Abstract
High mobility group box 1 protein (HMGB1), a critical proinflammatory cytokine, has recently been identified to be an immunostimulatory signal involved in sepsis-related immune dysfunction when released extracellularly, but the potential mechanism involved remains elusive. Here, we showed that the treatment with HMGB1 in vitro inhibited T lymphocyte immune response and expression of mitofusin-2 (Mfn-2; a member of the mitofusin family) in a dose- and time-dependent manner. Upregulation of Mfn-2 expression attenuated the suppressive effect of HMGB1 on T cell immune function. The phosphorylation of both extracellular signal-regulated kinase (ERK)1/2 and p38 mitogen-activated protein kinase (MAPK) was markedly upregulated by treating with high amount of HMGB1, while pretreatment with ERK1/2 and p38 MAPK-specific inhibitors (U0126 and SB203580) could attenuate suppression of T cell immune function and nuclear factor of activated T cell (NFAT) activation induced by HMGB1, respectively. HMGB1-induced activity of ERK1/2 and p38 was not fully inhibited in the presence of U0126 or SB203580. Interestingly, overexpression of Mfn-2 had no marked effect on HMGB1-mediated activation of MAPK, but could attenuate the suppressive effect of HMGB1 on the activity of NFAT. Thus, the mechanisms involved in HMGB1-induced T cell immune dysfunction in vitro at least partly include suppression of Mfn-2 expression, overactivation of ERK1/2, p38 MAPK, and intervention of NFAT activation, while the protective effect of Mfn-2 on T cell immune dysfunction induced by HMGB1 is dependent on other signaling pathway associated with NFAT, but not MAPK. Taken together, we conclude that overactivation of MAPK and suppression of Mfn-2 expression are two independent events in HMGB1-mediated T cell immune dysfunction.
Introduction
High mobility group box-1 protein (HMGB1) is a nonhistone DNA-binding protein that is commonly stored in the nucleus and plays a critical role in regulating gene transcription. Recently, HMGB1 was identified to be present extracellularly after active secretion (particularly by inflammatory cells) or passive release by necrotic cells, and it functions as a late proinflammatory cytokine involved in severe sepsis (Wang and others 1999; Huang and others 2010). Simultaneously, HMGB1 acts as a chemoattractant and activator of immune cells, such as dendritic cells (DCs) (Yang and others 2007), and is thus increasingly recognized as an immune alarmin. Further, evidence suggests that the excessive release of HMGB1 contributes to the development of immunosuppressive state after sepsis or severe trauma (Lantos and others 2010). T cells are major effectors and modulators of many immune responses and play a pivotal role in the development of various immune diseases. Dysfunction of T cell-mediated immunity has been increasingly recognized as an important step in the pathogenesis of severe trauma and sepsis, which contributes to diminished host resistance to infection (Kerksiek and Pamer 1999; Sir and others 2000). Our previous data showed that high levels of systemic HMGB1 were closely associated with T cell-mediated immunosuppression in both thermal injured animals and septic patients (Yao and Lin 2008; Zhang and others 2008). We also found that HMGB1 had a dual regulatory effect on immune functions of mice CD4+ T cell in vitro along with the different concentrations and stimulated duration (Zhao and others 2012). Additionally, HMGB1 is necessary for proliferation, survival, and polarization of naive CD4+ T cells after activation by allogeneic DCs (Zetterström and others 2002). In spite of these evidence that HMGB1 might exert a profound influence on immune functions of T cells, the precise regulatory mechanisms involved have not been clarified.
Mitofusin-2 (Mfn-2), a highly conserved transmembrane GTPase, localizes in outer membrane of mitochondria and plays a critical role in mitochondrial fusion process. Recent data have demonstrated that Mfn-2 is involved in the regulation of several crucial cellular pathways beyond fusion, including mitochondrial metabolism, cellular signaling cascade, apoptosis, and proliferation (de Brito and Scorrano 2008). Moreover, it was reported that Mfn-2 acted as an endogenous Ras inhibitor and inhibited Ras-activated mitogen-activated protein kinase (MAPK) signaling in vascular smooth muscle cells and cancer cell lines (Chen and others 2004). Although our previous study demonstrated that upregulation of Mfn-2 expression attenuated the immunosuppressive effect of HMGB1 on CD4+ T lymphocytes (Zhao and others 2012), the regulative signaling pathways of Mfn-2 in HMGB1-induced T cells immune dysfunction remain unclear.
MAPK is a key player in cellular signaling pathway and can be divided into several subgroups, among which extracellular signal-regulated kinase (ERK)1/2, c-jun amino-terminal kinase (JNK)/SAPK, and p38 are the three best characterized ones (Chang and Karin 2001). Many studies have implicated MAPK pathways as pivotal signaling pathways involved in thymocyte differentiation and T cell responses (Rincón and others 2000; Zhang and Dong 2005). MAPK activation is associated with inflammatory cytokines, genotoxic agents, ultraviolet light, and heat shock proteins (Seger and Krebs 1995; Chen and Thorner 2007). It has previously also been demonstrated that HMGB1 induces a transient phosphorylation of MAPKs and a nuclear translocation of nuclear factor kappaB (NF-κB) in outgrowing neurites, certain tumor cells, and human microvascular endothelial cells (Huttunen and others 1999; Taguchi and others 2000; Fiuza and others 2003).
In the present study, we investigated that the role of MAPK in T cell-mediated immune property induced by HMGB1 in vitro, and determined whether the regulatory role of Mfn-2 in HMGB1-mediated T cells immune dysfunction was associated with its inhibitory effect on activation of MAPK.
Materials and Methods
Materials
Phorbol-12-myristate-13-acetate (PMA), ionomycin, and Mfn-2 primary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). RPMI 1640 medium and other cell culture products were obtained from TianRunShanda Biotech Co. Ltd (Beijing, China). Fetal bovine serum was obtained from HyClone Laboratories (Logan, UT). U0126, SP600125, and SB203580 were purchased from Merck (Rahway, NJ). Recombinant human HMGB1 was purchased from R&D Systems (Minneapolis, MN). Interleukin (IL)-2, IL-4, and interferon (IFN)-γ enzyme-linked immunosorbent assay (ELISA) kits were obtained from Biosource (Worcester, MA). ERK1/2, p38, and JNK fast-activated cell-based ELISA (FACE™) kits were obtained from Active Motif (Carlsbad, CA). Monoclonal antibody to β-actin, poly-l-lysine, and methyl-thiazolyl-tetrazolium (MTT) was purchased from Sigma-Aldrich (St. Louis, MO). Trizol reagent was obtained from Invitrogen (California, CA). The phycoerythrin (PE) Annexin V apoptosis detection kit (annexin-V-PE and 7-amino-actinomycin D) was purchased from BD/PharMingen (San Diego, CA).
Cell culture and treatment
Jurkat E6-1 cells (purchased from the Cell Resource Center, Chinese Academy of Medical Sciences, Beijing, China) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, and 2 mM l-glutamine at 37°C in a humidified incubator with 5% CO2. For experiment, Jurkat cells were stimulated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, then recombinant HMGBl in different amount or phosphate buffered saline (PBS) was added to the T cell suspension, or cells were pretreated for 1 h with p38 MAPK inhibitor SB203580 (5 μM), ERK1/2 inhibitor U0126 (5 μM), or dimethyl sulfoxide (1 μL) before HMGB1 stimulation. The final concentration and duration of HMGB1 treatment in the present study were set as follows: 10, 100, and 1,000 ng/mL for 24 h, or 100 ng/mL HMGB1 for 12, 24, and 48 h as indicated in each experiment, respectively. All materials and compounds used in these experiments were sterile and endotoxin-free.
Lentiviral transduction
A full-length human Mfn-2 cDNA was obtained from Genscrip Corporation (Piscataway, NJ). Lentrivirus vectors expressing the DNA fragments encoding green fluorescent protein (GFP)-tagged full-length Mfn-2 of human (Lv-Mfn-2) were constructed, packed, and purified by GeneChem Co., Ltd. (Shanghai, China). Lentiviral transduction of Jurkat cells was done according to the protocol provided by the manufacturer. In brief, cells were grown to log phase, harvested, and washed twice with PBS. Cells were resuspended to 1×106 per mL in 4 mL of complete medium (RPMI 1640 with 10% fetal bovine serum) in 25-cm2 dishes. Lentivirus was added to a final multiplicity of infection (MOI) of 50 colony-forming units per cell. In initial experiments, polybrene was added to give a final concentration of 8 μg/mL. The cells were then incubated for a further 8 h at 37°C in CO2 incubator before the cells were washed and replated in fresh media without polybrene. After 48 h of culture to allow gene expression, transduced cells were resuspended to 5×105 per mL in fresh medium and placed in 96-well round-bottom plates.
T cell proliferation assay
Jurkat E6-1 cells (5×105 cells/mL) were inoculated to 96-well plates with 0.2 mL per well and treated with or without PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, recombinant HMGBl in different amount or PBS were added to the cell suspension. Then, 100 μL supernatant was procured after 10, 100, and 1,000 ng/mL HMGB1 for 24 h, or 100 ng/mL HMGB1 for 12, 24, and 48 h. About 20 μL MTT was added to each well. After culturing for 4 h, about 100 μL Triton-ISOP solution was added to dissolve the MTT crystals. When all the crystals had been dissolved through repeated blowing with a pipet, the optical density was measured by a microplate reader (Spectra MR; Dynex, Richfield, MN) at a wavelength of 540 nm.
Enzyme-linked immunosorbent assay
IL-2, IL-4, and IFN-γ levels in culture supernatants were quantitated by commercially available ELISA kits for human. ELISA was performed strictly following the protocols provided by the manufacturer. Plates were read in a microplate reader (Spectra MR; Dynex).
Assessment of MAPK expression and activation
ERK1/2, p38, and JNK phosphorylation levels in culture Jurkat cells stimulated by HMGB1 were measured by commercially available fast-activated cell-based ELISA (FACE) kits. ELISA was performed strictly following the protocols provided by the manufacturer, according to the methods previously described (Versteeg and others 2000). The absorbance of each well in plates was measured in a microplate reader (Spectra MR; Dynex) at a wavelength of 450 nm. Data were corrected with cell number performed through use of Crystal Violet.
Western blotting
Cell lysate that contained 40 μg of protein in sodium dodecyl sulfate (SDS)-Laemmli loading buffer per lane was separated by 8%–10% SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After transfer, membranes were blocked in Tris-buffered saline [20 mM Trisbase (pH 7.6), 150 mM sodium chloride] containing 5% nonfat milk (BioRad, Hercules, CA) and incubated for 1 h with primary antibodies, followed by incubation with a secondary antibody. The blots were detected with an ECL system (Amersham Biosciences, Uppsala, Sweden). The protein bands were quantified by densitometry using National Institutes of Health ImageJ software.
Real-time polymerase chain reaction
Total RNA isolation system and reverse transcription system were purchased from Promega Corp. (Madison, WI). Total RNA was extracted from cultured cells using Trizol reagent according to the manufacturer's instruction. The concentration of purified total RNA was determined spectrophotometrically at 260 nm. The mRNA for Mfn-2 and β-actin was quantified in duplicate by SYBR Green 2-step, real-time reverse transcription polymerase chain reaction (PCR). After removal of potentially contaminating DNA with DNase I, 1 μg of total RNA from each sample was used for reverse transcription with an oligo dt and a Superscript II to generate first-strand cDNA. PCR mixture was prepared using SYBR Green PCR Master Mix with the following primers: Mfn-2, 5′-TGG CTC AAG ACT ATA AGC TGC G-3′ (forward) and 5′-GAG GAC TAC TGG AGA AGG GTG G-3′ (reverse); β-actin, 5′-TGA CGT GGA CAT CCG CAA AG-3′ (forward) and 5′-CTG GAA GGT GGA CAG CGA GG-3′ (reverse). Thermal cycling conditions were 20 s at 95°C followed by 40 cycles of 95°C for 3 s, 60°C for 30 s on a sequence detection system (ABI RPISM 7500; Applied Biosystems, Foster City, CA). Mfn-2 gene expression was normalized with β-actin mRNA content.
Assay of nuclear factor of activated T cell activity
Nuclear protein was extracted from cultured cells using nuclear extract kit (Active Motif ) and the protocol was based on samples of 107 cells, avoiding freeze/thaw cycles. Cells were washed, and collected in ice-cold PBS in the presence of phosphatase inhibitors to limit further protein modifications. Then, cells were resuspended in a hypotonic buffer and treated with detergent to allow leakage of the cytoplasmic proteins into the supernatant. After collection of the cytoplasmic fraction, the nuclei were lysed, and nuclear proteins were solubilized in lysis buffer containing protease inhibition cocktail. Protein concentrations were determined by the Bradford protein assay kit (Applygen Technologies Inc., Beijing, China). The ELISA-based electrophoretic mobility shift assay (transAM™ NFAT kit; Active Motif ) was used to quantify the amount of active nuclear factor of activated T cell (NFAT) in nuclei (6 μg). Briefly, the NFAT activity was purified from a nuclear lysate upon binding an immobilized oligonucleotide containing a 5′-AGGAAA-3′ motif, and detected by ELISA.
Statistical analysis
All results were expressed as mean±standard deviation (SD) of more than three independent experiments conducted in triplicate, and analyzed with a one-way analysis of variance. Fisher's least significant difference was used to evaluate significant differences between groups. P values <0.05 were considered statistically significant.
Results
The effect of HMGB1 stimulation on immune dysfunction of T lymphocytes
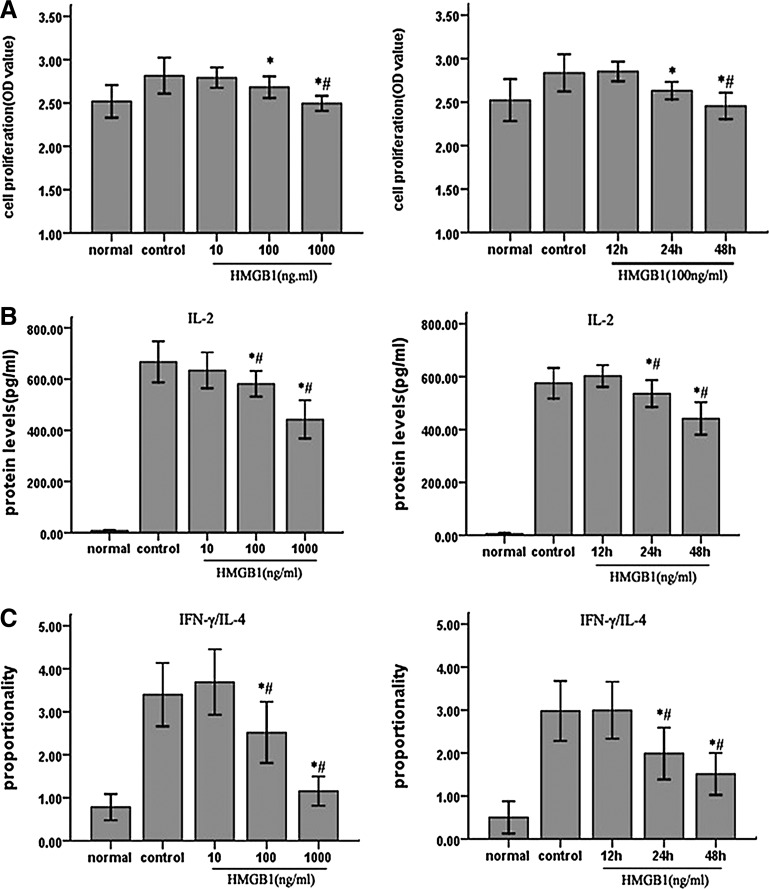
To investigate the influence of HMGB1 on T cell-mediated immune function, Jurkat cells were treated with different concentrations of HMGB1 for different duration. In our experiments, the immune function of T lymphocytes included T cell proliferation, IL-2 production, and ratio of IFN-γ (Th1 cytokine)/IL-4 (Th2 cytokine), which, respectively, reflecting differentiation of T lymphocytes, and was assessed by MTT and ELISA, respectively. As shown in Fig. 1, treatment with HMGB1 for 24 h resulted in a dose-dependent immunosuppression of T lymphocytes, which was in agreement with our previous results. Cells were treated with PMA/ionomycin and without HMGB1 as control, and T cells incubated with increasing concentrations of HMGB1 (10, 100, 1,000 ng/mL) for 24 h resulted in suppression of proliferation (P<0.05) as well as IL-2 production (P<0.05), and a decrease in IFN-γ/IL-4 ratio (P<0.05). When T cells were exposed to 100 ng/mL HMGB1 for different duration (12, 24, 48 h), immune function of T lymphocytes was markedly inhibited (P<0.05). Thus, cultured cells were exposed to HMGB1 at 100 ng/mL for 24 h in the following experiments.
FIG. 1.
The effect of HMGB1 on immune function of Jurkat cells in response to PMA/ionomycin. Jurkat cells were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with different dosages of HMGB1 (10, 100 and 1,000 ng/mL) for 24 h or with 100 ng/mL HMGB1 for different duration (12, 24, and 48 h). Cells cultured under normal condition served as baseline, and cells treated with PMA/ionomycin and 0 ng/mL HMGB1 or 100 ng/mL HMGB1 for 0 h served as controls. Methyl-thiazolyl-tetrazolium cell proliferation assay was used to assess Jurkat cell proliferative activity after HMGB1 stimulation, respectively (A). In addition, the levels of IL-2, IL-4, and IFN-γ were determined by enzyme-linked immunosorbent assay (B, C). Results of four independent experiments were shown as mean±standard deviation. *Statistically significant difference when compared with the controls (P<0.05). #Statistically significant difference when compared with HMGB1-10 ng/mL group (P<0.05). HMGB1, high mobility group box 1 protein; PMA, phorbol-12-myristate-13-acetate; IL, interleukin; IFN, interferon.
Upregulation of Mfn-2 expression attenuated the suppressive effect of HMGB1 on T cell immune function
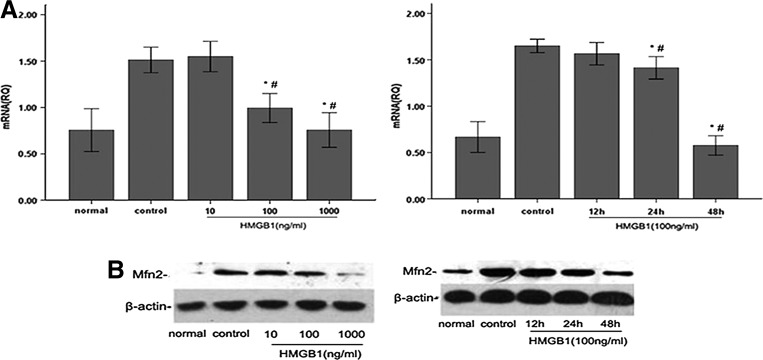
Previously, we reported that the expression of Mfn-2 was decreased in mouse CD4+ T lymphocytes when stimulated with a high dose of HMGB1. On the other hand, upregulation of Mfn-2 could diminish the suppressive effect of HMGB1 on CD4+ T lymphocytes (Zhao and others 2012). To assess whether Mfn-2 can exert the similar effect on human T cells, we detected the Mfn-2 expression by real-time PCR and western blot after stimulation of T cells with various doses of HMGB1 for different duration. Fig. 2A and 2B illustrated that incubation of Jurkat cells with HMGB1 (10, 100, 1,000 ng/mL) for 24 h induced dose-dependent reduction in Mfn-2 mRNA and protein expression, reaching their minimum with 1,000 ng/mL HMGB1. Moreover, the results also revealed the time-dependent inhibitory effect of Mfn-2 expression in Jurkat cells treated by HMGB1 (100 ng/mL), and the inhibitory effect of Mfn-2 expression reached its minimum after 48 h.
FIG. 2.
Effect of HMGB1 on Mfn-2 expression in response to PMA/ionomycin in Jurkat cells. Jurkat cells were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with different dosages of HMGB1 (10, 100, and 1,000 ng/mL) for 24 h or with 100 ng/mL HMGB1 for different duration (12, 24, and 48 h). Cells cultured normally served as baseline and cells treated with PMA/ionomycin and 0 ng/mL HMGB1 or 100 ng/mL HMGB1 for 0 h served as controls. Mfn-2 mRNA expression by real-time PCR in Jurkat cells in response to PMA/ionomycin with increasing dosages of HMGB1 for 24 h or with 100 ng/mL HMGB1 for different duration (n=4 for each time point), respectively (A). A typical western blot and the average increase in Mfn-2 protein abundance in response to PMA/ionomycin with different concentrations of HMGB1 for 24 h or with 100 ng/mL HMGB1 for different duration (n=4 for each time point), respectively (B). Results were shown as mean±standard deviation. *Statistically significant difference when compared with the controls (P<0.05). #Statistically significant difference when compared with HMGB1-10 ng/mL group or HMGB1-12 h group (P<0.05). Mfn-2, mitofusin-2; PCR, polymerase chain reaction.
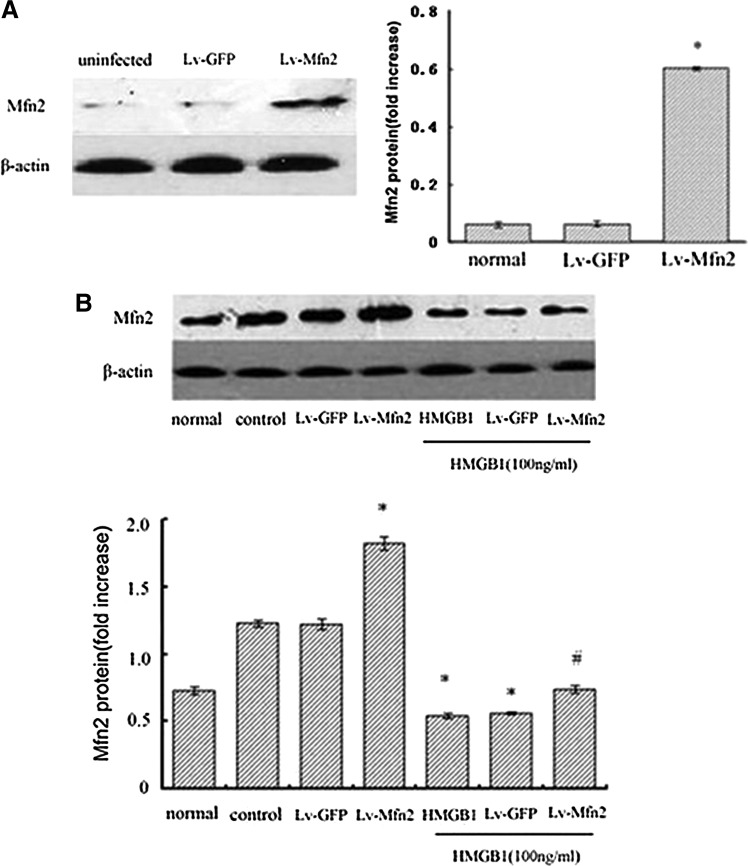
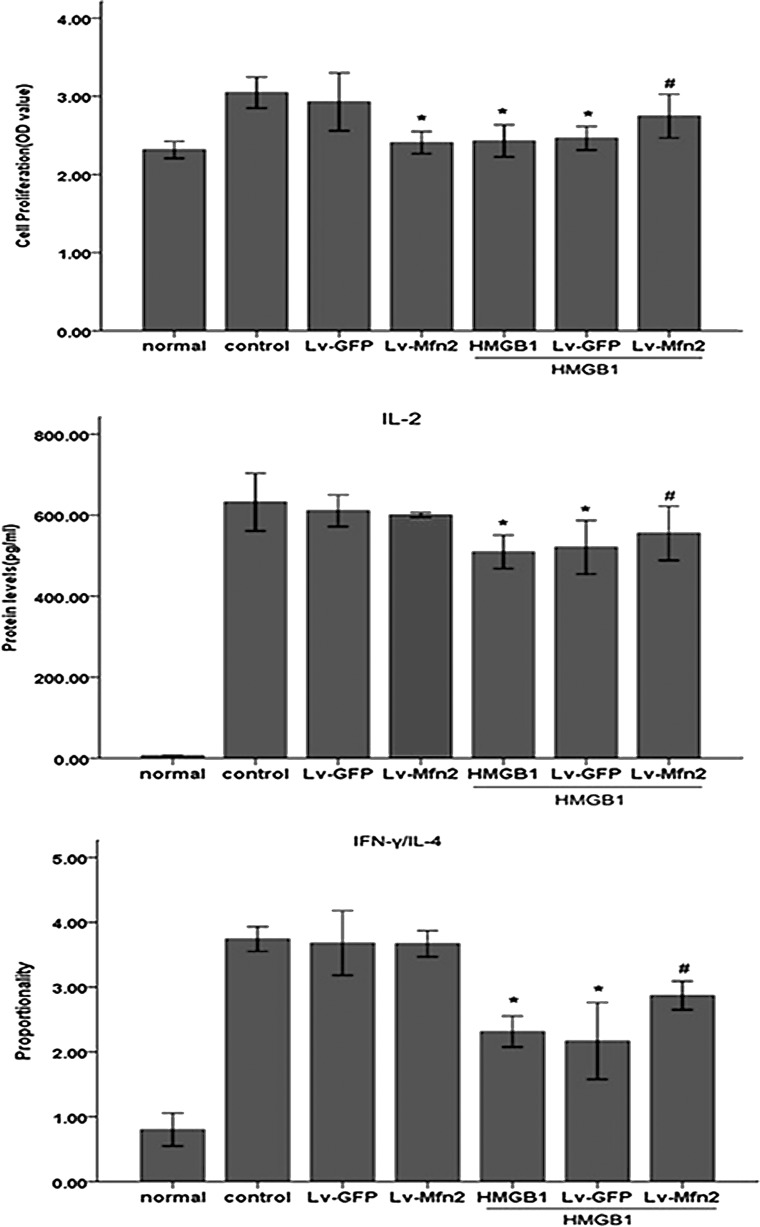
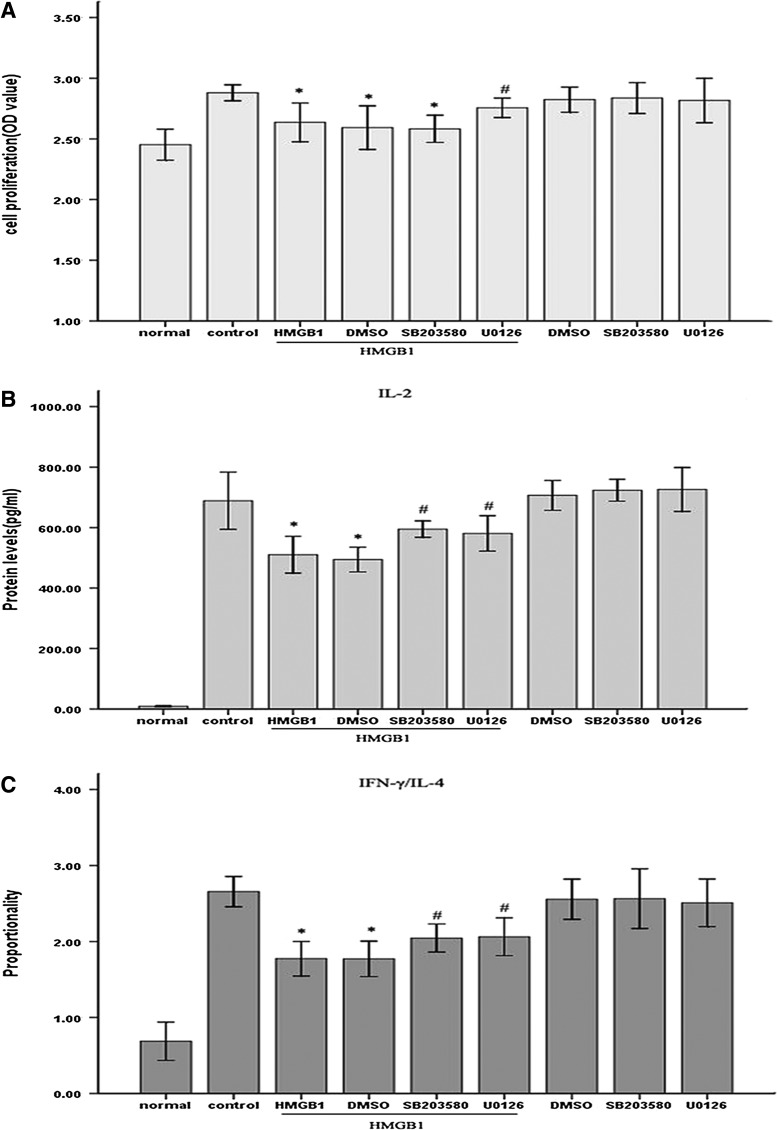
Subsequently, we made an attempt to determine whether Mfn-2 is necessary for HMGB1-induced T cell immune dysfunction by using lentivirus-mediated overexpression of Mfn-2. As shown in Fig. 3, infection of Jurkat cells with Lv-Mfn-2 (MOI=50) displayed a profound increase in Mfn-2 expression and did not completely prevent but attenuated HMGB1-induced downregulation of Mfn-2 compared with uninfected cells or those infected with the empty lentiviral vector, Lv-GFP (MOI=50). More importantly, we found that HMGB1-mediated inhibition of T cell proliferation and IL-2 production and decrease in IFN-γ/IL-4 were obviously attenuated in cells infected with Lv-Mfn-2 compared with uninfected cells and cells infected with Lv-GFP (Fig. 4) (all P<0.05), indicating that Mfn-2 is required for HMGB1-induced T cell immune dysfunction and upregulation of Mfn-2 expression markedly protected T cells against HMGB1-induced immunosuppression.
FIG. 3.
Effect of Lv-Mfn-2 transfection on Mfn-2 expression in Jurkat cells. Overexpression of Mfn-2 by lentivirus gene transfer (MOI=50) was shown by western blot with an anti-Mfn-2 antibody (n=4). *Statistically significant difference when compared with the controls or Lv-GFP group (P<0.05) (A). Jurkat cells transfected with Lv-Mfn-2 or Lv-GFP (MOI=50) were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with HMGB1 (100 ng/mL for 24 h) or without HMGB1. Mfn-2 protein abundance in Jurkat cells in presence or absence of HMGB1 were assessed by a typical western blot and average values, respectively (B). Mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1 treated group (P<0.05). MOI, multiplicity of infection; GFP, green fluorescent protein.
FIG. 4.
Overexpression of Mfn-2 attenuated HMGB1-induced immune dysfunction of Jurkat cells. Jurkat cells transfected with Lv-Mfn-2 or Lv-GFP (MOI=50) were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with HMGB1 (100 ng/mL for 24 h) or without HMGB1. Methyl-thiazolyl-tetrazolium cell proliferation assay was used to assess Jurkat cell proliferative activity after HMGB1 stimulation. In addition, levels of IL-2, IL-4, as well as IFN-γ were determined by enzyme-linked immunosorbent assay. Mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1-treated group (P<0.05).
HMGB1 induced immunosuppression of T lymphocytes through ERK1/2 and p38 MAPK but not JNK MAPK
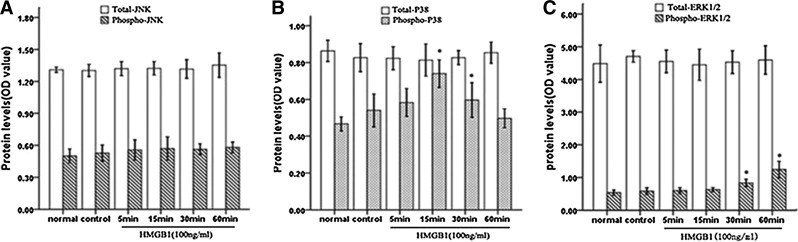
To determine whether treatment with HMGB1 could activate MAPK in Jurkat cells, we examined the phosphorylation of ERK1/2, p38 MAPK, and SAPK/JNK after HMGB1 stimulation with fast-activated cell-based (FACE) ELISA. As shown in Fig. 5, it was revealed that the exposure of cells to HMGB1 induced the activation of both ERK1/2 and p38 MAPK in a time-dependent manner, without obvious changes in phosphorylation levels of JNK. Simultaneously, no significant changes were observed in whole protein levels of these MAPKs. Treatment with HMGB1 induced the progressive accumulation of phosphorylated ERK1/2 starting from 30 min to 1 h (all P<0.05). In contrast, we detected a rapid activation of p38 MAPK. Indeed, there was an increase in phosphorylation levels of p38 MAPK within 5 min after HMGB1 exposure and retained its activity for 30 min after HMGB1 treatment, peaking at 15 min (P<0.05).
FIG. 5.
HMGB1 induced a transient phosphorylation of ERK1/2 and p38 MAPK. Jurkat cells were cultured in 96-well plates with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with HMGB1 (100 ng/mL) for different duration or without HMGB1 (control). The phosphorylation of JNK (A), p38 MAPK (B), and ERK1/2 (C) was assayed in triplicate using the phosphor-specific JNK, ERK1/2, and p38 antibody (from FACE kits) at various time points (5, 15, 30, or 60 min), respectively, while total JNK (A), p38 MAPK (B), and ERK1/2 (C) was determined using the total-specific JNK, ERK1/2, and p38 antibody from the FACE kits. Cell numbers were normalized using crystal violet. Mean values of three individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). ERK1/2, extracellular signal-regulated kinase 1/2; JNK, c-jun amino-terminal kinase; MAPK, mitogen-activated protein kinase.
To clearly elucidate the potential role of ERK1/2 and p38 MAPK in HMGB1-induced T cell immunosuppression, immune function of T cells was measured after HMGB1 stimulation in the presence or absence of ERK1/2-specific inhibitor, 5 μM of U0126 and p38 specific inhibitor, or 5 μM of SB203580. HMGB1-induced T cells immunosuppression were markedly attenuated by inhibiting the activation of ERK1/2 or p38 MAPK (Fig. 6A–C). Pretreatment with U0126 notably attenuated HMGB1-mediated inhibition of T cell proliferation (Fig. 6A) and IL-2 production (Fig. 6B), and lowering of IFN-γ/IL-4 (all P<0.05) (Fig. 6C), while SB203580 exerted no marked influence on HMGB1-mediated inhibition of cell proliferation (Fig. 6A) but was able to ameliorate suppression of IL-2 production (Fig. 6B) as well as IFN-γ/IL-4 ratio (Fig. 6C) (all P<0.05). Further, 5 μM U0126 or SB203580 had no effect on T cell immune function without HMGB1 stimulation. These data indicated that ERK1/2 and p38 MAPK pathways might participate in HMGB1-mediated immune dysfunction of T lymphocytes.
FIG. 6.
Effects of MAPK inhibitors on HMGB1-induced suppression of T cell immune response. Jurkat cells were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then pretreated with ERK1/2 inhibitor (5 μM U0126), p38 inhibitor (5 μM SB203580), or DMSO for 1 h before stimulation with HMGB1 (100 ng/mL, 24 h) or without HMGB1. Cells treated with PMA/ionomycin alone were used as controls. Cell proliferative activity and levels of IL-2, IL-4, as well as IFN-γ were determined by methyl-thiazolyl-tetrazolium cell proliferation assay and enzyme-linked immunosorbent assay after HMGB1 stimulation for 24 h, respectively (A–C). The mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1-treated group (P<0.05). DMSO, dimethyl sulfoxide.
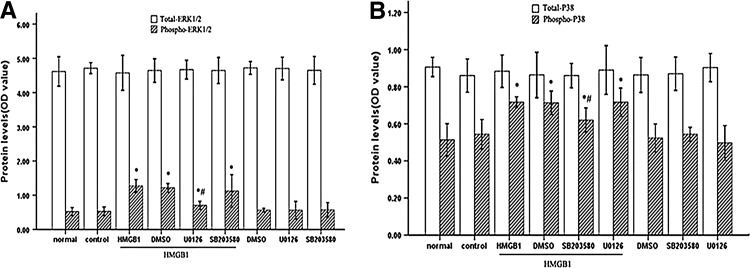
U0126 and SB203580 exerted their effect by moderately diminishing HMGB1-induced activation of ERK1/2 and p38 MAPK
It has been documented that inhibition of ERK1/2 decreased proliferation and IL-2 production of T cells (DeSilva and others 1998), and blockade of p38 MAPK interfered with Th1 cytokine production (Rincón and others 1998), we tried to evaluate ERK1/2 and p38 MAPK activation levels stimulated by HMGB1 in the presence of U0126 and SB203580 (Fig. 7). As expected, HMGB1-induced ERK1/2 activation was strongly reduced by pretreatment with U0126 (P<0.05) without being affected by SB203580, whereas SB203580 preincubation specifically interfered with phosphorylation of p38 MAPK (P<0.05) without affecting ERK1/2. Interestingly, it was of note that certain levels of ERK1/2 and p38 MAPK activation remained in cultures exposed to HMGB1 in the presence of U0126 or of SB203580 (both P<0.05). Taken together, these results implied that HMGB1-mediated T cell immunosuppression appeared to be associated with overactivation of ERK1/2 and p38 MAPK induced by HMGB1.
FIG. 7.
MAPK inhibitors exerted protective effects on HMGB1-induced suppression of T cell immune response through moderately diminishing phosphorylation of ERK1/2 and p38 MAPK. Jurkat cells were stimulated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then incubated with ERK1/2 inhibitor, p38 inhibitor, or DMSO at indicated concentrations for 1 h before stimulation with HMGB1 or without HMGB1. Cells treated with PMA/ionomycin alone were used as controls. The phosphorylation levels of ERK1/2 (A) and p38 MAPK (B) were assayed by indicated methods after HMGB1 (100 ng/mL) stimulation for 60 min (A) or 15 min (B), respectively. Mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1 treated group (P<0.05).
NFAT might be a potential target of ERK1/2 and p38 MAPK in HMGB1-mediated immunosuppression of T lymphocytes
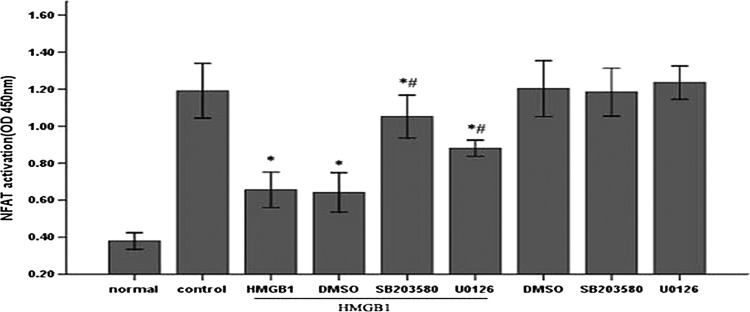
NFAT, a key transcription factor in T lymphocytes, is negatively regulated by various upstream signaling kinases such as MAPK, casein kinase 1 (CK1), and glycogen synthase kinase-3 (GSK-3) (Nel 2002). For this reason, we further examined the effect of HMGB1 on NFAT activation in the presence or absence of U0126 and SB203580. As shown in Fig. 8, HMGB1 significantly inhibited PMA/ionomycin- induced activation of NFAT (P<0.05), whereas pretreatment with U0126 and SB203580 could partially abrogate inhibitory effect of HMGB1 on NFAT activity, respectively (P<0.05). Similarly, 5 μM U0126 or SB203580 had no marked influence on NFAT activation without HMGB1 stimulation. These data indicated that NFAT might be a potential downstream transcription factor of ERK1/2 and p38 MAPK pathways in HMGB1-mediated immunosuppression of T lymphocytes.
FIG. 8.
NFAT served as a potential target of MAPK on HMGB1-mediated immunosuppression of T lymphocytes. Jurkat cells were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then pretreated with ERK1/2 inhibitor (5 μM U0126), p38 inhibitor (5 μM SB203580) or DMSO for 1 h before stimulation with HMGB1 (100 ng/mL, 24 h). Cells treated with PMA/ionomycin alone were used as controls. NFAT activity in the nuclear fraction of Jurkat cells was measured by enzyme-linked immunosorbent assay. Mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1 treated group (P<0.05). NFAT, nuclear factor of activated T cell.
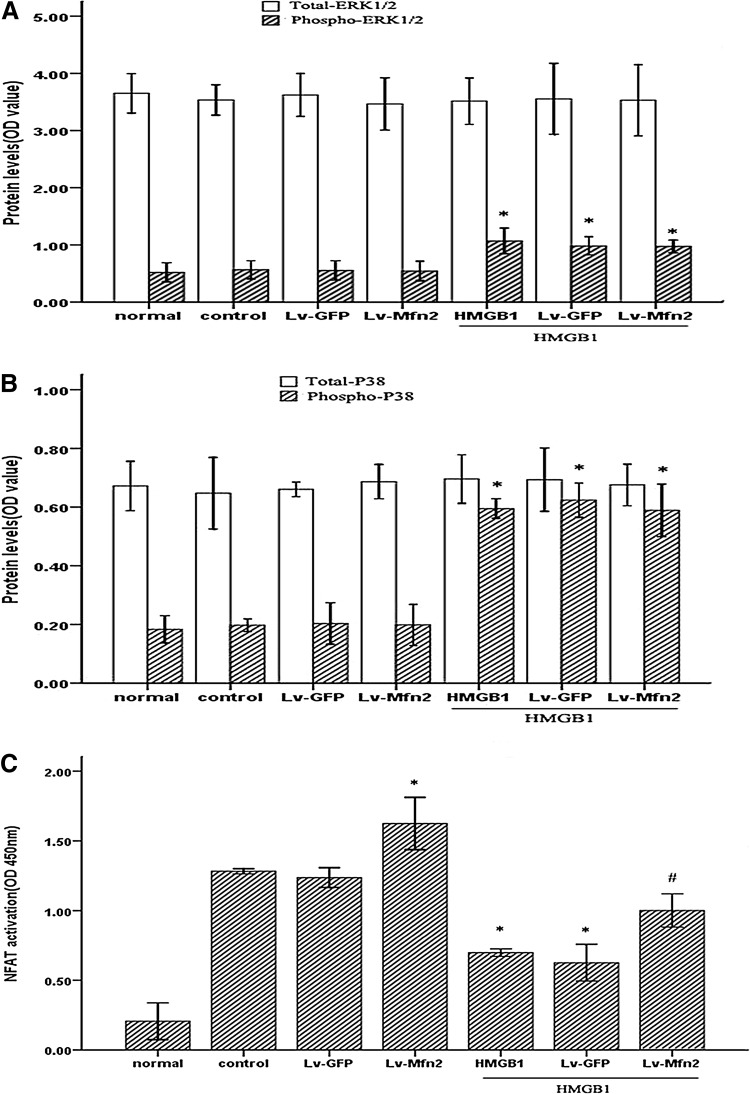
Overexpression of Mfn-2 dampened the suppression of HMGB1 on NFAT activation, but had no effect on HMGB1-induced activation of MAPK
It has been reported that Mfn-2 is able to bind to Ras and inhibit Ras-activated MAPK signaling in vascular smooth muscle cells and cancer cell lines (Chen and others 2004). Thus, hereupon we investigated the possible effect of Mfn-2 on the activation of ERK1/2 and p38 MAPK induced by HMGB1 in Jurkat cells. Unexpectedly, overexpression of Mfn-2 had no detectable effect on HMGB1-mediated activation of ERK1/2 or p38 MAPK in cultured Jurkat cells under our experimental condition (Fig. 9A, B). Next, we examined the impact of Mfn-2 on activation of NFAT in cultured cells with HMGB1 stimulation. Interestingly, as shown in Fig. 9C, upregulation of Mfn-2 expression markedly enhanced the NFAT activity of the cells with or without HMGB1 treatment (all P<0.05). Thus, these results indicated that the protective effect of Mfn-2 in HMGB1-mediated Jurkat cell immune dysfunction might be associated with other signaling pathways related to NFAT, with the exception of MAPK signal pathways.
FIG. 9.
Overexpression of Mfn-2 attenuated the suppressive effect of HMGB1 on NFAT activation, but exerted no effect on the phosphorylation of ERK1/2 and p38 MAPK induced by HMGB1 in Jurkat cells. Jurkat cells transfected with Lv-Mfn-2 or Lv-GFP (MOI=50) were incubated with PMA (50 ng/mL) plus ionomycin (1 μM) for 12 h, and then stimulated with HMGB1 (100 ng/mL) or without HMGB1. The phosphorylation levels of ERK1/2 (A) and p38 MAPK (B) were assayed by indicated methods after HMGB1 (100 ng/mL) stimulation for 60 min (A) or 15 min (B), respectively. Mean values of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). NFAT activity in the nuclear fraction of Jurkat cells measured by enzyme-linked immunosorbent assay after HMGB1 (100 ng/mL) stimulation for 24 h (C). Results of four individual experiments were shown as mean±standard deviation. *Statistically significant difference when compared with control group (P<0.05). #Statistically significant difference when compared with HMGB1-treated group (P<0.05).
Discussion
We previously reported that treatment with a high dosage of HMGB1 induced CD4+ T cell immunosuppression including inhibition of cell proliferation, IL-2 production and differentiation to Th2, and upregulation of Mfn-2, a mitochondrial sharping protein, and it could attenuate the suppressive effect of HMGB1 on T cell-mediated immune response; however, the signaling pathways involved remains to be elucidated. In the current study, we demonstrated that the immunosuppressive effects of HMGB1 on T cells in vitro were at least partly mediated by overactivating ERK1/2 as well as p38 MAPK pathways, and interfering with NFAT activation, and suppression of Mfn-2. Meanwhile, these data also showed that the protective effect of Mfn-2 on HMGB1-mediated T cell immunosuppression might be independent on MAPK signal pathways. Conclusively, we demonstrated that overactivation of MAPK and suppression of Mfn-2 expression appeared to be two independent events in HMGB1-mediated immune dysfunction of T cells. Moreover, the results provided an explanation for our previous findings that HMGB1 had a dual regulatory effect on immune functions of T cells with different concentrations and stimulation duration.
Immunosuppression of T cells following trauma or sepsis impairs cellular immune defenses, which can result in posttraumatic infectious complications and severe sepsis, which are known to be the leading causes of death in trauma patients (Oberholzer and others 2001; Patenaude and others 2005; Phan and others 2005). Although the mechanistic and molecular bases for sepsis-induced T cell immunosuppression have not yet been entirely elucidated, the main features of the condition have already been described. During sepsis, T cell proliferative responses and cytokine production (IL-2, tumor necrosis factor-α were significantly depressed, leading to an imbalance in T helper (Th) cell functions caused by a phenotypic imbalance in the regulation of Th1 and Th2 immune response (Ayala and others 1994; O'Sullivan and others 1995; Ferguson and others 1999; Heidecke and others 1999). HMGB1, an important extracellular mediator of inflammation described recently, was proven to be involved in maturation and activation of DCs (Yang and others 2007). Moreover, it had been recognized that a high level of systemic HMGB1 contributed to the impairment of cellular immune function in thermal injured animals and septic patients (Lantos and others 2010), and inhibition of HMGB1 secretion was shown to improve septic survival and T cell function (Zhang and others 2008; Lantos and others 2010). Thus, these findings support the view that HMGB1 release might be associated with the suppression of T lymphocytes after severe thermal or septic episode. Our current findings further strengthened the concept that HMGB1 had a direct influence on immune function of T lymphocytes, which might contribute to the anti-inflammatory immunosuppressive state in severe sepsis. However, when compared to our previous studies (Zhao and others 2012), it was not exhibited that a low dose of HMGB1 promoted the production of Th1 cytokines with proinflammatory properties in our experiment. The reason for this difference is unclear, but it is likely due to the distinction of cell types or experimental conditions.
Mfn-2 is an outer mitochondrial membrane protein and ubiquitously expressed. In addition to its well-established functional role in mitochondrial fusion, accumulating evidence reveals that this mitochondria-shaping protein plays a role in regulating mitochondrial metabolism, apoptosis, and even progression through cell cycle (de Brito and Scorrano 2008). Although our current and previous data have demonstrated that Mfn-2 is an important regulator of HMGB1-mediated T cell immunosuppression in vitro (Zhao and others 2012), the precise mechanisms as well as signal transduction underlying the immuno-regulatory effect of Mfn-2 on T cells remain poorly understood.
To understand the mechanisms underlying HMGB1-induced suppression of T cell-mediated immunity and the immuno-regulatory effect of Mfn-2 on T cells, we determined the effect of HMGB1 on the activation of MAPK isoforms. It was demonstrated that ERK1/2 and p38 might be the major MAPKs associated with HMGB1-mediated immunosuppressive effect on T cells, and such a conclusion was based on several independent lines of evidence. First, as it has previously been demonstrated that HMGB1 induces a transient phosphorylation of MAPK in outgrowing neurites, certain tumor cells and human microvascular endothelial cells (Huttunen and others 1999; Taguchi and others 2000; Fiuza and others 2003), our data showed that the HMGB1 treatment of T cells obviously led to the phosphorylation of both ERK1/2 and p38 MAPK, whereas the phosphorylation levels of JNK were not obviously affected. Second, the intervention of U0126 (ERK1/2-specific inhibitor) and SB203580 (p38 MAPK-specific inhibitor) could notably improve the inhibitory effect of HMGB1 on T cell-mediated immune response. More importantly, HMGB1-induced activity of ERK1/2 and p38 was not completely blocked by the presence of U0126 or of SB203580 in the present experiment. Therefore, our study provided the important information herein that HMGB1-mediated overphosphorylation of ERK1/2 and p38 might play a critical role in the suppressive effect of HMGB1 on T cell-mediated immunity. However, our result was in disagreement with previous studies which demonstrated that p38 MAPK and ERK1/2 positively regulated the differentiation of CD4+ T cells to Th1 but not Th2 (Rincón and Pedraza-Alva 2003; Dodeller and others 2005) and proliferation of T cells (DeSilva and others 1998), respectively. Further, p38 MAPK has been demonstrated to phosphorylate substrates directly, thus activating the key cell-cycle regulators p53 and p73, which subsequently lead to cell cycle arrest, apoptosis, cytokine production, regulation of RNA splicing, and cell differentiation (Bulavin and others 1999; Hildesheim and others 2002; Sanchez-Prieto and others 2002). Additionally, intense ERK activation throughout G1 leads to the accumulation of p21cip1 that inhibits cyclinE/CDK2 complexes to block S-phase entry (Chambard and others 2007). Thus, we speculate that overactivation of p38 and ERK1/2 stimulated by HMGB1 might induce T cell cycle arrest or apoptosis, which resulted in T cell-mediated immunosuppression. Nevertheless, other reasons cannot be excluded, and further investigation is required to define the apoptosis of T cells treatment with HMGB1 to support our hypothesis.
Cytokines secreted by T lymphocytes play pivotal roles in the regulation of T cell proliferation as well as differentiation. These cytokines, including IL-2, IL-4, IL-6, and IFN-γ, are regulated chiefly at the level of transcription of their genes and dependent on the activation of several obligatory transcription factors, such as NFAT, NF-κB, AP-1, and Oct-1 (Kitamura and others 2005). These factors collaborate to form a multifactor complex that binds the enhancer region in a stable manner and initiates transcription. Among them, NFAT is a major transcription factor participating in the transcriptional induction of various immune response genes in T lymphocytes. In the current experiment, our findings are in agreement with previous observation showing that HMGB1 stimulation significantly decreased the activation of NFAT during T cell activation. Of note, the data presented here indicated that pretreatment with either U0126 or SB203580 could partly abrogate inhibitory effect of HMGB1 on NFAT activation, implying that NFAT might be a potential downstream target of ERK1/2 and p38 MAPK during the suppression of T cell-mediated immune response induced by HMGB1. Besides the Ca2+/calmodulin-dependent Ser/Thr phosphatase, calcineurin can dephosphorylate the NFAT homology (NFAT-h) domain, leading to unmasking of the nuclear localization sequences and translocation of NFAT to the nucleus, and many protein kinases, including MAPK, CK1, and GSK-3, have been reported to negatively regulate NFAT activation by phosphorylating different members of NFAT at different serine residues (Beals and others 1997; Chow and others 2000). Thus, it is possible that HMGB1-induced overactivation of ERK1/2 and p38 MAPK might directly phosphorylate NFAT-h, resulting in an increase of its nuclear export. However, further studies are necessary to explore the exact mechanism of HMGB1-induced inhibition of NFAT activation, as NFAT activation is a complicated course and the NFAT family contains at least five isoforms, NFAT1 to NFAT5, and they play diverse roles in T cell-mediated immunity. For example, NFAT4 primarily regulates T cell differentiation in the thymus, whereas NFAT1 and NFAT2 are more specifically involved in regulating mature T cell activation and differentiation (Oukka and others 1998).
One of the major upstream proteins activating MAPK is the GTP-binding proteins, including the Ras family, which can be activated when the GTP-bound form is generated. Raf binds to Ras-GTP via its Ras-binding domain and is brought to the plasma membrane to be activated. Active Raf in turn phosphorylates MAPK/ERK kinase, and the latter activates ERK1/2 by tyrosine and threonine phosphorylation (Ray and Sturgill 1988; Cobb and others 1991; Huang and others 1993). Recently, Mfn-2 was reported to be an endogenous Ras inhibitor that was able to physically bind and sequester Ras, thereby inhibiting the downstream Ras signaling pathway, inactivating the ERK1/2 cascade, and eventually arresting the cell cycle in the G0/G1 phase in multiple tumor cell lines and rat vascular smooth muscle cells (Chen and others 2004). Therefore, we hypothesized that the protective effect of Mfn-2 on HMGB1-mediated impairment of T cellular immunity might be associated with its inhibitory role in MAPK activity. Unexpectedly, lentivirus-mediated overexpression of Mfn-2 had no detectable effect on either basal or HMGB1-induced activation of ERK1/2 and p38 MAPK in cultured Jurkat cells in our experimentation system. Thus, Mfn-2-mediated immune-regulating effect on T cells is unlikely attributable to blockade of Ras-Raf-ERK1/2 MAPK signaling pathway. Because the Ras–phosphatidylinositol 3-kinase–Akt pathway was also reported to be inhibited by Mfn-2 (Shen and others 2007), we speculate that this action of Mfn-2 is dependent on other Ras-dependent signals or independent on Ras-dependent signal pathways. In our current study, upregulation of Mfn-2 expression profoundly alleviated HMGB1-mediated suppressive effect on NFAT activity. Further, our previous study had demonstrated that upregulation of Mfn-2 expression in CD4+ T cells significantly attenuated the suppressive response of HMGB1 on calcium concentration and NFAT activation (Zhao and others 2012). In view of the above evidence, it might be reasonable to speculate that the protective effect of Mfn-2 on T cell-mediated immune response is primarily dependent on its protective effect on the Ca2+-NFAT signaling pathway, but not through inhibiting MAPK signaling pathway.
In conclusion, we demonstrated that HMGB1 could inhibit T cell-mediated immune response in vitro via overphosphorylation of ERK1/2 and p38 MAPK and intervention of NFAT activation. In addition, our data further strengthened the concept that downregulation of Mfn-2 and overphosphorylation of MAPK appeared to be two critical and independent events in HMGB1-mediated immunosuppression of T cells, and the protective effect of Mfn-2 on T cell immune function might be dependent on another signaling pathways related to NFAT, but not MAPK signal pathways. Obviously, further investigation is required to define the exact mechanisms underlying the immuno-regulatory effect of Mfn-2 on T lymphocyte. Thus, elucidation of the role of MAPK signaling and Mfn-2 in regulation of immune property of T lymphocytes induced by HMGB1 might provide promising strategy in the treatment of sepsis or severe trauma in future.
Acknowledgments
This work was supported, in part, by grants from the National Natural Science Foundation (81071545, 81071593, 81130035, 81121004), the National Basic Research Program of China (2012CB518102), and the Medical Key Subject (innovative research) of Zhejiang Province.
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- Ayala A. Deol ZK. Lehman DL. Herdon CD. Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-[gamma] release while increasing IL-4/IL-10 production. J Surg Res. 1994;56(6):579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- Beals CR. Sheridan CM. Turck CW. Gardner P. Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275(5308):1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Bulavin DV. Saito S. Hollander MC. Sakaguchi K. Anderson CW, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18(23):6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard JC. Lefloch R. Pouysségur J. Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773(8):1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chang L. Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen KH. Guo X. Ma D. Guo Y. Li Q, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6(9):872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- Chen RE. Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773(8):1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW. Dong C. Flavell RA. Davis RJ. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol Cell Biol. 2000;20(14):5227–5234. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH. Boulton TG. Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM. Scorrano L. Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid Redox Signal. 2008;10(3):621–633. doi: 10.1089/ars.2007.1934. [DOI] [PubMed] [Google Scholar]
- DeSilva DR. Jones EA. Favata MF. Jaffee BD. Magolda RL, et al. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160(9):4175–4181. [PubMed] [Google Scholar]
- Dodeller F. Skapenko A. Kalden JR. Lipsky PE. Schulze-Koops H. The p38 mitogen-activated protein kinase regulates effector functions of primary human CD4 T cells. Eur J Immunol. 2005;35(12):3631–3642. doi: 10.1002/eji.200535029. [DOI] [PubMed] [Google Scholar]
- Ferguson NR. Galley HF. Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999;25(1):106–109. doi: 10.1007/s001340050795. [DOI] [PubMed] [Google Scholar]
- Fiuza C. Bustin M. Talwar S. Tropea M. Gerstenberger E, et al. Inflammatory promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101(7):2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Heidecke CD. Hensler T. Weighardt H. Zantl N. Wagner H, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178(4):288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- Hildesheim J. Bulavin DV. Anver MR. Alvord WG. Hollander MC, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62(24):7305–7315. [PubMed] [Google Scholar]
- Huang W. Alessandrini A. Crews CM. Erikson RL. Raf-1 forms a stable complex with Mek1 and activates Mek1 by serine phosphorylation. Proc Natl Acad Sci U S A. 1993;90(23):10947–10951. doi: 10.1073/pnas.90.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Tang Y. Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ. Fages C. Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274(28):19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- Kerksiek KM. Pamer EG. T cell responses to bacterial infection. Curr Opin Immunol. 1999;11(4):400–405. doi: 10.1016/S0952-7915(99)80067-3. [DOI] [PubMed] [Google Scholar]
- Kitamura N. Kaminuma O. Mori A. Hashimoto T. Kitamura F, et al. Correlation between mRNA expression of Th1/Th2 cytokines and their specific transcription factors in human helper T-cell clones. Immunol Cell Biol. 2005;83(5):536–541. doi: 10.1111/j.1440-1711.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Lantos J. Földi V. Roth E. Wéber G. Bogár L, et al. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33(6):562–567. doi: 10.1097/SHK.0b013e3181cd8c88. [DOI] [PubMed] [Google Scholar]
- Nel AE. T-cell activation through the antigen receptor. Part 1: signaling components, signaling pathways, and signal integration at the T-cell antigen receptor synapse. J Allergy Clin Immunol. 2002;109(5):758–770. doi: 10.1067/mai.2002.124259. [DOI] [PubMed] [Google Scholar]
- Oberholzer A. Oberholzer C. Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16(2):83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- O'Sullivan ST. Lederer JA. Horgan AF. Chin DH. Mannick JA, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukka M. Ho IC. de la Brousse FC. Hoey T. Grusby MJ, et al. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9(3):295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- Patenaude J. D'Elia M. Hamelin C. Garrel D. Bernier J. Burn injury induces a change in T cell homeostasis affecting preferentially CD4+ T cells. J Leukoc Biol. 2005;77(2):141–150. doi: 10.1189/jlb.0703314. [DOI] [PubMed] [Google Scholar]
- Phan HH. Cho K. Nelson HA. Shin S. Jeong J, et al. Down-regulation of NF-kappaB activity associated with alteration in proliferative response in the spleen after burn injury. Shock. 2005;23(1):73–79. doi: 10.1097/01.shk.0000148052.66645.67. [DOI] [PubMed] [Google Scholar]
- Ray LB. Sturgill TW. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M. Enslen H. Raingeaud J. Recht M. Zapton T, et al. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17(10):2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M. Flavell RA. Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med. 2000;28(9):1328–1337. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- Rincón M. Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol Rev. 2003;192(1):131–142. doi: 10.1034/j.1600-065x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R. Sanchez-Arevalo VJ. Servitja JM. Gutkind JS. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene. 2002;21(6):974–979. doi: 10.1038/sj.onc.1205134. [DOI] [PubMed] [Google Scholar]
- Seger R. Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- Shen T. Zheng M. Cao C. Chen C. Tang J, et al. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282(32):23354–23361. doi: 10.1074/jbc.M702657200. [DOI] [PubMed] [Google Scholar]
- Sir O. Fazal N. Choudhry MA. Goris RJ. Gamelli RL, et al. Role of neutrophils in burn-induced microvascular injury in the intestine. Shock. 2000;14(2):113–117. doi: 10.1097/00024382-200014020-00006. [DOI] [PubMed] [Google Scholar]
- Taguchi A. Blood DC. del Toro G. Canet A. Lee DC, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Versteeg HH. Nijhuis E. van den Brink GR. Evertzen M. Pynaert GN, et al. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J. 2000;350(Pt 3):717–722. [PMC free article] [PubMed] [Google Scholar]
- Wang H. Bloom O. Zhang M. Vishnubhakat JM. Ombrellino M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Yang D. Chen Q. Yang H. Tracey KJ. Bustin M, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81(1):59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- Yao YM. Lin HY. The potential role of high mobility group box 1 protein in immune dysfunction and its regulatory mechanism after major trauma. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20(9):513–515. [PubMed] [Google Scholar]
- Zetterström CK. Bergman T. Rynnel-Dagöö B. Erlandsson Harris H. Soder O, et al. High mobility group box chromosomal protein 1 (HMGB1) is an antibacterial factor produced by the human adenoid. Pediatr Res. 2002;52(2):148–154. doi: 10.1203/00006450-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Zhang LT. Yao YM. Dong YQ. Dong N. Yu Y, et al. Relationship between high-mobility group box 1 protein release and T-cell suppression in rats after thermal injury. Shock. 2008;30(4):449–455. doi: 10.1097/SHK.0b013e3181672495. [DOI] [PubMed] [Google Scholar]
- Zhang YL. Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2(1):20–27. [PubMed] [Google Scholar]
- Zhao GJ. Yao YM. Lu ZQ. Hong GL. Zhu XM, et al. Up-regulation of mitofusin-2 protects CD4+ T cells from HMGB1-mediated immune dysfunction partly through Ca2+-NFAT signaling pathway. Cytokine. 2012;59(1):79–85. doi: 10.1016/j.cyto.2012.03.026. [DOI] [PubMed] [Google Scholar]