Abstract
Objective
To characterize relationships associated with adverse endometrial development in patients undergoing IUI for unexplained infertility.
Design
A retrospective review of 2,929 patients from 2004–2011.
Setting
Large metropolitan infertility practice.
Patient(s)
Patients with unexplained infertility undergoing first IUI cycle at age less than 43 years, with a total motile sperm count ≥8 million.
Intervention(s)
Clomiphene citrate (CC) with FSH stimulation followed by IUI.
Main Outcome Measure(s)
Endometrial thickness, serum E2 (in picograms per milliliter) levels on the day of hCG trigger administration, body mass index (BMI) (in kilograms per meter squared), total motile sperm, follicle number, and clinical pregnancy.
Result(s)
Of the 2,929 patients who met the inclusion criteria, 466 (15.9 %) achieved a clinical pregnancy. Pregnancy rates (PRs) increased significantly with increasing endometrial thickness on the day of hCG administration and with increasing serum E2 level, but were not significantly related to age, BMI, or follicle numbers according to multiple logistic regression modeling. Peak endometrial thickness declined with age and increasing E2 levels. The BMI was associated with thicker endometrium, but it was also associated with lower peak E2 levels.
Conclusion(s)
The impact of “endometrial factor” infertility may be underappreciated in IUI therapy. Targeted therapies to optimize the endometrium represent an important new area to improve in current fertility success rates.
Keywords: Endometrium, intrauterine insemination, assisted reproductive technologies
During the past 40 years, there has been a steady increase in fertility treatment success rates due to improvements such as embryo culture techniques and intracytoplasmic sperm injection (ICSI). Historically, when assisted reproductive technology (ART) fails, the assumption has been that the embryo was nonviable. However, there is increasing awareness that implantation failure of otherwise viable embryos may be responsible for a significant portion of IVF failures. New clinical treatments, such as therapeutic endometrial biopsy/injury, have gained recent attention to correct the so-called endometrial factor infertility (1, 2). However, endometrial development during IUI cycles has been less studied.
One of the strongest predictors of implantation is endometrial thickness. A number of reports have shown that embryo implantation and clinical pregnancy rates (PRs) are significantly higher in patients with an endometrial thickness >9 mm (3–5). Thin endometria, generally measuring <7 mm, are thought to be less able to support implantation and pregnancy (6).
The thickness of the endometrium is dependent on several influences including reproductive age, phase of menstrual cycle, ovarian hormone (estrogen [E2] and progesterone [P]) concentration, and endometrial hormone receptor density (7, 8). Infertility diagnoses, such as polycystic ovarian syndrome (PCOS), endometriosis, and recurrent pregnancy loss, have been associated with thin endometria and lower PRs (9). Available treatments for thin, unresponsive endometrium are limited and largely empiric or experimental including high doses of E, hCG, piroxicam, and granulocyte colony stimulating factor (10). Treatments, such as acetylsalicyclic acid, have also been proposed. The results of this intervention are mixed (11–13). Most of these methods are proposed to function in a similar manner, by increasing blood flow to the endometrium and allowing for its thickening and development. Vaginal sildenafil citrate (Viagra; Pfizer) is another treatment that has been suggested for its ability to relax vascular smooth muscle through a cyclic guanosine monophosphate-mediated pathway, and improve uterine artery blood flow (14).
However, the impact of endometrial thickness on PRs has not been studied extensively in an unexplained infertile population of patients undergoing IUI. Here we characterize the endometrial characteristics of a large unexplained infertile population undergoing IUI to explore the link between endometrial thickness and PRs, and to improve the understanding of the influences affecting endometrial development during IUI cycles.
MATERIALS AND METHODS
In this retrospective analysis, we identified 2,929 initial, consecutive, completed IUI cycles from 2004 through 2011 at a large private infertility practice for which we had complete clinical pregnancy outcomes. All consecutive patients less than 43 years of age with a diagnosis of unexplained infertility and undergoing their first cycle of clomiphene citrate (CC)/FSH IUI with more than 8 million total motile sperm were included in this retrospective review under an approved Institutional Review Board protocol. Those with total motile sperm <8 million were excluded to minimize the effect of male factor infertility on clinical PRs, as this threshold has previously been shown to be associated with PRs in our practice (15). Typically, patients received 100 mg of CC on menstrual cycle days 3–7, followed by 150 U of FSH on cycle day 9. Ultrasound and blood monitoring of E2 were performed on cycle day 13. Once follicles reached 18 mm, trigger was induced with 10,000 U of SC hCG.
A series of univariate and multiple logistic regression analyses were conducted to clarify the complex relationships among patient age, body mass index (BMI, in kilograms per meter squared), trigger day follicle number (≥14 mm), trigger day serum E2 concentration (in picograms per milliliter), and trigger day endometrial thickness (in millimeters). In these analyses, all other variables were considered to be potentially dependent in relation to patient age. The BMI was treated as an independent variable in relation to trigger day follicle number, serum E2, and endometrial thickness. Serum E2 was treated as a dependent variable in relation to follicle numbers, as it is the maturing follicles that are the source of E2 production. Endometrial thickness was considered to be potentially dependent in relation to E2 concentration.
The relationships between endometrial thickness on the day of hCG trigger, as well as the potentially confounding variables, and clinical pregnancy (defined as ultrasound identification of a gestational sac) were evaluated by univariate logistic regression analyses. Ectopic pregnancies (EPs) were included as a negative clinical pregnancy. The independent association between endometrial thickness and clinical pregnancy, after adjusting for potential confounding variables, was investigated using multiple logistic regression analysis.
RESULTS
A total of 2,929 eligible treatment cycles were available for analysis. Mean demographic characteristics and treatment cycle outcomes are summarized in Table 1. Sixteen percent of treatment cycles resulted in a clinical pregnancy.
TABLE 1.
Mean (SD) patient and cycle characteristics.
| Subjects (patients) | 2,929 |
| Female age (y) | 34.8 ± 3.9 |
| Female BMI | 24.5 ± 5.0 |
| Endometrial thickness at trigger | 8.8 ± 2.2 |
| Serum E2 at trigger | 701.0 ± 393.0 |
| Follicles ≥14 mm at trigger | 2.6 ± 1.3 |
| Insemination total motile sperm (millions) | 24.1 ± 18.7 |
| Clinical pregnancy | 466 (15.9%) |
| Multiple pregnancy | 85 (18.2%) |
| Twin pregnancies | 74 (15.9%) |
| Triplet pregnancies | 11 (2.4%) |
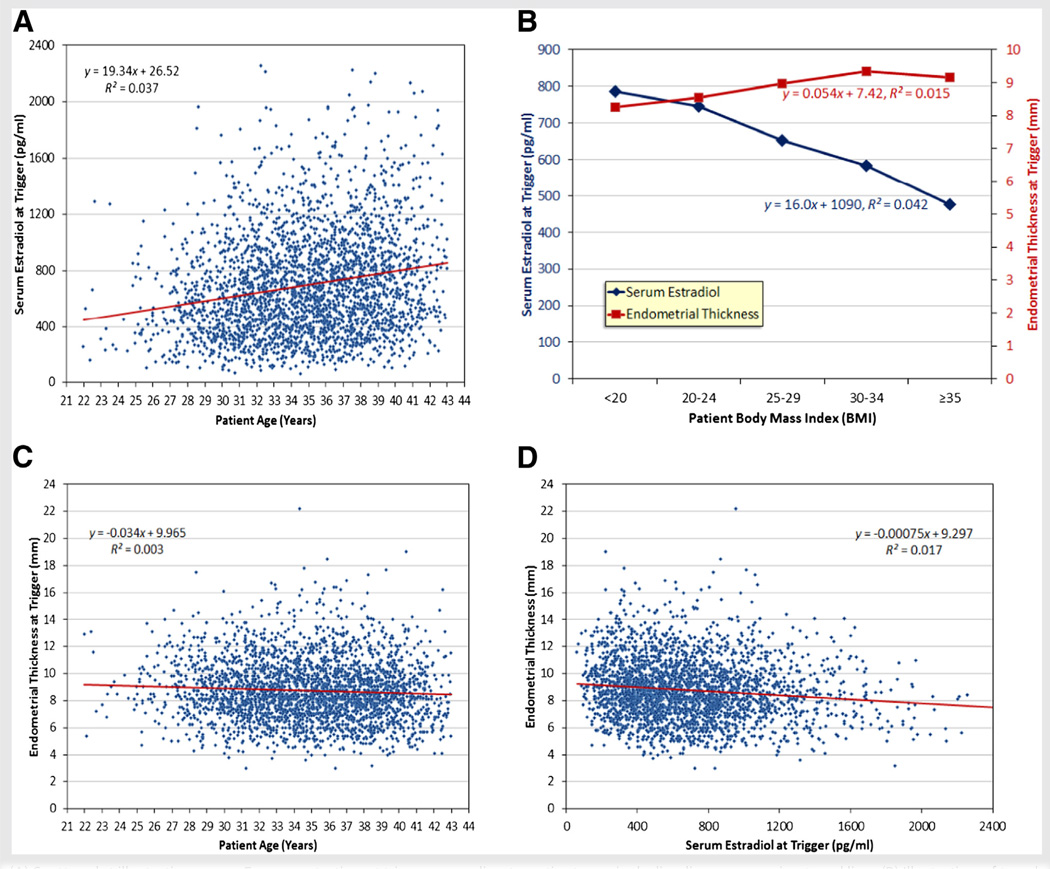
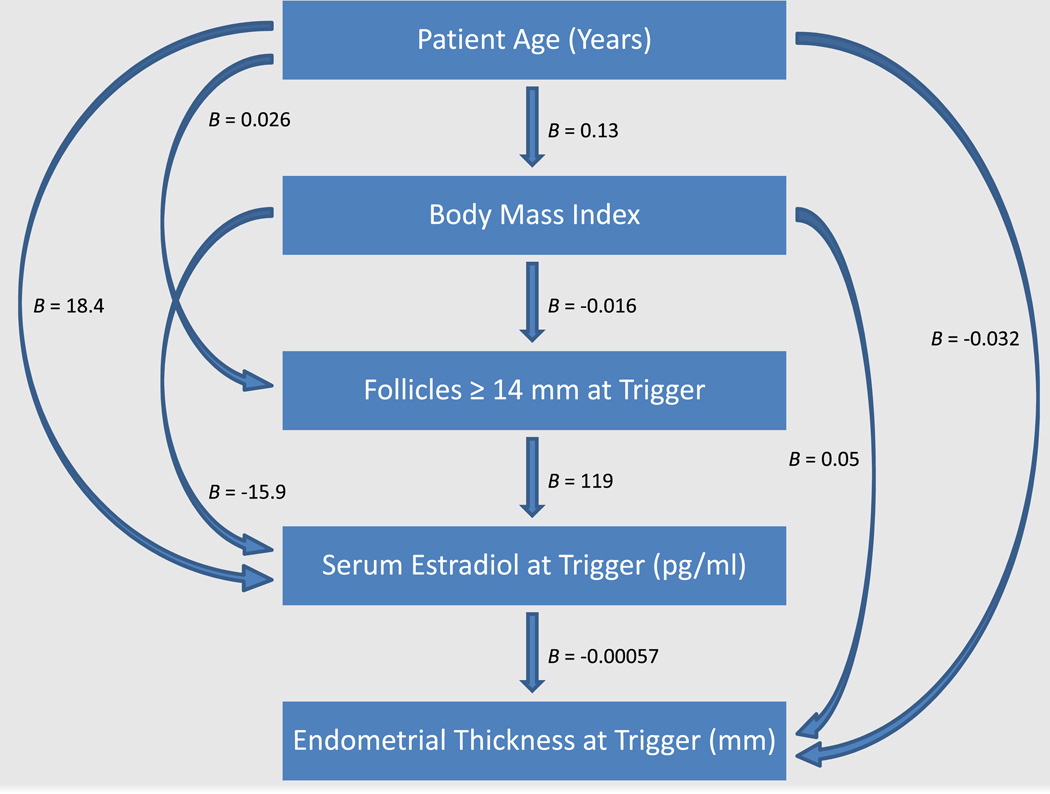
Figure 1 illustrates a flow diagram indicating all of the statistically significant independent associations among patient age, BMI, follicle number, serum E2 concentration, and endometrial thickness as determined by multiple regression analysis adjusting for other correlated variables. Unadjusted univariate relationships between selected pairs of these variables are illustrated in Figure 2. Age, BMI, and follicle numbers each contributed independently to serum E2 concentrations (Fig. 1; model R2 = 0.24). Serum E2 concentrations at trigger increased by approximately 18 pg/mL per year of age (P<.0001; Fig. 1; unadjusted univariate relationship illustrated in Fig. 2A), decreased by approximately 16 pg/mL per unit increase in BMI (P<.0001; Fig. 1; unadjusted univariate relationship illustrated in Fig. 2B), and increased by approximately 119 pg/mL per additional mature follicle (P<.0001; Fig. 1). Age, BMI, and serum E2 were each independently associated with endometrial thickness (Fig. 1; model R2 = 0.03). Endometrial thickness decreased by approximately 0.032 mm per year of age (P=.004; Fig. 1; unadjusted univariate relationship illustrated in Fig. 2C), increased by approximately 0.05 mm per unit increase in BMI (P<.0001; Fig. 1; unadjusted univariate relationship illustrated in Fig. 2B), and decreased by approximately 0.057 mm per 100 pg/mL increase in E2 (P<.0001; Fig. 1; unadjusted univariate relationship illustrated in Fig. 2D).
FIGURE 1.
(A) Scatter plot illustrating serum E2 concentration at trigger according to patient age, including linear regression trend line. (B) Illustration of trends in serum E2 concentration and endometrial thickness according to body mass index (BMI) categorization (<20 = underweight; 20–24 = normal weight; 25–29 = overweight; 30–34 = obese; >35 = very obese), with linear regression models indicated. (C) Scatter plot illustrating endometrial thickness at trigger according to patient age, including linear regression trend line. (D) Scatter plot illustrating endometrial thickness at trigger according to serum E2 concentration, including linear regression trend line.
FIGURE 2.
Diagram of patterns of association among age, body mass index (BMI), mature follicles, serum E2, and endometrial thickness. Arrows indicate statistically significant associations between independent and dependent variables. Partial regression coefficients were derived from multiple regression models and indicate estimated unit changes in dependent variables per unit change in independent variables.
Not unexpectedly, there was modest and weak but statistically significant increase in BMI with increasing age. A 10-year increase in age was associated with an approximate increase of 1.3 BMI units (P<.0001; R2 = 0.01; Fig. 1). Age and BMI were also independently associated with mature follicle numbers at trigger, albeit only very weakly (model R2 = 0.009), with follicle numbers increasing by approximately 0.26 per 10-year increase in age (P<.0001; Fig. 1), whereas decreasing by approximately 0.16 per 10 unit increase in BMI (P=.0009; Fig. 1).
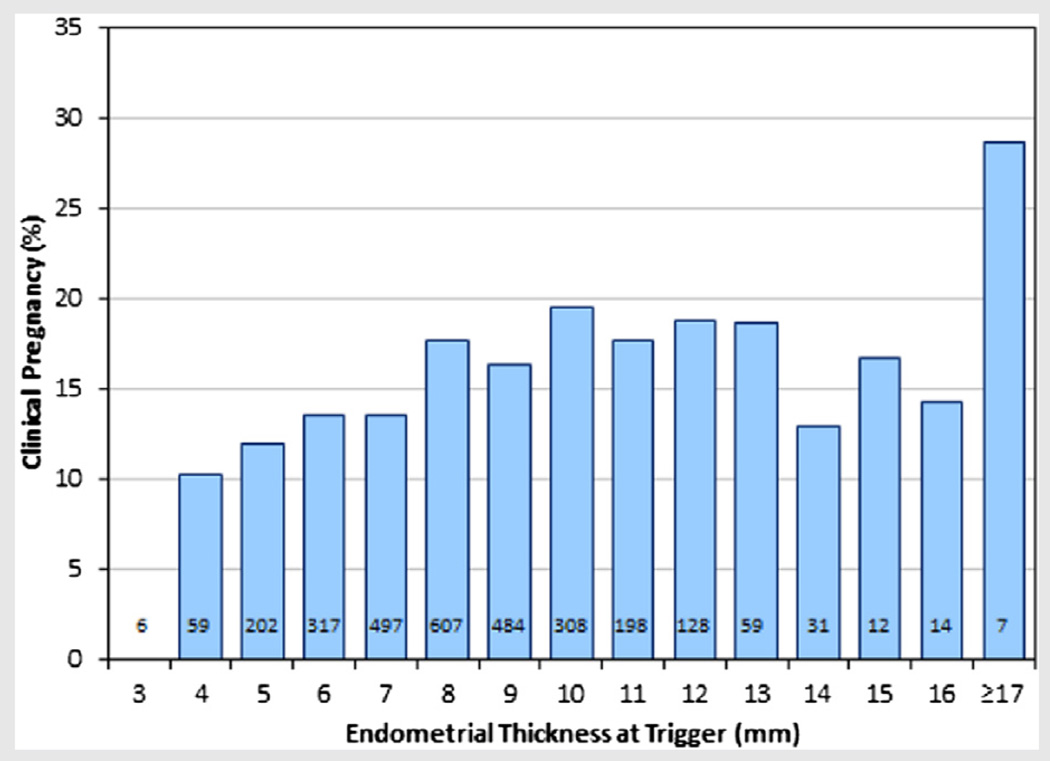
By univariate logistic regression analysis, endometrial thickness was significantly predictive of clinical pregnancy (P=.004). As illustrated in Figure 3, PRs increase gradually with increasing endometrial thickness through 10 mm. In the present study there were no pregnancies among cycles with an endometrial thickness of less than 4 mm.
FIGURE 3.
Illustration of clinical pregnancy rates (PR) according to endometrial thickness at trigger, with sample sizes indicated.
Univariate logistic regression analyses also indicated that clinical PRs were positively associated with both serum E2 (rising from 9.5% with serum E2 <200 pg/mL to 17.5% with serum E2 ≥500 g/mL; P=.027) and follicle numbers (rising from 12% with one mature follicle to 19% with four or more mature follicles; P=.018). However, clinical pregnancy was not significantly associated with patient age (P=.36), BMI (P=.16), or total motile sperm (P=.24) according to univariate logistic regression analyses.
In a multiple logistic regression model (P=.0013, R2 = 0.0086) adjusting for age (P=.26), BMI (P=.087), serum E2 (P=.0027), and follicles ≥14 mm (P=.18), the association between endometrial thickness and pregnancy remained significant (P=.0034). Total motile sperm was not correlated with endometrial thickness after adjusting for other variables (P=.34) and was not included in this multivariate model.
The only variable that was predictive of multiple pregnancy was the number of mature follicles (P=.004). Only 7% of all clinical pregnancies were multiples when only one mature follicle was noted on the day of trigger, but multiple PRs increased gradually with each additional mature follicle (Supplemental Fig. 1, available online). Most triplet pregnancies occurred when there were four or more follicles. None of the other variables evaluated (age, BMI, serum E2, endometrial thickness, or total motile sperm) approached statistical significance in relation to multiple pregnancy (P>.3 for all) in either univariate analyses or multivariate analysis adjusting for follicle numbers.
DISCUSSION
Achieving adequate endometrial thickness is widely considered an important element for success of infertility treatments. The thickness and sonographic pattern of the endometrium has been shown to impact implantation, and ultimately, clinical PRs (16, 17). It has been suggested that there may be an “all-or-none” phenomenon of endometrial receptivity, supported by elective single ET studies noting that increasing the number of embryos transferred only increases the multiple gestation rate, yet not increasing the overall PR (18).
Numerous studies have published results affirming a positive association between endometrial thickness and PRs in IVF populations (3–5, 8, 19). Here we provide evidence to conclude that endometrial thickness is also a significant predictor of clinical pregnancy in IUI cycles. Pregnancy rates were found to increase gradually with increasing endometrial thickness through 10 mm, beyond which there was a plateau in this rate. Adverse outcomes were not seen for an endometrium that measured more than 14mm (Fig. 3), as previously reported (20, 21). Despite widely contrasting results published for PRs among cycles with an endometrial thickness ≥14 mm (22), our data provide further evidence of nondetrimental effects of very thick endometrial linings (>14 mm) for IUI cycles.
Although endometrial thickness is significantly associated with pregnancy, we did observe that PRs were still more than 10%, even among the cycles with thin endometria (4–7 mm), supporting the notion that a thin endometrium can still support implantation and pregnancy. In this study there were no pregnancies among cycles with an endometrial thickness of less than 4 mm, although our sample included only six such cycles and therefore we cannot conclude that pregnancy is not possible even among patients with these thinnest linings. We have in fact noted successful pregnancies and births resulting from IUI in patients with endometrial thickness less than 4 mm among our patients not meeting the inclusion criteria for this particular study.
Consistent with previous reports, BMI was correlated with increased endometrial thickness (23). Overproliferation of the endometrium leading to atypical hyperplasia and cancer is widely accepted as a risk of obesity; however, the mechanism is typically thought to be excess E2 (24). Generally, obesity is thought of as a hyperestrogenic state, and therefore the observed endometrial responses (increase thickness and endometrial hyperplasia or cancer) could be due to this estrogenic effect. However, it somewhat counterintuitive that increasing BMI was associated with decreasing serum E2 concentrations in our study. The increased risk of endometrial hyperplasia and cancer is classically thought to be due increased E2 effect in obesity due to peripheral conversion of androgens. However, these results are consistent with previous groups who have shown that serum E2 concentrations negatively correlate with BMI (23, 25). This relationship warrants further investigation to examine the E2 independent proliferative effect of obesity, and could suggest that in obese women, endometrial growth may be more strongly influenced by influences other than peak serum E2.
Age was associated with surprising findings in this cohort: increasing E2 level at trigger, increasing numbers of mature follicles at trigger, and lack of correlation with clinical pregnancy. A higher number of mature follicles (and therefore E2 levels) would likely be tolerated during IUI stimulation in an older patient, who generally has a lower risk for high-order multiples with similar number of mature follicles compared with younger patients. Furthermore, the difference in E2 level and mature follicle number with age could be attributed to cancellation due to over-response in younger patients and increased likelihood of cancellation of poor response in older patients, skewing this comparison. This clinical management of pushing older patients to have more follicles may also explain why PRs are maintained despite increasing age.
Alternately, similar PRs across groups could suggest a common source of age-independent infertility (i.e., not age-related decline in oocyte quality) common to an unexplained infertility cohort such as fertilization defects or implantation abnormalities. Other possibilities include underlying etiologies of infertility readily amenable to clomid/IUI therapy, and these good prognosis patients are successfully treated in their first cycle of IUI at similar rates between younger and older women. Previous studies have shown that age is significantly associated with PR after IUI in a general infertility population (26, 27). One study found an inverse association between age and PRs in an unexplained population undergoing IUI cycles, but this study was limited by a smaller sample size, the inclusion of multiple IUI cycles for each patient, and a limitation to age less than 35 years old (28). One strength of our study was the inclusion of only the first IUI cycle for each patient with unexplained infertility through age 42 years, which has not been extensively studied previously. It is possible that age may not be as critical for IUI outcome in an unexplained infertility population, as women with diminished ovarian reserve were excluded in this analysis.
Here we present a comprehensive evaluation of a large unexplained infertility population to synthesize a new understanding of the complex interactions affecting endometrial development and treatment success. We provide a model to help clinicians understand normative data in patients undergoing IUI. The strengths of our study include the large population studied, and the use of an unexplained infertility population to elucidate aspects of putative endometrial factor infertility. The limitations of our study include lack of a prospective design, instead relying on a retrospective analysis.
In conclusion, the influence of the endometrium on fertility treatment success may be under-recognized. Here we further characterize relationships influencing endometrial development in a large unexplained infertility cohort undergoing IUI. Our findings demonstrate that PRs increase as endometrial thickness increased from 4–10 mm beyond which there was a plateau in the rate. It is possible that future therapies might optimize endometrial receptivity to improve on current fertility success rates.
Supplementary Material
Acknowledgments
Supported, in part, by the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/wolffef-endometrium-intrauterine-insemination-art/
E.F.W. has nothing to disclose. N.V. has nothing to disclose. C.A. has nothing to disclose. K.R. has nothing to disclose. E.W. holds stock in Natera Inc. and Counsyl Inc.
REFERENCES
- 1.Raziel A, Schachter M, Strassburger D, Bern O, Ron-El R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil Steril. 2007;87:198–201. doi: 10.1016/j.fertnstert.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 2.Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril. 2010;94:2030–2036. doi: 10.1016/j.fertnstert.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter K, Bugge K, Bromer J, Levy M. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril. 2007;87:53–59. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 4.Noyes N, Liu HC, Sultan K, Schattman G, Rosenwaks Z. Endometrial thickness appears to be a significant factor in embryo implantation in in-vitro fertilization. Hum Reprod. 1995;10:919–922. doi: 10.1093/oxfordjournals.humrep.a136061. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18:2337–2341. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs JD, Wells CS, Williams DB, Odem RR, Gast MJ, Strickler RC. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril. 1996;65:262–266. doi: 10.1016/s0015-0282(16)58082-0. [DOI] [PubMed] [Google Scholar]
- 7.Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril. 2011;96:530–535. doi: 10.1016/j.fertnstert.2011.07.1097. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Chen CH, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:336–340. doi: 10.1016/j.fertnstert.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Bromer J, Aldad T, Taylor H. Defining the proliferative phase endometrial defect. Fertil Steril. 2009;91:698–704. doi: 10.1016/j.fertnstert.2007.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95:2123.e13–2123.e17. doi: 10.1016/j.fertnstert.2011.01.143. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh YY, Tsai HD, Chang CC, Lo HY, Chen CL. Low-dose aspirin for infertile women with thin endometrium receiving intrauterine insemination: a prospective, randomized study. J Assist Reprod Genet. 2000;17:174–177. doi: 10.1023/A:1009474307376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Päkkilä M, Räsänen J, Heinonen S, Tinkanen H, Tuomivaara L, Mäkikallio K, et al. Low-dose aspirin does not improve ovarian responsiveness or pregnancy rate in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Hum Reprod. 2005;20:2211–2214. doi: 10.1093/humrep/dei020. [DOI] [PubMed] [Google Scholar]
- 13.Hurst BS, Bhojwani JT, Marshburn PB, Papadakis MA, Loeb TA, Matthews ML. Low-dose aspirin does not improve ovarian stimulation, endometrial response, or pregnancy rates for in vitro fertilization. J Exp Clin Assist Reprod. 2005;2:8. doi: 10.1186/1743-1050-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sher G, Fisch JD. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum Reprod. 2000;15:806–809. doi: 10.1093/humrep/15.4.806. [DOI] [PubMed] [Google Scholar]
- 15.Biggs JRK, Osheroff J, Widra EA. Relationship between semen parameters and outcome of intrauterine insemination (IUI) Fertil Steril. 2010;94:S236. [Google Scholar]
- 16.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97:1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Gonen Y, Casper RF, Jacobson W, Blankier J. Endometrial thickness and growth during ovarian stimulation: a possible predictor of implantation in in vitro fertilization. Fertil Steril. 1989;52:446–450. doi: 10.1016/s0015-0282(16)60916-0. [DOI] [PubMed] [Google Scholar]
- 18.Stillman R, Richter K, Banks N, Graham J. Elective single embryo transfer: a 6-year progressive implementation of 784 single blastocyst transfers and the influence of payment method on patient choice. Fertil Steril. 2009;92:1895–1906. doi: 10.1016/j.fertnstert.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ghamdi A, Coskun S, Al-Hassan S, Al-Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37. doi: 10.1186/1477-7827-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissman A, Gotlieb L, Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril. 1999;71:147–149. doi: 10.1016/s0015-0282(98)00413-0. [DOI] [PubMed] [Google Scholar]
- 21.Rashidi BH, Sadeghi M, Jafarabadi M, Tehrani Nejad ES. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol. 2005;120:179–184. doi: 10.1016/j.ejogrb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Dietterich C, Check JH, Choe JK, Nazari A, Lurie D. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertil Steril. 2002;77:781–786. doi: 10.1016/s0015-0282(01)03276-9. [DOI] [PubMed] [Google Scholar]
- 23.Souter I, Baltagi LM, Kuleta D, Meeker JD, Petrozza JC. Women, weight, and fertility: the effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertil Steril. 2011;95:1042–1047. doi: 10.1016/j.fertnstert.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 24.Antunes CM, Strolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, et al. Endometrial cancer and estrogen use. Report of a large case-control study. N Engl J Med. 1979;300:9–13. doi: 10.1056/NEJM197901043000103. [DOI] [PubMed] [Google Scholar]
- 25.Dodson WC, Kunselman AR, Legro RS. Association of obesity with treatment outcomes in ovulatory infertile women undergoing superovulation and intrauterine insemination. Fertil Steril. 2006;86:642–646. doi: 10.1016/j.fertnstert.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93:79–88. doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 27.Esmailzadeh S, Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil Steril. 2007;88:432–437. doi: 10.1016/j.fertnstert.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Demir B, Dilbaz B, Cinar O, Karadag B, Tasci Y, Kocak M, et al. Factors affecting pregnancy outcome of intrauterine insemination cycles in couples with favourable female characteristics. J Obstet Gynaecol. 2011;31:420–423. doi: 10.3109/01443615.2011.569780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.