Abstract
Background & Aims
Interferon-γ (IFN-γ), a cytokine produced by activated natural killer cell (NK) and T lymphocytes, is an important regulator of innate and adaptive immunity during hepatitis C virus (HCV) infection. However, the cellular sources and mechanisms of IFN-γ induction in HCV-infection are not fully understood.
Methods
We cultured normal human peripheral blood mononuclear cells (PBMCs) with different populations of immune cells and JFH-1 HCV-infected Huh7.5 (JFH-1/Huh7.5) cells.
Results
We found that PBMCs produced large amounts of IFN-γ after co-culture with JFH-1/Huh7.5 cells. Using intracellular cytokine staining we confirmed that NK cells and NKT cells (to a lesser extent) were the major IFN-γ producers within PBMCs. Purified NK/NKT cells did not produce IFN-γ in response to JFH-1/Huh7.5 cells and depletion of accessory (HLA-DR+) cells prevented IFN-γ induction in PBMCs. Through selective cell depletion of dendritic cells or monocytes from PBMCs, we determined that plasmacytoid dendritic cells (pDCs) were indispensable for NK-IFN-γ induction and the presence of monocytes was needed for maximal NK-IFN-γ induction. We further revealed that NK-IFN-γ induction depended on pDC-derived IFN-α while other IFN-γ inducing cytokines, IL-12 and IL-18, played minimal roles. Close contact between JFH-1/Huh7.5 cells and NK cells was required for IFN-γ production and monocyte-derived IL-15, significantly augmented IFN-γ induction.
Conclusions
We discovered a novel mechanism where NK cells interact with pDCs and monocytes, efficiently producing IFN-γ in response to HCV-infected cells. This indicates that co-operation between NK cells and accessory cells is critical for IFN-γ production and regulators of immunity during HCV infection.
Keywords: NK, pDC, monocyte, IFN-α
Introduction
HCV infection is a major health burden, leading to severe liver diseases in millions of people worldwide [1]. Immune responses play a crucial role in virus control and affect the disease progression, although the underlying mechanism is not completely understood [2]. Recently, several studies showed that natural killer (NK) cells, an important innate immune cell population, are involved in anti-HCV immune responses in both acute and chronic hepatitis C infection [3–7]. Increased NK cell cytotoxicity against HCV-infected cells mediated by type I IFNs has been shown by several studies [8–10], yet, it is unclear whether NK cells produce immunoregulatory IFN-γ in response to HCV-infection.
The activation of NK cells is mainly regulated by the balance of inhibitory and activating signals triggered by a series of surface receptors [11], however, NK cells can also respond to signals derived from activated accessory cells [12, 13]. It has been well documented that accessory cell-derived IL-12 is crucial for NK cell-IFN-γ production in response to various TLR agonists and infectious agents; in contrast, pDC-derived IFN-α increases NK cell cytotoxicity [14]. Although previous reports show that NK cells themselves do not respond to HCV virions or HCV-infected cells [15, 16], recent reports revealed that human dendritic cells can be activated to produce IFNs in response to HCVinfected cells [17, 18]. Based on these findings, we hypothesized that human NK cells can respond to HCV-infected cells and this might be facilitated by accessory cells.
In this study, we evaluated the roles of distinct accessory cell populations involved in NK-IFN-γ induction and their interacting mechanisms in response to HCV-infected cells. For the first time we showed that NK cells produced IFN-γ in response to HCV-infected cells through a pDC- and type I IFN- dependent manner and that monocyte-derived IL-15 augmented this process. These results suggest that IFN-γ produced by NK cells could play a crucial antiviral role during HCV-infection and NK cells are part of the immune network in response to HCV infection.
Materials and methods
Cells, replicons, and viruses
Huh7.5 cells and full-length genomic replicon cells (FL) were kindly provided by Dr. C. Rice. Transfection of in vitro synthesized JFH-1 RNA constructs and production of JFH-1 virus stock were performed as previously described [19]. Detailed information and protocols are described in the Supplementary Materials and Methods section.
Preparation of human PBMCs, DC subsets and co-culture experiments
PBMCs were isolated using Ficoll-Hypaque density centrifugation. Informed consent was obtained according to procedures approved by the Committee for Protection of Human Subjects in Research at the University of Massachusetts Medical School. Detailed protocols for different cell isolation and co-culture experiments are described in the Supplementary Materials and Methods section.
Reagents, Enzyme-Linked Immunosorbent Assay, RNA Quantification, Flow Cytometry, and Statistical Analysis
See the Supplementary Materials and Methods section for more detail.
Results
Human NK cells produce IFN-γ in response to HCV-infected cells
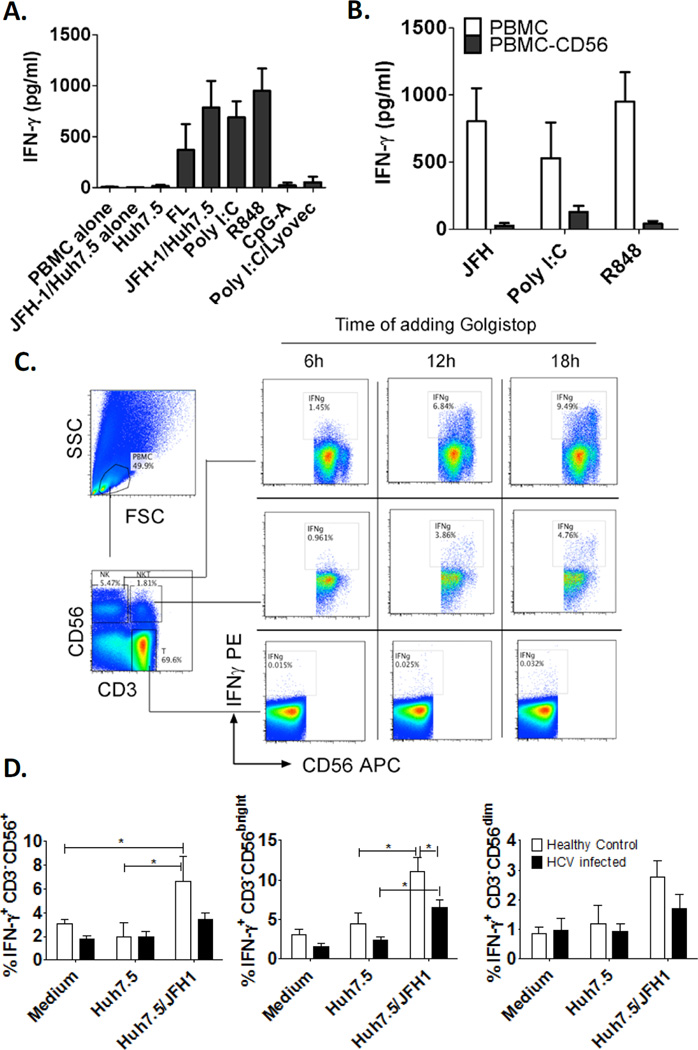
To test whether IFN-γ was induced from human immune cells in response to HCV infection, human PBMCs were co-cultured with HCV-infected Huh7.5 hepatoma cells (JFH-1/Huh7.5) or stimulated with canonical PAMPs mimicking the viral ligands. We found that large amount of IFN-γ was induced in PBMCs by HCV-containing full-length replicon cells (FL) or JFH-1/Huh7.5 cells and not by uninfected Huh7.5 cells or PBMCs alone (Fig. 1A). The TLR3 ligand, Poly I:C, and TLR7/8 ligand, R848, also stimulated IFN-γ production while TLR9 ligand, CpG-A, and RIG-I ligand, Poly I:C/lyovec, did not or only weakly induced IFN-γ production. Next, we tested whether NK cells represent IFN-γ producers in response to HCV-infected cells, Poly I:C or R848 and found that depletion of CD56+ cells (human NK and NKT marker) in PBMCs abolished IFN-γ production in all scenarios (Fig. 1B). Furthermore, using intracellular IFN-γ staining we observed 10% of NK cells (CD3−CD56+) and 5% of NKT cells (CD3+CD56+) producing IFN-γ in response to HCV-infected cells, while conventional T cells (CD3+CD56−) failed to produce IFN-γ (Fig. 1C). Poly I:C and R848 also stimulated NK and NKT cells to produce IFN-γ, and IFN-γ production was more rapidly induced in response to Poly I:C and R848 than HCV-infected cells (Supporting Fig. 1A). In addition, a small percentage of conventional T cells also responded to Poly I:C with IFN-γ production (Supporting Fig. 1B). These observations suggested different mechanisms responsible for IFN-γ production in response to different stimulations.
Figure 1. Human NK cells produce IFN-γ in response to HCV-infected hepatoma cells.
(A) Human PBMCs (4×106) were co-cultured with JFH-1/Huh7.5 cells or full-length HCV replicons (2.5×105) or stimulated with TLR3 ligand - poly I:C (20µg/mL), TLR7/8 ligand - R848 (2µg/mL), TLR9 ligand - CpG-A (2µM) or RIG-I ligand poly I:C/Lyovec (1µg/mL) for 24 hours. (B) Human PBMCs or PBMCs depleted of CD56+ cells were co-cultured with JFH-1/Huh7.5 cells or stimulated with poly I:C or R848 for 24 hours, IFN-γ production in the supernatants was measured by ELISA. (Mean±SD, n=3–6) (C) Human PBMCs were co-cultured with JFH-1/Huh7.5 cells and Golgistop was added at different timepoints afterwards. 24 hours after co-culture, intracellular IFN-γ production in NK, NKT and T cells were measured by flow cytometry. One representative data of three independent experiments was shown. (D) PBMCs and JFH-1/Huh7.5 cells were co-cultured and were treated with Golgistop after 6 hours and harvested after 24 hours to measure intracellular IFN-γ. The data is represented as Mean±SD, Healthy control, n=7; HCV-infected, n=7,*P<0.05.
Human NK cells consist of two major subsets: CD56 bright, regulatory and less cytotoxic subset, and CD56 dim, cytotoxic and less regulatory subset [22]. CD56 bright NK cells are enriched in human liver. Here, we found more IFN-γ positivity in the CD56 bright compared to the CD56 dim subset in response to HCV-infected cells suggesting that the CD56 bright subset may play a major role in IFN-γ production in HCV-infected livers (Fig. 1D and Supporting Fig. 2C, D). We also observed less IFN-γ induction by JFH-1/Huh7.5-infected hepatoma cells and in HCV-infected patients compared to healthy donors (Fig. 1D and Supporting Fig. 2 A, B).
Plasmacytoid dendritic cells are required for NK cell IFN-γ production in response to HCV-infected cells
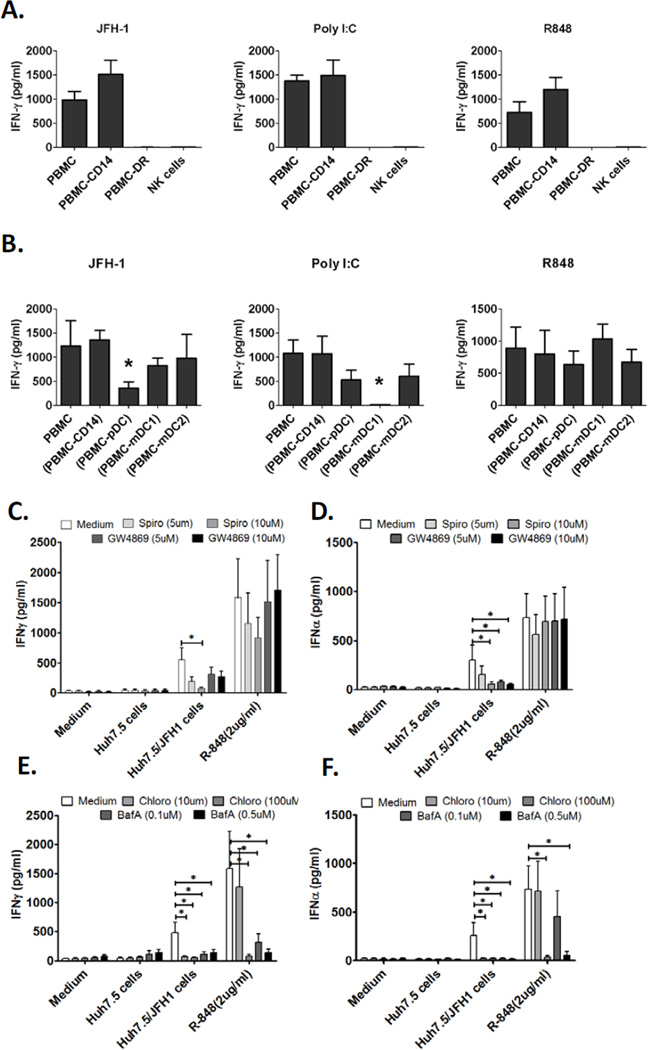
Next, we examined whether NK cells alone could produce IFN-γ in response to HCV-infected cells. We found that purified NK cells did not produce IFN-γ when co-cultured with JFH-1/Huh7.5 cells. Furthermore, depletion of accessory cells (HLA-DR+) from PBMCs also prevented IFN-γ induction by HCV-infected cells (Fig. 2A), indicating that NK-IFN-γ production in response to HCV-infected cells totally depended on accessory cells. Likewise, IFN-γ induction by Poly I:C and R848 required the presence of accessory cells (Fig. 2A).
Figure 2. Human NK cells produce IFN-γ in response to HCV-infected hepatoma cells in an accessory cell dependent manner.
(A) Human PBMCs, PBMCs depleted of CD14+ monocytes (PBMC-CD14), PBMCs depleted of HLA-DR+ cells (PBMC-DR) or purified NK cells were co-cultured with JFH-1/Huh7.5 cells or stimulated with poly I:C or R848 for 24 hours, IFN- γ production in the supernatants was measured by ELISA (Mean±SD, n=3). (B) Human PBMCs, PBMCs depleted of CD14+ monocytes (PBMC-CD14), PBMCs depleted of pDCs (PBMC-pDC), PBMCs depleted of mDC1s (PBMC mDC1) or PBMCs depleted of mDC2s (PBMC-mDC2) were co-cultured with JFH-1/Huh7.5cells or stimulated with poly I:C or R848 for 24 hours, IFN-γ production in the supernatants was measured by ELISA (Mean±SD, n=3). *P<0.05 versus other groups. (C, D) PBMCs from healthy donors were co-cultured with JFH-1/Huh7.5 cells or stimulated with R848 for 24 hours in the presence or absence of exosome inhibitors as indicated. IFN-γ and IFN-α production in the supernatants was measured by ELISA (Mean±SD, n=6, *P<0.05). (E, F) IFN-γ and IFN-α release was measured by ELISA in the presence of endosome inhibitors, chloroquine and BafilomycinA. (Mean±SD, n=6, *P<0.05).
To identify the indispensable accessory cell population(s) for NK-IFN-γ production, we depleted the distinct accessory cell subsets from PBMCs to test their distinctive roles in NK-IFN-γ induction by HCV-infected cells. We found that depletion of CD14+ monocytes, CD1c+ mDC1s or BDCA3+ mDC2s did not prevent IFN-γ induction in response to HCV-infected cells, while depletion of pDCs significantly reduced IFN-γ production (Fig. 2B), suggesting that pDCs played an essential role in NK-cell activation by HCV-infected cells. We also found that CD1c+ mDC1s and monocytes were involved in NK-IFN-γ production in response to Poly I:C and R848 stimulation, respectively (Fig.2B and Supporting Fig. 3), suggesting that a unique mechanism, different from Poly I:C and R848 induced NK activation, was involved in NK-IFN-γ induction in response to HCV-infected cells.
HCV-RNA containing exosomes from HCV-infected cells may activate pDC to produce IFN- [23]. To test whether this process was also required for NK-IFN-γ production, we studied the effects of two structurally unrelated neutral sphingomyelinase inhibitors (Spiroepoxide and GW4869) that inhibit exosome release. As shown in the Figure 2C, Spiroepoxide significantly reduced the IFN-γ secretion in the co-cultures of PBMCs with JFH-1/Huh7.5. GW4869 also lowered the IFN-γ levels but to a less extent. Both Sprioepoxide and GW4869 significantly reduced IFN-α secretion in a dose dependent manner (Figure 2D). Neither compound inhibited IFN-α nor IFN-γ production mediated by R-848.
HCV-infected cells activate pDCs through triggering TLR7 [17, 18]. Hence, we treated the co-cultures of PBMC and JFH-1/Huh7.5 with endosome acidification inhibitors, chloroquine and Bafilomycin A. As shown in Figure 2E and F, we observed that both inhibitors abolished the IFN-α and IFN-γ induction in the co-cultures or the R-848 treated PBMCs, indicating that TLR-7/8 and endosome pathways played essential roles in triggering NK-cells to produce IFN-γ.
Monocytes are required for optimal IFN-γ induction in response to HCV-infected cells
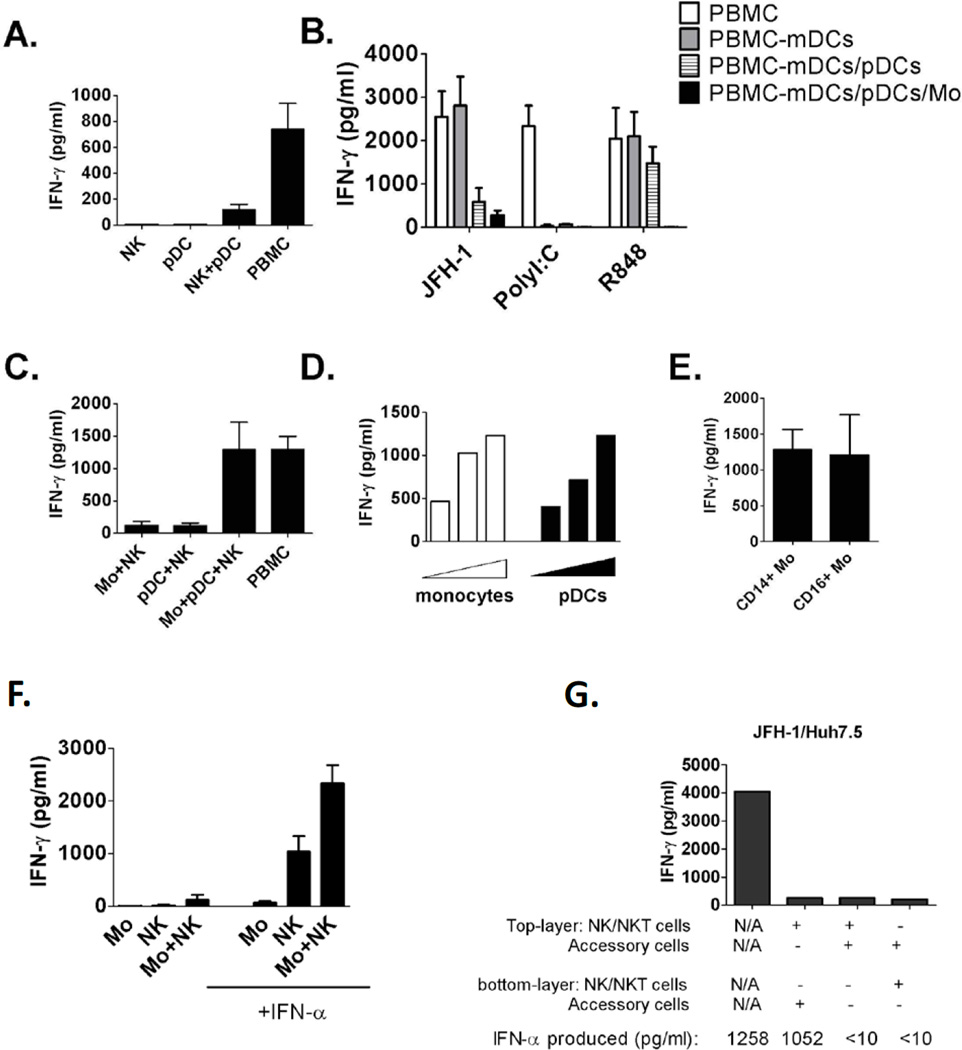
To further evaluate the requirement of HCV-induced IFN-γ induction in NK cells and the role of pDC in this process, we tested co-cultures of isolated pDCs and NK cells with HCV-infected Huh7.5 cells. However, isolated pDCs plus NK cells only produced minimal IFN-γ in response to HCV-infected cells compared to PBMCs (Fig. 3A), suggesting the necessity for other cell populations for optimal NK-IFN-γ induction. We further found that depletion of mDCs did not affect NK-IFN-γ induction; however, depletion of both pDCs and monocytes abolished NK cell-IFN-γ induction (Fig. 3B), suggesting the involvement of both pDCs and monocytes in optimal IFN-γ induction in response to HCV-infected cells. We also confirmed that mDCs and monocytes were necessary for NK-IFN-γ induction by Poly I:C and R848 respectively (Fig. 3B).
Figure 3. Both human pDC and monocytes are required for optimal IFN-γ induction in NK cells in response to HCV-infected cells.
(A) Purified human NK cells, pDCs, NKs and pDCs or PBMCs were co-cultured with JFH-1/Huh7.5cells for 24 hours. (B) Human PBMCs or PBMCs depleted of accessory cell subsets as indicated were co-cultured with JFH-1/Huh7.5cells for 24 hours. (C) Human monocytes, pDCs and NK cells were co-cultured with JFH-1/Huh7.5 cells for 24 hours. (D) Increased amounts of monocytes or pDCs were co-cultured with NKs and JFH-1/Huh7.5 cells for 24 hours. (E) Purified CD14+ or CD16+ monocytes were co-cultured with NKs and JFH1/Huh7.5cells for 24 hours. (F) Human monocytes and NK cells were co-cultured with JFH-1/Huh7.5 cells in the presence or absence of exogenous IFN-α for 24 hours. Data from (A–F) is represented as Mean±SD. (G) Accessory cells and NK cells were separated with co-cultured JFH-1/Huh7.5 cells using transwell insert as indicated, one representative data of three experiments was shown. In all experiments (A-G), IFN-γ production was measured by ELISA 24 hours after co-cultures.
To evaluate the importance of monocytes, we isolated CD14+ monocytes, pDCs and NK cells, then co-cultured them with JFH-1/Huh7.5 cells. We found that monocytes-NK or pDCs-NK co-cultures produced minimal IFN-γ, while a mixture of monocytes, pDCs and NK cells synergistically resulted in comparable level of IFN-γ as PBMCs in response to HCV-infected cells (Fig. 3C). We further showed a dose-dependent increase in NK-IFN-γ induction with increasing number of monocytes or pDCs in cocultures (Fig. 3D). Finally, we tested the role of both CD14+ monocytes and CD16+ monocytes and found that both cell populations comparably enhanced NK-IFN-γ production (Fig. 3E). Since the type I IFN was produced by pDCs in co-cultures, we examined whether the dependence on pDC could be replaced by exogenous type I IFN. We found that adding recombinant IFN-α-2a to co-cultures of NK cells and HCV-infected Huh7.5 cells induced IFN-γ production although at a relatively low level (Fig. 3F), while the presence of monocytes in co-cultures plus exogenous IFN-α synergistically increased NK-IFN-γ production (Fig. 3F). In contrast, type I IFN and monocytes did not affect Poly I:C induced NK-IFN-γ production (Supporting Fig. 4). We also determined that cell-cell contact between NK cell and HCV-infected cells was required for IFN-γ induction in co-cultures. Separation between NK cells, accessory cells, and HCV-infected Huh7.5 cells by transwell disrupted NK-IFN-γ production even in the presence of type I IFN production (Fig. 3G).
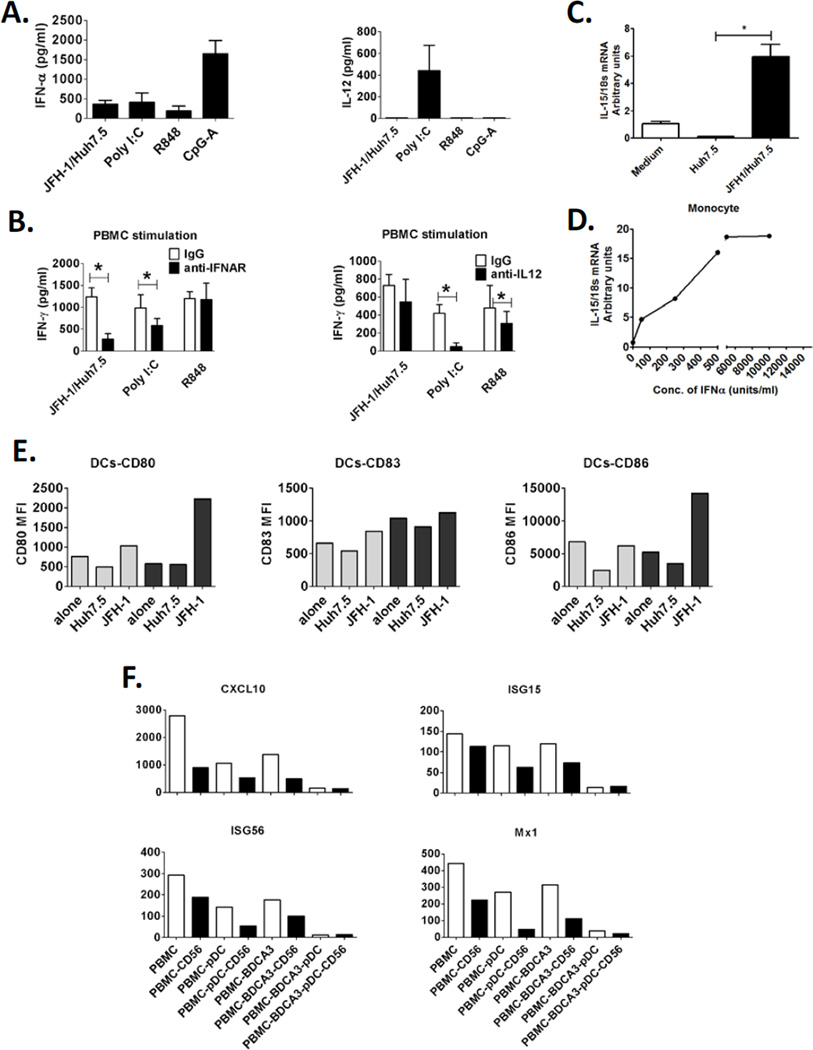
Mechanisms involved in NK-IFN-γ induction in response to HCV-infected cells To search for underlying mechanism in IFN-γ induction, we studied the potential cytokine involvement in NK-IFN-γ productions in co-cultures, including the IFN-γ inducing cytokines, IL-12 and IL-18, and the pDC specific cytokine, IFN-α. We found substantial IFN-α induction and minimal induction of IL-12 and IL-18 in co-cultures of human PBMCs and JFH-1/Huh7.5 cells (Fig. 4A and data not shown), indicating the involvement of IFN-α and not IL-12 or IL-18 in NK-IFN-γ induction in response to HCV-infected cells. We further showed that anti-IL12 blocking antibody did not affect IFN-γ induction while anti-IFNAR blocking antibody significantly reduced IFN-γ induction in the co-cultures (Fig.4B), confirming the essential role of type I IFN and not IL-12 in NK-IFN-γ induction in response to HCV-infected cells. In contrast, IL-12 was substantially induced by Poly I:C stimulation and anti-IL-12 blocking antibody completely prevented NK-IFN-γ production in response to Poly I:C, while R848 induced IFN-γ production only partially depended on IL-12 (Fig. 4B).
Figure 4. IFN-α and not IL-12 is involved in IFN-γ induction in NK cells in response to HCV-infected cells.
(A) Human PBMCs were co-cultured with JFH-1/Huh7.5cells or stimulated with poly I:C, R848 and CpG-A for 24 hours. IFN-α and IL-12 production were measured by ELISA. (B) Human PBMCs were co-cultured with JFH-1/Huh7.5cells or stimulated with poly I:C or R848 in the presence or absence of anti-IFNAR or anti-IL-12 antibody IFN-γ production was measured by ELISA. (Mean±SD, n=3), *P<0.05. (E) Human PBMCs (grey bar) or PBMCs depleted of CD56+ cells (black bar) were co-cultured with JFH1/Huh7.5 cells for 24 hours. Co-cultured PBMCs were collected and expression of co-stimulatory molecules, CD80, CD83 and CD86, on DCs (Lin1- HLA-DR+ population) were analyzed by flow cytometry. One representative data of two experiments was shown. (F) Human immune cells (white bar) or their counterparts depleted of CD56+ cells (black bar) were co-cultured with JFH-1/Huh7.5 cells for 12 hours. Co-cultured cells were collected and ISGs (CXCL10, ISG15, ISG56 and MX1) expression levels were measured by realtime PCR. One representative data of two experiments was shown.
Since cytokines, IL-12 and IL-18 were not involved in NK-IFN-γ induction by HCV-infected cells; pDCs and monocytes may assist NK activation via IL-15 that can be induced by IFN-α on antigen presenting cells and activate proximal NK cells through a trans-presentation way [24, 25]. We found here that monocytes expressed higher levels of maturation markers, CD80, CD86, and also IL-15Rα in co-cultures with HCV-infected cells, indicating that monocytes were activated in co-cultures and may use IL-15 to signal NK-IFN-γ production (Supporting Figure 5). Indeed, when we measured IL-15 transcript levels in monocytes in the presence of JFH-1/Huh7.5 or IFN-α, it was elevated in both the conditions (Fig. 4C, D)
IFN-γ produced by NK cells induces potential antiviral immune responses
Finally, we studied the antiviral functions of NK-produced IFN-γ. We found that depletion of NK/NKT cells from PBMCs not only resulted in significantly lower expression of costimulatory molecules, such as CD80 and CD86, on dendritic cells in response to HCV-infected cells (Fig. 4E), but also lead to decreased induction of ISGs expression in co-cultures, including CXCL10, ISG15, ISG56 and MX1 (Fig. 4F). Finally, consistent with previous reports, we showed that in the presence of pDCs, NK cells induced massive cell death of HCV-infected Huh7.5 cells (Supporting Fig. 6), probably through the TRAIL-apoptotic pathway. Based on these data, here we try to build a novel model reflecting the cell interaction mechanism leading to NK-IFN-γ production in response to HCV-infection, where pDC derived or exogenous IFN-α sensitized NK cells actively recognize HCV-infected hepatocytes and produce IFN-γ in response, while monocytic cells, such as monocytes or liver Kupffer cells synergistically enhance IFN-γ induction through an IL-15 mediated mechanism (Supporting Fig. 7). IFN-γ from NK cells has important immunoregulatory roles in enhancing antiviral status in HCV-infected hepatocytes and maturation of antigen presenting cell populations.
Discussion
Recent reports showed increased NK cytotoxicity induced by type I IFN pathway during HCV-infection or after IFN-α based therapy. Type I IFN activated NK cells were found to induce apoptosis of HCV-infected hepatoma cells through a TRAIL-triggered cell death pathway [3, 4, 6, 8–10]. However, it is still unclear whether another important aspect of NK cells, IFN-γ production, is induced and whether NK cell-derived cytokines play any roles in response to hepatitis C infection [26]. Here using co-cultures of human immune cells and JFH-1 infected hepatoma cells, we revealed a novel mechanism in which NK cells produced IFN-γ in response to HCV-infected cells through a pDC-type I IFN dependent mechanism. We also demonstrated that the optimal NK-IFN-γ production depended on the presence of monocytes. We further show that NK cell-derived IFN-γ had a synergistic effect in inducing interferon stimulated genes (ISGs) expression and maturation of dendritic cells (DCs) in response to HCV-infected cells. Our results strongly suggest that NK cells and IFN-γ play an active role in orchestration of innate immune activation in addition to their increased cytotoxicity during HCV-infection.
NK cell activity is regulated through two major ways: first, the balance between numerous inhibitory and activating receptors on NK cell surface and second, is the crosstalk with other cells, especially with the dendritic cells [11]. Although it is tempting to speculate that NK cells respond to HCV virions or HCV-infected cells directly, our results do not support this hypothesis. Consistently, earlier reports even showed that NK cell activity was compromised after exposure to HCV virions or HCV-infected cells [16, 18, 27]. Here, we show for the first time that NK cells respond to HCV-infected cells and produce IFN-γ requiring the presence of accessory cells. Crosstalk between NK cells and dendritic cells has been recognized in many studies, especially in response to PAMPs or infections [12]. One canonical crosstalk mechanism repeatedly corroborated in different models is that increased NK cytotoxicity depends on pDC-derived type I IFN while increased NK-IFN-γ production depends on mDC-derived IL-12 [14]. However, different from this paradigm, we show novel evidence that increased NK-IFN-γ induction by HCV-infected cells depends on pDCs and type I IFN and not on mDCs and IL-12. We found that human PBMCs produced all three types of IFNs and minimal inflammatory cytokine production, including IL-12 and IL-18. Indeed, neutralizing anti-IL-12 antibody or depletion of mDCs failed to prevent NK-IFN-γ induction in response to HCV-infected cells in our experiments, while neutralizing anti-IFNAR antibody or depletion of pDCs significantly decreased NK-IFN-γ production. While we identified a novel role of pDCs in NK-IFN-γ production, we also demonstrated that pDCs can be fully replaced by exogenous IFN-α treatment, indicating that the close contact between pDCs and NK cells was not necessary. In contrast, close contact between accessory cells and NK cells was indispensible for optimal IFN-γ induction in response to poly I:C or R848. Our finding of increased human NK-IFN-γ production by type I IFN was unexpected as exogenous IFN-α is insufficient to induce IFN-γ from NK cells even in the presence of poly I:C and R848, suggesting the existence of an unknown activating mechanism by type I IFN-activated NK cells in response to HCV-infected cells. Our results suggest that monocytes or mDCs fulfill this unique function.
In our experiments, IFN-α-treated NK cells produced only low levels of IFN-γ in response to HCV-infected cells, while optimal IFN-γ induction required both pDC-derived type I IFNs and interaction with monocytes. We discovered that monocytes are involved in maximal NK-IFN-γ induction in response to HCV virus infection. Although the exact mechanism needs to be further elucidated, monocyes expressed higher levels of maturation markers, CD80, CD86, and also IL-15Rα in co-cultures with HCV-infected cells, so they might boost NK-IFN-γ production through the IL-15 pathway. This finding has important implications during hepatitis C infection. Based on our findings, we propose a model where in the HCV-infected liver, human dendritic cells first recognize HCV-infected hepatocytes and produce type I IFNs which not only increase NK cell cytotoxicity but also sensitize IFN-γ induction in NK cells by HCV-infected cells (Supporting Fig. 7). The large numbers of NK cells and Kupffer cells in the human liver [28] allows ample interactions between IFN-α sensitized NK cells and “liver-resident macrophages”, Kupffer cells, in the local liver environment to produce IFN-γ in response to hepatitis C infection. Most likely the same mechanism will also function during IFN-α therapy for chronic-HCV infected patients and account for at least some of the anti-viral effects mediated by peg-IFN-α. The limitation of our study is that it characterized functions of circulating NK cells that should be carefully interpreted regarding the function of NK cells in the HCV infected liver.
Our novel findings reveal a sequential type I IFN and type II induction from human immune cells in response to hepatitis C infection. We propose that the subsequent NK derived type II IFN production plays a role in maintaining, amplifying and supplementing the initial type I IFN response. As reported previously, we confirmed that type I IFN and type II IFN have synergistic effects in inducing expressions of many interferon stimulating genes in HCV-infected hepatoma cells [29]. More importantly, although type I IFN generally has a stronger anti-viral effect than type II IFN, type II IFN plays an important role in regulation of immune responses [30]. We noticed that depletion of NK cells prevented maturation of dendritic cells in response to HCV-infected cells, probably through IFN-γ, recapitulating their well recognized immunoregulatory effects on antigen presenting cells.
Finally, although our results suggest that TLR3 and TLR8 ligands can partially reproduce IFN-γ induction in PBMCs seen in the presence of HCV-infected hepatoma cells, the mechanisms seem to be different from those invovled in HCV-induced NK cell activation. Our data indicate that the accessory cell requirement was different in support of a TLR3- or TLR8-induced NK-IFN-γ response where the presence of mDC1 supported poly I:C-induced and monocytes supported the R848-induced IFN-γ productiont, while pDC derived IFN- and monocytes together were required for HCV-induced IFN-γ production. However, it remains to be determined whether the combination of these or related events contributes to IFN-γ induction by HCV-infected cells in PBMCs.
In conclusion, we show a unique mechanism that human NK cells actively produce IFN-γ in response to HCV-infected cells through a type I IFN and monocytes dependent pathway. Our results suggest that in addition to cytotoxicity, NK cells are actively involved in anti-HCV immunity during hepatitis C infection via production of IFN-γ.
Supplementary Material
Acknowledgements
This work was supported by NIH grant R37AA014372 (GS). The authors thank Drs Charles M.Rice, Takaji Wakita, Christoph Seeger for kindly providing reagents.
Abbreviations used in this paper
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- HCV
hepatitis C virus
- IFN
interferon
- ISG
interferon stimulated gene
- mDC
myeloid dendritic cell
- NK
natural killer
- NKT
natural killer T
- PAMPs
pathogen associated molecular patterns
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- pDC
plasmacytoid dendritic cell
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Thomas D, Zoulim F. New challenges in viral hepatitis. Gut. 2012;61(Suppl 1):i1–i5. doi: 10.1136/gutjnl-2012-302122. [DOI] [PubMed] [Google Scholar]
- 2.Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–1239. 1239, e1231–e1232. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlich B, Ahlenstiel G, Azpiroz AZ, Stoltzfus J, Noureddin M, Serti E, et al. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39–48. doi: 10.1002/hep.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. 1160, e1151–e1157. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Dessouki O, Kamiya Y, Nagahama H, Tanaka M, Suzu S, Sasaki Y, et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: Reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331–337. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. e321–e322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelletier S, Drouin C, Bedard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreira da Silva R, Munz C. Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals. Cell Mol Life Sci. 2011;68:3505–3518. doi: 10.1007/s00018-011-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 14.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JC, Lim JB, Park JH, Lee JM. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85:12557–12569. doi: 10.1128/JVI.00838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Kodys K, Babcock GJ, Szabo G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of HCV-infected cells and induction of IFNalpha. Hepatology. 2012 doi: 10.1002/hep.25827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S, Kodys K, Szabo G. Impaired expression and function of toll-like receptor 7 in hepatitis C virus infection in human hepatoma cells. Hepatology. 2010;51:35–42. doi: 10.1002/hep.23256. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in HCV-infected hepatocytes depends on TLR3 sensing of HCV dsRNA intermediates. Hepatology. 2011;55:666–675. doi: 10.1002/hep.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaji K, Nabeshima S, Murata M, Chong Y, Furusyo N, Ikematsu H, et al. Interferon-alpha/beta upregulate IL-15 expression in vitro and in vivo: analysis in human hepatocellular carcinoma cell lines and in chronic hepatitis C patients during interferon-alpha/beta treatment. Cancer Immunol Immunother. 2006;55:394–403. doi: 10.1007/s00262-005-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoukry NH, Pelletier S, Chang KM. A view to natural killer cells in hepatitis C. Gastroenterology. 2011;141:1144–1148. doi: 10.1053/j.gastro.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183–190. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 29.Okuse C, Rinaudo JA, Farrar K, Wells F, Korba BE. Enhancement of antiviral activity against hepatitis C virus in vitro by interferon combination therapy. Antiviral Res. 2005;65:23–34. doi: 10.1016/j.antiviral.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.