone (median: 77 % for both groups). Fewer neurons were positive for Islet-1 alone (18%–22%), or both markers (1%–2%; Fig. 1G). The median number of ChAT-positive neurons, Islet-1-positive neurons, and neurons positive for both of these motoneuron markers was similar for transplants of Pure and Ventral preparations. These results suggest that transplanting more motoneurons into peripheral nerve (Pure vs. Ventral preparations) did not alter the number of neurons that expressed either single or dual motoneuron markers 10 weeks later.
For all motoneuron markers (ChAT and/or Islet-1), mean motoneuron survival was significantly higher for Ventral preparations (3.4% ± 0.7%) vs. Pure preparations (1.5% ± 0.2%, p = 0.021). These results suggest many of the smaller neurons initially present in Ventral preparations expressed motoneuron markers at 10 weeks.
Regenerating Axons Formed Neuromuscular Junctions But the End Plates Lacked Complexity
Axons that regenerated from the transplants formed neuromuscular junctions but the general morphology and size of reinnervated and normally innervated motor end plates differed (Fig. 1H–N). Most reinnervated end plates were simple plaques (81%–85% across groups; Fig. 1H, K). Some reinnervated end plates had a dispersed, fragmented shape (10%–14%; Fig. 1I, M). Only 5% to 6% of reinnervated end plates had an elaborate, complex distribution of acetylcholine receptors with a pretzel-like structure (Fig. 1L), similar to intact end plates (Fig. 1N). The proportion of end plate types differed for reinnervated vs. control muscles (p < 0.001, Fig. 1O). Total end plate area, estimated from α-bungarotoxin staining, was significantly smaller in reinnervated compared to intact muscles, irrespective of group (p = 0.006; median across groups: 319–367 μm2; control: 441 μm2). Both the simple structure and small size of reinnervated end plates suggest that they were immature. Nevertheless, a profusion of positive immunostaining for SV2 (a protein localized to vertebrate synaptic vesicles [21]) at the pre-synaptic nerve terminals of reinnervated muscles suggests the potential for synaptic communication (Fig. 1J).
Small Diameter Axons Regenerated from Transplanted Neurons and Became Myelinated
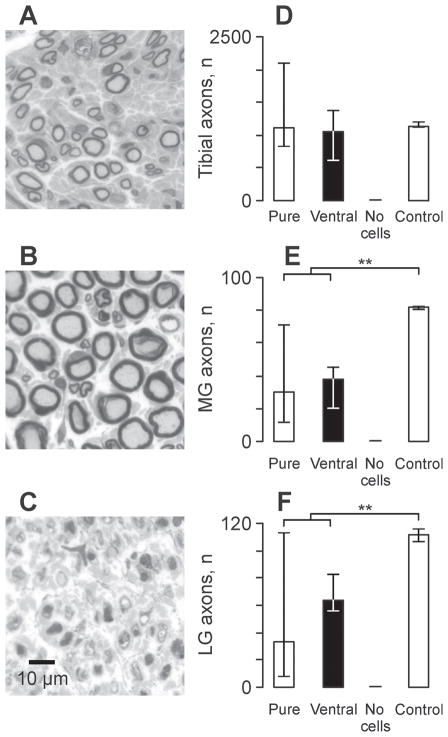
Myelinated axons were evident in the nerves to MG, LG and in the tibial nerve distal to these branches in all animals that received a cell transplant (Fig. 2A). Axons that grew from cell transplants were smaller in diameter than axons in nerves of control animals (Fig. 2B). No axons were present in nerves to muscles of animals that had injection of medium only so these muscles remained denervated (Fig. 2C). For each group, the median number of myelinated axons in the tibial nerve was comparable to values for control animals (Fig. 2D). However, for MG and LG the median axon count for each experimental group was significantly lower than control values (Fig. 2E, F; p = 0.002 and p = 0.01, respectively).
Figure 2.
Axons regenerate from the cell transplants. (A–C) Toluidine blue-stained cross-sections of nerves to lateral gastrocnemius (LG) in an animal that received a transplant purified for motoneurons (A) or a transplant of medium without cells (C) compared to a nerve from a naïve rat (B). (D–F) Median (25th and 75th percentile) number of myelinated axons that grew from the transplant in the tibial nerve (D), and into the nerves to medial gastrocnemius (MG) (E) and (LG) (F) muscles 10 weeks after transplantation. Statistical significance is shown with asterisks (**p ≤ 0.01). Pure = preparations purified for motoneurons; Ventral = ventral preparations.
Reinnervation Reduced Muscle Atrophy and Restored Muscle Excitability
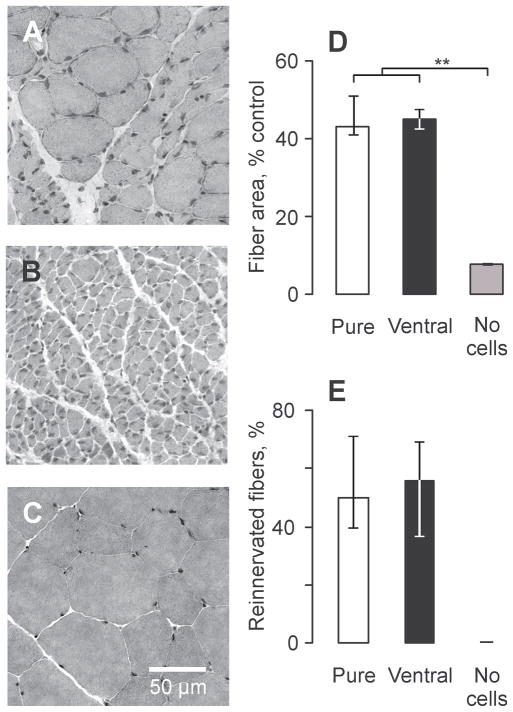
Transplantation of embryonic cells into the tibial nerve significantly reduced muscle atrophy compared to long-term denervation (Fig. 3A, B). The median areas for reinnervated muscle fibers were comparable across groups (Pure: 1125 μm2, Ventral: 1176 μm2), significantly larger than denervated fibers (237 μm2), but smaller than fibers in intact muscles (2539 μm2, p ≤ 0.001, Fig. 3C, D).
Figure 3.
Muscle reinnervation reduces muscle fiber atrophy. (A–C) Cross sections of medial gastrocnemius muscles stained with hematoxylin and eosin taken from an animal that received a transplant of neurons purified for motoneurons (A), a transplant of medium (B) compared to muscle from a naïve animal (C). (D, E) Median (25th and 75th percentile) muscle fiber areas (D) and percentage of reinnervated fibers (E) across groups. Statistical significance is shown with asterisks (**p ≤ 0.01). Pure = preparations purified for motoneurons; Ventral = ventral preparations.
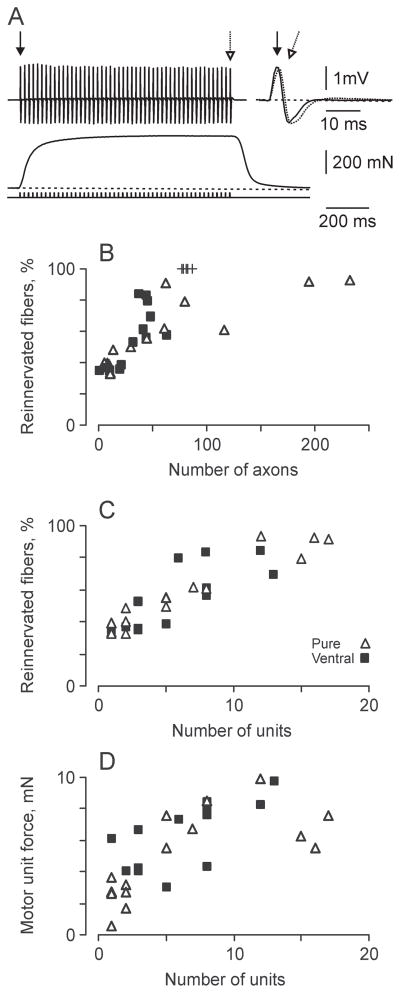
The functional effectiveness of the cell transplantation was assessed after 10 weeks by electrically stimulating the transplants. Visible contractions were evoked in all MG, LG and plantaris muscles when ventral spinal cord cells were transplanted and in 93% of these muscles for purified transplants. Both the EMG and force evoked in MG by electrical stimulation of the transplants were maintained well during stimulation at 50 Hz, suggesting secure neuromuscular transmission (Fig. 4A). Muscle contractions were eliminated following section of the nerves to these muscles, establishing that the evoked muscle responses depended on exciting axons that grew from the transplant. No EMG or force was elicited in muscles of animals that received only medium and no cells, even when the transplants were stimulated with 150V and 1-ms duration pulses, physiological confirmation that these muscles remained denervated long-term.
Figure 4.
Reinnervation is a function of axon and motor unit numbers. (A) Electromyographic activity (EMG) (upper) and force (lower) evoked from a medial gastrocnemius muscle reinnervated from a transplant of purified motoneurons. Different arrows mark the first and last EMG potentials that are also overlaid (right). (B, C) Percentage of reinnervated muscle fibers in relation to number of axons (B) and motor unit number (C). + = Data from control animals. Pure = preparations purified for motoneurons; Ventral = ventral preparations. (D) Mean motor unit force per medial gastrocnemius muscle compared to motor unit number.
The median number of reinnervated MG motor units was comparable across groups even though approximately twice as many motoneurons were transplanted with purified preparations versus Ventral spinal cord cell preparations (median for purified preparations: 6 units, Ventral: 7. Uninjured MG muscles contain a median of 82 motor units, obtained by counting the total number of large diameter (>6 μm) myelinated axons in nerves to MG and halving this value to account for the presence of both motor and sensory axons (5).
Intramuscular Axon Sprouting Facilitated Muscle Reinnervation
The extent of muscle reinnervation was comparable across groups. Half of the MG muscle fibers were reinnervated following transplantation of purified preparations (median: 50%). The corresponding data for Ventral preparations was 56% (Fig. 3E). For a given MG muscle, the extent of reinnervation depended on the number of myelinated axons (Fig. 4B) and reinnervated motor units (Fig. 4C), irrespective of the type of transplant. Linear regression analysis suggested that the entire MG muscle could be reinnervated by 18 motor units following transplantation of either Pure or Ventral cell preparations. Thus, few motoneurons were needed to reinnervate MG muscles completely because intramuscular sprouting of axons was extensive. The limits to axon sprouting may also have been met as average unit forces plateaued at 10 mN when there were 12 to 13 reinnervated units per muscle (Fig. 4D).
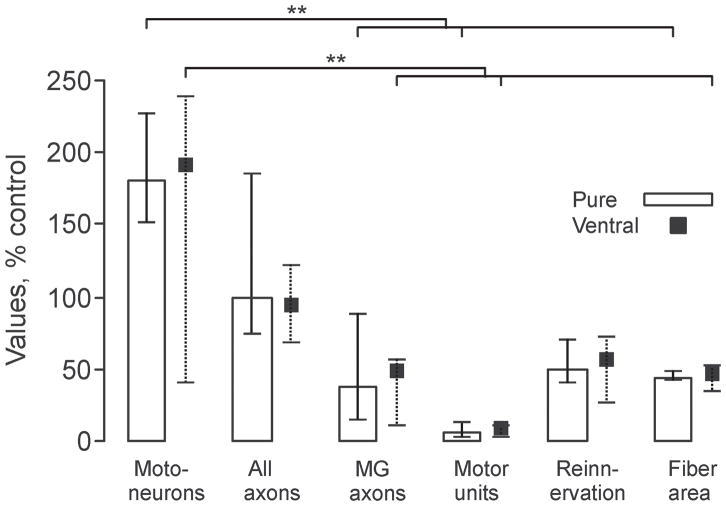
Neuron Survival Exceeded the Number of Reinnervated Motor Units
Figure 5 highlights how each of the measurements varies and contributes to functional outcome. It also identifies the sites of deficits. Median motoneuron survival exceeded the MG axon count, reinnervated motor unit count and reinnervated muscle area for each transplant group (p ≤ 0.001, Fig. 5). The number of myelinated axons in the nerves to MG muscles also exceeded the reinnervated motor unit count by approximately 5 times irrespective of the type of transplant. Despite the small percentage of reinnervated motor units relative to control values, there were significant and similar amounts of muscle reinnervation and recovery of reinnervated muscle fiber areas across the different transplants.
Figure 5.
Motoneuron survival exceeds the motor unit count for each transplant type. Median (25th and 75th percentile) number of neurons positive for choline acetyl transferase (ChAT) and/or Islet-1 (Motoneurons), axons growing from the transplant (All axons) and into the nerve to medial gastrocnemius (MG) axons, MG motor units (Motor units), percentage of reinnervated fibers (Reinnervation) and area of reinnervated muscle fibers (Fiber area) across groups, all expressed relative to values for naïve control animals. Statistical significance is shown with asterisks (**p ≤ 0.01). Pure = preparations purified for motoneurons; Ventral = ventral preparations.
DISCUSSION
Motoneuron survival always exceeded the number of reinnervated motor units when embryonic neurons were transplanted into peripheral nerve. This was the case when ventral spinal cord cells were transplanted or when these cells were purified to increase motoneuron numbers approximately 2-fold (Fig. 5). Thus, muscle reinnervation and function were not limited by the number of motoneurons that were transplanted, or by motoneuron survival. Transplantation of larger neurons, as occurred in purified preparations, also conferred no functional advantage.
An Incomplete Motoneuron Identity May Limit Muscle Reinnervation and Function
A mismatch between the numbers of motoneurons and reinnervated motor units could arise if the transplanted neurons fail to retain a motoneuron identity sufficient to reinnervate muscle, as described in zebrafish (25). During development, transient and selective expression of several transcription factors establishes which neurons become motoneurons (26). This differentiation occurs before motor axon initiation, i.e. largely before we isolated ventral spinal cord cells at E14-15. Differentiation status and ChAT expression can be diminished by lack of trophic support or activity (27–31). Peripheral nerve may not contain all of the factors needed to promote motoneuron differentiation and ChAT expression. The transplanted neurons did not receive supraspinal, spinal, or afferent inputs either. Thus, both inadequate trophic support and activity may constrain or misdirect the identity of some motoneurons after they are transplanted into peripheral nerve such that they become incapable of reinnervating muscle.
The progressive birth of neurons during development (32, 33) also meant that some neurons were less mature when transplanted. These neurons may fail to completely differentiate into motoneurons if all of the signaling required for this is not present in peripheral nerve. Identification of relatively large Islet-1-immunopositive cells that were ChAT-negative (Fig. 1B) suggests incomplete motoneuron differentiation. Islet-1 by itself is inadequate to prompt complete motoneuron differentiation (34). Thus, if muscle reinnervation from neurons in peripheral nerve is to be improved, single or double transduction of genes that control late stage motoneuron differentiation may be needed to overcome the lack of appropriate extrinsic cues (26, 35, 36). Consistent with this suggestion, in vitro studies show that motoneurons isolated from developing spinal cords survive but most of them fail to differentiate completely and establish neuromuscular connections when cultured with myotubes (37, 28).
Motoneuron counts would also exceed motor unit numbers if neurons are unable to produce axons, and axons may also fail to exit the transplant. Local synapses form in the transplant, which will reduce axon and motor unit numbers (14). Some smaller ChAT-positive neurons may be interneurons with short axons that only contribute to neuron counts (38). Growth factors can facilitate motoneuron survival independently of axon regeneration (39), consistent with higher numbers of motoneurons vs. axons in our Pure and Ventral cell transplants (Fig. 5). Long-term delivery of factors or alternative substrates may be necessary to facilitate axon regeneration itself, growth over longer distances (40), or growth within the peripheral nerve environment of an adult animal. If the failure to regenerate axons relates to an inhibitory environment, it may be necessary to neutralize inflammatory cytokines or to digest proteoglycans with chondroitinase, a treatment shown to increase peripheral axon growth several-fold (41, 42).
Immature Neuromuscular Junctions May Limit Muscle Reinnervation and Function
In this study, some muscle fibers were reinnervated 33% to 93% across muscles, whereas other fibers remained denervated. Even though the amount of reinnervation was proportional to the number of myelinated axons and motor units (Fig. 4), myelinated axon numbers were approximately 5 times higher than the motor unit counts (Fig. 5). These results suggest that only axons belonging to certain neurons reinnervated the muscle. Our data suggest that it is the cholinergic neurons that reinnervate muscle. Neurons traced retrograde from our reinnervated muscles are ChAT-positive (13). Reinnervated muscles also have acetylcholinesterase-positive end plates and clustering of acetylcholine receptors, indicative of cholinergic signaling at motor end plates (5, 43–45).
Transplantation of embryonic neurons outside the developing nervous system could also alter neurotransmitter specification (46). Spinal motoneurons can release glutamate and γ-aminobutyric acid, at least transiently (47, 48), and glutaminergic terminals have been observed in adult rat skeletal muscle (49). Thus, growth of non-cholinergic axons into muscle may contribute to the differences in motoneuron, axon and motor unit numbers. Expression of non-cholinergic transmitters could also contribute to the many immature end plates seen in our reinnervated muscles (Fig. 1) and the low numbers of functional motor units.
Another question is whether all of the neuromuscular junctions that formed in our muscles were sufficiently mature to function. When neuromuscular junctions are formed initially, they are simple plaque-like end plates (Fig. 1). Maturation involves pruning to a single axon contact and specialization of the nerve terminal and acetylcholine receptors to form complex end plate structures (50). Only 5% to 6% of our reinnervated end plates had an elaborate, complex distribution of acetylcholine receptors with a pretzel-like structure similar to intact end plates (Fig. 1). Nevertheless, some junctions obviously formed and functioned well. One highlight of this study was the maintenance of evoked EMG and force upon transplant stimulation (Fig. 4A), consistent with the fatigue resistance of muscles reinnervated from embryonic motoneurons in nerve (7, 4).
Muscle activity also plays integral roles in the formation and maturation of neuromuscular junctions (51, 52). There is exchange of signals between the nerve terminal, the Schwann cell, and the muscle fiber during neuromuscular junction formation. These interactions occur over short distances in the embryo and are critical for differentiation of both the post-synaptic muscle cell and the pre-synaptic motoneuron (53, 54). The absence of supraspinal and afferent inputs in our model and the requirement for axon growth over greater distances may mean motoneuron-muscle interactions were suboptimal, limiting muscle reinnervation and function.
Reinnervation Reduced Atrophy and Restored Muscle Function
Of considerable clinical relevance is the extent to which muscle atrophy was reduced by reinnervation. Denervated muscle fiber areas were only 8% of initial 10 weeks after transplantation of medium without cells. In contrast, median areas for reinnervated MG fibers varied from 41% to 45% control across groups (Fig. 3D). These fiber areas are similar to innervated muscle fiber areas after spinal isolation (55), a model that also creates inactive target muscles (56). Linear regression analysis also showed that complete muscle reinnervation was possible with 18 motor units with each type of transplant. Thus, considerable reinnervation occurs from intramuscular axon sprouting (57), a process that may be facilitated in our model since collateral branching of terminal sprouts is more abundant in inactive muscles (58).
The presence of only a few functional motor units following transplantation (Fig. 4) emphasizes that sprouting of motor axons within muscle is fundamental to reinnervation and recovery of muscle function. Low motor unit counts change how muscles function (1). Force gradation (control) will be coarse in a muscle controlled by few motoneurons. Functional reserve will be limited because there are few new motor units to recruit to continue a task, resulting in premature muscle fatigue. Increasing the number of transplanted motoneurons that form functional motor units is therefore an important mechanism to defer weakness and fatigue in these reinnervated muscles.
Neural Replacement Strategies Are Influenced by the Local Environment
Overall, our data suggest that the local peripheral nerve environment shapes motoneuron survival and imposes constraints on motoneuron differentiation, as well as the formation and maturation of neuromuscular junctions. Understanding the regulatory influences of activity on these processes, as well as transmitter specification (59, 29), may improve muscle reinnervation and function. This knowledge is broadly relevant to studies of neuron replacement after injury or disease where there is progressive dysfunction and death of motoneurons (e.g. amyotrophic lateral sclerosis, spinal muscular atrophy). Some neural stem cell transplants do include mixed populations of cells at different stages of differentiation (60–65). A greater understanding of the biology of immature neurons transplanted into the adult peripheral nerve is therefore important clinically because of the ability of these neurons to reinnervate muscle and rescue it from atrophy. Restoration of function by electrical stimulation is a rehabilitation strategy that people with spinal cord injury value (66–67).
In conclusion, we demonstrate that the number of embyronic neurons transplanted into peripheral nerve and motoneuron survival does not limit muscle reinnervation. The low number of reinnervated motor units, and the prevalence of simple motor end plates, suggest muscle reinnervation and function may be limited by incomplete motoneuron differentiation and immature neuromuscular junctions.
Acknowledgments
This research was funded by USPHS grant NS-39098, The Miami Project to Cure Paralysis, and the State of Florida. We acknowledge the Developmental Studies Hybridoma Bank, developed under the auspices of NICHD and maintained by the University of Iowa, for supplying monoclonal antibodies.
The authors thank Ms. Adriana Martinez for completing the references.
References
- 1.Thomas CK, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve. 2006;33:21–41. doi: 10.1002/mus.20400. [DOI] [PubMed] [Google Scholar]
- 2.Schmalbruch H, Al Amood WS, Lewis DM. Morphology of long-term denervated rat soleus muscle and the effect of chronic electrical stimulation. J Physiol. 1991;441:233–41. doi: 10.1113/jphysiol.1991.sp018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–7. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CK, Erb DE, Grumbles RM, et al. Embryonic cord transplants in peripheral nerve restore skeletal muscle function. J Neurophysiol. 2000;84:591–5. doi: 10.1152/jn.2000.84.1.591. [DOI] [PubMed] [Google Scholar]
- 5.Grumbles RM, Wood P, Rudinsky M, et al. Muscle reinnervation with delayed or immediate transplant of embryonic ventral spinal cord cells into adult rat peripheral nerve. Cell Transplant. 2002;11:241–50. [PubMed] [Google Scholar]
- 6.Thomas CK, Sesodia S, Erb DE, et al. Properties of medial gastrocnemius motor units and muscle fibers reinnervated by embryonic ventral spinal cord cells. Exp Neurol. 2003;180:25–31. doi: 10.1016/s0014-4886(02)00024-9. [DOI] [PubMed] [Google Scholar]
- 7.Grumbles RM, Casella GT, Rudinsky MJ, et al. The immunophilin ligand FK506, but not the P38 kinase inhibitor SB203580, improves function of adult rat muscle reinnervated from transplants of embryonic neurons. Neuroscience. 2005;130:619–30. doi: 10.1016/j.neuroscience.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Front Biosci. 2003;8:s522–s532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- 9.Emgard M, Hallin U, Karlsson J, et al. Both apoptosis and necrosis occur early after intracerebral grafting of ventral mesencephalic tissue: a role for protease activation. J Neurochem. 2003;86:1223–32. doi: 10.1046/j.1471-4159.2003.01931.x. [DOI] [PubMed] [Google Scholar]
- 10.Zietlow R, Lane EL, Dunnett SB, et al. Human stem cells for CNS repair. Cell Tissue Res. 2008;331:301–22. doi: 10.1007/s00441-007-0488-1. [DOI] [PubMed] [Google Scholar]
- 11.Hanson MG, Jr, Shen S, Wiemelt AP, et al. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–71. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–40. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 13.Casella GT, Almeida VW, Grumbles RM, et al. Neurotrophic factors improve muscle reinnervation from embryonic neuron. Muscle Nerve. 2010;42:788–97. doi: 10.1002/mus.21757. [DOI] [PubMed] [Google Scholar]
- 14.Grumbles RM, Sesodia S, Wood PM, et al. Neurotrophic factors improve motoneuron survival and function of muscle reinnervated by embryonic neurons. J Neuropathol Exp Neurol. 2009;68:736–46. doi: 10.1097/NEN.0b013e3181a9360f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J Neurosci Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 16.Duong FH, Warter JM, Poindron P, et al. Effect of the nonpeptide neurotrophic compound SR 57746A on the phenotypic survival of purified mouse motoneurons. Br J Pharmacol. 1999;128:1385–92. doi: 10.1038/sj.bjp.0702910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haastert K, Grosskreutz J, Jaeckel M, et al. Rat embryonic motoneurons in long-term co-culture with Schwann cells-a system to investigate motoneuron diseases on a cellular level in vitro. J Neurosci Methods. 2005;142:275–84. doi: 10.1016/j.jneumeth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–30. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basgen JM, Nicholas SB, Mauer M, et al. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148. doi: 10.1159/000092905. [DOI] [PubMed] [Google Scholar]
- 20.Howard CU, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. Oxford: BIOS Scientific Publishers Ltd; 1998. [Google Scholar]
- 21.Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–94. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grumbles RM, Almeida VW, Thomas CK. Embryonic neurons transplanted into the tibial nerve reinnervate muscle and reduce atrophy but NCAM expression persists. Neurol Res. 2008;30:183–9. doi: 10.1179/174313208X281073. [DOI] [PubMed] [Google Scholar]
- 23.Kernell D. The motoneurone and its muscle fibres-Monographs of the Physiological Society. New York: Oxford University Press, Inc; 2006. [Google Scholar]
- 24.Vult von Steyern F, Martinov V, Rabben I, et al. The homeodomain transcription factors Islet 1 and HB9 are expressed in adult alpha and gamma motoneurons identified by selective retrograde tracing. Eur J Neurosci. 1999;11:2093–102. doi: 10.1046/j.1460-9568.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 25.Reimer MM, Sorensen I, Kuscha V, et al. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510–6. doi: 10.1523/JNEUROSCI.1189-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol. 2008;18:36–43. doi: 10.1016/j.conb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–5. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol Rev. 1998;78:143–70. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 29.Root CM, Velazquez-Ulloa NA, Monsalve GC, et al. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–84. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer NC, Gu X, Olson E. Action potentials, calcium transients and the control of differentiation of excitable cells. Curr Opin Neurobiol. 1994;4:70–7. doi: 10.1016/0959-4388(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Nagao M, Sugimori M, et al. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci. 2001;21:9814–23. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nornes HO, Das GD. Temporal pattern of neurogenesis in spinal cord: cytoarchitecture and directed growth of axons. Proc Natl Acad Sci USA. 1972;69:1962–6. doi: 10.1073/pnas.69.7.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Henderson CE. Patterns of programmed cell death in populations of developing spinal motoneurons in chicken, mouse, and rat. Dev Biol. 1999;214:60–71. doi: 10.1006/dbio.1999.9413. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- 35.Roybon L, Hjalt T, Christophersen NS, et al. Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, Msx1, Ngn2, and Pitx3. J Neurosci. 2008;28:3644–56. doi: 10.1523/JNEUROSCI.0311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijeyekoon R, Barker RA. Cell replacement therapy for Parkinson’s disease. Biochim Biophys Acta. 2009;1792:688–702. doi: 10.1016/j.bbadis.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Berg DK, Fischbach GD. Enrichment of spinal cord cell cultures with motoneurons. J Cell Biol. 1978;77:83–98. doi: 10.1083/jcb.77.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber RP, Phelps PE, Houser CR, et al. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–46. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg JL, Espinosa JS, Xu Y, et al. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–92. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krekoski CA, Neubauer D, Zuo J, et al. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–13. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muir D. The potentiation of peripheral nerve sheaths in regeneration and repair. Exp Neurol. 2010;223:102–11. doi: 10.1016/j.expneurol.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 43.Erb DE, Mora RJ, Bunge RP. Reinnervation of adult rat gastrocnemius muscle by embryonic motoneurons transplanted into the axotomized tibial nerve. Exp Neurol. 1993;124:372–6. doi: 10.1006/exnr.1993.1208. [DOI] [PubMed] [Google Scholar]
- 44.Cohen MW, Weldon PR. Localization of acetylcholine receptors and synaptic ultrastructure at nerve-muscle contacts in culture: dependence on nerve type. J Cell Biol. 1980;86:388–401. doi: 10.1083/jcb.86.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–21. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Ma W, Behar T, Barker JL. Transient expression of GABA immunoreactivity in the developing rat spinal cord. J Comp Neurol. 1992;325:271–90. doi: 10.1002/cne.903250210. [DOI] [PubMed] [Google Scholar]
- 48.Mentis GZ, Alvarez FJ, Bonnot A, et al. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci USA. 2005;102:7344–9. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunelli G, Spano P, Barlati S, et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci USA. 2005;102:8752–7. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slater CR. Neuromuscular Junction (NMJ): Mammalian Development. In: Larry R, editor. Encyclopedia of Neuroscience. Squire Academic Press; 2009. pp. 585–93. [Google Scholar]
- 51.Nick TA, Ribera AB. Synaptic activity modulates presynaptic excitability. Nat Neurosci. 2000;3:142–9. doi: 10.1038/72082. [DOI] [PubMed] [Google Scholar]
- 52.Thompson W, Jansen JK. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2:523–35. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- 53.Cao G, Ko CP. Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses. J Neurosci. 2007;27:6712–22. doi: 10.1523/JNEUROSCI.1329-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 55.Tower SS. Function and structure in the chronically isolated lumbo-sacral spinal cord of the dog. J Comp Neurol. 1937;67:109–31. [Google Scholar]
- 56.Pierotti DJ, Roy RR, Bodine-Fowler SC, et al. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J Physiol. 1991;444:175–92. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tam SL, Archibald V, Jassar B, et al. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–67. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eldridge L, Liebhold M, Steinbach JH. Alterations in cat skeletal neuromuscular junctions following prolonged inactivity. J Physiol. 1981;313:529–45. doi: 10.1113/jphysiol.1981.sp013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okado N, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J Neurosci. 1984;4:1639–52. doi: 10.1523/JNEUROSCI.04-06-01639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garbuzova-Davis S, Sanberg PR. Feasibility of cell therapy for amyotrophic lateral sclerosis. Exp Neurol. 2009;216:3–6. doi: 10.1016/j.expneurol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Lowry N, Goderie SK, Adamo M, et al. Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol. 2008;209:510–22. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Magnus T, Liu Y, Parker GC, et al. Stem cell myths. Philos Trans R Soc Lond B Biol Sci. 2008;363:9–22. doi: 10.1098/rstb.2006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronsyn MW, Berneman ZN, Van TV, et al. Can cell therapy heal a spinal cord injury? Spinal Cord. 2008;46:532–9. doi: 10.1038/sc.2008.13. [DOI] [PubMed] [Google Scholar]
- 64.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:1–22. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang SC, Li XJ, Johnson MA, et al. Human embryonic stem cells for brain repair? Philos Trans R Soc Lond B Biol Sci. 2008;363:87–99. doi: 10.1098/rstb.2006.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KD. Consideration of user priorities when developing neural prosthetics. J Neural Eng. 2009;6:55003. doi: 10.1088/1741-2560/6/5/055003. [DOI] [PubMed] [Google Scholar]
- 67.Brown-Triolo DL, Roach MJ, Nelson K, et al. Consumer perspectives on mobility: implications for neuroprosthesis design. J Rehabil Res Dev. 2002;39:659–69. [PubMed] [Google Scholar]